Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry
Abstract
1. Introduction
2. Results
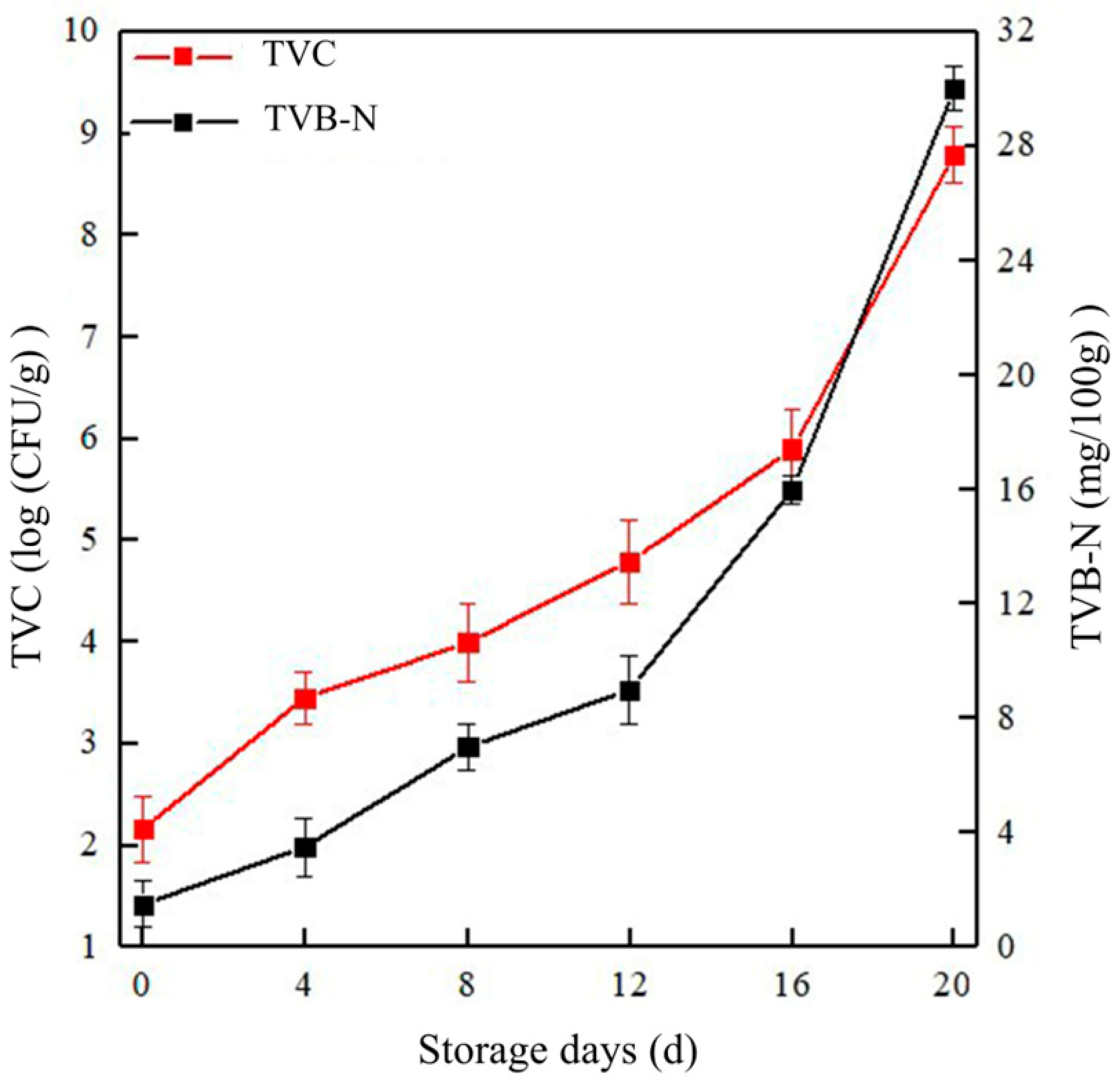
2.1. Experimental Results Obtained for TVB-N and TVC
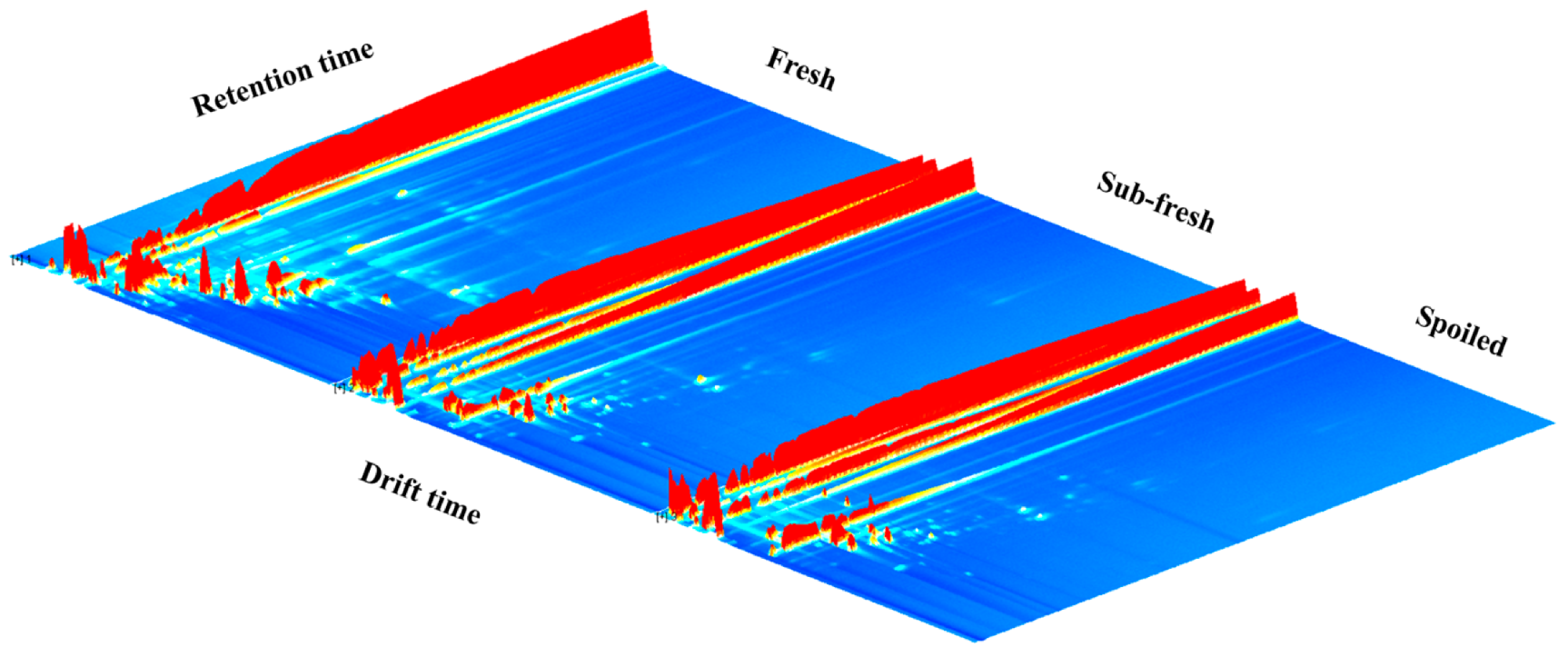
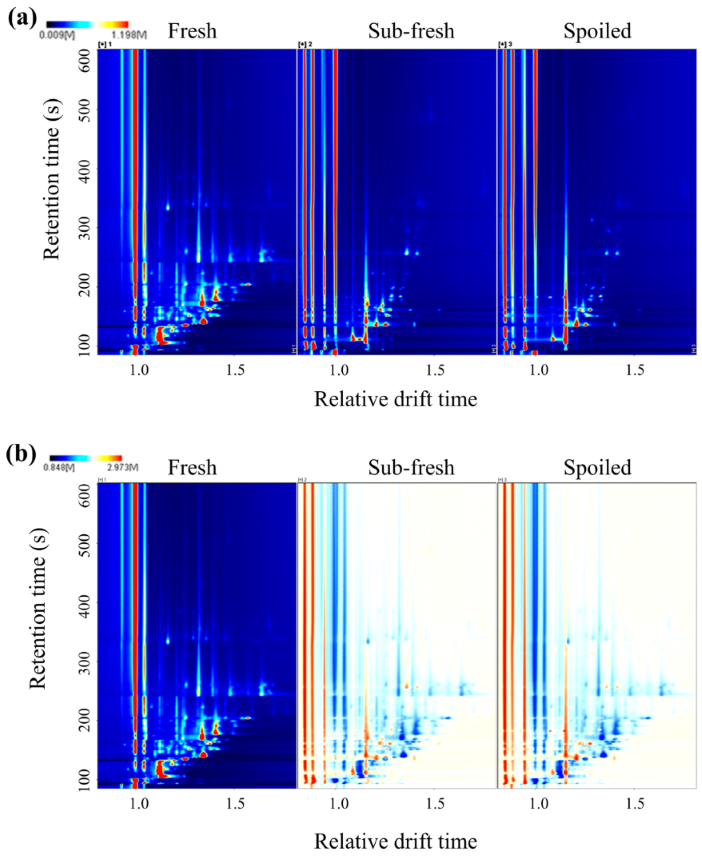
2.2. GC-IMS Atlas Analysis
2.3. Analysis of the VOCs in Goat Meat
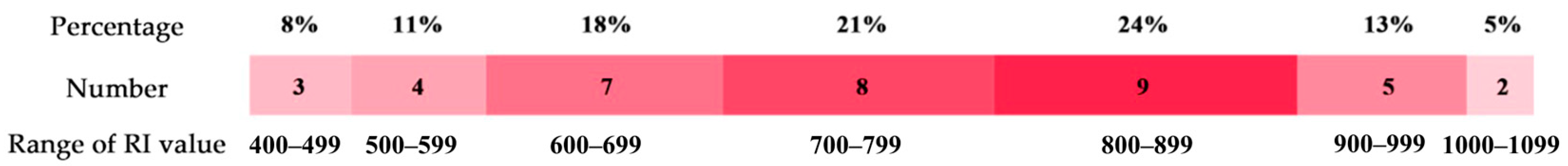
2.3.1. Retention Index Distribution of VOCs in Goat Meat
2.3.2. Characteristic Distribution of VOCs in Goat Meat
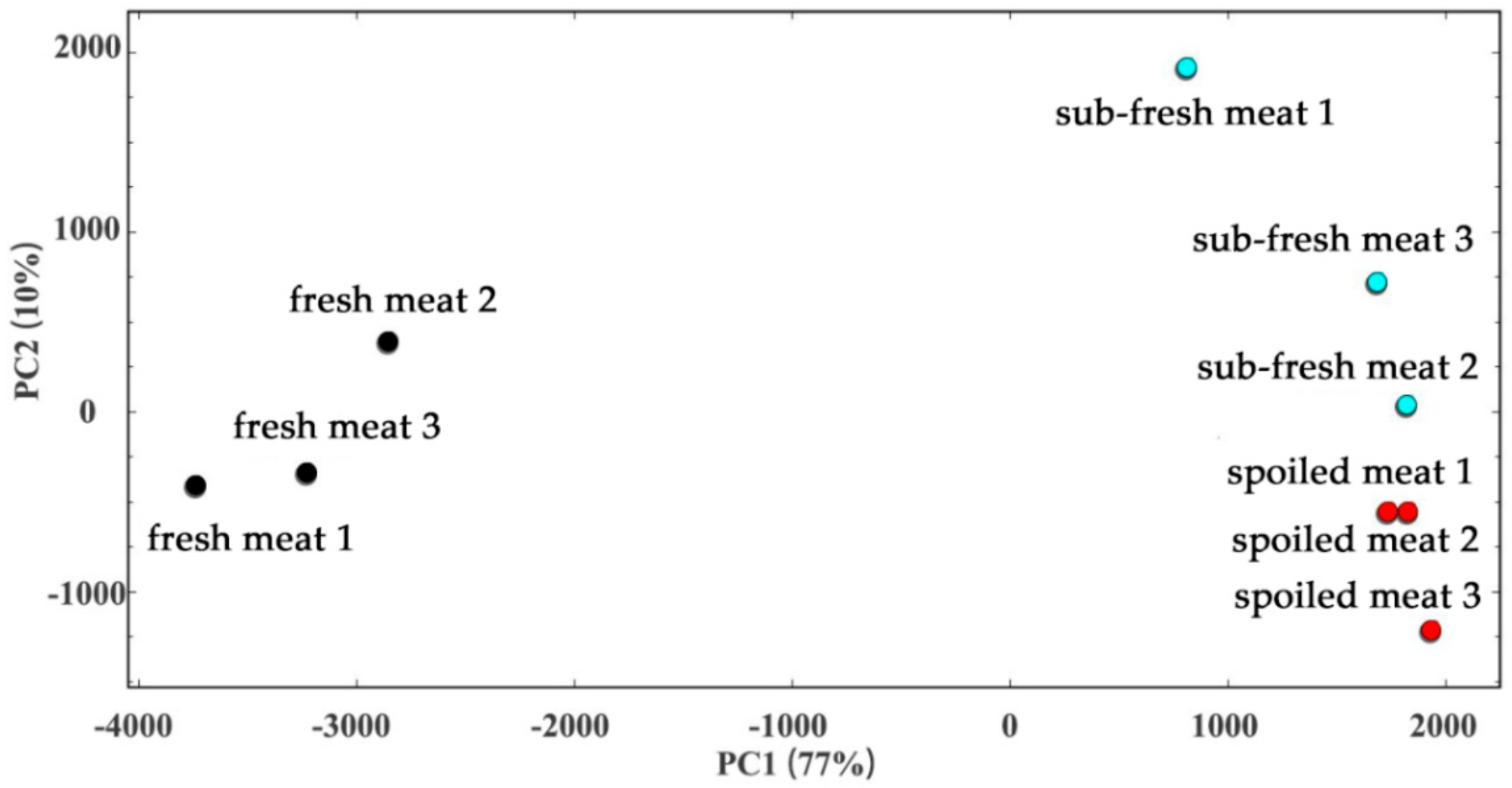
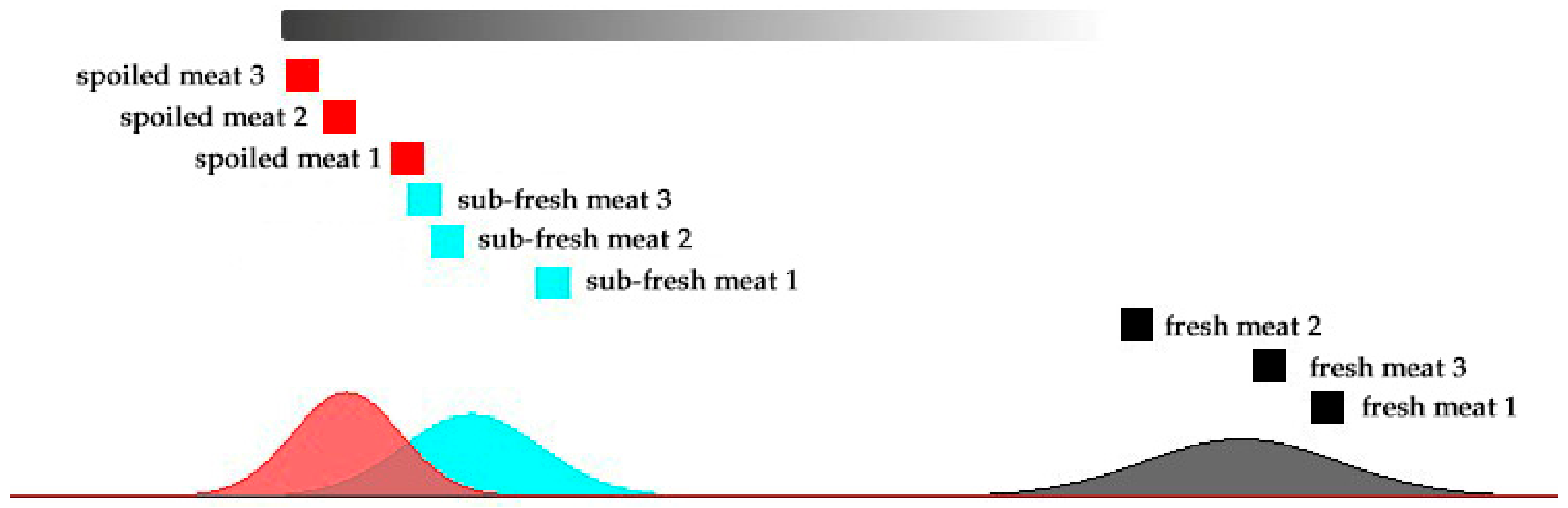
2.4. Cluster Analysis of the Meat Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Processing
4.3. Determination of TVB-N and TVCs
4.4. The experimental Method of GC-IMS
4.4.1. Instrumentation
4.4.2. GC-IMS Conditions
4.4.3. Detection Method
4.4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jia, W.; Di, C.; Shi, L. Applications of Lipidomics in Goat Meat Products: Biomarkers, Structure, Nutrition Interface and Future Perspectives. J. Proteom. 2023, 270, 104753. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, S.; Pavlović, M.; Pavlović, I.; Tasić, A.; Janjić, J.; Baltić, M.Ž. Influence of Breed on Selected Quality Parameters of Fresh Goat Meat. Arch. Anim. Breed. 2020, 63, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The Potential of Goat Meat in the Red Meat Industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat Spoilage during Distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat Quality of Santa Inês Lamb Chilled-Then-Frozen Storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef]
- Yan, T.; Hou, C.; Wang, Z.; Li, X.; Chen, L.; Liang, C.; Xu, Y.; Zhang, D. Effects of Chilling Rate on Progression of Rigor Mortis in Postmortem Lamb Meat. Food Chem. 2022, 373, 131463. [Google Scholar] [CrossRef]
- Liu, H.; Saito, Y.; Riza, D.F.A.; Kondo, N.; Yang, X.; Han, D. Rapid Evaluation of Quality Deterioration and Freshness of Beef during Low Temperature Storage Using Three-Dimensional Fluorescence Spectroscopy. Food Chem. 2019, 287, 369–374. [Google Scholar] [CrossRef]
