Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum
Abstract
1. Introduction
2. Results and Discussion
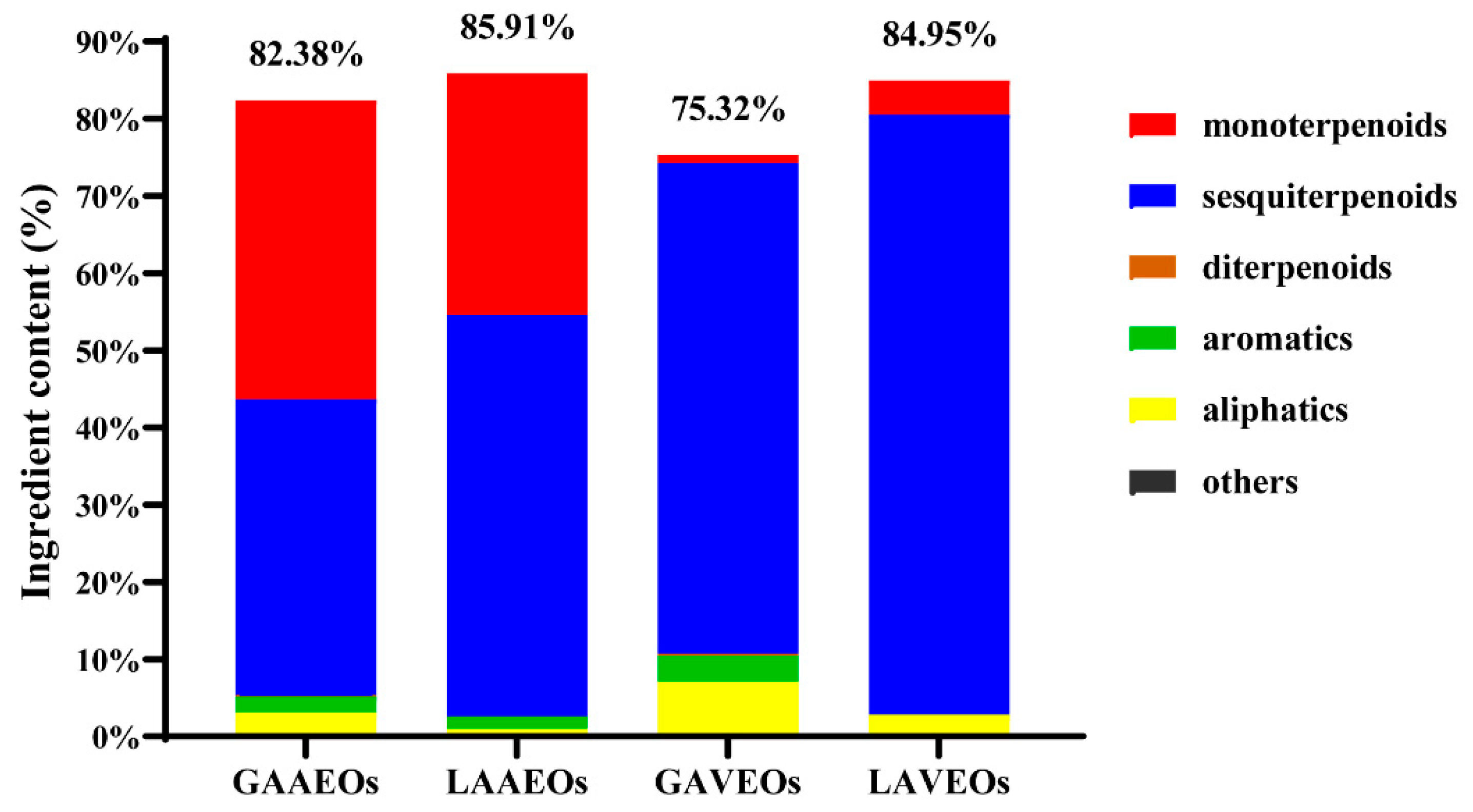
2.1. Chemical Compositions of EOs from Different parts of A. argyi and A. verlotorum
2.1.1. Comparison and Analysis of the Chemical Composition of AAEOs and AVEOs
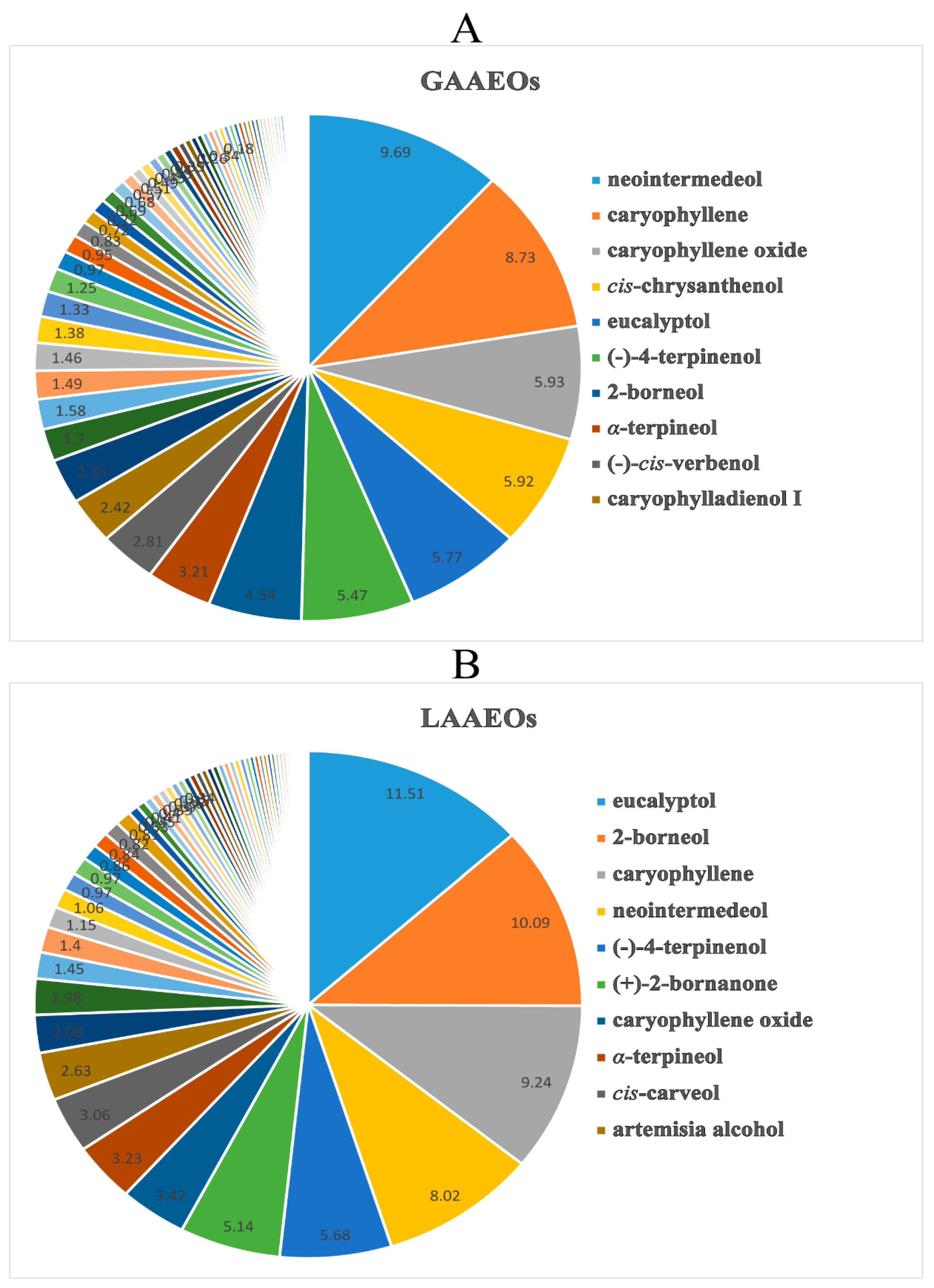
2.1.2. Comparison and Analysis of the EOs from Whole Grass and Leaves of A. Argyi
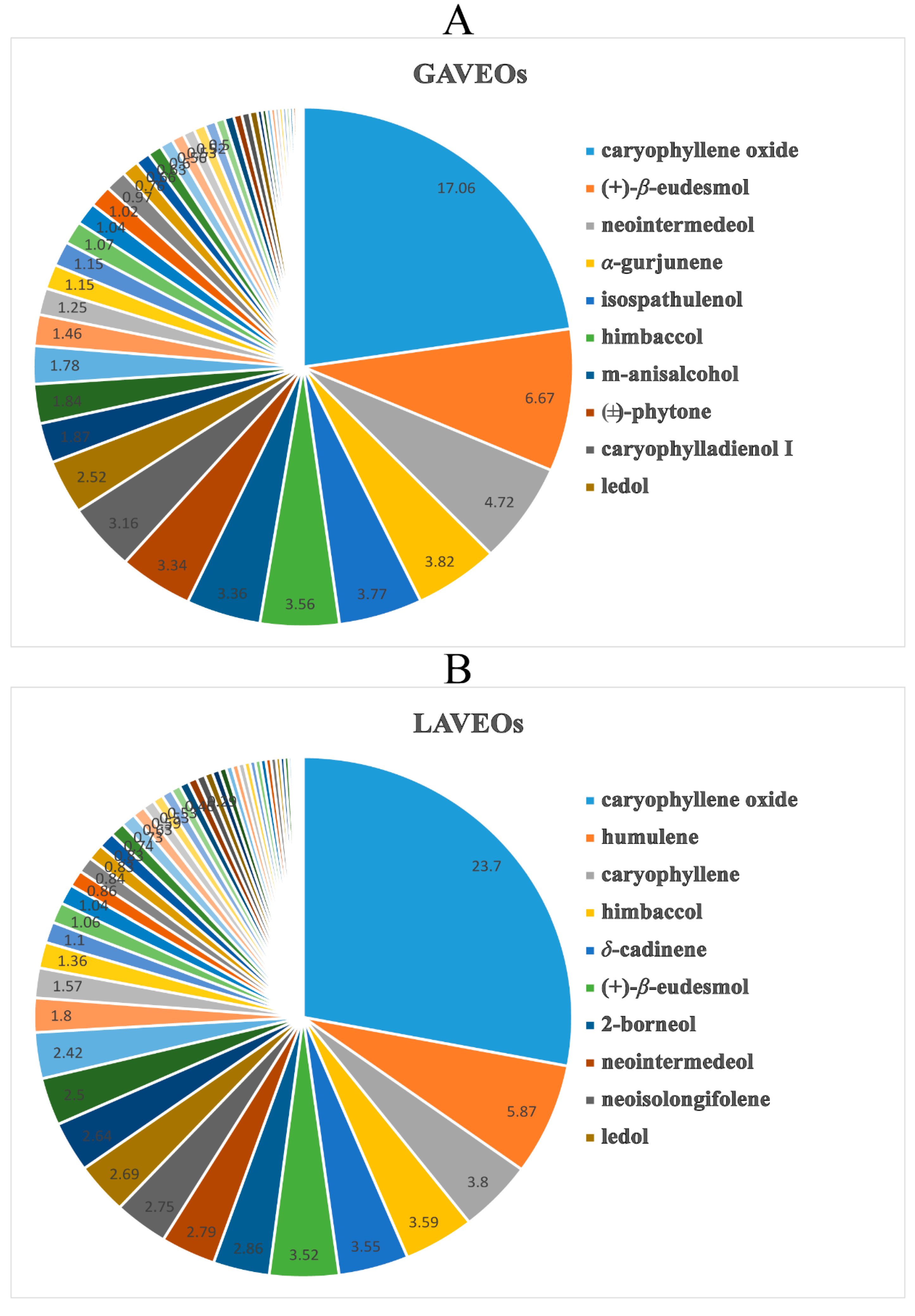
2.1.3. Comparison and Analysis of the EOs from Whole grass and Leaves of A. verlotorum
2.2. Analysis of the Main Active Ingredients and the Potential Effect of EOs from A. argyi and A. verlotorum Using the Ingredient-Target-Pathway Networking
2.2.1. Comparison and Analysis of Main Active Ingredients of AAEOs and AVEOs
2.2.2. Comparison and Analysis of Key Proteins of AAEOs and AVEOs
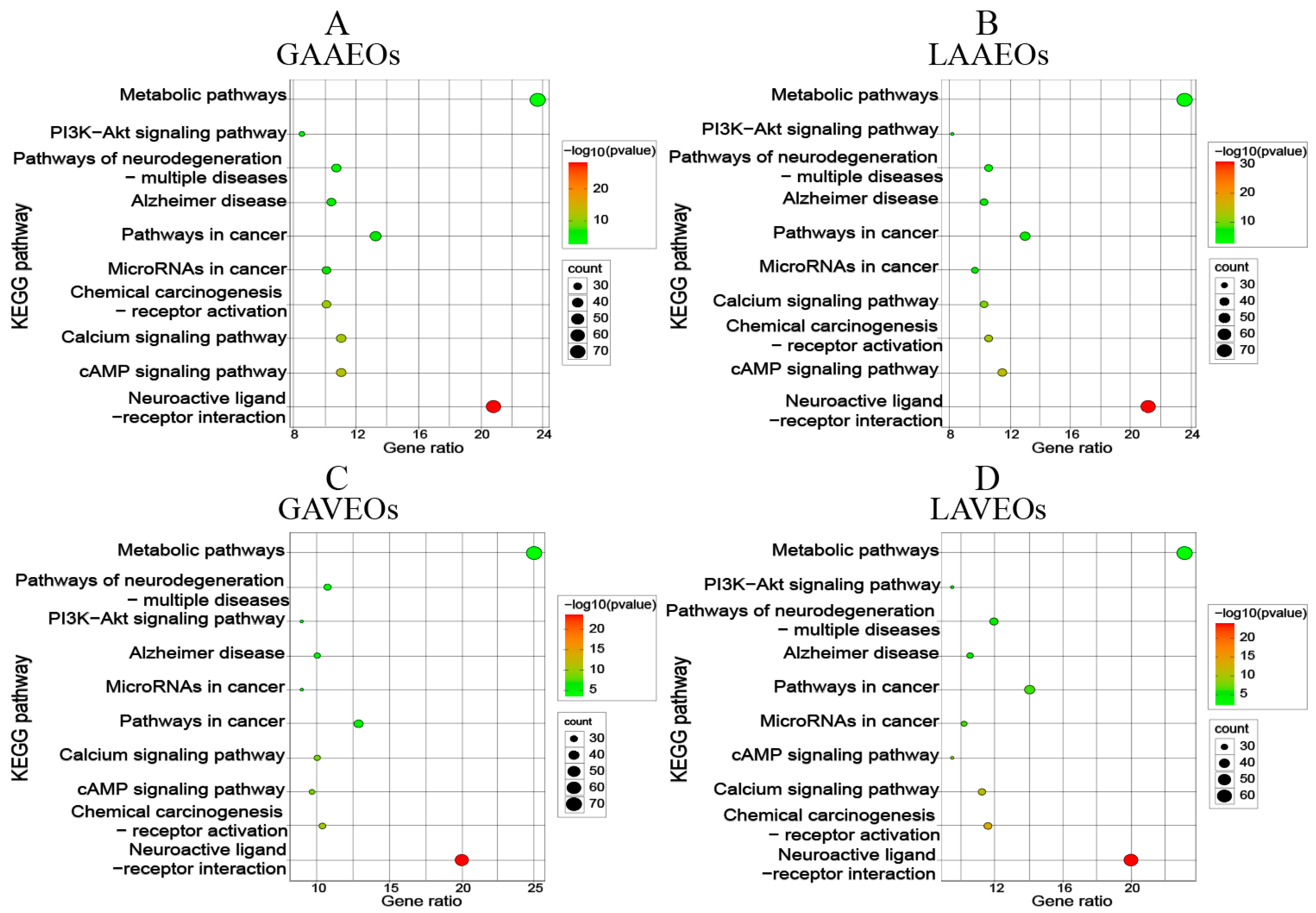
2.2.3. GO Enrichment Analysis and KEGG Pathway Annotation
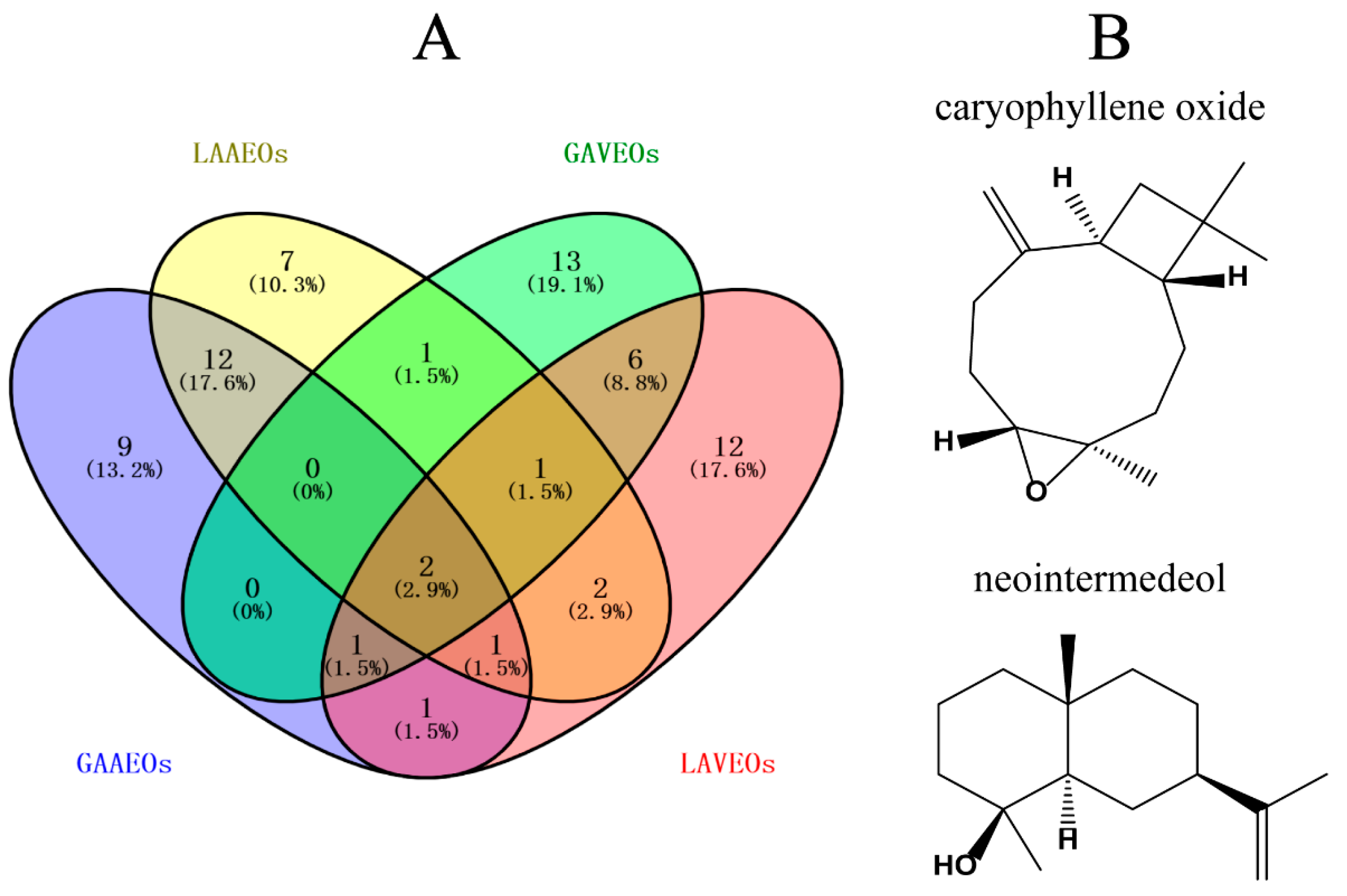
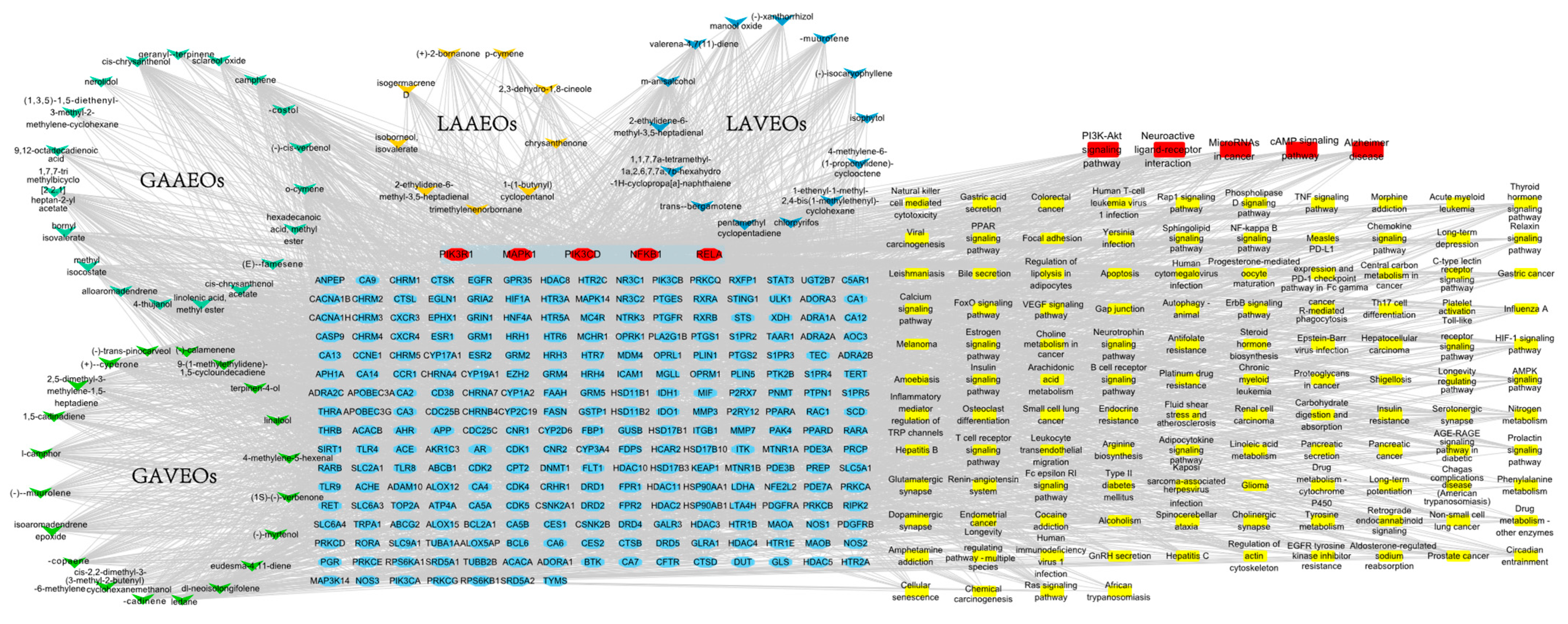
2.2.4. Network Analysis of the Unique Components of the Four Artemisia Essential Oils
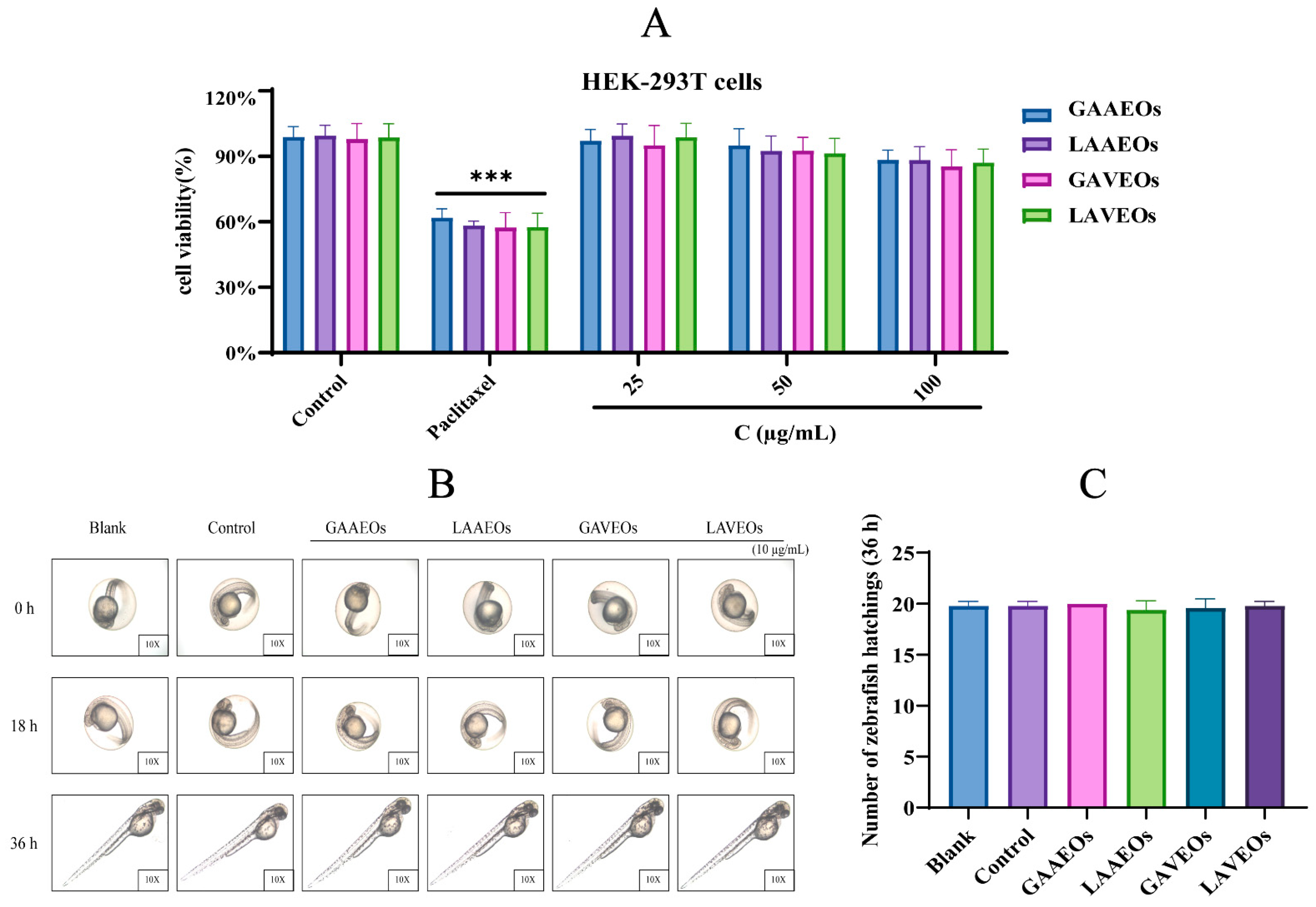
2.3. In Vitro and In Vivo Toxicity of Artemisia Essential Oils
3. Conclusions
4. Materials and Methods
4.1. Plant Materials and Reagent
4.2. Extraction of EOs A. verlotorum
4.3. GC-MS Analysis
4.4. Chemical Ingredients Database Building of EOs
4.5. Collection of Target Proteins and Pathways of the EOs
4.6. Networking Construction
4.7. Gene Ontology and Pathway Enrichment Analysis
4.8. Toxicity Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.T.d. Mining the essential oils of the Anthemideae. Afr. J. Biotechno. 2004, 3, 706–720. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Wen, W.; Xu, P.; Xiang, H.; Wen, M.; Ye, X.; Chu, C.; Tong, S. Comprehensive two-dimensional countercurrent chromatography × gas chromatography characterization of Artemisia argyi essential oil. Anal. Chim. Acta. 2023, 1237, 340614–340624. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015; p. 89. [Google Scholar]
- Xiang, F.; Bai, J.H.; Tan, X.B.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var argyi cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. Dual bioactivities of essential oil extracted from the leaves of Artemisia argyi as an antimelanogenic versus antioxidant agent and chemical composition analysis by GC/MS. Int. J. Mol. Sci. 2012, 13, 14679–14697. [Google Scholar] [CrossRef]
- Campelo-Felix, P.H.; Souza, H.J.; Figueiredo, J.A.; Fernandes, R.V.; Botrel, D.A.; de Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and anti-oxidant properties of lime essential oil microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, H.J.; Chao, J.; Liu, J.F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017, 204, 107–117. [Google Scholar] [CrossRef]
