Interactions between Layered Double Hydroxide Nanoparticles and Egg Yolk Lecithin Liposome Membranes
Abstract
1. Introduction
2. Results and Discussion
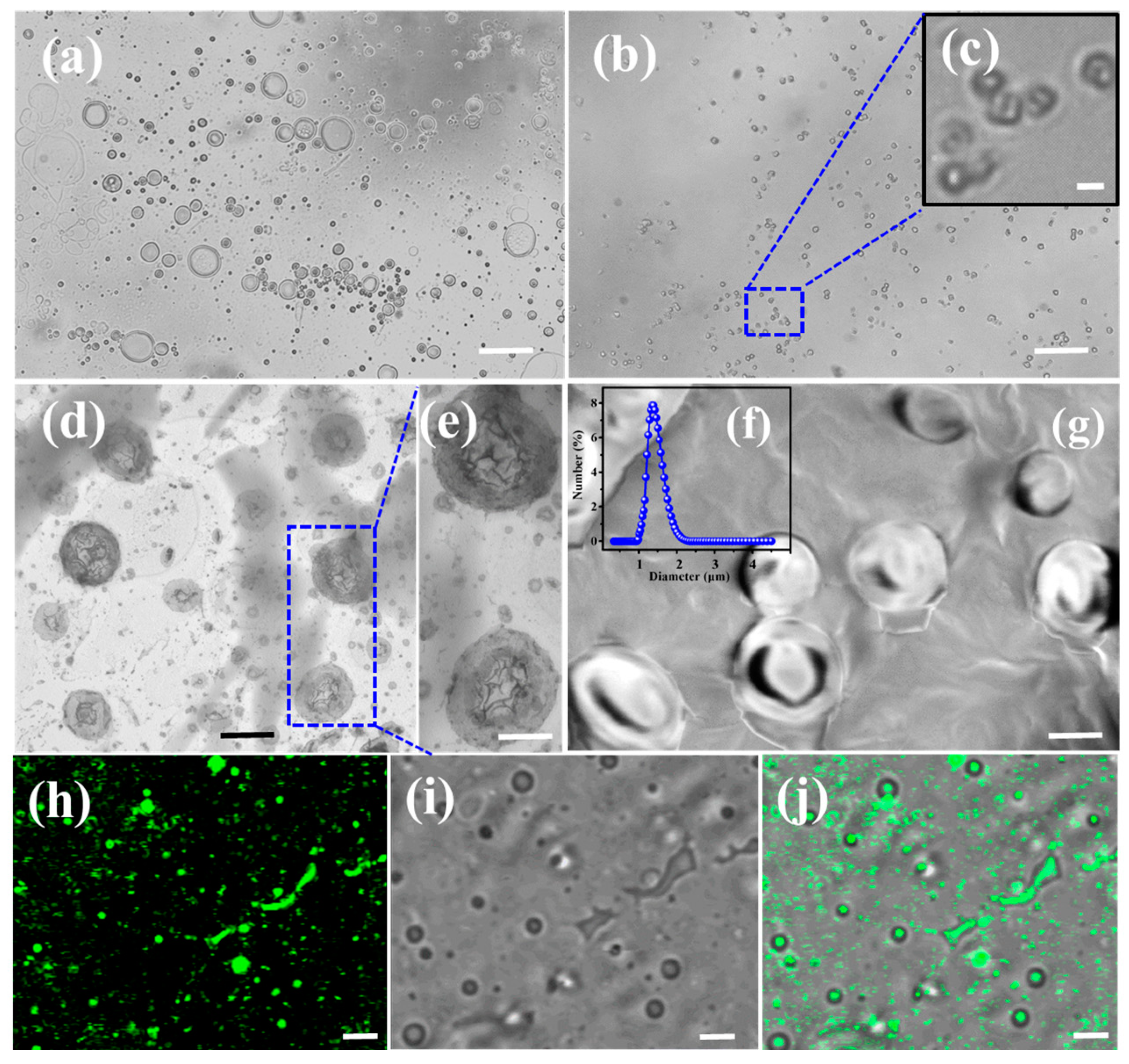
2.1. The Formation of EYL
2.2. The Formation of LHDs and Calcein-Loaded LDHs
2.3. The Permeability of Calcein and Nile Red to the Protocell Membranes
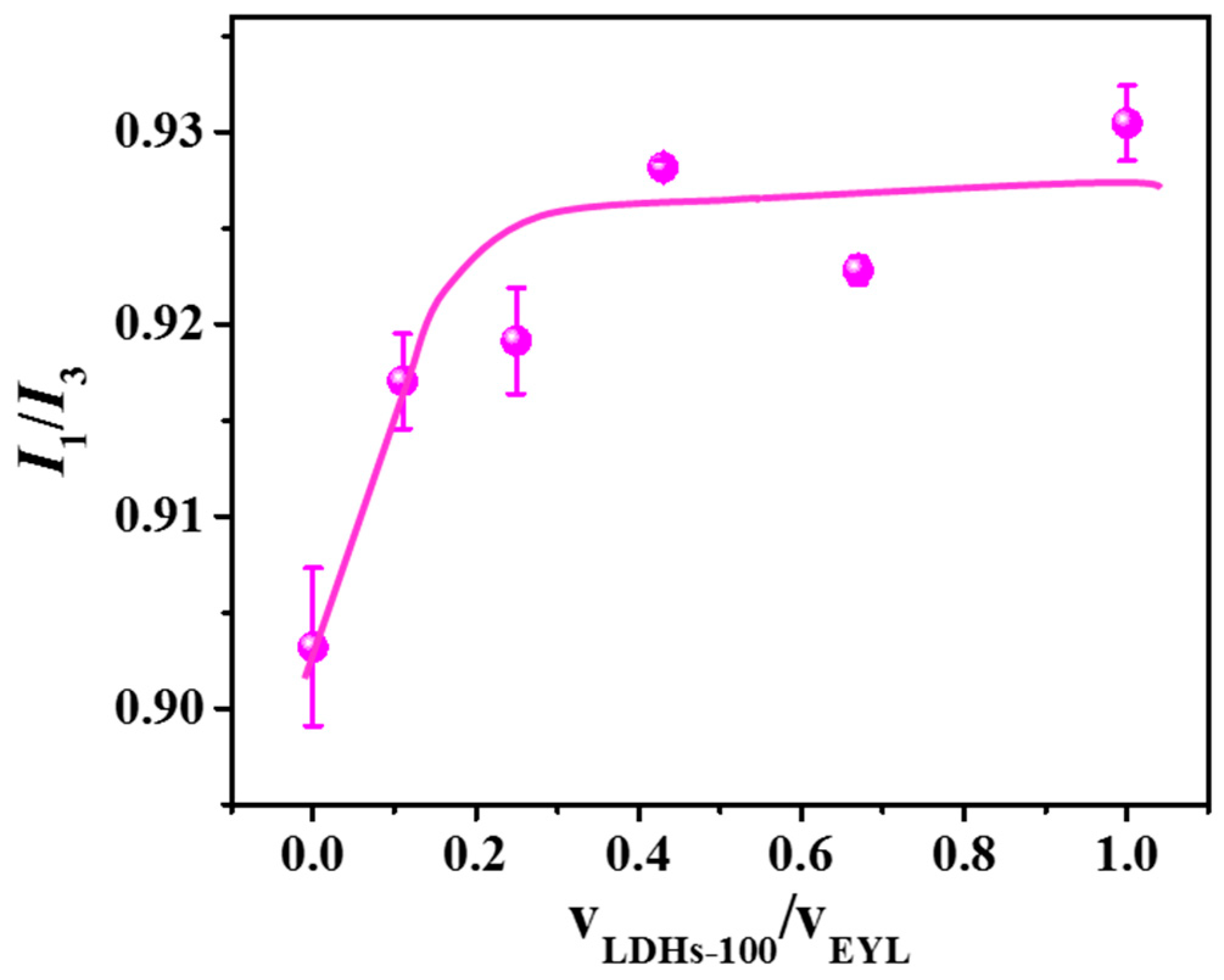
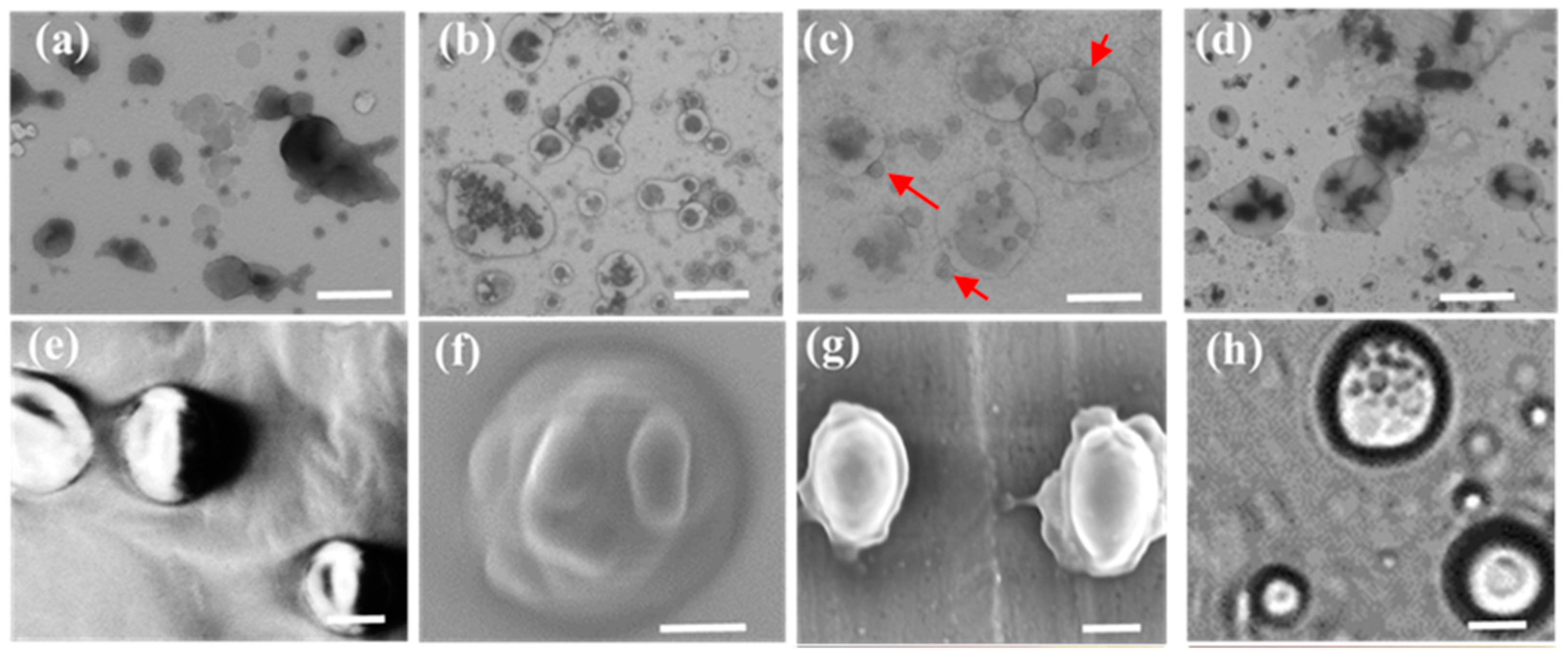
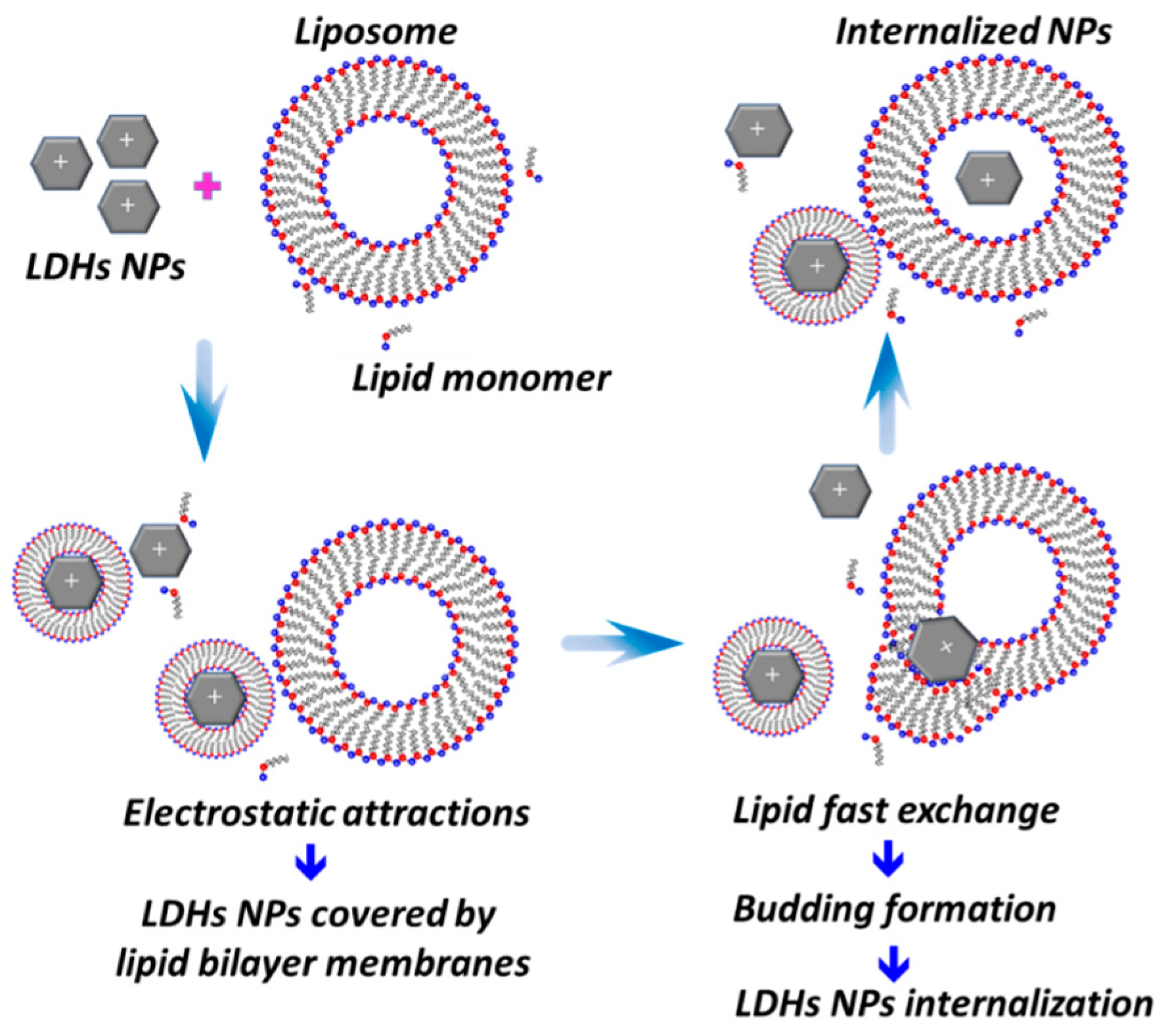
2.4. The Interaction between LDHs Particles and Liposome Membranes
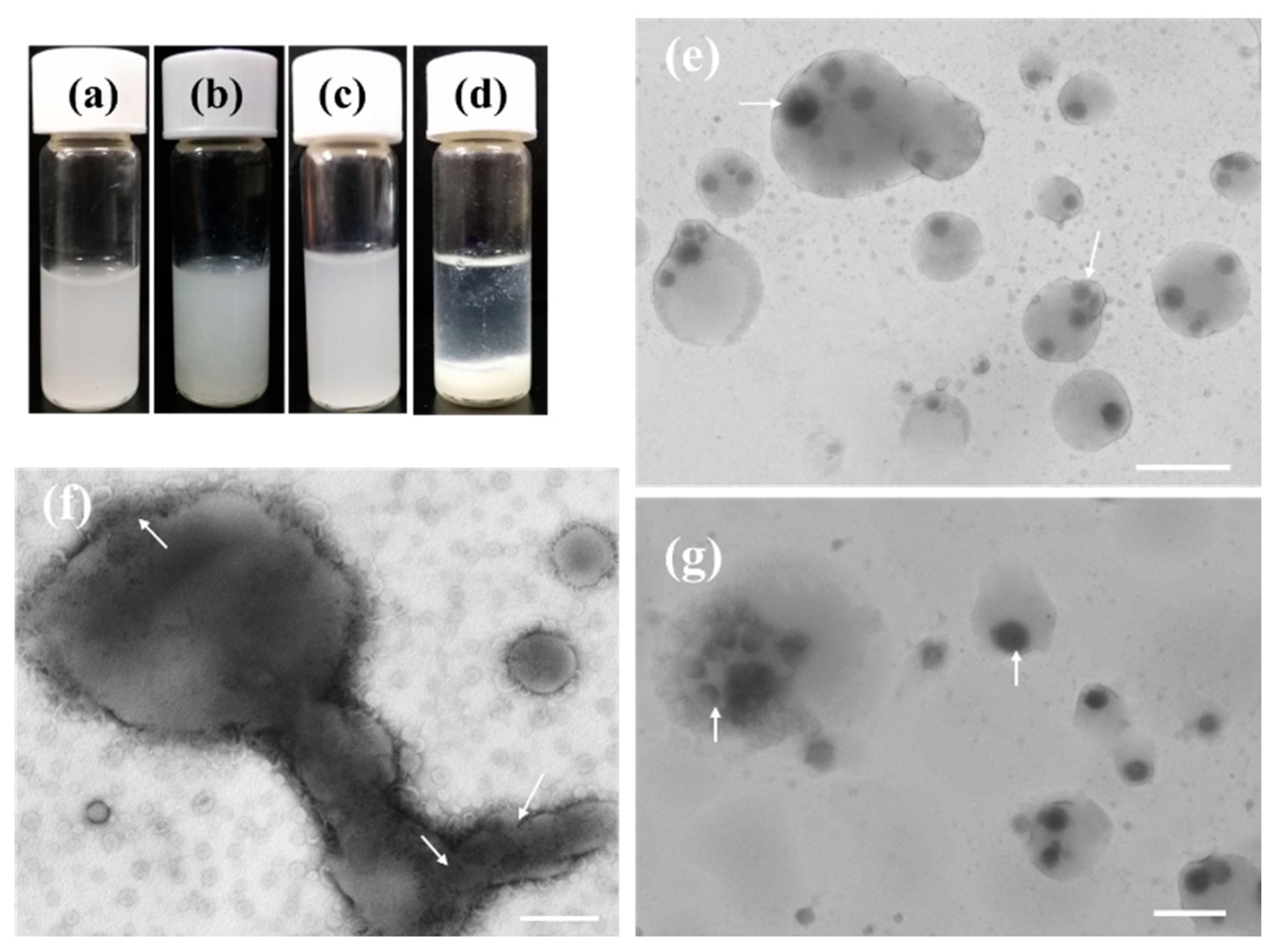
2.5. The Stability of the LDHs-100@EYL Dispersion
2.6. The Possible Trans-Membrane Mechanism of LDHs Particles
3. Materials and Methods
3.1. Materials
3.2. Preparation of Calcein-Loaded Liposomes (Denoted as CE@EYL)
3.3. LDHs Nanoparticle Synthesis
3.4. Intercalation of Calcein into LDHs-100 (Denoted as CE-LDHs-100)
3.5. Characterization of Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Michel, R.; Gradzielski, M. Experimental aspects of colloidal interactions in mixed systems of liposome and inorganic nanoparticle and their applications. Int. J. Mol. Sci. 2012, 13, 11610–11642. [Google Scholar]
