Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles
Abstract
1. Introduction
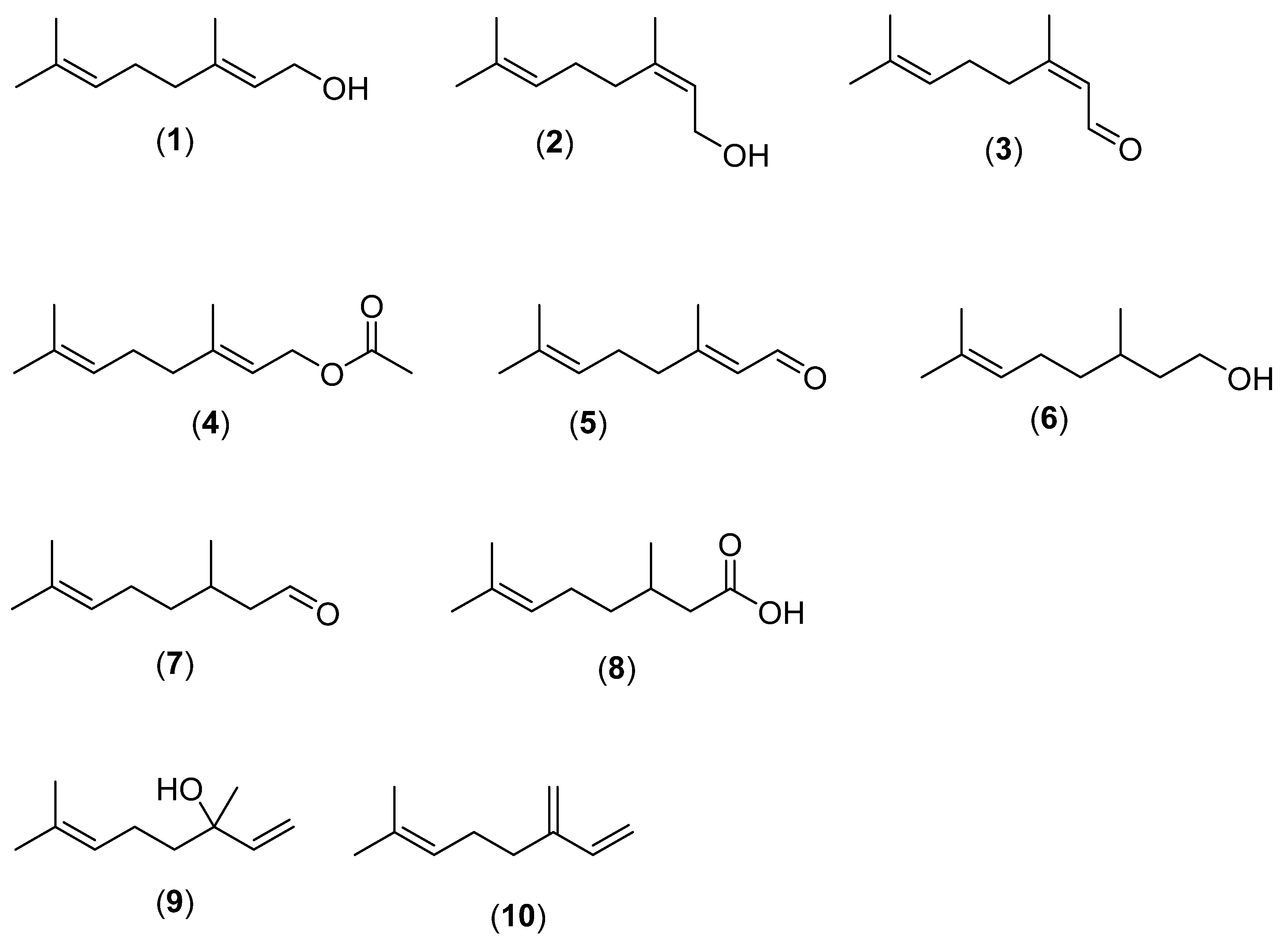
2. Monoterpenes
2.1. Linear Monoterpenes
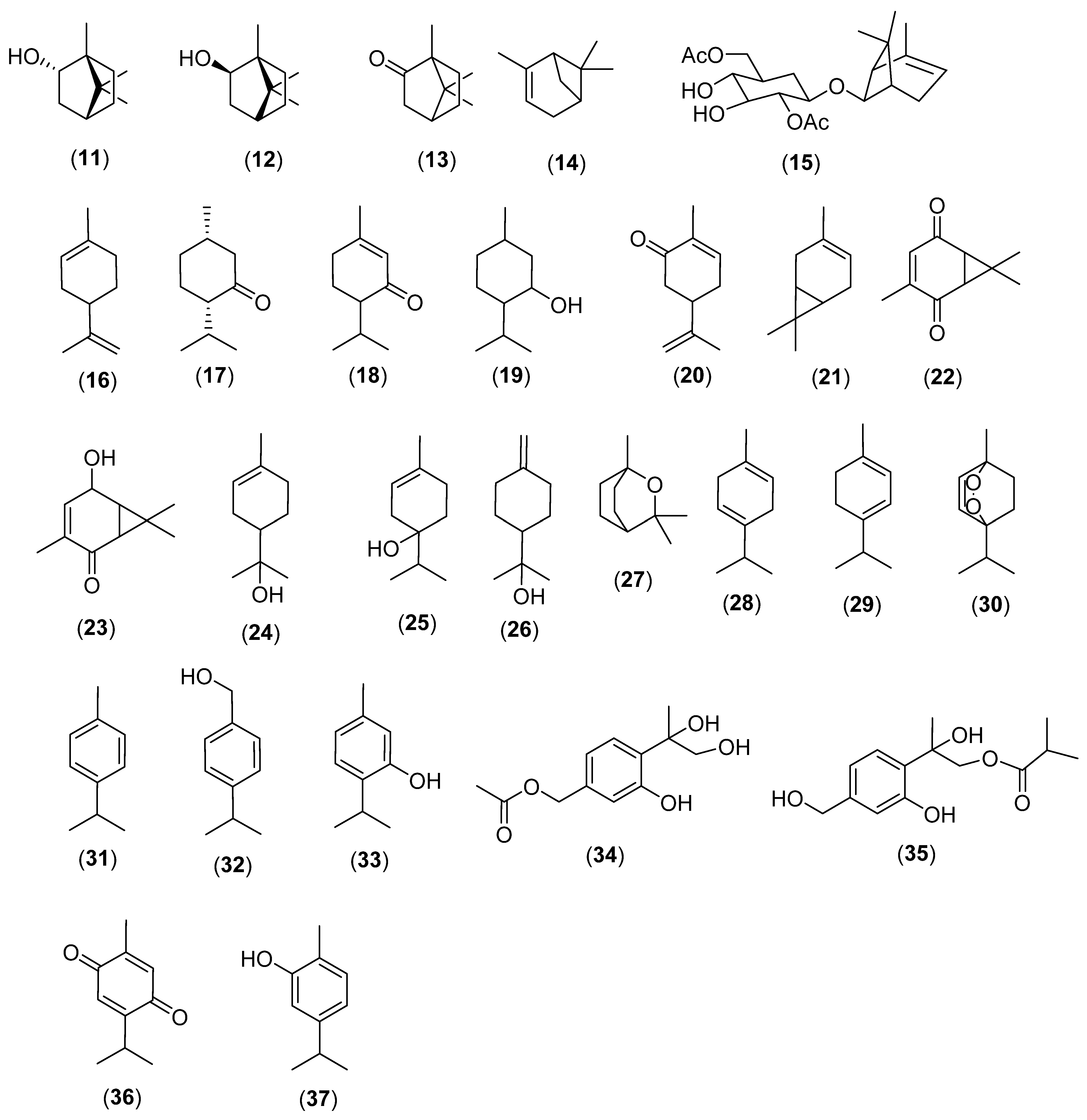
2.2. Cyclic Monoterpenes
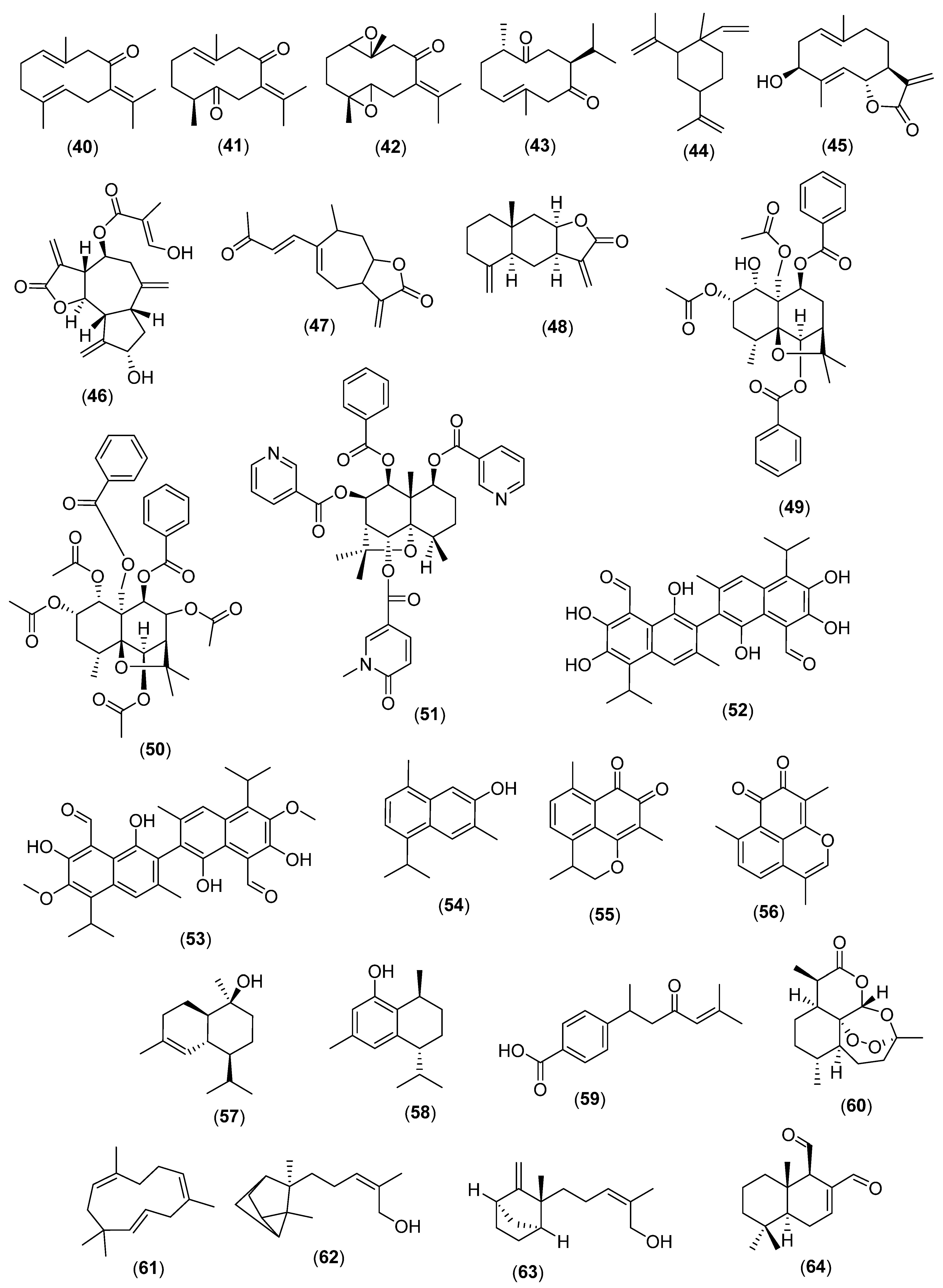
3. Sesquiterpenes
3.1. Linear Sesquiterpenes
3.2. Cyclic Sesquiterpenes
3.3. Miscellaneous
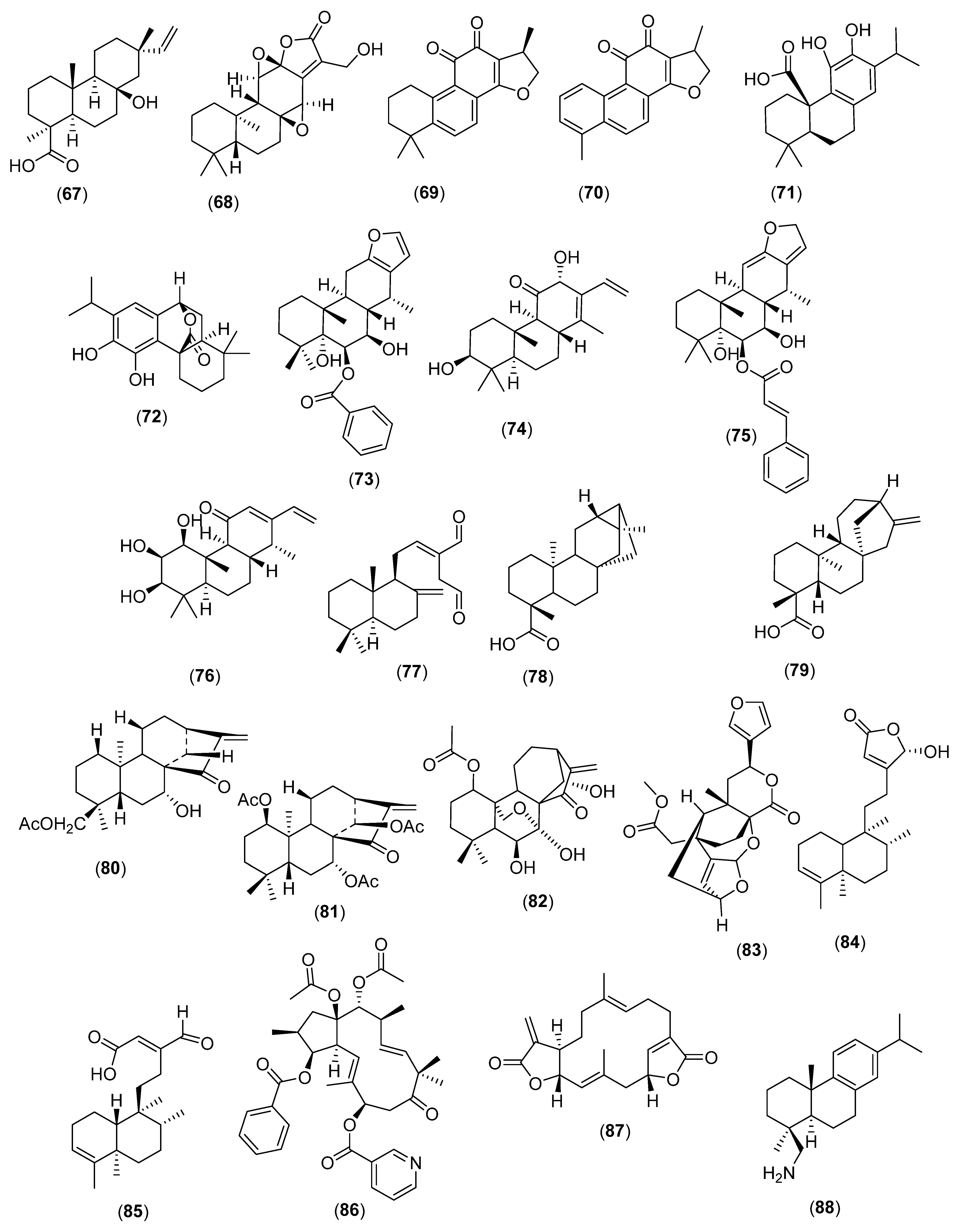
4. Diterpenes
4.1. Linear Diterpenes
4.2. Cyclic Diterpenes
4.3. Miscellaneous
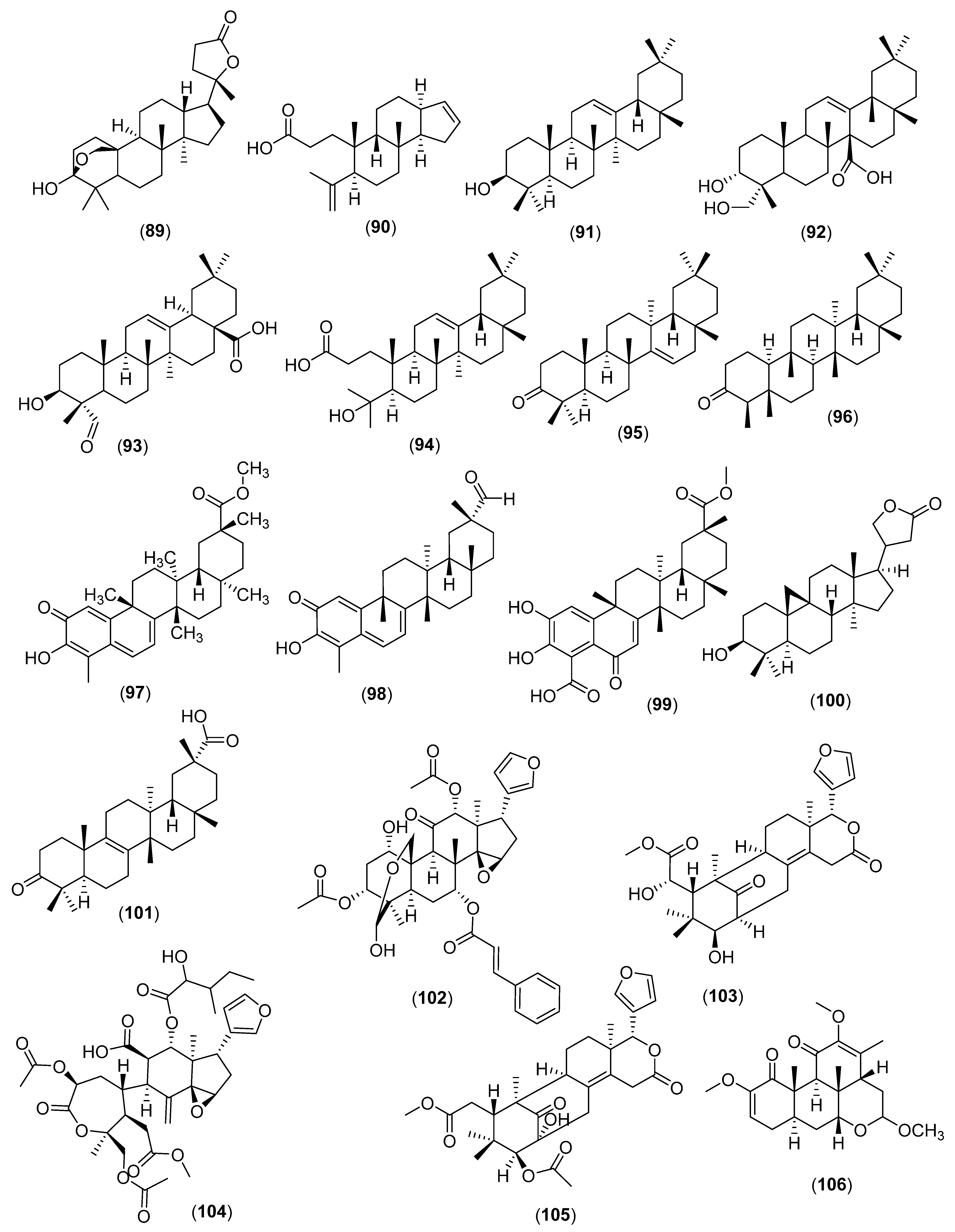
5. Triterpenes
5.1. Cyclic Triterpenes
5.2. Miscellaneous
6. The Distribution of Antibacterial and Antifungal Terpenes
7. Antibacterial and Antifungal Strength and Spectrum of Terpenes
8. Influence of Molecular Mass
9. Influence of Solubility and Polar Surface Area
10. Structure Activity and Mechanism of Action
11. Antibiotic and Antifungal Potentiating Effects
12. The Safety Issues of Terpenes with Respect on Human Health
13. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. 2016, 181, 1–20. [Google Scholar]
- Tiku, A.R. Antimicrobial Compounds (Phytoanticipins and Phytoalexins) and Their Role in Plant Defense, in Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 845–868. [Google Scholar]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Maillard, J.Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.V.D. Bacterial cleanup: Lateral diffusion of hydrophobic molecules through protein channel walls. Biomol. 2010, 1, 263–270. [Google Scholar]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spect. 2017, 5, 3. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Szubzda, B. Novel permittivity test for determination of yeast surface charge and flocculation abilities. J. Ind. Microbiol. 2012, 39, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Zgurskaya, Y.H. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 2001, 3, 215–218. [Google Scholar]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Ramak, P.; Osaloo, S.K.; Sharifi, M.; Ebrahimzadeh HBehmanesh, M. Biosynthesis, regulation and properties of plant monoterpenoids. J. Med. Plant Res. 2014, 8, 983–991. [Google Scholar]
- Lis-Balchin, M.; Buchbauer, G.; Ribisch, K.; Wenger, M.T. Comparative antibacterial effects of novel Pelargonium essential oils and solvent extracts. Lett. Appl. Microbiol. 1998, 27, 135–141. [Google Scholar] [CrossRef]
- Onawunmi, G.O.; Yisak, W.-A.; Ogunlana, E. Antibacterial constituents in the essential oil of Cymbopogon citratus (DC). Stapf. J. Ethnopharmacol. 1984, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Vitali, C.; De Laurentis, N.; Armenise DMilillo, M.A. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, M.; Narita, T.; Ichikawa, H.; Kawakami, J.; Nakane, A. Antibacterial and antifungal activities of isoprenoids. Trans. Matter. Res. Soc. Jpn. 2011, 36, 55–58. [Google Scholar] [CrossRef]
- Orhan, İ.E.; Özcelik, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Tampieri, M.P.; Galuppi, R.; Macchioni, F.; Carelle, M.S.; Falcioni, L.; Cioni, P.L.; Morelli, I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005, 159, 339–345. [Google Scholar] [CrossRef]
- Borges, A.; Alves, A.C.; Simões, M. Effect of selected terpenoids on antibiotic potentiation and eradication of Staphylococcus aureus biofilms—A structure activity relationship study. In Proceedings of the Biofilms 9 Conference, Karlsruhe, Germany, 29 September–1 October 2020. [Google Scholar]
- Sonboli, A.; Mirjalili, M.H.; Hadian, J.; Ebrahimi, S.N.; Yousefzadi, M. Antibacterial activity and composition of the essential oil of Ziziphora clinopodioides subsp. bungeana (Juz.) Rech. f. from Iran. Z. Naturforsch. C 2006, 61, 677–680. [Google Scholar] [CrossRef]
- Radulović, N.; Mišić, M.; Aleksić, J.; Đoković, D.; Palić Stojanović, G. Antimicrobial synergism and antagonism of salicylaldehyde in Filipendula vulgaris essential oil. Fitoterapia 2007, 78, 565–570. [Google Scholar] [CrossRef]
- Park, S.N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander essential oil and linalool–interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. C 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Guo, D.L.; Liu, W.W.; Liu, Y.X. Synergistic antimicrobial activity of berberine hydrochloride, baicalein and borneol against Candida albicans. Chin. Herb. Med. 2017, 9, 353–357. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.; Sanglard, D.; Soković, M. Camphor and eucalyptol—Anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Wong, J.; Rideout, R.A. Monoterpene glycoside and flavonoids from Blumea lacera. J. Nat. Med. 2007, 61, 474–475. [Google Scholar] [CrossRef]
- Hyatt, D.C.; Youn, B.; Zhao, Y.; Santhamma, B.; Coates, R.M.; Croteau, R.B.; Kang, C. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Cheallaigh, A.N.; Mansell, D.J.; Toogood, H.S.; Tait, S.; Lygidakis, A.; Scrutton, N.S.; Gardiner, J.M. Chemoenzymatic synthesis of the intermediates in the peppermint monoterpenoid biosynthetic pathway. J. Nat. Prod. 2018, 81, 1546–1552. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. JMM 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; Sousa, D.P.D.; Santos, A.G.D. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci. 2018, 53. [Google Scholar] [CrossRef]
- Oh, J.; Hwang, I.H.; Kim, D.C.; Kang, S.C.; Jang, T.S.; Lee, S.H.; Na, M. Anti-listerial compounds from Asari Radix. Arch. Pharm. Res. 2010, 33, 1339–1345. [Google Scholar] [CrossRef]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, W.; Yoo, J.G.; Ebersole, J.L.; Huang, C.B. Antibacterial compounds from Siraitia grosvenorii leaves. Nat. Prod. Res. 2011, 25, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control. 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Pinto, E.; Gonçalves, M.J.; Oliveira, P.; Coelho, J.; Cavaleiro, C.; Salgueiro, L. Activity of Thymus caespititius essential oil and α-terpineol against yeasts and filamentous fungi. Ind. Crops Prod. 2014, 62, 107–112. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lower-Nedza, A.D.; Hong, M.; Jiec, S.; Wang, Z.; Yingmao, D.; Tschiggerl, C.; Bucar, F.; Brantner, A.H. Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat. Prod. Commun. 2013, 8, 523–526. [Google Scholar] [CrossRef]
- Pare, P.W.; Zajicek, J.; Ferracini, V.L.; Melo, I.S. Antifungal terpenoids from Chenopodium ambrosioides. Biochem. Syst. Ecol. 1993, 21, 649–653. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.C.; Zhou, X.R.; Xu, X.J.; Fu, Q.Y.; Liu, C.Z. Two thymol derivatives from the flower buds of Lonicera japonica and their antibacterial activity. Nat. Prod. Res. 2018, 32, 2238–2243. [Google Scholar] [CrossRef]
- Haieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar]
- Dey, D.; Ray, R.; Hazra, H. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef]
- Mahmoud, H.; Sepahvand, A.; Jahanbakhsh, S.; Ezatpour, B.; Mousavi, S.A. Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. JMM 2014, 24, e155–e161. [Google Scholar]