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical composition and antimicrobial activities of Artemisia argyi Levl. et Vant essential oils extracted by simultaneous distillation-extraction, subcritical extraction and hydrodistillation. Molecules 2019, 24, 483. [Google Scholar] [CrossRef]
- Wenqiang, G.; Shufen, L.; Ruixiang, Y.; Yanfeng, H. Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat. Prod. Res. 2006, 20, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hou, A.; Xiang, Z. Study on Artemisia Argyi essential oil: Anti-inflammatory, anti-anaphylatic and analgesic effects. J. N. Med. 2005, 15, 36–39. [Google Scholar]
- Bao, X.; Yuan, H.; Wang, C.; Liu, J.; Lan, M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr. Polym. 2013, 98, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Martinotti, E.; Baragatti, B.; Breschi, M.C.; Morelli, I. Vascular effects of aqueous crude extracts of Artemisia verlotorum Lamotte (Compositae): In vivo and in vitro pharmacological studies in rats. Phytother. Res. 1999, 13, 645–648. [Google Scholar] [CrossRef]
- de Lima, T.C.; Morato, G.S.; Takahashi, R.N. Evaluation of the central properties of Artemisia verlotorum. Planta. Med. 1993, 59, 326–329. [Google Scholar] [CrossRef]
- Moran, A.; Montero, M.J.; Martin, M.L.; San, R.L. Pharmacological screening and antimicrobial activity of the essential oil of Artemisia caerulescens subsp. gallica. J. Ethnopharmacol. 1989, 26, 197–203. [Google Scholar] [CrossRef]
- Song, X.W.; Wen, X.; He, J.W.; Zhao, H.; Li, S.M.; Wang, M.Y. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168–184. [Google Scholar] [CrossRef]
- Charrier, N.; Clarke, B.; Cutler, L.; Demont, E.; Dingwall, C.; Dunsdon, R.; Hawkins, J.; Howes, C.; Hubbard, J.; Hussain, I.; et al. Second generation of BACE-1 inhibitors. Part 1: The need for improved pharmacokinetics. Bioorg. Med. Chem. Lett. 2009, 19, 3664–3668. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, E.C.; Lim, H.J.; Choi, S.J.; Shin, D.H. Characterization of nutritional composition, anti-oxidative capacity, and sensory attributes of seomae mugwort, a native korean variety of Artemisia argyi H. Lév. & Vaniot. J. Anal. Methods. Chem. 2015, 2015, 1–9. [Google Scholar]
- Zhang, W.J.; You, C.X.; Yang, K.; Chen, R.; Wang, Y.; Wu, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Su, Y.; et al. Bioactivity of essential oil of Artemisia argyi Levl. et Van. and its main compounds against Lasioderma serricorne. J. Oleo. Sci. 2014, 63, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, W.B.; Guo, Y.; Li, Y.; Cao, X.F.; Xu, W.; Yang, L.; Chen, F.L. Aqueous enzyme-ultrasonic pretreatment for efficient isolation of essential oil from Artemisia argyi and investigation on its chemical composition and biological activity. Ind. Crop. Prod. 2020, 158, 113031–113041. [Google Scholar] [CrossRef]