- Wang, F.; Liu, J. Liposome supported metal oxide nanoparticles: Interaction mechanism, light controlled content release, and intracellular delivery. Small 2014, 10, 3927–3931. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size dependency of gold nanoparticles interacting with model membranes. Commun. Chem. 2020, 3, 130. [Google Scholar] [PubMed]
- Feng, Y.; Zhang, Y.; Liu, G.; Liu, X.; Gao, S. Interaction of graphene oxide with artificial cell membranes: Role of anionic phospholipid and cholesterol in nanoparticle attachment and membrane disruption. Colloids Surf. B 2021, 202, 111685. [Google Scholar]
- Sun, J.; Liu, X.R.; Li, S.; He, P.; Li, W.; Gross, M.L. Nanoparticles and photochemistry for native-like transmembrane protein footprinting. Nat. Commun. 2021, 12, 7270. [Google Scholar]
- Liu, J. Interfacing zwitterionic liposomes with inorganic nanomaterials: Surface forces, membrane integrity, and applications. Langmuir 2016, 32, 4393–4404. [Google Scholar]
- Dalai, P.; Sahai, N. Mineral-lipid interactions in the origins of Life. Trends Biochem. Sci. 2019, 44, 331–341. [Google Scholar] [PubMed]
- Martin, M.; Hanczyc, S.M.F.; Jack, W.; Szostak, J.W. Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division. Science 2003, 302, 618–622. [Google Scholar]
- Hanczyc, M.M.; Mansy, S.S.; Szostak, J.W. Mineral surface directed membrane assembly. Orig. Life Evol. Biosph. 2007, 37, 67–82. [Google Scholar]