- Almshawit, H.; Macreadie, I. Fungicidal effect of thymoquinone involves generation of oxidative stress in Candida glabrata. Microbiol. Res. 2017, 195, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, J.; Di Girolamo, A.; Bouwmeester, H.J.; de Ridder, D.; Beekwilder, J.; van Dijk, A.D. An analysis of characterized plant sesquiterpene synthases. Phytochemistry 2019, 158, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Vattekkatte, A.; Garms, S.; Brandt, W.; Boland, W. Enhanced structural diversity in terpenoid biosynthesis: Enzymes, substrates and cofactors. Org. Biomol. Chem. 2018, 16, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Diastuti, H.; Syah, Y.M.; Juliawaty, L.D.; Singgih, M. Antibacterial activity of germacrane type sesquiterpenes from Curcuma heyneana Rhizomes. Indones. J. Chem. 2014, 14, 32–36. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Abdullah Al-Harbi, N.; Ignacimuthu, S.; Muthukumar, C. Antimicrobial activity of sesquiterpene lactones from traditional medicinal plant, Costus speciosus (Koen ex. Retz.) Sm. BMC Complement. Altern. Med. 2012, 12, 13. [Google Scholar]
- Saeed, M.A.; Sabir, A. Antibacterial activities of some constituents from oleo-gum-resin of Commiphora mukul. Fitoterapia 2004, 75, 204–208. [Google Scholar] [CrossRef]
- Erasto, P.; Grierson, D.; Afolayan, A. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol. 2006, 106, 117–120. [Google Scholar] [CrossRef]
- de Kraker, J.W.; Franssen, M.C.; de Groot, A.; König, W.A.; Bouwmeester, H.J. (+)-Germacrene A biosynthesis: The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 1998, 117, 1381–1392. [Google Scholar] [CrossRef]
- Shakeri, A.; Amini, E.; Asili, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen, L. Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2018, 32, 1436–1440. [Google Scholar]
- Cho, W.I.; Choi, J.B.; Lee, K.; Chung, M.S.; Pyun, Y.R. Antimicrobial activity of torilin from Torilis japonica fruit against Bacillus subtilis. J. Food Sci. 2008, 73, M37–M46. [Google Scholar] [CrossRef]
- Iranshahi, M.; Hosseini, S.T.; Shahverdi, A.R.; Molazade, K.; Khan, S.S.; Ahmad, V.U. Diversolides A–G, guaianolides from the roots of Ferula diversivittata. Phytochemistry 2008, 69, 2753–2757. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Shim, C.K.; Bae, D.W.; Kawk, Y.S.; Yang, M.S.; Kim, H.K. Identification and biological characteristics of an antifungal compound extracted from Cocklebur (Xanthium Strumarium) against Phytophthora drechsleri. Plant Pathol. J. 2002, 18, 288–292. [Google Scholar] [CrossRef]
- Fukuyama, N.; Ino, C.; Suzuki, Y.; Kobayashi, N.; Hamamoto, H.; Sekimizu, K.; Orihara, Y. Antimicrobial sesquiterpenoids from Laurus nobilis L. Nat. Prod. Res. 2011, 25, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Guoruoluo, Y.; Zhou, H.; Zhou, J.; Zhao, H.; Aisa, H.A.; Yao, G. Isolation and characterization of sesquiterpenoids from Cassia buds and their antimicrobial activities. J. Agric. Food. Chem. 2017, 65, 5614–5619. [Google Scholar] [CrossRef] [PubMed]
- Picman, A.K.; Schneider, E.F. Inhibition of fungal growth by selected sesquiterpene lactones. Biochem. Syst. Ecol. 1993, 21, 307–314. [Google Scholar] [CrossRef]
- Liu, C.; Mishra, A.K.; He, B.; Tan, R. Antimicrobial activities of isoalantolactone, a major sesquiterpene lactone of Inula racemosa. Sci. Bull. 2001, 46, 498–501. [Google Scholar] [CrossRef]
- Tissandié, L.; Viciana, S.; Brevard, H.; Meierhenrich, U.J.; Filippi, J.J. Towards a complete characterization of guaiac wood oil. Phytochemistry 2018, 149, 64–81. [Google Scholar] [CrossRef]
- Chen, J.J.; Kuo, W.L.; Chen, I.S.; Peng, C.F.; Sung, P.J.; Cheng, M.J.; Lim, Y.P. Microjaponin, a new dihydroagarofuranoid sesquiterpene from the stem of Microtropis japonica with antituberculosis activity. Chem. Biodivers. 2014, 11, 1241–1246. [Google Scholar] [CrossRef]
- Chou, T.H.; Chen, I.S.; Peng, C.F.; Sung, P.J.; Chen, J.J. A new dihydroagarofuranoid sesquiterpene and antituberculosis constituents from the root of Microtropis japonica. Chem. Biodivers. 2008, 5, 1412–1418. [Google Scholar] [CrossRef]
- Chen, J.J.; Yang, C.S.; Peng, C.F.; Chen, I.S.; Miaw, C.L. Dihydroagarofuranoid sesquiterpenes, a lignan derivative, a benzenoid, and antitubercular constituents from the stem of Microtropis japonica. J. Nat. Prod. 2008, 71, 1016–1021. [Google Scholar] [CrossRef]
- Chou, T.H.; Chen, I.S.; Sung, P.J.; Peng, C.F.; Shieh, P.C.; Chen, J.J. A new dihydroagarofuranoid sesquiterpene from Microtropis fokienensis with antituberculosis activity. Chem. Biodivers. 2007, 4, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Q.; Ren, Q.; Kong, X.; Wang, L.; Wang, H.; Xu, J.; Guo, Y. Isolation and characterization of sesquiterpenes from Celastrus orbiculatus and their antifungal activities against phytopathogenic fungi. J. Agric. Food Chem. 2014, 62, 10945–10953. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, S.; Wang, Q.; Yin, M.; Jia, X.; Li, L.; Feng, X. Two new dihydro-β-agarofuran sesquiterpenes from Monimopetalum chinense. Phytochem. Lett. 2019, 34, 108–112. [Google Scholar] [CrossRef]
- Limberger, R.P.; Simões-Pires, C.A.; Sobral, M.; Menut, C.; Bessiere, J.M.; Henriques, A.T. Essential oils from Calyptranthes concinna, C. lucida and C. rubella (Myrtaceae). Rev. Cienc. Farm. Basica Apl. 2002, 38, 355–360. [Google Scholar] [CrossRef]
- Boonsri, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, S.; Kanjana-Opas, A. Cytotoxic and antibacterial sesquiterpenes from Thespesia populnea. J. Nat. Prod. 2008, 71, 1173–1177. [Google Scholar] [CrossRef]
- Przybylski, P.; Pyta, K.; Stefańska, J.; Ratajczak-Sitarz, M.; Katrusiak, A.; Huczyński, A.; Brzezinski, B. Synthesis, crystal structures and antibacterial activity studies of aza-derivatives of phytoalexin from cotton plant–gossypol. Eur. J. Med. Chem. 2009, 44, 4393–4403. [Google Scholar] [CrossRef] [PubMed]
- Masila, V.M.; Midiwo, J.O.; Zhang, J.; Gisacho, B.M.; Munayi, R.; Omosa, L.K.; Wiggers, F.T.; Jacob, M.R.; Walker, L.A.; Muhammad, I. Anti-vancomycin-resistant Enterococcus faecium and E. faecalis activities of (-)-gossypol and derivatives from Thespesia garckeana. Nat. Prod. Comm. 2015, 10, 613–616. [Google Scholar]
- Anuthara, R.; Midhun, S.J.; Mathew, L. An in vitro and in silico study of anti-dermatophytic activity of gossypol from fruits of Thespesia populnea (L.) Sol. ex Correa. Asian Pac. J. Trop. Biomed. 2021, 11, 543. [Google Scholar]
- Mongkol, R.; Chavasiri, W. Antimicrobial, herbicidal and antifeedant activities of mansonone E from the heartwoods of Mansonia gagei Drumm. J. Integr. Agric. 2016, 15, 2795–2802. [Google Scholar] [CrossRef]