- Pan, J.G.; Xu, Z.L.; Ji, L. Chemical studies on essential oils from 6 Artemisia species. China J. Chin. Mater. Med. 1993, 17, 741–744. [Google Scholar]
- Shabana, M.M.; Haggag, M.Y.; Hilal, S.H. Phytochemical study of Chinese herbal drugs. I. Volatile oil of Artemisia argyi Levl et Vant. Egy. J. Pharm. Sci. 1980, 19, 271–280. [Google Scholar]
- Wan, L.; Lu, J.; Guo, S. GC-MS fingerprint of volatile oil of Artemisia argyi. Med. Plant. 2016, 7, 1–4. [Google Scholar]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kroner, L.U. Absinthe–a review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Essential oil composition of Aegle marmelos (L.) Correa: Chemotypic and seasonal variations. J. Sci. Food. Agric. 2014, 94, 1904–1913. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Wu, S.; Wang, C.; Guo, X.; Wu, J.; Zhou, M. De novo assembly and analysis of the Artemisia argyi transcriptome and identification of genes involved in terpenoid biosynthesis. Sci. Rep. 2018, 8, 5824–5835. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Volatile constituents of the leaf oil of Artemisia verlotiorum Lamotte and Ambrosia tenuifolia Sprengel (Syn.: Artemisia psilostachyaauct. non L.). J. Essent. Oil. Res. 1996, 8, 559–561. [Google Scholar] [CrossRef]
- Vernin, G.A. GC/MS Analysis of Artemisia verlotiorum Lamotte Oil. J. Essent. Oil. Res. 2011, 12, 143–146. [Google Scholar] [CrossRef]
- Yano, S.; Suzuki, Y.; Yuzurihara, M.; Kase, Y.; Takeda, S.; Watanabe, S.; Aburada, M.; Miyamoto, K.-I. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur. J. Pharmacol. 2006, 553, 99–103. [Google Scholar] [PubMed]
- Alanís, R.M.; Kennedy, J.F. Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Carbohydr. Polym. 2005, 62, 88–96. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, S.K.; Cheng, X.L.; Tian, X.W.; Wang, J. Caryophyllene oxide exhibits anti-cancer effects in MG-63 human osteosarcoma cells via the inhibition of cell migration, generation of reactive oxygen species and induction of apoptosis. Bangl. J. Pharmacol. 2016, 11, 817–823. [Google Scholar] [CrossRef]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.-C.; Lee, D.-S.; Ahn, K.S.; Cho, S.K. Cytotoxic activity of β-Caryophyllene oxide isolated from Jeju Guava (Psidium cattleianum Sabine) leaf. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- Dougnon, G.; Ito, M. Essential oil from the leaves of chromolaena odorata, and sesquiterpene caryophyllene oxide induce sedative activity in mice. Pharmaceuticals 2021, 14, 651. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and caryophyllene oxide: A variety of chemical transformations and biological activities. Chem. Pap. 2021, 76, 1–39. [Google Scholar] [CrossRef]