- Stephane, M.; Olivier, L.; Etienne, D.; Alain, B. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005, 5, 281–285. [Google Scholar]
- Michel, R.; Kesselman, E.; PLoStica, T.; Danino, D.; Gradzielski, M. Internalization of silica nanoparticles into fluid liposomes: Formation of interesting hybrid colloids. Angew. Chem. Int. Ed. Engl. 2014, 53, 12441–12445. [Google Scholar]
- Ghosh, P.; Bag, S.; Roy, P.; Chakraborty, I.; Dasgupta, S. Permeation of flavonoid loaded human serum albumin nanoparticles across model membrane bilayers. Int. J. Biol. Macromol. 2022, 222, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: Membrane-loaded and janus vesicles. Nano Lett. 2010, 10, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Haffner, S.; Nystrom, L.; Nordstrom, R.; Xu, Z.P.; Davoudi, M.; Schmidtchen, A.; Malmsten, M. Membrane interactions and antimicrobial effects of layered double hydroxide nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 23832–23842. [Google Scholar]
- Raval, J.; Gongadze, E.; Bencina, M.; Junkar, I.; Rawat, N.; Mesarec, L.; Kralj-Iglic, V.; Gozdz, W.; Iglic, A. Mechanical and electrical interaction of biological membranes with nanoparticles and nanostructured surfaces. Membranes 2021, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar]