- Suh, Y.G.; Kim, S.N.; Shin, D.Y.; Hyun, S.S.; Lee, D.S.; Min, K.H.; Han, S.M.; Li, F.; Choi, E.C.; Choi, S.H. The structure–activity relationships of mansonone F, a potent anti-MRSA sesquiterpenoid quinone: SAR studies on the C6 and C9 analogs. Bioorg. Med. Chem. Lett. 2006, 16, 142–145. [Google Scholar] [CrossRef]
- Nagasampagi, B.; Yankov, L.; Dev, S. Sesquiterpenoids from the wood of Cedrela toona Roxb; partial synthesis of t-muurolol, t-cadinol and cubenol; structures of δ-cadinene and δ-cadinol. Tetrahedron Lett. 1968, 9, 1913–1918. [Google Scholar] [CrossRef]
- Claeson, P.; Rådström, P.; Sköld, O.; Nilsson, Å.; Höglund, S. Bactericidal effect of the sesquiterpene T-cadinol on Staphylococcus aureus. Phytother. Res. 1992, 6, 94–98. [Google Scholar] [CrossRef]
- Nishizawa, M.; Inoue, A.; Sastrapradja, S.; Hayashi, Y. (+)-8-Hydroxycalamenene: A fish-poison principle of Dysoxylum acutangulum and D. Alliaceum. Phytochemistry 1983, 22, 2083–2085. [Google Scholar] [CrossRef]
- Datta, B.K.; Mukhlesur Rahman, M.; Gray, A.I.; Nahar, L.; Hossein, S.A.; Auzi, A.A.; Sarker, S.D. Polygosumic acid, a new cadinane sesquiterpene from Polygonum viscosum, inhibits the growth of drug-resistant Escherichia coli and Staphylococcus aureus (MRSA) in vitro. J. Nat. Med. 2007, 61, 391–396. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Kumarihamy, B.M.; Jayarathna, K.N.; Udishani, N.G.; Bandara, B.R.; Hara, N.; Fujimoto, Y. Antifungal constituents of the stem bark of Bridelia retusa. Phytochemistry 2003, 62, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Case, R.J.; Inui, T.; Wang, Y.; Cho, S.; Fischer, N.H.; Franzblau, S.G. New perspectives on natural products in TB drug research. Life Sci. 2005, 78, 485–494. [Google Scholar] [CrossRef]
- Adekenov, S. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia 2017, 121, 16–30. [Google Scholar] [CrossRef]
- Chung, I.Y.; Jang, H.J.; Yoo, Y.J.; Hur, J.; Oh, H.Y.; Kim, S.H.; Cho, Y.H. Artemisinin displays bactericidal activity via copper-mediated DNA damage. Virulence 2022, 13, 149–159. [Google Scholar] [CrossRef]
- Rahman, A.; Shanta, Z.S.; Rashid, M.A.; Parvin, T.; Afrin, S.; Khatun, M.K.; Sattar, M.A. In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arab. J. Chem. 2016, 9, S475–S479. [Google Scholar] [CrossRef]
- Kumar, S.S.; Srinivas, P.; Negi, P.S.; Bettadaiah, B.K. Antibacterial and antimutagenic activities of novel zerumbone analogues. Food Chem. 2013, 141, 1097–1103. [Google Scholar] [CrossRef]
- Shin, D.S.; Eom, Y.B. Zerumbone inhibits Candida albicans biofilm formation and hyphal growth. Can. J. Microbiol. 2019, 65, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Lekphrom, R. Bioactive constituents of the roots of Polyalthia cerasoides. J. Nat. Prod. 2007, 70, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Moreira da Silva, T.; Pinheiro, C.D.; Puccinelli Orlandi, P.; Pinheiro, C.C.; Soares Pontes, G. Zerumbone from Zingiber zerumbet (L.) smith: A potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2018, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Jassal, K.; Kaushal, S.; Rashmi Rani, R. Antifungal potential of guava (Psidium guajava) leaves essential oil, major compounds: Beta-caryophyllene and caryophyllene oxide. Arch. Phytopathol. Pflanzenschutz 2021, 54, 2034–2050. [Google Scholar] [CrossRef]
- Kawabata, J.; Tahara, S.; Mizutani, J. Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae). Agric. Biol. Chem. 1981, 45, 1447–1453. [Google Scholar]
- Kim, T.H.; Hatano, T.; Okamoto, K.; Yoshida, T.; Kanzaki, H.; Arita, M.; Ito, H. Antifungal and ichthyotoxic sesquiterpenoids from Santalum album heartwood. Molecules 2017, 22, 1139. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from East Indian sandalwood tree (Santalum album L.). Lett. Appl. Microbiol. 2012, 55, 476–486. [Google Scholar] [CrossRef]
- Phongmaykin, J.; Kumamoto, T.; Ishikawa, T.; Suttisri, R.; Saifah, E. A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch. Pharm. Res. 2008, 31, 21–27. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Lee, S.H.; Ha, T.J. Antibacterial activity of polygodial. Phytother. Res. 2005, 19, 1013–1017. [Google Scholar] [CrossRef]
- Kubo, I.; Taniguchi, M. Polygodial, an antifungal potentiator. J. Nat. Prod. 1988, 51, 22–29. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Indwar, F.; Ignacimuthu, S. Antimicrobial activity of confertifolin from Polygonum hydropiper. Pharm. Biol. 2010, 48, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Gray, A.I.; Waterman, G. A new antibacterial sesquiterpene from Premna oligotricha. J. Nat. Prod. 1993, 56, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Sotanaphun, U.; Lipipun, V.; Suttisri, R.; Bavovada, R. A new antiviral and antimicrobial sesquiterpene from Glyptopetalum sclerocarpum. Planta Med. 1999, 65, 257–258. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Saludes, J.P.; Garson, M.J.; Franzblau, S.G.; Aguinaldo, A.M. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother. Res. 2002, 16, 683–685. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.Y.; Wu, J. Chemical constituents and some biological activities of plants from the genus Ceriops. Chem. Biodivers. 2012, 9, 1–11. [Google Scholar] [CrossRef]
- Chen, H.D.; Yang, S.P.; Wu, Y.; Dong, L.; Yue, J.M. Terpenoids from Toona ciliata. J. Nat. Prod. 2009, 72, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Yan, Q.L.; Ma, Y.F.; Sun, C.P.; Chen, C.M.; Tian, X.G.; Han, X.Y.; Wang, C.; Deng, S.; Ma, X.C. ent-Abietane and tigliane diterpenoids from the roots of Euphorbia fischeriana and their inhibitory effects against Mycobacterium smegmatis. J. Nat. Prod. 2017, 80, 1248–1254. [Google Scholar] [CrossRef]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef]
- Han, Y.; Joo, I. Antifungal effect of tanshinone from Salvia miltiorrhiza against disseminated candidiasis. Yakhak Hoeji 2013, 57, 119–124. [Google Scholar]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Souza, M.G.; Andrade Silva, M.L.; da Silva Filho, A.A.; Martins, C.H.G.; Miller Crotti, A.E.; Pauletti, P.M.; Groppo, M.; et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: Relevance of carnosic acid and carnosol. Chem. Biodivers. 2010, 7, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sui, Y.; Zhong, L.; Ma, T.; Ma, Z.; Liu, X. Carnosol inhibits the growth and biofilm of Candida albicans. JMM 2022, 32, 101234. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, F.A. Antimicrobial activity of carnosic acid isolated from Rosmarinus officinalis L. leaves. Tikrit J. Pure Sci. 2011, 16, 1662–1813. [Google Scholar]
- Promsawan, N.; Kittakoop, P.; Boonphong, S.; Nongkunsarn, P. Antitubercular cassane furanoditerpenoids from the roots of Caesalpinia pulcherrima. Planta Med. 2003, 69, 776–777. [Google Scholar]
- Eldeen, I.; Van Heerden, F.; Van Staden, J. In vitro biological activities of niloticane, a new bioactive cassane diterpene from the bark of Acacia nilotica subsp. kraussiana. J. Ethnopharmacol. 2010, 128, 555–560. [Google Scholar] [CrossRef]
