Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield of the Assayed Extraction Conditions
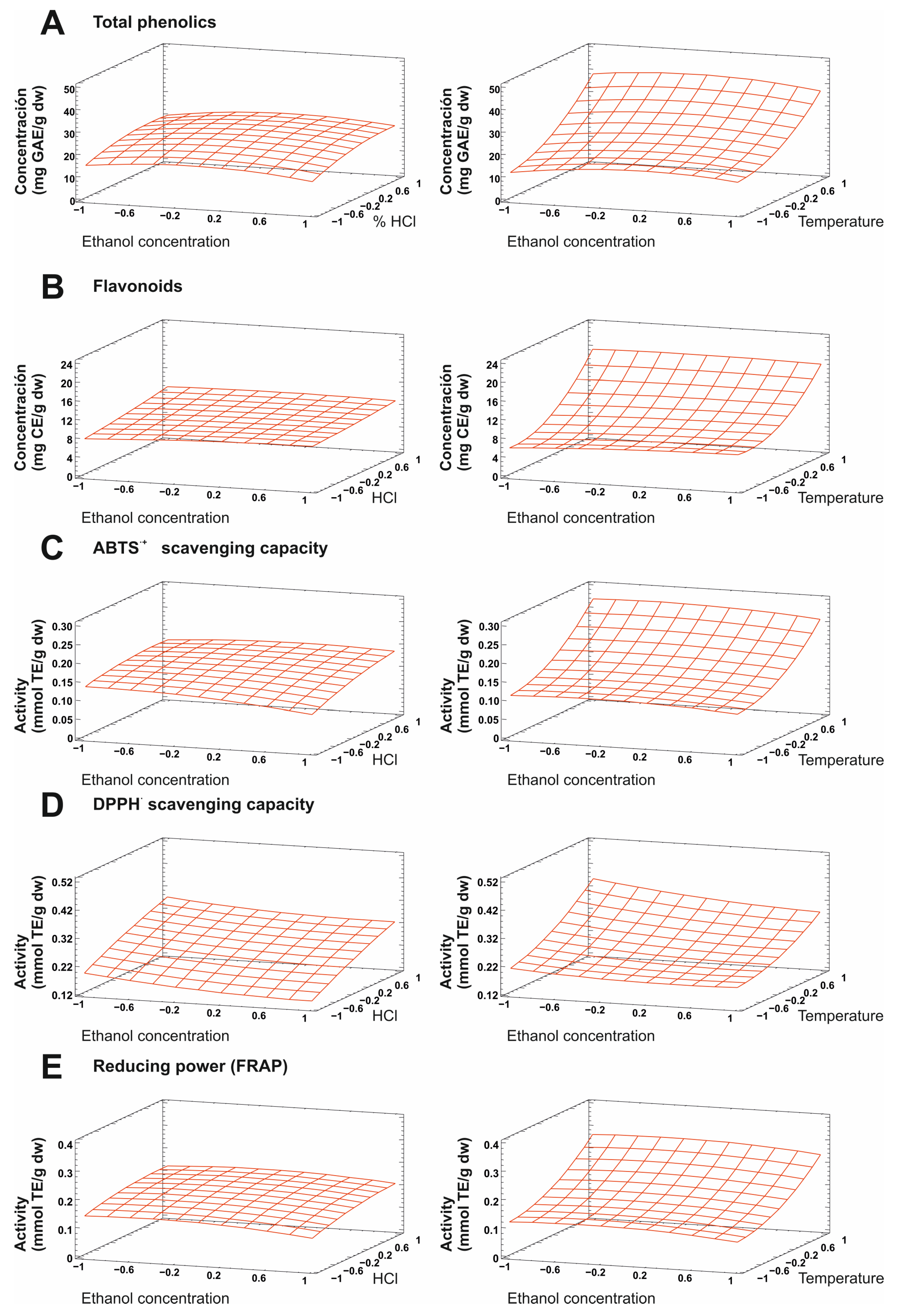
2.2. Model Fitting
2.3. Validation of the Predictive Models Developed
2.4. Quantitative Phenolic Profile via HPLC–DAD–ESI-MS/MS
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction Procedure
3.4. Experimental Design
3.5. Total Phenolics and Flavonoids
3.6. Radical Scavenging Capacity
3.7. HPLC-DAD-ESI-MS/MS Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Queiroz, M.; Oppolzer, D.; Gouvinhas, I.; Silva, A.M.; Barros, A.I.R.N.A.; Domínguez-Perles, R. New grape stems’ isolated phenolic compounds modulate reactive oxygen species, glutathione, and lipid peroxidation in vitro: Combined formulations with vitamins C and E. Fitoterapia 2017, 120, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pérez, A.; Medina, S.; Sánchez-Bravo, P.; Domínguez-Perles, R.; García-Viguera, C. The (Poly)phenolic Profile of Separate Winery By-Products Reveals Potential Antioxidant Synergies. Molecules 2023, 28, 2081. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; Deng, G.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021, 759, 143543. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Texeira, A.; Baenas, N.; Domínguez-Perles, R. Grape stems as a source of bioactive compounds: Application towards added-value commodities and significance for human health. Phytochem. Rev. 2015, 14, 921–931. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, F.; Cañas-Sarazúa, R.; Briones-Labarca, V. Pisco grape pomace: Iron/copper speciation and antioxidant properties, towards their comprehensive utilization. Food Biosci. 2022, 47, 101781. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and antimicrobial properties of wine byproducts and their potential uses in the food industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. FRIN 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Optimization of the extraction of phenolic compounds from red grape marc (Vitis vinifera L.) using response surface methodology. J. Wine Res. 2018, 29, 26–36. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Pereira, D.; de Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. J. Food Meas. Charact. 2019, 13, 1120–1129. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–323. [Google Scholar]