- Bansal, H.; Pravallika, V.S.S.; Srivastava, G.; Ganjewala, D. Bioactivity assessment of essential oils of Cymbopogon species using a network pharmacology approach. Biol. Futur. 2022, 73, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, T.; Perkins, N.D.; C, L.W. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Grumont, R.J.; Hochrein, H.; Fuchsberger, M.; Gugasyan, R.; Vremec, D.; Shortman, K.; Gerondakis, S. Distinct roles for the NF-κB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood 2005, 106, 3457–3464. [Google Scholar] [CrossRef]
- Wilson, C.L.; Jurk, D.; Fullard, N.; Banks, P.; Page, A.; Luli, S.; Elsharkawy, A.M.; Gieling, R.G.; Chakraborty, J.B.; Fox, C.; et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 2015, 6, 6818–6831. [Google Scholar] [CrossRef]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 2, 4172–4186. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Ma, L.; Wu, X.; Mu, Y.; Pan, J.; Hu, J.; Hu, T.; Fu, Q.; Guo, D. The DEAD-box RNA helicase DDX1 interacts with RelA and enhances nuclear factor kappaB-mediated transcription. J. Cell Biochem. 2009, 106, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S. RelA life and death decisions. Mol. Cell 2004, 13, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.J.; Rocha, S.; Perkins, N.D. Active repression of anti-apoptotic gene expression by RelA(p65) NF-κB. Mol. Cell 2004, 13, 853–865. [Google Scholar] [CrossRef]
- Lee, J.I.; Burckart, G.J. Nuclear factor kappa B: Important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998, 38, 981–993. [Google Scholar] [CrossRef]
- Winnay, J.N.; Boucher, J.; Mori, M.A.; Ueki, K.; Kahn, C.R. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box–binding protein-1 to modulate the unfolded protein response. Nat. Med. 2010, 16, 438–445. [Google Scholar] [CrossRef]
- Argetsinger, L.S.; Norstedt, G.; Billestrup, N.; White, M.F.; Carter-Su, C. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J. Biol. Chem. 1996, 271, 29415–29421. [Google Scholar] [CrossRef]
- Yun, C.; Jung, Y.; Chun, W.; Yang, B.; Ryu, J.; Lim, C.; Kim, J.H.; Kim, H.; Cho, S.I. Anti-inflammatory effects of Artemisia leaf extract in mice with contact dermatitis in vitro and in vivo. Mediat. Inflamm. 2016, 2016, 8027537–8027554. [Google Scholar] [CrossRef]
- Zeng, K.W.; Wang, S.; Dong, X.; Jiang, Y.; Tu, P.F. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-kappaB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine 2014, 21, 298–306. [Google Scholar] [CrossRef]
- Xiao, J.Q.; Liu, W.Y.; Sun, H.P.; Li, W.; Koike, K.; Kikuchi, T.; Yamada, T.; Li, D.; Feng, F.; Zhang, J. Bioactivity-based analysis and chemical characterization of hypoglycemic and antioxidant components from Artemisia argyi. Bioorg. Chem. 2019, 92, 103268–103284. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chin. J. Cancer Res. 2013, 25, 536–543. [Google Scholar] [PubMed]