- Ata, A.; Udenigwe, C.C.; Gale, E.M.; Samarasekera, R. Minor chemical constituents of Caesalpinia bonduc. Nat. Prod. Commun. 2009, 4, 311–314. [Google Scholar] [CrossRef]
- Koga, H.; Nakayachi, O. Morphological studies on attachment of spores of Magnaporthe grisea to the leaf surface of rice. J. Gen. Plant Pathol. 2004, 70, 11–15. [Google Scholar] [CrossRef]
- Ghosh, S.; Indukuri, K.; Bondalapati, S.; Saikia, A.K.; Rangan, L. Unveiling the mode of action of antibacterial labdane diterpenes from Alpinia nigra (Gaertn.) Bl Burtt seeds. Eur. J. Med. Chem. 2013, 66, 101–105. [Google Scholar] [CrossRef]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Yamamura, Y.; Kurosaki, F.; Lee, J.B. Elucidation of terpenoid metabolism in Scoparia dulcis by RNA-seq analysis. Sci. Rep. 2017, 7, 43311. [Google Scholar] [CrossRef]
- Phan, M.G.; Phan, T.S.; Matsunami, K.; Otsuka, H. Chemical and biological evaluation on scopadulane-type diterpenoids from Scoparia dulcis of Vietnamese origin. Chem. Pharm. Bull. 2006, 54, 546–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zulfiker, A.H.M.; Siddiqua, M.; Nahar, L.; Habib, M.R.; Uddin, N.; Hasan, N.; Rana, M.S. In vitro antibacterial, antifungal and cytotoxic activity of Scoparia dulcis L. Int. J. Pharmacol. Pharm. Sci. 2011, 3, 198–203. [Google Scholar]
- Zgoda-Pols, J.R.; Freyer, A.J.; Killmer, L.B.; Porter, J.R. Antimicrobial diterpenes from the stem bark of Mitrephora celebica. Fitoterapia 2002, 73, 434–438. [Google Scholar] [CrossRef]
- Hernández, D.M.; Díaz-Ruiz, G.; Rivero-Cruz, B.E.; Bye, R.A.; Aguilar, M.I.; Rivero-Cruz, J.F. Ent-trachyloban-19-oic acid from Iostephane heterophylla as a promising antibacterial agent against Streptococcus mutans biofilms. Fitoterapia 2012, 83, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Otomo, K.; Kenmoku, H.; Oikawa, H.; König, W.A.; Toshima, H.; Mitsuhashi, W.; Yamane, H.; Sassa, T.; Toyomasu, T. Biological functions of ent-and syn-copalyl diphosphate synthases in rice: Key enzymes for the branch point of gibberellin and phytoalexin biosynthesis. Plant J. 2004, 39, 886–893. [Google Scholar] [CrossRef]
- Okada, K.; Kawaide, H.; Miyamoto, K.; Miyazaki, S.; Kainuma, R.; Kimura, H.; Fujiwara, K.; Natsume, M.; Nojiri, H.; Nakajima, M.; et al. HpDTC1, a stress-inducible bifunctional diterpene cyclase involved in momilactone biosynthesis, functions in chemical defense in the moss Hypnum plumaeforme. Sci. Rep. 2016, 6, 25316. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Shi, Y.; Liang, J.Y.; Min, Z.-D. Antibacterial ent-rosane and ent-kaurane diterpenoids from Sagittaria trifolia var. sinensis. CJNM 2009, 7, 341–345. [Google Scholar] [CrossRef]
- Jang, W.S.; Jyoti, M.A.; Kim, S.; Nam, K.W.; Ha, T.K.Q.; Oh, W.K.; Song, H.Y. In vitro antituberculosis activity of diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J. Nat. Med. 2016, 70, 127–132. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, D.; Zou, L.; Yang, B.; Zhao, M. Antibacterial activity-guided purification and identification of a novel C-20 oxygenated ent-kaurane from Rabdosia serra (Maxim.) Hara. Food Chem. 2013, 139, 902–909. [Google Scholar] [CrossRef]
- Pelot, K.A.; Mitchell, R.; Kwon, M.; Hagelthorn, L.M.; Wardman, J.F.; Chiang, A.; Bohlmann, J.; Ro, D.K.; Zerbe, P. Biosynthesis of the psychotropic plant diterpene salvinorin A: Discovery and characterization of the Salvia divinorum clerodienyl diphosphate synthase. TPJ 2017, 89, 885–897. [Google Scholar] [CrossRef]
- Shriram, V.; Jahagirdar, S.; Latha, C.; Kumar, V.; Puranik, V.; Rojatkar, S.; Dhakephalkar, P.K.; Shitole, M.G. A potential plasmid-curing agent, 8-epidiosbulbin E acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. IJAA 2008, 32, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Betrand Teponno, R.; Mbaveng, A.T.; Tapondjou, L.A.; Meyer, J.J.M.; Barboni, L.; Lall, N. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement. Altern. Med. 2012, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Murthy, M.M.; Subramanyam, M.; Bindu, M.H.; Annapurna, J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005, 76, 336–339. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Shukla, P. Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula. Nat. Prod. Commun. 2009, 4, 327–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Xu, J.B.; Zhao, J.X.; Xu, C.H.; Dong, L.; Ding, J.; Yue, J.M. Diterpenoids from Croton laui and their cytotoxic and antimicrobial activities. J. Nat. Prod. 2014, 77, 1013–1020. [Google Scholar] [CrossRef]
- Khan, S.; Jabbar, A.; Hasan, C.M.; Rashid, M.A. Antibacterial activity of Barringtonia racemosa. Fitoterapia 2001, 72, 162–164. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Halbert, G.W.; Waterman, P.G. A novel antibacterial diterpene from Premna schimperi. Planta Med. 1990, 56, 187–189. [Google Scholar] [CrossRef]
- Khurram, M.; Lawton, L.A.; Edwards, C.; Iriti, M.; Hameed, A.; Khan, M.A.; Khan FA ur Rahman, S. Rapid bioassay-guided isolation of antibacterial clerodane type diterpenoid from Dodonaea viscosa (L.) Jacq. Int. J. Mol. Sci. 2015, 16, 20290–20307. [Google Scholar] [CrossRef]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and rearranged jatrophane-type diterpenes: Biogenesis, structure, isolation, biological activity and SARs (1984–2019). Phytochem. Rev. 2020, 19, 265–336. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Peng, S.; He, W.J.; Liu, Y.H.; Wang, J.F.; Zhou, X.J. Antitubercular and cytotoxic tigliane-type diterpenoids from Croton tiglium. Bioorg. Med. Chem. Lett. 2016, 26, 4996–4999. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Feng, J.E.; Duan, L.K.; Liu, C.J.; Li, X.F.; Huang, C.Q.; Shi, S.L.; Wang, R.R.; Zuo, A.X.; He, H.P. Tigliane diterpenoids with larvicidal, antifungal, and α-glucosidase inhibitory activities from Croton damayeshu. J. Nat. Prod. 2022, 85, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Mongkolvisut, W.; Sutthivaiyakit, S. Antimalarial and antituberculous poly-O-acylated jatrophane diterpenoids from Pedilanthus tithymaloides. J. Nat. Prod. 2007, 70, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Kaemchantuek, P.; Chokchaisiri, R.; Prabpai, S.; Kongsaeree, P.; Chunglok, W.; Utaipan, T.; Chamulitrat, W.; Suksamrarn, A. Terpenoids with potent antimycobacterial activity against Mycobacterium tuberculosis from Trigonostemon reidioides roots. Tetrahedron 2017, 73, 1594–1601. [Google Scholar] [CrossRef]
- Lien, H.M.; Wu, H.Y.; Hung, C.L.; Chen, C.J.; Wu, C.L.; Chen, K.W.; Huang, C.L.; Chang, S.J.; Chen, C.C.; Lin, H.J.; et al. Antibacterial activity of ovatodiolide from Anisomeles indica against Helicobacter pylori. Sci. Rep. 2019, 9, 4205. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.N. In vitro antibacterial activity of saffron (Crocus sativus L.) extract and its two major constituents against Helicobacter pylori. Planta Med. 2010, 76, 496. [Google Scholar] [CrossRef]