- Predescu, N.C.; Papuc, C.; Nicorescu, V.; Gajaila, A.; Goran, G.V.; Petcu, C.D.; Stefan, T.A. The Influence of Solid-to-Solvent Ratio and Extraction Methodon Total Phenolic Content, Flavonoid Content and AntioxidantProperties of Some Ethanolic Plant Extracts. Rev. Chim. Bucharest 2016, 67, 1922–1927. [Google Scholar]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Morelli, L.L.L.; Prado, M.A. Extraction optimization for antioxidant phenolic compounds in red grape jam using ultrasound with a response surface methodology. Ultrason. Sonochem. 2012, 19, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5. [Google Scholar] [CrossRef]
- Türker, N.; Erdoğdu, F. Effects of pH and temperature of extraction medium on effective diffusion coefficient of anthocynanin pigments of black carrot (Daucus carota var. L.). J. Food Eng. 2006, 76, 579–583. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Spigno, G. Grape marc phenolics: Extraction kinetics, quality and stability of extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Librán, C.M.; Mayor, L.; M. Garcia-Castello, E.; Vidal-Brotons, D. Polyphenol extraction from grape wastes: Solvent and pH effect. Agric. Sci. 2013, 04, 56–62. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T. Deployment of response surface methodology to optimize recovery of grape (Vitis vinifera) stem and seed polyphenols. Procedia Food Sci. 2011, 1, 1686–1693. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ghafoor, K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018, 55, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.H.; da Silva Lindemann, I.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramás, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research—Plant fortification and bioavailability issues. Trends Food Sci. Technol. 2021, 117, 92–105. [Google Scholar] [CrossRef]
- Vámos-Vigyázó, L.; Haard, N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. C R C Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Havlikovfi, L.; Mková, K. Heat Stability of Anthocyanins. Z. Lebensm. Unters. Forsch. 1985, 181, 427–432. [Google Scholar] [CrossRef]
- Abraão, A.S.; Fernandes, N.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus lusitanica L. Fruits as a Novel Source of Bioactive Compounds with Antioxidant Potential: Exploring the Unknown. Antioxidants 2022, 11, 1738. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gongaza, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.d.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Validation of a HPLC-DAD-ESI/MS n method for caffeoylquinic acids separation, quantification and identification in medicinal Helichrysum species from Macaronesia. Food Res. Int. 2012, 45, 362–368. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Maria Carvalho, A.; Santos-Buelga, C. Use of HPLC-DAD-ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Shaheen, F.; Ali, L.; Ali, S.; Erdemoglu, N.; Sener, B. Antioxidant flavonoids from Tamus communis ssp. cretica. Chem. Nat. Compd. 2009, 45, 346–349. [Google Scholar] [CrossRef]
- Potential, R.; Synergies, A.; Costa-p, A.; Medina, S.; Paola, S. The (Poly) Phenolic Profile of Separate Winery By-Products; 2023; pp. 1–26. [Google Scholar]
- Zhao, X.; Zhang, S.S.; Zhang, X.K.; He, F.; Duan, C.Q. An effective method for the semi-preparative isolation of high-purity anthocyanin monomers from grape pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Pan, Q.; Cui, X.; Duan, C. Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four. Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-Color Related Phenolic Composition of Garnacha Tintorera (Vitis vinifera L.) Grapes and Red Wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Trikas, E.D.; Melidou, M.; Papi, R.M.; Zachariadis, G.A.; Kyriakidis, D.A. Extraction, separation and identification of anthocyanins from red wine by-product and their biological activities. J. Funct. Foods 2016, 25, 548–558. [Google Scholar] [CrossRef]
- Maier, T.; Göppert, A.; Kammerer, D.R.; Schieber, A.; Carle, R. Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. Eur. Food Res. Technol. 2008, 227, 267–275. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; Martínez-Valverde, I.; Chesson, A.; Provan, G. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Garcia, J.; Granato, D.; Barros, A. Seed Phytochemical Profiling of Three Olive Cultivars, Antioxidant Capacity, Enzymatic Inhibition, and Effects on Human Neuroblastoma Cells (SH-SY5Y). Molecules 2022, 27, 5057. [Google Scholar] [CrossRef]
| Assay | Coded Level | TPC (mg GAE/g dw) | FC (mg CE/g dw) | ABTS (mmol TEAC/g dw) | DPPH (mmol TEAC/g dw) | FRAP (mmol TEAC/g dw) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol Concentration (%) | pH (% of HCl) | Temperature (°C) | Observed | Predicted | Observed | Predicted | Observed | Predicted | Observed | Predicted | Observed | Predicted | |
| 1 | −1 (50) | −1 (0.5) | −1 (20) | 8.84 | 8.49 | 5.93 | 5.18 | 0.09 | 0.10 | 0.09 | 0.10 | 0.11 | 0.11 |
| 2 | −1 (50) | −1 (0.5) | 0 (40) | 15.77 | 14.81 | 8.49 | 7.45 | 0.15 | 0.14 | 0.15 | 0.14 | 0.15 | 0.14 |