- Weng, X.; Luan, X.; Kong, C.; Chang, Z.; Li, Y.; Zhang, S.; Al-Majeed, S.; Xiao, Y. A Comprehensive Method for Assessing Meat Freshness Using Fusing Electronic Nose, Computer Vision, and Artificial Tactile Technologies. J. Sens. 2020, 2020, e8838535. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Bazar, G.; Gillay, Z.; Kovacs, Z. Emerging Trends of Advanced Sensor Based Instruments for Meat, Poultry and Fish Quality—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3443–3460. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Delgado-Pando, G. Sensory Analysis and Consumer Research in New Meat Products Development. Foods 2021, 10, 429. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of Electronic Nose (e-Nose) and Electronic Tongue (e-Tongue) in Food Quality-Related Properties Determination: A Review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Roy, M.; Yadav, B.K. Electronic Nose for Detection of Food Adulteration: A Review. J. Food Sci. Technol. 2022, 59, 846–858. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman Spectroscopy Coupled with Chemometrics for Food Authentication: A Review. TrAC Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ara, P.; Ehsani, R.J. A Comprehensive Review on Food Applications of Terahertz Spectroscopy and Imaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1563–1621. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, Y.; Han, Y.; Li, Z.; Yang, Z.; Chai, Q.; Wang, W.; Zhang, Y.; Fu, C. A Review of the Discriminant Analysis Methods for Food Quality Based on Near-Infrared Spectroscopy and Pattern Recognition. Molecules 2021, 26, 749. [Google Scholar] [CrossRef]
- Steinhaus, M. Chapter 9: Gas Chromatography–Olfactometry: Principles, Practical Aspects and Applications in Food Analysis. In Advanced Gas Chromatography in Food Analysis; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 337–399. [Google Scholar]
- Egea, M.B.; Bertolo, M.R.V.; de Oliveira Filho, J.G.; Lemes, A.C. A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules 2021, 26, 5181. [Google Scholar] [CrossRef]
- Raimbault, A.; Noireau, A.; West, C. Analysis of Free Amino Acids with Unified Chromatography-Mass Spectrometry—Application to Food Supplements. J. Chromatogr. A 2020, 1616, 460772. [Google Scholar] [CrossRef]