- Jin-Ho, C.; Seo-Young, K.; Jong-Sang, P.; Yong-Joo, J.; Josik, P. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar]
- Bégu, S.; Aubert-Pouëssel, A.; Polexe, R.; Leitmanova, E.; Lerner, D.A.; Devoisselle, J.-M.; Tichit, D. New layered double hydroxides/phospholipid bilayer hybrid material with strong potential for sustained drug delivery system. Chem. Mater. 2009, 21, 2679–2687. [Google Scholar]
- Du, N.; Hou, W.G.H.; Song, S.E. A novel composite: Layered double hydrxides encapsulated in vesicles. J. Phys. Chem. B 2007, 111, 13909–13913. [Google Scholar] [CrossRef]
- Nie, H.Q.; Hou, W.G. Vesicle formation induced by layered double hydroxides in the catanionic surfactant solution composed of sodium dodecyl sulfate and dodecyltrimethylammonium bromide. Colloid Polym. Sci. 2011, 289, 775–782. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Du, N.; Zhang, R.; Hou, W. Large-scale aqueous synthesis of layered double hydroxide single-layer nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 49–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Du, N.; Song, S.; Hou, W. Betamethasone dipropionate intercalated layered double hydroxide and the composite with liposome for improved water dispersity. Appl. Clay Sci. 2017, 143, 336–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Mi, Y.; Li, H.; Hou, W. Engineering