- Hussein, R.A.; Salih, N.A.; Eman Thabit, N. Bioactivity of crocin pigment of saffron plant. Plant Arch. 2018, 18, 357–364. [Google Scholar]
- Atta-ur-Rahman; Nasreen, A.; Akhtar, F.; Shekhani, M.S.; Clardy, J.; Parvez, M.; Choudhary, M.I. Antifungal diterpenoid alkaloids from Delphinium denudatum. J. Nat. Prod. 1997, 60, 472–474. [Google Scholar] [CrossRef]
- Sj, A.R.; Ck, R. Dehydroabietylamine, a diterpene from Carthamus tinctorious L. showing antibacterial and anthelmintic affects with computational evidence. Curr. Comput. Aided Drug. Des. 2020, 16, 231–237. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, M.; Islam, M. Antibacterial, antifungal and cytotoxic activities of amblyone from Amorphophallus campanulatus. Ind. J. Pharmacol. 2008, 40, 41. [Google Scholar] [CrossRef]
- Lee, D.G.; Chang, Y.S.; Park, Y.K.; Hahm, K.S.; Woo, E.R. Antimicrobial effects of ocotillone from stem bark of Ailanthus altisshima. J. Microbiol. Biotechnol. 2002, 12, 854–857. [Google Scholar]
- Michael, A.S.; Thompson, C.G.; Abramovitz, M. Artemia salina as a test organism for bioassay. Science 1956, 123, 464. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, A.; Haque, M.E.; Rahman, M.M. Antimicrobial and cytotoxic activities of Laportea crenulata. Fitoterapia 2008, 79, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Biosynthesis of structurally diverse triterpenes in plants: The role of oxidosqualene cyclases. Proc. Indian Natl. Sci. Acad. 2016, 82, 1189–1210. [Google Scholar] [CrossRef]
- Nick, A.; Wright, A.D.; Rali, T.; Sticher, O. Antibacterial triterpenoids from Dillenia papuana and their structure-activity relationships. Phytochemistry 1995, 40, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Ankeo, S.B.; Damen, F.; Sandjo, L.P.; Celik, I.; Tane, P.; Kuete, V. Antibacterial activities of the methanol extracts, fractions and compounds from Harungana madagascariensis Lam. ex Poir. (Hypericaceae). J. Ethnopharmacol. 2016, 190, 100–105. [Google Scholar]
- Chander, M.P.; Vinod Kumar, K.; Lall, C.; Vimal Raj, R.; Vijayachari, P. GC/MS profiling, in vitro anti-leptospiral and haemolytic activities of Boesenbergia rotunda (L.) Mansf. used as a medicinal plant by Nicobarese of Andaman and Nicobar Islands. Nat. Prod. Res. 2016, 30, 1190–1192. [Google Scholar] [CrossRef]
- Kongcharoensuntorn, W.; Naengchomnong, W.; Samae, A. Synergistic antibacterial effect of lup-20 (29)-ene-3α, 23-diol from Glochidion daltonii (Mull. Arg.) Kurz. antibiotics on opportunistic bacteria. Chonburi Hosp. J. 2019, 44, 207. [Google Scholar]
- Harizon; Pujiastuti, B.; Kurnia, D.; Sumiarsa, D.; Shiono, Y.; Supratman, U. Antibacterial triterpenoids from the bark of Sonneratia alba (Lythraceae). Nat. Prod. Commun. 2015, 10, 277–280. [Google Scholar] [CrossRef]
- Saeed, M.A.; Sabir, A. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia 2001, 72, 807–809. [Google Scholar] [CrossRef]
- Bibi, N.; Tanoli, S.A.K.; Farheen, S.; Afza, N.; Siddiqi, S.; Zhang, Y.; Kazmi, S.U.; Malik, A. In vitro antituberculosis activities of the constituents from Haloxylon salicornicum. Bioorg. Med. Chem. Lett. 2010, 20, 4173–4176. [Google Scholar] [CrossRef]
- Bonvicini, F.; Antognoni, F.; Mandrone, M.; Protti, M.; Mercolini, L.; Lianza, M.; Gentilomi, G.A.; Poli, F. Phytochemical analysis and antibacterial activity towards methicillin-resistant Staphylococcus aureus of leaf extracts from Argania spinosa (L.) Skeels. Plant Biosyst. 2017, 151, 649–656. [Google Scholar] [CrossRef]
- Zheng, C.J.; Sohn, M.J.; Kim, K.Y.; Yu, H.E.; Kim, W.G. Olean-27-carboxylic acid-type triterpenes with potent antibacterial activity from Aceriphyllum rossii. J. Agric. Food Chem. 2008, 56, 11752–11756. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.C.; Xiao, J.H.; Tseng, Y.H.; Kuo, Y.H.; Wang, S.Y. Composition and antifungal activity of balsam from Liquidambar formosana Hance. Holzforsch. 2013, 67, 345–351. [Google Scholar] [CrossRef]
- Emirdağ-Öztürk, S.; Karayıldırım, T.; Çapcı-Karagöz, A.; Alankuş-Çalışkan, Ö.; Özmen, A.; Poyrazoğlu-Çoban, E. Synthesis, antimicrobial and cytotoxic activities, and structure–activity relationships of gypsogenin derivatives against human cancer cells. Eur. J. Med. Chem. 2014, 82, 565–573. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Wang, X.N.; Yin, S.; Dong, L.; Yue, J.M. Ring A-seco triterpenoids with antibacterial activity from Dysoxylum hainanense. Bioorg. Med. Chem. Lett. 2011, 21, 125–129. [Google Scholar] [CrossRef]
- Polacheck, I.; Kwon-Chung, K.J. Canavanine resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1986, 29, 468–473. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, Y.Y.; Jiao, Q.Y.; Khan, A.; Li, F.; Han, D.F.; Cao, G.D.; Lou, H.X. Triterpenoid saponins from the pulp of Sapindus mukorossi and their antifungal activities. Phytochemistry 2018, 147, 1–8. [Google Scholar] [CrossRef]
- Ali, B.; Tabassum, R.; Riaz, N.; Yaqoob, A.; Khatoon, T.; Tareen, R.B.; Jabbar, A.; Nasim, F.U.H.; Saleem, M. Bioactive triterpenoids from Atriplex lasiantha. J. Asian Nat. Prod. Res. 2015, 17, 843–850. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, Y.; Yang, J.; Li, X.N.; San, M.M.; Oo, T.N.; Wang, Y.; Yang, X. Triterpenoids and their glycosides from Glinus oppositifolius with antifungal activities against Microsporum gypseum and Trichophyton rubrum. Molecules 2019, 24, 2206. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mukherjee, K.; Roy, A. Two novel antifungals, acornine 1 and acornine 2, from the bark of mangrove plant Aegiceras corniculatum (Linn.) Blanco from Sundarban Estuary. Pharmacogn. Mag. 2014, 10 (Suppl. 2), S342. [Google Scholar]
- Brahmachari, G.; Mandal, N.C.; Roy, R.; Ghosh, R.; Barman, S.; Sarkar, S.; Jash, S.K.; Mondal, S. A new pentacyclic triterpene with potent antibacterial activity from Limnophila indica Linn. (Druce). Fitoterapia 2013, 90, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; Leon-Rivera, I.; Rios, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef]
- Manríquez-Torres, J.J.; Zúñiga-Estrada, A.; González-Ledesma, M.; Torres-Valencia, J.M. The antibacterial metabolites and proacacipetalin from Acacia cochliacantha. J. Mex. Chem. Soc. 2007, 51, 228–231. [Google Scholar]
- Ghosh, P.; Chakraborty, P.; Mandal, A.; Rasul, M.G.; Chakraborty, M.; Saha, A. Triterpenoids from Schleichera oleosa of Darjeeling foothills and their antimicrobial activity. Ind. J. Pharm. Sci. 2011, 73, 231. [Google Scholar] [CrossRef]
- Mehta, A.; Srivastva, G.; Kachhwaha, S.; Sharma, M.; Kothari, S.L. Antimycobacterial activity of Citrullus colocynthis (L.) Schrad. against drug sensitive and drug resistant Mycobacterium tuberculosis and MOTT clinical isolates. J. Ethnopharmacol. 2013, 149, 195–200. [Google Scholar] [CrossRef]