- Putri, S.P.; Ikram, M.M.M.; Sato, A.; Dahlan, H.A.; Rahmawati, D.; Ohto, Y.; Fukusaki, E. Application of Gas Chromatography-Mass Spectrometry-Based Metabolomics in Food Science and Technology. J. Biosci. Bioeng. 2022, 133, 425–435. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current Role of Modern Chromatography and Mass Spectrometry in the Analysis of Mycotoxins in Food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Xiao, L.; Zhang, X.; Yang, C.; Li, Z.; Zhu, M.; Liu, Z.; Wang, Y. Discrimination and Characterization of the Volatile Profiles of Five Fu Brick Teas from Different Manufacturing Regions by Using HS–SPME/GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H.; et al. Application and Development Trends of Gas Chromatography–Ion Mobility Spectrometry for Traditional Chinese Medicine, Clinical, Food and Environmental Analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Zhu, M.; He, C.; Li, Z.; Wang, Y.; Liu, Z. Characteristic Fingerprints and Change of Volatile Organic Compounds of Dark Teas during Solid-State Fermentation with Eurotium Cristatum by Using HS-GC-IMS, HS-SPME-GC-MS, E-Nose and Sensory Evaluation. LWT 2022, 169, 113925. [Google Scholar] [CrossRef]
- Castell, A.; Arroyo-Manzanares, N.; de Dios Hernandez, J.; Guillén, I.; Vizcaíno, P.; López-García, I.; Hernández-Córdoba, M.; Viñas, P. Ion Mobility Spectrometry as an Emerging Tool for Characterization of the Volatile Profile and Identification of Microbial Growth in Pomegranate Juice. Microchem. J. 2022, 174, 107099. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.; Du, D. Recent Development of HS-GC-IMS Technology in Rapid and Non-Destructive Detection of Quality and Contamination in Agri-Food Products. TrAC Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS Based Volatile Profile Characterization of the Chinese Dry-Cured Hams from Different Regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and Characterization of the Volatile Organic Compounds in Eight Kinds of Huajiao with Geographical Indication of China Using Electronic Nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, C.; Gao, L.; Zhuang, H.; Feng, T.; Xu, G. Analysis of Volatile Flavor Compounds of Green Wheat under Different Treatments by GC-MS and GC-IMS. J. Food Biochem. 2022, 46, e13875. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N.; Zhang, H. Analysis of Flavor Formation during Production of Dezhou Braised Chicken Using Headspace-Gas Chromatography-Ion Mobility Spec-Trometry (HS-GC-IMS). Food Chem. 2022, 370, 130989. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic Fingerprints and Volatile Flavor Compound Variations in Liuyang Douchi during Fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Tian, L.; Zeng, Y.; Zheng, X.; Chiu, Y.; Liu, T. Detection of Peanut Oil Adulteration Mixed with Rapeseed Oil Using Gas Chromatography and Gas Chromatography–Ion Mobility Spectrometry. Food Anal. Methods 2019, 12, 2282–2292. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Cui, D.; Fang, X.; Gao, J.; Liu, Y. Fast and Non-Destructive Profiling of Commercial Coffee Aroma under Three Conditions (Beans, Powder, and Brews) Using GC-IMS. Molecules 2022, 27, 6262. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of Volatile Compounds and Flavor Fingerprint in Jingyuan Lamb of Different Ages Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Han, W.; Gui, L.; Yuan, Z.; Hou, S.; Wang, Z.; Yang, B.; Raza, S.H.A.; Alowais, A.F.S.; et al. Effect of Different Heat Treatments on the Quality and Flavor Compounds of Black Tibetan Sheep Meat by HS-GC-IMS Coupled with Multivariate Analysis. Molecules 2023, 28, 165. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Yuan, C.; Xi, B.; Yang, B.; Meng, X.; Guo, T.; Yue, Y.; Gao, Y.; Liu, J.; et al. Evaluation of Mutton Quality Characteristics of Dongxiang Tribute Sheep Based on Membership Function and Gas Chromatography and Ion Mobility Spectrometry. Front. Nutr. 2022, 9, 852399. [Google Scholar] [CrossRef]
- He, X.; Wang, H.; Yang, X.; Xi, B.; Li, W.; Wang, F.; Li, H.; Gao, Y. The Effects of Frozen Storage on Fatty Acids, Amino Acids, and Volatile Compounds in Mutton Stored for 90 Days. J. Food Process. Preserv. 2022, 46, e16518. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Zhao, X.; He, Y. Application of ε-Polylysine in Extending the Storage Period of Pork Jerky. Food Sci. Nutr. 2021, 9, 3250–3257. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, D.; Zheng, X.; Wen, X.; Yan, T.; Zhang, Z.; Hou, C. Effects of Different Storage Temperatures on the Physicochemical Properties and Bacterial Community Structure of Fresh Lamb Meat. Food Sci. Anim. Resour. 2021, 41, 509–526. [Google Scholar] [CrossRef]
- Wan, J.; Liu, Q.; Ma, C.; Muhoza, B.; Huang, Y.; Sun, M.; Song, S.; Ho, C.-T. Characteristic Flavor Fingerprint Disclosure of Dzo Beef in Tibet by Applying SAFE-GC-O-MS and HS-GC-IMS Technology. Food Res. Int. 2023, 166, 112581. [Google Scholar] [CrossRef]