of (10-hydroxycamptothecin intercalated layered double hydroxide)@liposome nanocomposites with excellent water dispersity. J. Phys. Chem. Solids 2017, 108, 125–132. [Google Scholar]
- Hou, W.G.; Su, Y.L.; Sun, D.J.; Zhang, C.G. Studies on zero point of charge and permanent charge density of Mg-Fe hydrotalcite-like compounds. Langmuir 2001, 17, 1885–1887. [Google Scholar] [CrossRef]
- Oh, J.M.; Choi, S.J.; Kim, S.T.; Choy, J.H. Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates enhanced efficacy due to clathrin-mediated endocytosis. Bioconjug. Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, M.; Li, H.; Liu, J.; Yuan, S.; Du, N.; Hou, W. Model of protocell compartments—Dodecyl hydrogen sulfate vesicles. Phys. Chem. Chem. Phys. 2018, 20, 1332–1336. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Szostak, J.W. Replicating vesicles as models of primitive cell growth and division. Curr. Opin. Chem. Biol. 2004, 8, 660–664. [Google Scholar] [CrossRef]
- Svetina, S. Vesicle budding and the origin of cellular life. Chemphyschem 2009, 10, 2769–2776. [Google Scholar] [CrossRef]
- Du, N.; Song, R.; Li, H.; Song, S.; Zhang, R.; Hou, W. A nonconventional model of protocell-like vesicles: Anionic clay surface-mediated formation from a single-tailed amphiphile. Langmuir 2015, 31, 12579–12586. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, L.; Yi, Y.; Wang, X.; Yu, Y. Rupture of lipid membranes induced by amphiphilic Janus nanoparticles. ACS Nano 2018, 12, 3646–3657. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, V.; Gorbenko, G.P. Lysozyme effect on structural state of model membranes as revealed by pyrene excimerization studies. Biophys. Chem. 2005, 114, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; An, X.; Chen, Z.; Ma, X. Microstructure study of liposomes decorated by hydrophobic magnetic nanoparticles. Chem. Phys. Lipids 2012, 165, 563–570. [Google Scholar] [CrossRef]
- Michel, R.; Plostica, T.; Abezgauz, L.; Danino, D.; Gradzielski, M. Control of the stability and structure of liposomes by means of nanoparticles. Soft Matter 2013, 9, 4167–4177. [Google Scholar] [CrossRef]
- Savarala, S.; Ahmed, S.; Ilies, M.A.; Wunder, S.L. Formation and colloidal stability of DMPC supported lipid bilayers on SiO2 nanobeads. Langmuir 2010, 26, 12081–12088. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Hong, S.; Mecke, A.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. Nanoparticle interaction with biological membranes does nanotechnology present a Janus face. Acc. Chem. Res. 2007, 40, 335–342. [Google