Charge Transfer Complex of Lorlatinib with Chloranilic Acid: Characterization and Application to the Development of a Novel 96-Microwell Spectrophotometric Assay with High Throughput
Abstract
1. Introduction
2. Results and Discussion
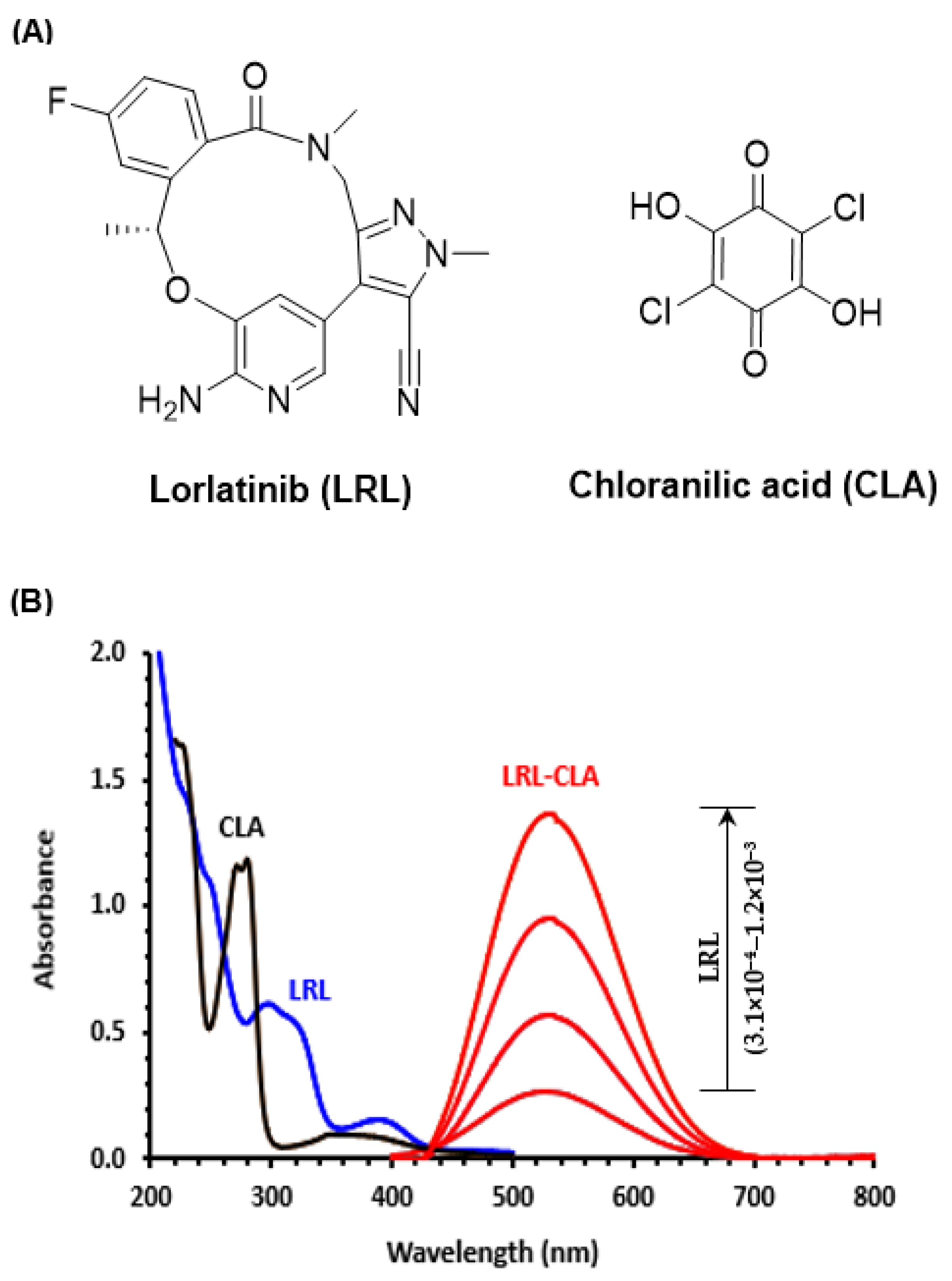
2.1. UV-Visible Absorption Spectra and Band Gap Energy