- Yuan, J.Q.; Yang, X.Z.; Miao, J.H.; Tang, C.P.; Ke, C.Q.; Zhang, J.B.; Ma, X.J.; Ye, Y. New triterpene glucosides from the roots of Rosa laevigata Michx. Molecules 2008, 13, 2229–2237. [Google Scholar] [CrossRef]
- Lahlou, E.H.; Hirai, N.; Kamo, T.; Tsuda, M.; Ohigashi, H. Actinidic acid, a new triterpene phytoalexin from unripe kiwi fruit. Biosci. Biotechnol. Biochem. 2001, 65, 480–483. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S. Acetyl-11-keto-β-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Res. Notes 2011, 4, 406. [Google Scholar] [CrossRef]
- Oluyori, A.P.; Shaw, A.K.; Preeti, R.; Reddy, S.; Atolani, O.; Olatunji, G.A.; Fabiyi, O.A. Natural antifungal compounds from the peels of Ipomoea batatas Lam. Nat. Prod. Res. 2016, 30, 2125–2129. [Google Scholar] [CrossRef]
- Wang, L.K.; Zheng, C.J.; Li, X.B.; Chen, G.Y.; Han, C.R.; Chen, W.H.; Song, X.P. Two new lanostane triterpenoids from the branches and leaves of Polyalthia oblique. Molecules 2014, 19, 7621–7628. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Nizer, W.S.; Ferraz, A.C.; Moraes, T.D.F.S.; Lima, W.G.; Dos Santos, J.P.; Duarte, L.P.; Ferreira, J.M.S.; de Brito Magalhães, C.L.; Vieira-Filho, S.A.; Andrade, A.C.D.S.P.; et al. Pristimerin from Salacia crassifolia (Mart. Ex. Schult.) G. Don. (Celastraceae) roots as a potential antibacterial agent against Staphylococcus aureus. J. Ethnopharmacol. 2021, 266, 113423. [Google Scholar] [CrossRef] [PubMed]
- Gullo, F.P.; Sardi, J.C.; Santos, V.A.; Sangalli-Leite, F.; Pitangui, N.S.; Rossi, S.A.; de Paula e Silva, A.C.; Soares, L.A.; Silva, J.F.; Oliveira, H.C.; et al. Antifungal activity of maytenin and pristimerin. eCAM 2012, 2012, 340787. [Google Scholar] [CrossRef]
- Padilla-Montaño, N.; de León Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone From Natural Sources. Foods 2021, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- De León, L.; Moujir, L. Activity and mechanism of the action of zeylasterone against Bacillus subtilis. J. Appl. Microbiol. 2008, 104, 1266–1274. [Google Scholar] [CrossRef]
- Singh, S.; Dubey, V.; Singh, D.K.; Fatima, K.; Ahmad, A.; Luqman, S. Antiproliferative and antimicrobial efficacy of the compounds from the roots of Oenothera biennis L. J. Pharm. Pharmacol. 2017, 69, 1230–1243. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tang, G.H.; Yuan, C.M.; Zhang, Y.; Zou, T.; Yu, C.; Zhao, Q.; Hao, X.J.; He, H.P. Aphagrandinoids A–D, cycloartane triterpenoids with antibacterial activities from Aphanamixis grandifolia. Fitoterapia 2013, 85, 64–68. [Google Scholar] [CrossRef]
- Tan, M.A.; Takayama, H.; Aimi, N.; Kitajima, M.; Franzblau, S.G.; Nonato, M.G. Antitubercular triterpenes and phytosterols from Pandanus tectorius Soland. var. laevis. J. Nat. Med. 2008, 62, 232–235. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Heliawati, L.; Syah, Y.M.; Bumi, M.B. Bryononic acid: Antibacterial compound from fruit hulls of S. koetjape Merr extract. J. Chem. Pharm. Sci. 2019, 12, 1–5. [Google Scholar] [CrossRef]
- Shi, Y.S.; Zhang, Y.; Li, H.T.; Wu, C.H.; El-Seedi, H.R.; Ye, W.K.; Wang, Z.W.; Li, C.B.; Zhang, X.F.; Kai, G.Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. JFF 2020, 75, 104213. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Li, H.; Mao, X.; Zhao, Y.; Shi, X.; Yang, B. Antibacterial and cytotoxic triterpenoids from the ethanol extract of Dysoxylum densiflorum (Blume) Miq. Phytochem. Lett. 2014, 10, 219–223. [Google Scholar] [CrossRef]
- Liang, X.; Li, B.; Wu, F.; Li, T.; Wang, Y.; Ma, Q.; Liang, S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement. Altern. Med. 2017, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, Y.; Liu, X.T.; Liang, J.Y.; Ip, N.Y.; Min, Z.D. Minor limonoids from Melia toosendan and their antibacterial activity. Planta Med. 2007, 73, 1298–1303. [Google Scholar] [CrossRef]
- Yuan, C.M.; Zhang, Y.; Tang, G.H.; Di, Y.T.; Cao, M.M.; Wang, X.Y.; Zuo, G.Y.; Li, S.L.; Hua, H.M.; He, H.P.; et al. Khayseneganins A–H, limonoids from Khaya senegalensis. J. Nat. Prod. 2013, 76, 327–333. [Google Scholar] [CrossRef]
- Rahman, A.S.; Chowdhury, A.A.; Ali, H.A.; Raihan, S.Z.; Ali, M.S.; Nahar, L.; Sarker, S.D. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J. Nat. Med. 2009, 63, 41–45. [Google Scholar] [CrossRef]
- Lin, B.D.; Yuan, T.; Zhang, C.R.; Dong, L.; Zhang, B.; Wu, Y.; Yue, J.M. Structurally diverse limonoids from the fruits of Swietenia mahagoni. J. Nat. Prod. 2009, 72, 2084–2090. [Google Scholar] [CrossRef]
- Lu, X.F.; Lin, P.C.; Zi, J.C.; Fan, X.N. Limonoids from seeds of Azadirachta indica and their antibacterial activity. Chin. Med. J. 2019, 44, 4864–4873. [Google Scholar]
- Lin, B.D.; Chen, H.D.; Liu, J.; Zhang, S.; Wu, Y.; Dong, L.; Yue, J.M. Mulavanins A–E: Limonoids from Munronia delavayi. Phytochemistry 2010, 71, 1596–1601. [Google Scholar] [CrossRef]
- Wong, C.P.; Nugroho, A.E.; Awouafack, M.D.; Win, Y.Y.; Win, N.N.; Ngwe, H.; Morita, H. Two new quassinoids and other constituents from Picrasma javanica wood, and their biological activities. J. Nat. Med. 2019, 73, 589–596. [Google Scholar]
- Nejma, A.B.; Nguir, A.; Jannet, H.B.; Daïch, A.; Othman, M.; Lawson, A.M. New septanoside and 20-hydroxyecdysone septanoside derivative from Atriplex portulacoides roots with preliminary biological activities. Bioorg. Med. Chem. Lett. 2015, 25, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Han, T.; Wu, J.Z.; Huang, B.K.; Qin, L.P. Antifungal secondary metabolites from endophytic Verticillium sp. Biochem. Syst. Ecol. 2009, 37, 162–165. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Rajab, M.S.; Franzblau, S.G.; Fronczek, F.R.; Fischer, N.H. Antimycobacterial ergosterol-5, 8-endoperoxide from Ajuga remota. Planta Med. 1999, 65, 732–734. [Google Scholar] [CrossRef]
- Januário, A.H.; Filho, E.R.; Pietro, R.C.L.R.; Kashima, S.; Sato, D.N.; França, S.C. Antimycobacterial physalins from Physalis angulata L. (Solanaceae). Phytother. Res. 2002, 16, 445–448. [Google Scholar] [CrossRef]
- Silva, M.T.; Simas, S.M.; Batista, T.G.; Cardarelli, P.; Tomassini, T.C. Studies on antimicrobial activity, in vitro, of Physalis angulata L. (Solanaceae) fraction and physalin B bringing out the importance of assay determination. Mem. Inst. Ozwaldo Cruz 2005, 100, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Gray, A.I. Antimicrobial constituents from the stem bark of Feronia limonia. Phytochemistry 2002, 59, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, D.S.; Oh, M.N.; Chung, S.C.; Lee, J.S.; Chang, I.M.; Oh, K.B. Inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by β-sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci. Biotechnol. Biochem. 2003, 67, 2477–2479. [Google Scholar] [CrossRef]
- Subramaniam, S.; Keerthiraja, M.; Sivasubramanian, A. Synergistic antibacterial action of β-sitosterol-D-glucopyranoside from Desmostachya bipinnata leaves with antibiotics against common human pathogens. Rev. Braz. Pharmacogn. 2014, 24, 44–50. [Google Scholar] [CrossRef]