- Seo, J.M.; Kang, H.M.; Son, K.H.; Kim, J.H.; Lee, C.W.; Kim, H.M.; Chang, S.I.; Kwon, B.M. Antitumor activity of flavones isolated from Artemisia argyi. Planta. Med. 2003, 69, 218–222. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, F.; Xu, R.; Yang, M.; Ci, Z.; Wu, Z.; Zhang, D.; Lin, J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H.Lev. & vaniot essential oil. J. Ethnopharmacol. 2021, 279, 114404–114417. [Google Scholar]
- Wortzel, I.; Seger, R. The ERK cascade: Distinct functions within various subcellular organelles. Genes. Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Scheife, R.T. Protein binding: What does it mean? DICP 1989, 23, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Morath, A.; Schamel, W.W.; Steinberger, P.; Leitner, J.; Huber, R.; Hamburger, M.; Grundemann, C. Immunosuppressive Activity of Artemisia argyi Extract and Isolated Compounds. Front. Pharmacol. 2020, 11, 402. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.B.; Yang, W.; Meng, D.L. Gastro-protective effect of edible plant Artemisia argyi in ethanol-induced rats via normalizing inflammatory responses and oxidative stress. J. Ethnopharmacol. 2018, 214, 207–217. [Google Scholar] [CrossRef]
- Chen, J.K.; Kuo, C.H.; Kuo, W.W.; Day, C.H.; Wang, T.F.; Ho, T.J.; Lin, P.Y.; Lin, S.Z.; Shih, T.C.; Shih, C.Y.; et al. Artemisia argyi extract ameliorates IL-17A-induced inflammatory response by regulation of NF-kappa B and Nrf2 expression in HIG-82 synoviocytes. Environ. Toxicol. 2022, 37, 2793–2803. [Google Scholar] [CrossRef]
- Ullah, I.; Subhan, F.; Ayaz, M.; Shah, R.; Ali, G.; Haq, I.U.; Ullah, S. Anti-emetic mechanisms of Zingiber officinale against cisplatin induced emesis in the pigeon; behavioral and neurochemical correlates. BMC Complement. Altern. Med. 2015, 15, 34–42. [Google Scholar] [CrossRef]
- Zhang, L.X.; Wei, Y.; Wang, W.; Fan, Y.; Li, F.F.; Li, Z.N.; Lin, A.Q.; Gu, H.K.; Song, M.F.; Wang, T.; et al. Quantitative fingerprint and antioxidative properties of Artemisia argyi leaves combined with chemometrics. J. Sep. Sci. 2023, 46, e220062–e220075. [Google Scholar] [CrossRef]
- Deng, J.; Ye, L.F.; Xu, G.H.; Ma, Z.G.; Cao, H.; Zhang, Y.; Wu, M.H. Quantitative and qualitative analysis of Artemisiae verlotori Folium and Artemisiae argyi Folium by high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Sep. Sci. 2023, 63, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Xiang, J.Y.; Han, J.X.; Yang, F.; Li, H.Z.; Chen, H.; Xu, M. Essential oils from spices inhibit cholinesterase activity and improve behavioral disorder in AlCl3 induced dementia. Chem. Biodivers. 2022, 19, e202100443–e202100452. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the rule of 5 and drugability. Adv. Drug. Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Ru, J.L.; Li, P.; Wang, J.N.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 2014, 6, 13–21. [Google Scholar] [CrossRef]