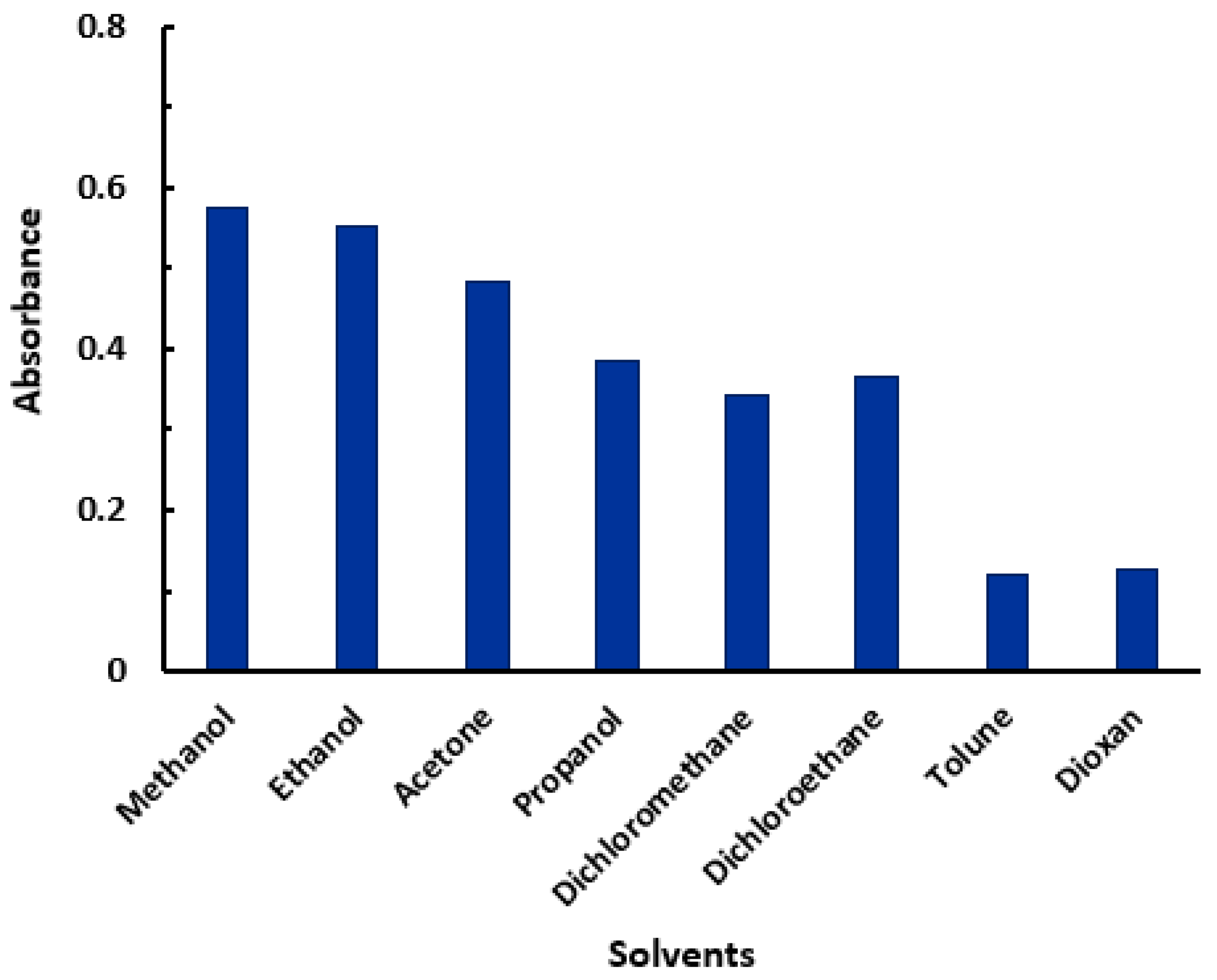
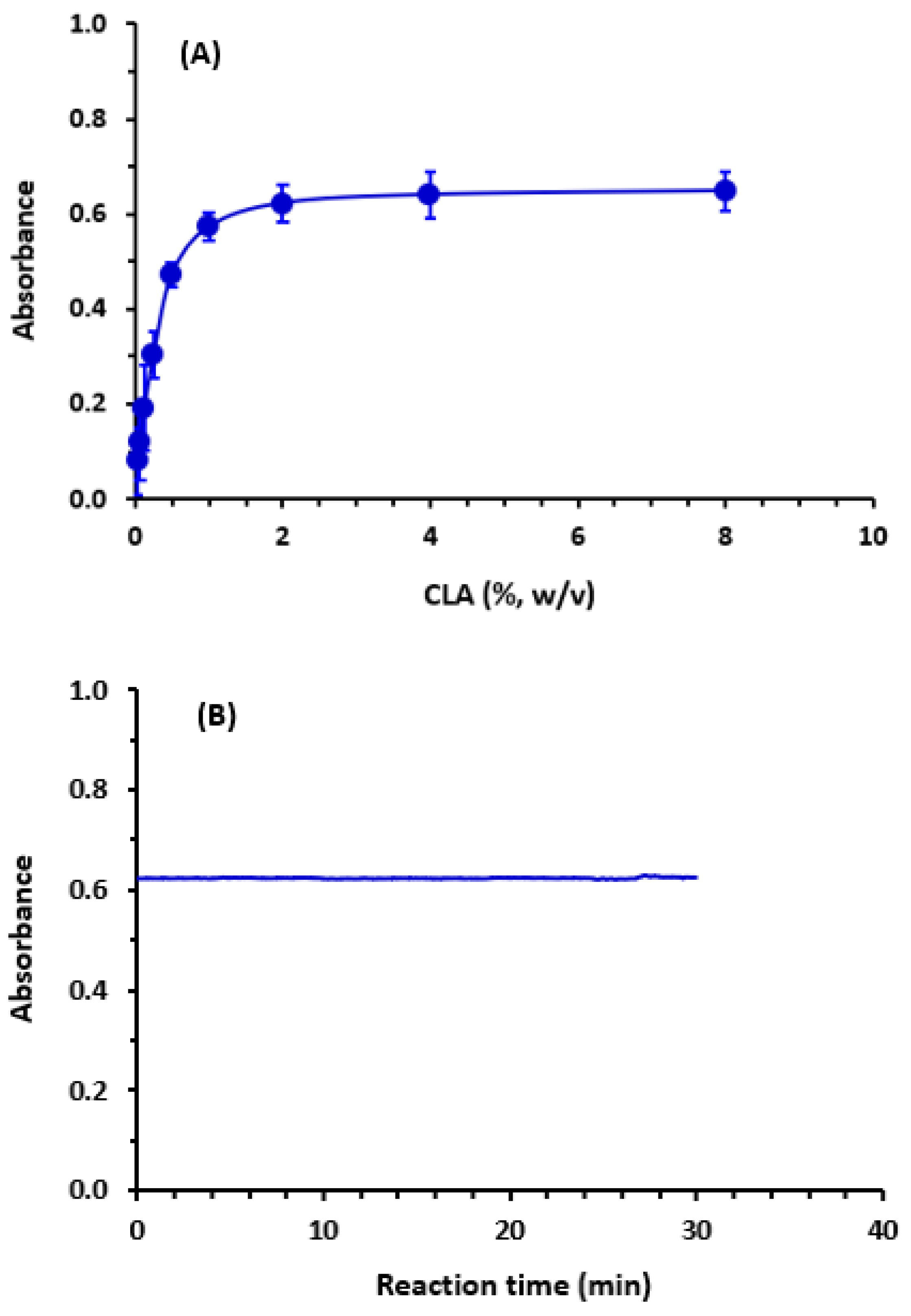
2.2. Optimization of the Reaction Conditions
2.3. Electronic Constants and Properties
2.3.1. Association