- de Moura, R.M.X.; Pereira, P.S.; Januario, A.H.; de Castro França SDias, D.A. Antimicrobial screening and quantitative determination of benzoic acid derivative of Gomphrena celosioides by TLC-densitometry. Chem. Pharm. Bull. 2004, 52, 1342–1344. [Google Scholar] [CrossRef][Green Version]
- Meerungrueang, W.; Panichayupakaranant, P. Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharm. Biol. 2014, 52, 1104–1109. [Google Scholar] [CrossRef]
- Thu, Z.M.; Oo, S.M.; Nwe, T.M.; Aung, H.T.; Armijos, C.; Hussain, F.H.; Vidari, G. Structures and bioactivities of steroidal saponins from the genera dracaena and sansevieria. Molecules 2021, 26, 1916. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Mitaine-Offer, A.C.; Miyamoto, T.; Dongmo, A.; Lacaille-Dubois, M.A. A new steroidal saponin from Dioscorea cayenensis. Chem. Pharm. Bull. 2004, 52, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Zhong, L.; Sui, Y.; Quan, G.; Huang, Y.; Wang, F.; Ma, T. Dioscin inhibits virulence factors of Candida albicans. BioMed Res. Int. 2018, 2018, 4651726. [Google Scholar] [CrossRef]
- Cho, J.; Choi, H.; Lee, J.; Kim, M.S.; Sohn, H.Y.; Lee, D.G. The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Lauren, D.R.; Cooney, J.M.; Jensen, D.J.; Wurms, K.V.; Upritchard, J.E.; Cannon, R.D.; Wang, M.Z.; Li, M.Z. Antifungal saponins from Paris polyphylla Smith. Planta Med. 2008, 74, 1397–1402. [Google Scholar] [CrossRef]
- Fouedjou, R.T.; Teponno, R.B.; Quassinti, L.; Bramucci, M.; Petrelli, D.; Vitali, L.A.; Fiorini, D.; Tapondjou, L.A.; Barboni, L. Steroidal saponins from the leaves of Cordyline fruticosa (L.) A. Chev. and their cytotoxic and antimicrobial activity. Phytochem. Lett. 2014, 7, 62–68. [Google Scholar] [CrossRef]
- Yang, C.R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.J.; Li, X.C. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef]
- Lin, T.C.; Fan, M.C.; Wang, S.Y.; Huang, J.W. Identification of the Solanum nigrum extract component involved in controlling cabbage black leaf spot disease. J. Agric. Food. Chem. 2011, 59, 1667–1672. [Google Scholar] [CrossRef]
- Yücesan, B. In Vitro Propagation and Cardiac Glycoside Production in Endemic Digitalis L. species of Anatolia. Ph.D. Thesis, Abant Izzet Bayzal University: Bolu, Türkiye, 2011. [Google Scholar]
- Srinivas, P.V.; Rao, R.R.; Rao, J.M. Two new tetracyclic triterpenes from the heartwood of Ailanthus excelsa Roxb. Chem. Biodivers. 2006, 3, 930–934. [Google Scholar] [CrossRef]
- Dong, S.H.; Zhang, C.R.; Dong, L.; Wu, Y.; Yue, J.M. Onoceranoid-type triterpenoids from Lansium domesticum. J. Nat. Prod. 2011, 74, 1042–1048. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoids from Lansium domesticum. Philip. Agric. Sci. 2006, 89, 101. [Google Scholar]
- Ji, C.J.; Zeng, G.Z.; Han, J.; He, W.J.; Zhang, Y.M.; Tan, N.H. Zizimauritic acids A–C, three novel nortriterpenes from Ziziphus mauritiana. Bioorg. Med. Chem. Lett. 2012, 22, 6377–6380. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Laughlin, T.F.; Kady, I.O. Thymoquinone inhibits Escherichia coli ATP synthase and cell growth. PLoS ONE 2015, 10, e0127802. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Swee-Hua-Erin, L.I.M.; Lai, K.S. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef]
- Lawrence, N.J.; McGown, A.T.; Nduka, J.; Hadfield, J.A.; Pritchard, R.G. Cytotoxic michael-type amine adducts of α-methylene lactones alantolactone and isoalantolactone. Bioog. Med. Chem. Lett. 2001, 11, 429–431. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Makino, T.; Yoshino, M. Prooxidant action of furanone compounds: Implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Food Chem. Toxicol. 2007, 45, 1258–1262. [Google Scholar] [CrossRef]
- Rosenberg, L.J.; Adlakha, R.C.; Desai, D.M.; Rao, P.N. Inhibition of DNA polymerase α by gossypol. BBA 1986, 866, 258–267. [Google Scholar] [CrossRef]
- Sun, D.A.; Starck, S.R.; Locke, E.P.; Hecht, S.M. DNA Polymerase β Inhibitors from Sandoricum koetjape. J. Nat. Prod. 1999, 62, 1110–1113. [Google Scholar] [CrossRef]
- Haraguchi, H.; Kataoka, S.; Okamoto, S.; Hanafi, M.; Shibata, K. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytother. Res. 1999, 13, 151–156. [Google Scholar] [CrossRef]
- Mert-Türk, F. Saponins versus plant fungal pathogens. J. Cell. Mol. Biol. 2006, 5, 13–17. [Google Scholar]
- Polacheck, I.; Levy, M.; Guizie, M.; Zehavi, U.; Naim, M.; Evron, R. Mode of action of the antimycotic agent G2 from alfalfa roots. Zentralbl Bakteriol. 1991, 275, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.T.; Zheng, L.H.; Bao, Y.L.; Yu, C.L.; Wu, Y.; Meng, X.Y.; Li, Y.X. Reversal effect of Dioscin on multidrug resistance in human hepatoma HepG2/adriamycin cells. Eur. J. Pharmacol. 2011, 654, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Lv, Q.; Sun, X.; Zhou, Y.; Guo, Y.; Qiu, J.; Zhang, P.; Wang, J. Isoalantolactone restores the sensitivity of gram-negative Enterobacteriaceae carrying MCR-1 to carbapenems. J. Cell. Mol. Med. 2020, 24, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.S.; Mistry, N.; Mehra, S. Repurposing artemisinin as an anti-mycobacterial agent in synergy with rifampicin. Tuberculosis 2019, 115, 146–153. [Google Scholar] [CrossRef]
- Puapairoj, P.; Naengchomnong, W.; Kijjoa, A.; Pinto, M.M.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.; Herz, W. Cytotoxic activity of lupane-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Med. 2005, 71, 208–213. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [Google Scholar] [CrossRef]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef]
- Weng, H.Z.; Tian, Y.; Zhang, J.S.; Huang, J.L.; Tang, G.H.; Yin, S. A new tigliane-type diterpenoid from Euphorbia tirucalli. Nat. Prod. Res. 2022, 36, 5380–5386. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Ind. J. Med. Res. 2019, 149, 129. [Google Scholar]
- Markham, P.N.; Neyfakh, A.A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1996, 40, 2673–2674. [Google Scholar] [CrossRef] [PubMed]
- Login, I.S.; Judd, A.M.; Cronin, M.J.; Yasumoto, T.; MacLeod, R.M. Reserpine is a calcium channel antagonist in normal and GH3 rat pituitary cells. Am. J. Physiol. Endocrinol. Metab. 1985, 248, E15–E19. [Google Scholar] [CrossRef]
- Gupta, S.; Cohen, K.A.; Winglee, K.; Maiga, M.; Diarra, B.; Bishai, W.R. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Machado, L.; Costa, S.; Cerca, P.; Spengler, G.; Viveiros, M.; Amaral, L. Role of calcium in the efflux system of Escherichia coli. IJAA 2011, 37, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef]
- Kwak, Y.G.; Kim, D.K.; Ma, T.Z.; Park, S.A.; Park, H.; Jung, Y.H.; Yoo, D.J.; Eun, J.S. Torilin fromTorilis japonica (Houtt.) DC. Blocks hKv1. 5 channel current. Arch. Pharmacal Res. 2006, 29, 834–839. [Google Scholar]