- Wang, L.; Tan, N.; Hu, J.; Wang, H.; Duan, D.; Ma, L.; Xiao, J.; Wang, X. Analysis of the main active ingredients and bioactivities of essential oil from Osmanthus fragrans Var. thunbergii using a complex network approach. BMC Syst. Biol. 2017, 11, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic. Acids. Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Clyne, A.; Yang, L.; Yang, M.; May, B.; Yang, A.W.H. Molecular docking and network connections of active compounds from the classical herbal formula Ding Chuan Tang. Peer. J. 2020, 8, e8685–e8697. [Google Scholar] [CrossRef] [PubMed]
- Traag, V.A.; Waltman, L.; van Eck, N.J. From Louvain to Leiden: Guaranteeing well-connected communities. Sci. Rep. 2019, 9, 5233–5245. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zheng, Y.; Yin-Yue, C.; Feng, Y.; Dai, S.X.; Zhao, S.; Chen, H.; Xu, M. Essential oil of lemon myrtle (Backhousia citriodora) induces S-phase cell cycle arrest and apoptosis in HepG2 cells. J. Ethnopharmacol. 2023, 312, 116493–116536. [Google Scholar] [CrossRef] [PubMed]


| No. | Latin Name | Plant Parts | Abbreviation | Appearance of Essential Oils | Collecting Locations | Yield (v/w, %) |
|---|---|---|---|---|---|---|
| YP-1 | A. argyi | whole grass | GAAEOs | blue | Nanyang, Henan | 0.15 |
| YP-2 | A. argyi | leaves | LAAEOs | blue | Nanyang, Henan | 0.34 |
| YP-3 | A. verlotorum | whole grass | GAVEOs | yellow-green | Luofo mountain, Guangdong | 0.015 |
| YP-4 | A. verlotorum | leaves | LAVEOs | yellow-green | Luofo mountain, Guangdong | 0.032 |
| GAAEOs | NO a | Scores b | LAAEOs | NO a | Scores b | GAVEOs | NO a | Scores b | LAVEOs | NO a | Scores b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| methyleugenol | C37 | 8462.68 | alloaromadendrene oxide | C44 | 8986.16 | m-anisalcohol | C34 | 11053.29 | 9-(1-methylethylidene)-1,5-cycloundecadiene | C14 | 6343.27 |
| 1-octen-3-ol | C9 | 7899.98 | methyleugenol | C26 | 8884.00 | alloaromadendrene oxide | C32 | 8097.39 | 4-methylene-5-hexenal | C13 | 5064.08 |
| o-cymene | C40 | 7509.06 | 1-octen-3-ol | C6 | 8458.30 | pentamethylcyclopentadiene | C35 | 7295.15 | β-elemene | C46 | 5060.33 |
| chamazulene | C22 | 6276.07 | chamazulene | C15 | 6509.30 | β-elemene | C29 | 6874.15 | β-calacorene | C44 | 4812.89 |
| eugenol | C32 | 5706.63 | p-cymene | C49 | 6502.40 | β-calacorene | C21 | 5972.17 | (+)-α-calacorene | C5 | 4325.45 |
| bornyl isovalerate | C16 | 4615.64 | trimethylenenorbornane | C50 | 5701.88 | neointermedeol | C17 | 5612.14 | bornyl acetate | C16 | 4218.15 |