- Zhu, W.; Benkwitz, F.; Kilmartin, P.A. Volatile-Based Prediction of Sauvignon Blanc Quality Gradings with Static Headspace–Gas Chromatography–Ion Mobility Spectrometry (SHS–GC–IMS) and Interpretable Machine Learning Techniques. J. Agric. Food Chem. 2021, 69, 3255–3265. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Arroyo-Manzanares, N.; Arce, C.; Arce, L. HS-GC-IMS and Chemometric Data Treatment for Food Authenticity Assessment: Olive Oil Mapping and Classification through Two Different Devices as an Example. Food Control 2019, 98, 82–93. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium macrocarpon Ait.) Using Gas Chromatography–Olfactometry (GC-O) and Odor Activity Value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of Volatile Compounds in Chilled Cod (Gadus morhua) Fillets by Gas Chromatography and Detection of Quality Indicators by an Electronic Nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, Y.; Xiao, Z. Evaluation of Perceptual Interactions between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model. Foods 2020, 9, 183. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Dai, Q.; Mo, H.; Liu, Z.; Pu, H.; Zhu, X.; Yao, L.; Xu, D.; Hu, L. Blended Cumin/Zanthoxylum Essential Oil Improve the Antibacterial, Fresh-Keeping Performance and Flavor of Chilled Fresh Mutton. Meat Sci. 2023, 200, 109173. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of Volatile Component Changes in Jujube Fruits during Cold Storage by Using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Ji, X.; Min, W.; Yan, W. HS-GC-IMS Detection of Volatile Organic Compounds in Yak Milk Powder Processed by Different Drying Methods. LWT 2021, 141, 110855. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; He, J.; Chen, L.; Zhang, J.; Jin, Y.; Zhou, J.; Zhang, Y. A Green Triple-Locked Strategy Based on Volatile-Compound Imaging, Chemometrics, and Markers to Discriminate Winter Honey and Sapium Honey Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Food Res. Int. 2019, 119, 960–967. [Google Scholar] [CrossRef]