- Habermehl, G.G.; Fliegner, W. Terpenes and their biological relevance. Stud. Nat. Prod. Chem. 1997, 20, 3–24. [Google Scholar]
- Matura, M.; Sköld, M.; Börje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Derm. 2005, 52, 320–328. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor air chemistry: Terpene reaction products and airway effects. Int. J. Hyg. Environ. Health 2020, 225, 113439. [Google Scholar] [CrossRef]
- Di Sotto, A.; Evandri, M.G.; Mazzanti, G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. MRGTEM 2008, 653, 130–133. [Google Scholar] [CrossRef]
- Agus, H.H. Terpene Toxicity and Oxidative Stress. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; pp. 33–42. [Google Scholar]
- Weinstein, L.I.; Albersheim, P. Host-pathogen interactions: XXIII. The mechanism of the antibacterial action of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol. 1983, 72, 557–563. [Google Scholar] [CrossRef] [PubMed]

| Groups of Angiosperms | Clades | Type of Terpenes | References |
|---|---|---|---|
| Basal Angiosperms | Protomagnoliids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Sesquiterpenes (Lindenanes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Magnoliids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Bisabolanes, Eudesmanes, Guaianes, Germacranes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Clerodanes, Kauranes, Trachylobanes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Monocots | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Guaianes, Germacranes, Humulanes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Clerodanes, Kauranes, Rosanes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Triterpenes (Dammaranes, Stigmastanes, Spirostanes, Tirucallanes) | |||
| Core Angiosperms | Eudicots | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Diterpenes (Diterpene alkaloids) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Core Eudicots | Monoterpenes | ||
| Triterpenes (Lupanes, Oleananes) | |||
| Fabids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Bisabolanes, Dihydroagarofurans) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Cassanes, Clerodanes, Jatrophanes, Kauranes, Pimaranes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Triterpenes (Cucurbitanes, Friedelanes, Lupanes, Oleananes, Stigmastanes, Ursanes) | |||
| Malvids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Cadinanes, Drimanes, Eudesmanes, Germacranes, Guaianes, Humulanes, Santalanes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Pimaranes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Triterpenes (Cholestanes, Dammaranes, Ergostanes, Lanostanes, Limonoids, Lupanes, Oleananes, Quassinoids, Stigmastanes, Taraxasteranes, Ursanes) | |||
| Upper Angiosperms | Asterids | Diterpenes (Cadinanes, Clerodanes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] |
| Triterpenes (Oleananes) | |||
| Lamiids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Eudesmanes, Guaianes, Humulanes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Cembranes, Clerodanes, Kauranes, Labdanes, Scopaludanes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Triterpenes (Cardenolides, Ergostanes, Oleananes, Spirostanes, Ursanes) | |||
| Campanuliids | Monoterpenes | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Sesquiterpenes (Bisabolanes, Eudesmanes, Germacranes, Guaianes) | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | ||
| Diterpenes (Diterpene alkaloids, Kauranes) | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] |
| Type of Terpenes | Name of Terpenes | MM (g/mol) | LogD | PSA (Ų) | Gram-Positive | Gram-Negative | Mycobacteria | Filamentous Fungi | Yeasts | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpenes | α-Terpineol (24) | 154.2 | 3 | 20 | S. aureus (*) | E. coli (*) | G. citri-aurantii (*) | [20,32] | ||
| Terpinen-4-ol (25) | 154.2 | 3 | S. aureus (*) | E. coli (*) S. enteritidis (*) | [33,36] | |||||
| Cuminol (32) | 150.2 | 2.3 | 20 | B. cereus | [15] | |||||
| Carvacrol (37) | 150.2 | 3 | 20 | B. subtilis | P. aeruginosa | [13,16] | ||||
| Sesquiterpenes | Gossypol (52) | 518.5 | 5.1 | 156 | B. cereus S. aureus S. epidermidis | [62,63,64,65,66,67,68,69,70] | ||||
| (+)-6,6′-Methoxygossypol (53) | 546.7 | n.a | n.a | E. faecalis | [62,63,64,65,66,67,68,69,70] | |||||
| 7-Hydroxycadalene (54) | 214.3 | 4.7 | 20 | B. cereus | [62,63,64,65,66,67,68,69,70] | |||||
| Mansonone F (56) | 240.2 | 2.5 | 43 | MRSA | [71] | |||||
| Polygodial (64) | 234.3 | 3.8 | 34 | S. libertiana | S. cerevisae H. anomala C. utilis | [90,92] | ||||
| Diterpenes | Geranylgeraniol (65) | 290.4 | 7.4 | 20 | S. aureus | [95] | ||||
| 17-Hydroxyjolkinolide B (68) | 346.4 | 2.6 | 72 | M. smegmatis | [99] | |||||
| ent-1β,14β-diacetoxy-7α-hydroxykaur-16-en-15-one (81) | n.a | n.a | n.a | M. tuberculosis | [119] | |||||
| 16α-Hydroxy-cleroda-3,13 (14)Z-diene-15,16-olide (84) | n.a | n.a | n.a | E. coli K. pneumoniae P. aeruginosa S. typhi | [119] | |||||
| Triterpenes | Dysoxyhainic acid I (94) | 458.7 | n.a | 57.5 | B. subtilis | [156] | ||||
| Pristimerin (97) | 464.2 | 7.1 | 64 | B. subtilis (°) S. epidermidis (°) | [172,173,174] | |||||
| Celastrol (98) | 450.6 | 4 | 75 | B. cereus (°) B. megaterium (°) B. pumilus (°) B. subtilis (°) S. aureus (°) S. epidermidis (°) | H. capsulatum (*) | C. neoformans (*) | [175] | |||
| (20R)-3β-Hydroxy-24,25,26,27-tetranor-5α cycloartan-23,21-olide (100) | n.a | n.a | n.a | MRSA | [178] | |||||
| Ergosterol-5,8-endoperoxide (107) | 412.6 | 6.9 | 29 | M. tuberculosis | [194,195,196,197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiart, C.; Kathirvalu, G.; Raju, C.S.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; Rajagopal, M.; Sathiya Seelan, J.S.; Rusdi, N.A.; Lanting, S.; et al. Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles. Molecules 2023, 28, 3873. https://doi.org/10.3390/molecules28093873
Wiart C, Kathirvalu G, Raju CS, Nissapatorn V, Rahmatullah M, Paul AK, Rajagopal M, Sathiya Seelan JS, Rusdi NA, Lanting S, et al. Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles. Molecules. 2023; 28(9):3873. https://doi.org/10.3390/molecules28093873
Chicago/Turabian StyleWiart, Christophe, Geethanjali Kathirvalu, Chandramathi Samudi Raju, Veeranoot Nissapatorn, Mohammed Rahmatullah, Alok K. Paul, Mogana Rajagopal, Jaya Seelan Sathiya Seelan, Nor Azizun Rusdi, Scholastica Lanting, and et al. 2023. "Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles" Molecules 28, no. 9: 3873. https://doi.org/10.3390/molecules28093873
APA StyleWiart, C., Kathirvalu, G., Raju, C. S., Nissapatorn, V., Rahmatullah, M., Paul, A. K., Rajagopal, M., Sathiya Seelan, J. S., Rusdi, N. A., Lanting, S., & Sulaiman, M. (2023). Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles. Molecules, 28(9), 3873. https://doi.org/10.3390/molecules28093873