| cis-carveol | C23 | 4358.01 | eugenol | C22 | 5568.67 | chlorpyrifos | C33 | 5080.56 | caryophyllene oxide | C20 | 4076.81 |
| junenol | C35 | 4222.82 | β-elemene | C53 | 5117.62 | (+)-α-calacorene | C1 | 4997.34 | 2,5-dimethyl-3-methylene-1,5-heptadiene | C11 | 4066.86 |
| neointermedeol | C38 | 3900.09 | junenol | C25 | 4547.44 | 2-borneol | C4 | 4197.71 | (−)-verbenone | C9 | 3884.48 |
| γ-pironene | C53 | 3777.84 | trans-4-thujanol | C31 | 4372.10 | caryophyllene oxide | C9 | 4072.41 | neointermedeol | C36 | 3875.57 |
| eucalyptol | C31 | 3512.37 | cis-carveol | C16 | 4218.90 | mustakone | C16 | 3428.25 | ledane | C32 | 3635.38 |
| copaene | C28 | 3422.12 | 2-ethylidene-6-methyl-3,5-heptadienal | C43 | 4017.18 | β-selinene | C23 | 3130.88 | isoaromadendrene epoxide | C28 | 3631.91 |
| camphor | C8 | 3298.98 | caryophyllene oxide | C14 | 3864.40 | (−)-xanthorrhizol | C27 | 2908.70 | 2-borneol | C12 | 3330.03 |
| methyl isocostate | C36 | 3249.50 | α-himachalene | C51 | 3523.03 | β-costol | C22 | 2801.19 | (+)-α-cyperone | C6 | 3159.83 |
| γ-costol | C51 | 3240.85 | γ-pironene | C37 | 3487.45 | himbaccol | C12 | 2651.97 | (−)-calamenene | C1 | 2998.45 |
| caryophyllene oxide | C21 | 3158.27 | neointermedeol | C27 | 3457.32 | ledol | C15 | 2651.97 | δ-cadinene | C49 | 2985.95 |
| 4-thujanol | C12 | 2986.18 | eucalyptol | C21 | 3336.46 | palustrol | C18 | 2397.18 | cis-carveol | C22 | 2899.12 |
| trans-4-thujanol | C44 | 2986.18 | bornyl acetate | C45 | 3168.03 | 4(15),5,10(14)-germacratrien-1-ol | C11 | 2333.46 | linalool | C34 | 2869.81 |
| (−)-β-bourbonene | C4 | 2968.32 | γ-terpinene | C38 | 3009.81 | isoledene | C13 | 2331.30 | mustakone | C35 | 2747.32 |
| 2-borneol | C10 | 2951.02 | chrysanthenone | C46 | 2935.24 | α-gurjunene | C19 | 2318.41 | α-himachalene | C42 | 2652.59 |
| camphene | C17 | 2884.04 | 1-(1-butynyl) cyclopentanol | C41 | 2756.51 | (±)-α-curcumene | C3 | 2280.32 | neoisolongifolene | C23 | 2606.73 |
| cis-p-menth-2-en-1-ol | C27 | 2594.42 | copaene | C19 | 2752.69 | τ-muurolol | C25 | 1888.10 | β-selinene | C47 | 2491.54 |
| (±)-piperitone | C6 | 2490.96 | cis-p-menth-2-en-1-ol | C18 | 2519.15 | α-neocallitropsene | C20 | 1815.24 | α-copaene | C40 | 2418.91 |
| (−)-carvone | C2 | 2489.47 | (−)-β-bourbonene | C3 | 2434.89 | α-muurolene | C38 | 1762.92 | himbaccol | C27 | 2395.18 |
| selina-4,11-dien | C42 | 2458.10 | yogomi alcohol | C32 | 2385.97 | ledol | C33 | 2395.18 | |||
| (+)-δ-cadinene | C11 | 2187.37 | selina-4,11-dien | C29 | 2376.64 | camphor | C31 | 2272.53 |
| GAAEOs | NO. c | Score d | LAAEOs | NO. c | Score d | GAVEOs | NO. c | Score d | LAVEOs | NO. c | Score d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NFKB1 | T26 | 14,010.38 | NFKB1 | T26 | 14,260.94 | NFKB1 | T35 | 13,462.24 | NFKB1 | T40 | 13,974.02 |