- Bassey, A.P.; Boateng, E.F.; Zhu, Z.; Zhou, T.; Nasiru, M.M.; Guo, Y.; Dou, H.; Ye, K.; Li, C.; Zhou, G. Volatilome Evaluation of Modified Atmosphere Packaged Chilled and Super-Chilled Pork Loins Using Electronic Nose and HS-GC-IMS Integration. Food Packag. Shelf Life 2022, 34, 100953. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Chu, J.; Sun, C.; Lin, S. Vegetable Extracts: Effective Inhibitors of Heterocyclic Aromatic Amines and Advanced Glycation End Products in Roasted Mackerel. Food Chem. 2023, 412, 135559. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, C.; Wang, Y.; Yang, X.; Zhang, J.; Ji, Z. Nondestructive Determination of Salmon Fillet Freshness during Storage at Different Temperatures by Electronic Nose System Combined with Radial Basis Function Neural Networks. Int. J. Food Sci. Technol. 2020, 55, 2080–2091. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, G.; Luo, Y.; Pu, Y.; Ge, C.; Liao, G. Effect of Natural Spices on Precursor Substances and Volatile Flavor Compounds of Boiled Wuding Chicken during Processing. Flavour Fragr. J. 2020, 35, 570–583. [Google Scholar] [CrossRef]
- Duan, S.; Tang, X.; Li, W.; Huang, X. Analysis of the Differences in Volatile Organic Compounds in Different Muscles of Pork by GC-IMS. Molecules 2023, 28, 1726. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of Volatile Flavor Compounds and Characterization of Aroma-Active Compounds of Water-Boiled Salted Duck Using GC–MS–O, GC–IMS, and E-Nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Liu, X.-S.; Liu, J.-B.; Yang, Z.-M.; Song, H.-L.; Liu, Y.; Zou, T.-T. Aroma-Active Compounds in Jinhua Ham Produced with Different Fermentation Periods. Molecules 2014, 19, 19097–19113. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of Aging on Volatile Compounds in Cooked Beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Wu, Q.; Gu, Q.; Chen, M.; Zhang, Y.; Sun, X.; Zhang, J. High-Throughput Sequencing Analysis of Bacterial Community Composition and Quality Characteristics in Refrigerated Pork during Storage. Food Microbiol. 2019, 83, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Du, Y.; Yang, C.; Li, L.; Wang, S.; Liu, Y.; Wang, W. Development of PVA/EVA-Based Bilayer Active Film and Its Application to Mutton. LWT 2020, 133, 110109. [Google Scholar] [CrossRef]


| Compounds | MW | RI | Rt (s) | Dt (RIPrel) | The Peak Intensity | ||
|---|---|---|---|---|---|---|---|
| Fresh Meat | Sub-Fresh Meat | Spoiled Meat | |||||