| 3 | −1 (50) | −1 (0.5) | 1 (60) | 31.88 | 31.72 | 15.11 | 16.77 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| 4 | −1 (50) | 0 (2.0) | −1 (20) | 10.56 | 11.64 | 6.38 | 5.84 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| 5 | −1 (50) | 0 (2.0) | 0 (40) | 18.34 | 19.85 | 8.83 | 8.75 | 0.15 | 0.16 | 0.15 | 0.16 | 0.17 | 0.17 |
| 6 | −1 (50) | 0 (2.0) | 1 (60) | 38.06 | 38.64 | 18.46 | 18.70 | 0.27 | 0.27 | 0.27 | 0.27 | 0.29 | 0.30 |
| 7 | −1 (50) | 1 (3.5) | −1 (20) | 11.97 | 10.21 | 6.35 | 6.87 | 0.12 | 0.11 | 0.12 | 0.11 | 0.12 | 0.12 |
| 8 | −1 (50) | 1 (3.5) | 0 (40) | 19.59 | 20.31 | 10.41 | 10.41 | 0.17 | 0.16 | 0.17 | 0.16 | 0.20 | 0.18 |
| 9 | −1 (50) | 1 (3.5) | 1 (60) | 41.64 | 40.98 | 7.40 | 21.00 | 0.28 | 0.28 | 0.28 | 0.28 | 0.31 | 0.32 |
| 10 | 0 (70) | −1 (0.5) | −1 (20) | 11.87 | 12.04 | 5.43 | 6.61 | 0.11 | 0.10 | 0.11 | 0.10 | 0.11 | 0.11 |
| 11 | 0 (70) | −1 (0.5) | 0 (40) | 16.75 | 17.53 | 7.47 | 8.44 | 0.13 | 0.13 | 0.13 | 0.13 | 0.14 | 0.14 |
| 12 | 0 (70) | −1 (0.5) | 1 (60) | 34.71 | 33.62 | 18.37 | 17.33 | 0.24 | 0.24 | 0.24 | 0.24 | 0.25 | 0.25 |
| 13 | 0 (70) | 0 (2.0) | −1 (20) | 14.63 | 15.79 | 5.24 | 6.91 | 0.11 | 0.12 | 0.11 | 0.12 | 0.12 | 0.13 |
| 14 | 0 (70) | 0 (2.0) | 0 (40) | 23.91 | 23.17 | 10.75 | 9.39 | 0.15 | 0.16 | 0.15 | 0.16 | 0.19 | 0.18 |
| 15 | 0 (70) | 0 (2.0) | 1 (60) | 41.72 | 41.14 | 20.11 | 18.90 | 0.28 | 0.27 | 0.28 | 0.27 | 0.31 | 0.30 |
| 16 | 0 (70) | 1 (3.5) | −1 (20) | 16.38 | 14.96 | 7.34 | 4.89 | 0.13 | 0.13 | 0.13 | 0.13 | 0.12 | 0.13 |
| 17 | 0 (70) | 1 (3.5) | 0 (40) | 22.48 | 24.21 | 11.15 | 1070 | 0.17 | 0.18 | 0.17 | 0.18 | 0.19 | 0.18 |
| 18 | 1 (70) | 1 (3.5) | 1 (60) | 44.93 | 40.71 | 22.95 | 20.09 | 0.30 | 0.29 | 0.30 | 0.29 | 0.36 | 0.31 |
| 19 | 1 (90) | −1 (0.5) | −1 (20) | 8.74 | 9.15 | 7.38 | 7.42 | 0.06 | 0.07 | 0.06 | 0.07 | 0.08 | 0.08 |
| 20 | 1 (90) | −1 (0.5) | 0 (40) | 15.03 | 13.82 | 9.27 | 8.83 | 0.11 | 0.10 | 0.11 | 0.10 | 0.12 | 0.11 |
| 21 | 1 (90) | −1 (0.5) | 1 (60) | 26.67 | 29.08 | 17.85 | 17.28 | 0.19 | 0.21 | 0.19 | 0.21 | 0.21 | 0.22 |
| 22 | 1 (90) | 0 (2.0) | −1 (20) | 12.77 | 13.49 | 8.16 | 7.38 | 0.11 | 0.10 | 0.11 | 0.10 | 0.12 | 0.10 |
| 23 | 1 (90) | 0 (2.0) | 0 (40) | 24.9 | 20.15 | 9.53 | 9.41 | 0.16 | 0.15 | 0.16 | 0.15 | 0.15 | 0.15 |
| 24 | 1 (90) | 0 (2.0) | 1 (60) | 36.07 | 37.19 | 16.31 | 18.50 | 0.26 | 0.26 | 0.26 | 0.26 | 0.27 | 0.28 |
| 25 | 1 (90) | 1 (3.5) | −1 (20) | 13.26 | 13.25 | 9.28 | 7.70 | 0.12 | 0.12 | 0.12 | 0.12 | 0.11 | 0.10 |