Scholar] [CrossRef]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: Interaction of polymers and nanoparticles with membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Ogawa, M.; Kaiho, H. Homogeneous precipitation of uniform hydrotalcite particles. Langmuir 2002, 18, 4240–4242. [Google Scholar] [CrossRef]
- Jin-Ho, C.; Seo-Young, K.; Yong-Joo, J. Inorganic layered double hydroxides as nonviral vectors. Angew. Chem. Int. Ed. 2000, 39, 4041–4045. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, Y.; Du, N. Interactions between Layered Double Hydroxide Nanoparticles and Egg Yolk Lecithin Liposome Membranes. Molecules 2023, 28, 3929. https://doi.org/10.3390/molecules28093929
Liu B, Wang Y, Du N. Interactions between Layered Double Hydroxide Nanoparticles and Egg Yolk Lecithin Liposome Membranes. Molecules. 2023; 28(9):3929. https://doi.org/10.3390/molecules28093929
Chicago/Turabian StyleLiu, Bin, Yanlan Wang, and Na Du. 2023. "Interactions between Layered Double Hydroxide Nanoparticles and Egg Yolk Lecithin Liposome Membranes" Molecules 28, no. 9: 3929. https://doi.org/10.3390/molecules28093929
APA StyleLiu, B., Wang, Y., & Du, N. (2023). Interactions between Layered Double Hydroxide Nanoparticles and Egg Yolk Lecithin Liposome Membranes. Molecules, 28(9), 3929. https://doi.org/10.3390/molecules28093929