| PIK3CD | T118 | 9959.54 | MAPK1 | T70 | 10,134.16 | MAPK1 | T171 | 10,188.79 | MAPK1 | T139 | 10,667.82 |
| MAPK1 | T70 | 9895.40 | PIK3CD | T105 | 9055.98 | PIK3CD | T161 | 6665.46 | PIK3CD | T110 | 6385.01 |
| PRKCA | T175 | 6076.31 | PIK3CA | T134 | 6661.89 | PRKCA | T120 | 6410.28 | PIK3CA | T260 | 4726.91 |
| PIK3CA | T144 | 5979.37 | PRKCA | T150 | 5812.37 | PIK3CA | T180 | 5693.44 | PIK3CB | T261 | 4431.85 |
| PIK3R1 | T156 | 5327.63 | PIK3CB | T263 | 5567.77 | PIK3CB | T181 | 5693.44 | PIK3R1 | T186 | 4424.04 |
| RELA | T215 | 5264.89 | RELA | T190 | 5363.08 | RELA | T46 | 4034.71 | NOS2 | T42 | 4065.17 |
| PIK3CB | T281 | 5001.83 | NOS2 | T47 | 4740.83 | PIK3R1 | T144 | 3792.55 | PRKCA | T197 | 3704.80 |
| NOS2 | T47 | 4484.77 | PIK3R1 | T201 | 3986.61 | NOS2 | T36 | 3742.43 | RELA | T120 | 3315.37 |
| PRKCB | T99 | 3924.41 | PRKCB | T245 | 3899.08 | CASP9 | T57 | 2926.28 | PRKCB | T164 | 3205.22 |
| CASP9 | T137 | 3217.58 | CASP9 | T127 | 3476.93 | ITGB3 | T219 | 2724.65 | CASP9 | T58 | 2908.34 |
| NOS3 | T27 | 2945.04 | NOS3 | T27 | 3296.31 | RAC1 | T266 | 2605.33 | NOS3 | T43 | 2799.68 |
| STAT3 | T125 | 2873.77 | IDH1 | T286 | 2816.61 | ADAM10 | T178 | 2482.65 | ACACA | T132 | 2548.51 |
| SLC9A1 | T122 | 2457.80 | CYP1A2 | T116 | 2592.61 | NOS3 | T37 | 2260.63 | ITGB1 | T135 | 2498.96 |
| TP53 | T230 | 2279.98 | ACACA | T63 | 2570.09 | PLA2G1B | T94 | 2167.69 | FBP1 | T283 | 2494.99 |
| CYP1A2 | T86 | 2186.07 | PLA2G1B | T144 | 2496.26 | MAOA | T32 | 2087.59 | PRKCG | T166 | 2431.40 |
| GUSB | T245 | 2182.93 | ENPP1 | T271 | 2386.76 | MAPK14 | T6 | 1975.62 | STAT3 | T86 | 2402.65 |
| MTOR | T201 | 2178.04 | TP53 | T197 | 2281.28 | CYP1A2 | T68 | 1920.10 | UGT2B7 | T255 | 2334.90 |
| ENPP1 | T290 | 2166.02 | MAOA | T66 | 2247.70 | MTOR | T158 | 1832.80 | CYP1A2 | T90 | 2251.76 |
| STAT3 | T126 | 1802.37 | TP53 | T239 | 2194.28 | ||||||
| UGT2B7 | T172 | 1774.58 | PLA2G1B | T76 | 2169.10 | ||||||
| SLC9A1 | T51 | 1773.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-F.; Zheng, Y.; Feng, Y.; Chen, H.; Dai, S.-X.; Wang, Y.; Xu, M. Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum. Molecules 2023, 28, 3927. https://doi.org/10.3390/molecules28093927
Wang Y-F, Zheng Y, Feng Y, Chen H, Dai S-X, Wang Y, Xu M. Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum. Molecules. 2023; 28(9):3927. https://doi.org/10.3390/molecules28093927
Chicago/Turabian StyleWang, Yun-Fen, Yang Zheng, Yang Feng, Hao Chen, Shao-Xing Dai, Yifei Wang, and Min Xu. 2023. "Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum" Molecules 28, no. 9: 3927. https://doi.org/10.3390/molecules28093927
APA StyleWang, Y.-F., Zheng, Y., Feng, Y., Chen, H., Dai, S.-X., Wang, Y., & Xu, M. (2023). Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum. Molecules, 28(9), 3927. https://doi.org/10.3390/molecules28093927