| 26 | 1 (90) | 1 (3.5) | 0 (40) | 18.66 | 21.69 | 7.84 | 10.38 | 0.16 | 0.17 | 0.16 | 0.17 | 0.14 | 0.17 |
| 27 | 1 (90) | 1 (3.5) | 1 (60) | 38.11 | 40.71 | 18.48 | 20.08 | 0.28 | 0.29 | 0.28 | 0.29 | 0.28 | 0.31 |
| Variable | Statistic | X1 | X2 | X3 | X1,2 | X1,3 | X2,3 | X12 | X22 | X32 | Model F-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | p-value | 0.851 | *** | *** | 0.358 | 0.205 | * | ** | * | *** | 0.16 |
| F-value | 0.04 | 39.58 | 569.91 | 0.89 | 1.74 | 8.32 | 11.26 | 6.31 | 33.61 | ||
| FC | p-value | 0.41 | * | * | 0.49 | 0.40 | 0.25 | 0.66 | 0.79 | *** | 0.20 |
| F-value | 0.71 | 7.41 | 209.99 | 0.51 | 0.76 | 1.45 | 0.20 | 0.08 | 27.46 | ||
| ABTS | p-value | ** | *** | *** | * | 0.652 | 0.063 | 0.070 | 0.078 | *** | 0.59 |
| F-value | 8.86 | 73.93 | 858.08 | 12.73 | 0.21 | 3.94 | 3.74 | 3.52 | 59.27 | ||
| DPPH | p-value | *** | *** | *** | 0.539 | * | 0.088 | 0.121 | 0.287 | ** | 0.89 |
| F-value | 32.34 | 208.97 | 270.04 | 0.40 | 6.79 | 3.36 | 2.72 | 1.23 | 15.82 | ||
| FRAP | p-value | * | *** | *** | 0.428 | 0.998 | ** | 0.072 | 0.141 | *** | 0.05 |
| F-value | 6.17 | 30.14 | 384.69 | 0.66 | 0.01 | 10.75 | 3.68 | 2.38 | 23.94 | ||
| Polynomial model | R2 | MAE | |||||||||
| TPC = 23.1678 + 0.0988027X1 + 3.33974X2 + 12.6736X3 − 3.21974X12 + 0.591537X1X2 − 0.825963X1X3 − 2.29359X22 + 1.88461X2X3 + 5.29474X32 | 0.962 | 0.010 | |||||||||
| FC = 9.38469 + 0.333288X1 + 1.12598X2 + 5.99543X3 − 0.305237X12 − 0.355901X1X2 − 0.435901X1X3 + 0.184871X22 + 0.636473X2X3 + 3.5232X32 | 0.909 | 0.989 | |||||||||
| ABTS = 0.161795 − 0.00761641X1 + 0.0226204X2 + 0.0770648X3 − 0.0092011X12 + 0.0110754X1X2 − 0.00142462X1X3 − 0.00849074X22 + 0.00643056X2X3 + 0.0348426X32 | 0.975 | 0.008 | |||||||||
| DPPH = 0.2413529 − 0.0218333X1 + 0.0690476X2 + 0.0784921X3 + 0.010625X12 + 0.00308333X1X2 − 0.01275X1X3 − 0.00761905X22 + 0.0119048X2X3 + 0.027381X32 | 0.958 | 0.100 | |||||||||
| FRAP = 0.178926 − 0.0105464X1 + 0.0239717X2 + 0.0856384X3 − 0.0151381X12 + 0.0041814X1X2 + 0.0000137318X1X3 − 0.0115839X22 + 0.0176242X2X3 + 0.0367495X32 | 0.945 | 0.010 | |||||||||
| Response | Process Variables | Predicted Values at the Optimal Conditions | ||
|---|---|---|---|---|
| Ethanol Concentration (%) | HCl Concentration (%) | Temperature (°C) | ||
| TPC (mg GAE/g dw) | 69.6 | 3.5 | 60.0 | 44.066 |
| FC (mg CE/g dw) | 55.1 | 3.5 | 60.0 | 21.022 |
| ABTS (mmol TEAC/g dw) | 72.1 | 3.5 | 60.0 | 0.294 |
| DPPH (mmol TEAC/g dw) | 53.0 | 3.5 | 60.0 | 0.456 |