| Nonanal | 142.2 | 1109.4 | 507.00 | 1.47224 | 134.67 ± 16.47 | 112.6 ± 14.37 | 100.36 ± 7.2 |
| 6-Methyl-5-hepten-2-one | 126.2 | 982 | 332.67 | 1.16872 | 819.63 ± 117.43 | 199.81 ± 26.1 | 292 ± 31.68 |
| 2-Pentylfuran | 138.2 | 994.4 | 343.20 | 1.26022 | 152.99 ± 57.26 | 20.31 ± 2.71 | 21.56 ± 4.07 |
| 2-Octanone | 128.2 | 988.9 | 338.52 | 1.35933 | 201.15 ± 53.1 | 30.03 ± 8.61 | 25.28 ± 4.49 |
| 2-Heptanone-M | 114.2 | 894.4 | 258.57 | 1.25869 | 277.81 ± 43.47 | 80.54 ± 15.88 | 67.6 ± 5.84 |
| 2-Heptanone-D | 114.2 | 894 | 258.18 | 1.63533 | 651.06 ± 315.24 | 35.53 ± 1.95 | 35.14 ± 7.79 |
| 1-Hexanol-M | 102.2 | 872.6 | 246.48 | 1.32121 | 1367.78 ± 204.33 | 138.8 ± 68.68 | 88.76 ± 11.05 |
| 1-Hexanol-D | 102.2 | 869.6 | 244.92 | 1.63838 | 1027.97 ± 298.11 | 36.27 ± 5.67 | 35.33 ± 6.1 |
| (E)-2-Heptenal | 112.2 | 954.4 | 309.27 | 1.26022 | 87.47 ± 17.56 | 17.1 ± 4.38 | 20.72 ± 5.89 |
| Furfural-M | 96.1 | 829.1 | 223.86 | 1.08333 | 228.79 ± 201.4 | 77.46 ± 14.29 | 59.18 ± 2.91 |
| Furfural-D | 96.1 | 828.3 | 223.47 | 1.34408 | 379.64 ± 260.16 | 38.96 ± 4.74 | 38.45 ± 4.11 |
| Hexanal-M | 100.2 | 791.3 | 204.23 | 1.25743 | 769.3 ± 78.18 | 108.25 ± 30.82 | 91.89 ± 28.01 |
| Hexanal-D | 100.2 | 791.3 | 204.23 | 1.56718 | 951.23 ± 239.23 | 21.78 ± 4.46 | 24.85 ± 1.3 |
| 2-Hexanone-M | 100.2 | 781.7 | 199.51 | 1.1807 | 67.42 ± 8.89 | 40.13 ± 28.33 | 15.4 ± 2.08 |
| 2-Hexanone-D | 100.2 | 781 | 199.27 | 1.49755 | 370.76 ± 77.81 | 19.05 ± 2.6 | 19.47 ± 2.54 |
| 1-Pentanol-M | 88.1 | 763.4 | 192.06 | 1.24606 | 506.38 ± 62.43 | 141.63 ± 74.77 | 69.22 ± 5.47 |
| 1-Pentanol-D | 88.1 | 759.7 | 190.57 | 1.51176 | 361.02 ± 52.76 | 19.02 ± 2.61 | 17.43 ± 4.73 |
| Isopentanol | 88.1 | 728 | 177.66 | 1.49613 | 332 ± 117.62 | 27.71 ± 18 | 27.62 ± 12.22 |
| 3-Hydroxy-2-butanone | 88.1 | 709.3 | 170.04 | 1.33844 | 3349.22 ± 1203.65 | 140.62 ± 26.97 | 110.76 ± 6.14 |
| Pentanal | 86.1 | 693.8 | 163.75 | 1.42336 | 97.36 ± 27.32 | 4.41 ± 2.02 | 4.22 ± 1.72 |
| 2-Pentanone | 86.1 | 693 | 163.40 | 1.36302 | 495.65 ± 176.86 | 19.57 ± 5.45 | 12.47 ± 2.47 |
| 1-Butanol | 74.1 | 685.4 | 160.79 | 1.37978 | 411.35 ± 57.75 | 76.87 ± 32.02 | 34.27 ± 7.71 |
| 3-Methylbutanal | 86.1 | 648.4 | 151.01 | 1.41777 | 320.47 ± 105.92 | 77.03 ± 80.15 | 36.6 ± 25.94 |