| FRAP (mmol TEAC/g dw) | 65.8 | 3.5 | 60.0 | 0.332 |
| Peak | Rt | λmax | [M–H]− m/z | MS2 [M–H]− (Relative Abundance) | Tentative Identification | Concentration (mg mL−1) |
|---|---|---|---|---|---|---|
| 1 | 4.08 | 321 | 353 | 191 (100), 179 (61), 173 (4), 161 (8), 135 (17) | 3-O-Caffeoylquinic acid | 1.082 ± 0.012 |
| 2 | 5.12 | 322 | 337 | 163 (100) | p-Coumaroylquinic acid | 0.072 ± 0.002 |
| 3 | 5.26 | 321 | 353 | 191 (100), 179 (23), 173 (31), 161 (9) | 5-O-Caffeoylquinic acid | 0.059 ± 0.001 |
| 4 | 5.75 | 335 | 401 | 269 (100) | Apigenin-O-pentoside | 0.104 ± 0.004 |
| 5 | 13.18 | 343 | 609 | 301 (100) | Quercetin-3-O-rutinoside | 0.377 ± 0.002 |
| 6 | 13.76 | 348 | 625 | 317 (100) | Myricetin-O-rutinoside | 0.203 ± 0.002 |
| 7 | 14.39 | 342 | 463 | 301 (100) | Quercetin-3-O-glucoside | 0.097 ± 0.002 |
| 8 | 14.94 | 336 | 577 | 431 (36), 285 (100) | Kaempferol 3′,4′-di-O-rhamnoside | 0.043 ± 0.002 |
| 9 | 16.24 | 347 | 433 | 301 (100) | Quercetin-O-pentoside | 0.312 ± 0.003 |
| 10 | 16.68 | 349 | 433 | 301 (100) | Quercetin-O-pentoside | 0.059 ± 0.002 |
| 11 | 18.35 | 520 | 465 | 303 (100) | Delphinidin-3-O-glucoside | 0.007 ± 0.000 |
| 12 | 19.57 | 520 | 463 | 301 (100) | Peonidin-3-O-glucoside | 0.016 ± 0.001 |
| 13 | 20.71 | 520 | 493 | 331 (100) | Malvidin-3-O-glucoside | 0.013 ± 0.001 |
| 14 | 25.35 | 520 | 625 | 317 (100) | Petunidin-3-(6″coumaroyl)-glucoside | 0.008 ± 0.000 |
| Total Phenolic acid | 1.212 ± 0.015 | |||||
| Total Flavonoids | 1.195 ± 0.017 | |||||
| Total Anthocyanins | 0.044 ± 0.002 | |||||
| Total Phenolic compounds | 2.407 ± 0.032 | |||||
| Independent Variables | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ethanol concentration (%) | X1 | 50 | 70 | 90 |
| pH (% of HCl) | X2 | 0.5 | 2.0 | 3.5 |
| Temperature (°C) | X3 | 20 | 40 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. https://doi.org/10.3390/molecules28093885
Moutinho J, Gouvinhas I, Domínguez-Perles R, Barros A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules. 2023; 28(9):3885. https://doi.org/10.3390/molecules28093885
Chicago/Turabian StyleMoutinho, Joana, Irene Gouvinhas, Raúl Domínguez-Perles, and Ana Barros. 2023. "Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications" Molecules 28, no. 9: 3885. https://doi.org/10.3390/molecules28093885
APA StyleMoutinho, J., Gouvinhas, I., Domínguez-Perles, R., & Barros, A. (2023). Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules, 28(9), 3885. https://doi.org/10.3390/molecules28093885