| Ethyl Acetate | 88.1 | 608.7 | 140.53 | 1.34403 | 4009.34 ± 1618.55 | 63.88 ± 7.93 | 71.83 ± 0.62 |
| 2-Butanone | 72.1 | 588.2 | 135.12 | 1.25353 | 3265.26 ± 130.9 | 2135.66 ± 826.26 | 1461.21 ± 234.95 |
| Methyl acetate | 74.1 | 551.2 | 125.34 | 1.18872 | 661.91 ± 39.34 | 89.72 ± 70.73 | 96.34 ± 62.91 |
| 2-Propanone | 58.1 | 500.9 | 112.06 | 1.12057 | 6216.66 ± 86.68 | 1936.03 ± 675.44 | 1212.88 ± 178.14 |
| Ethanol | 46.1 | 469.8 | 103.86 | 1.12839 | 3654.02 ± 57.73 | 263.72 ± 179.61 | 201.85 ± 59.64 |
| Acetic acid | 60.1 | 612.7 | 141.58 | 1.15967 | 224.97 ± 48.26 | 1711.15 ± 333.28 | 2011.53 ± 64.07 |
| Ethyl propanoate | 102.1 | 689.1 | 161.83 | 1.45576 | 7.21 ± 0.8 | 32.41 ± 42.79 | 9.53 ± 1.53 |
| Dimethylamine | 45.1 | 446 | 97.57 | 0.95409 | 163.36 ± 14.1 | 1005.22 ± 844.72 | 2576.22 ± 418.3 |
| Trimethylamine | 59.1 | 520.7 | 117.30 | 1.15855 | 1330.47 ± 138.29 | 2587.24 ± 1048.38 | 4036.3 ± 580.56 |
| Ammonia | 17 | 447.4 | 97.92 | 0.89375 | 392 ± 18.72 | 4593.52 ± 1263.04 | 3087.01 ± 1186.73 |
| Styrene | 104.2 | 891.1 | 256.14 | 1.41659 | 125.45 ± 15.61 | 262.75 ± 103.59 | 283.26 ± 60.9 |
| Octanal | 128.2 | 991.5 | 340.73 | 1.42632 | 120.71 ± 5.98 | 168.41 ± 35.68 | 126.78 ± 7.55 |
| (E)-2-Hexenal | 98.1 | 824.7 | 221.60 | 1.19013 | 24.45 ± 5.18 | 118.97 ± 29.93 | 57.1 ± 9.06 |
| Ethyl 2-methylbutanoate | 130.2 | 826.9 | 222.76 | 1.24848 | 103.69 ± 23.04 | 103.76 ± 19.55 | 62.29 ± 9.9 |
| Dipropyl disulfide | 150.3 | 1094 | 484.99 | 1.48917 | 47.67 ± 7.68 | 125.7 ± 20.48 | 73.99 ± 7.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhang, B.; Dong, X.; Wei, Y.; Li, H.; Tang, B. Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry. Molecules 2023, 28, 3874. https://doi.org/10.3390/molecules28093874
He S, Zhang B, Dong X, Wei Y, Li H, Tang B. Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry. Molecules. 2023; 28(9):3874. https://doi.org/10.3390/molecules28093874
Chicago/Turabian StyleHe, Shan, Bin Zhang, Xuan Dong, Yuqing Wei, Hongtu Li, and Bo Tang. 2023. "Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry" Molecules 28, no. 9: 3874. https://doi.org/10.3390/molecules28093874
APA StyleHe, S., Zhang, B., Dong, X., Wei, Y., Li, H., & Tang, B. (2023). Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry. Molecules, 28(9), 3874. https://doi.org/10.3390/molecules28093874