Constant, Free Energy Change, and Donor Ionization Potential
2.3.2. Oscillator Strength and Transition Dipole Moment
2.3.3. Resonance Energy
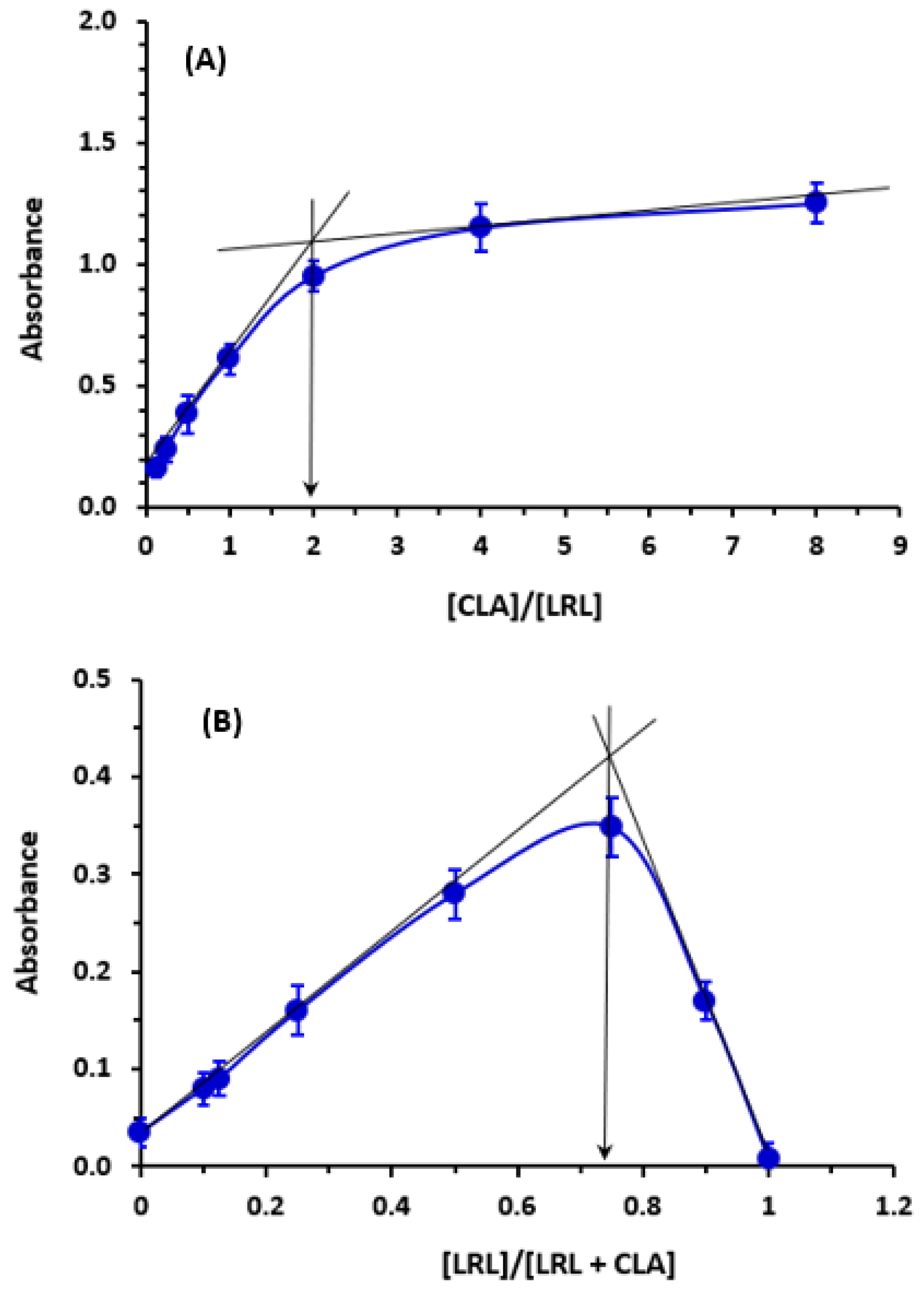
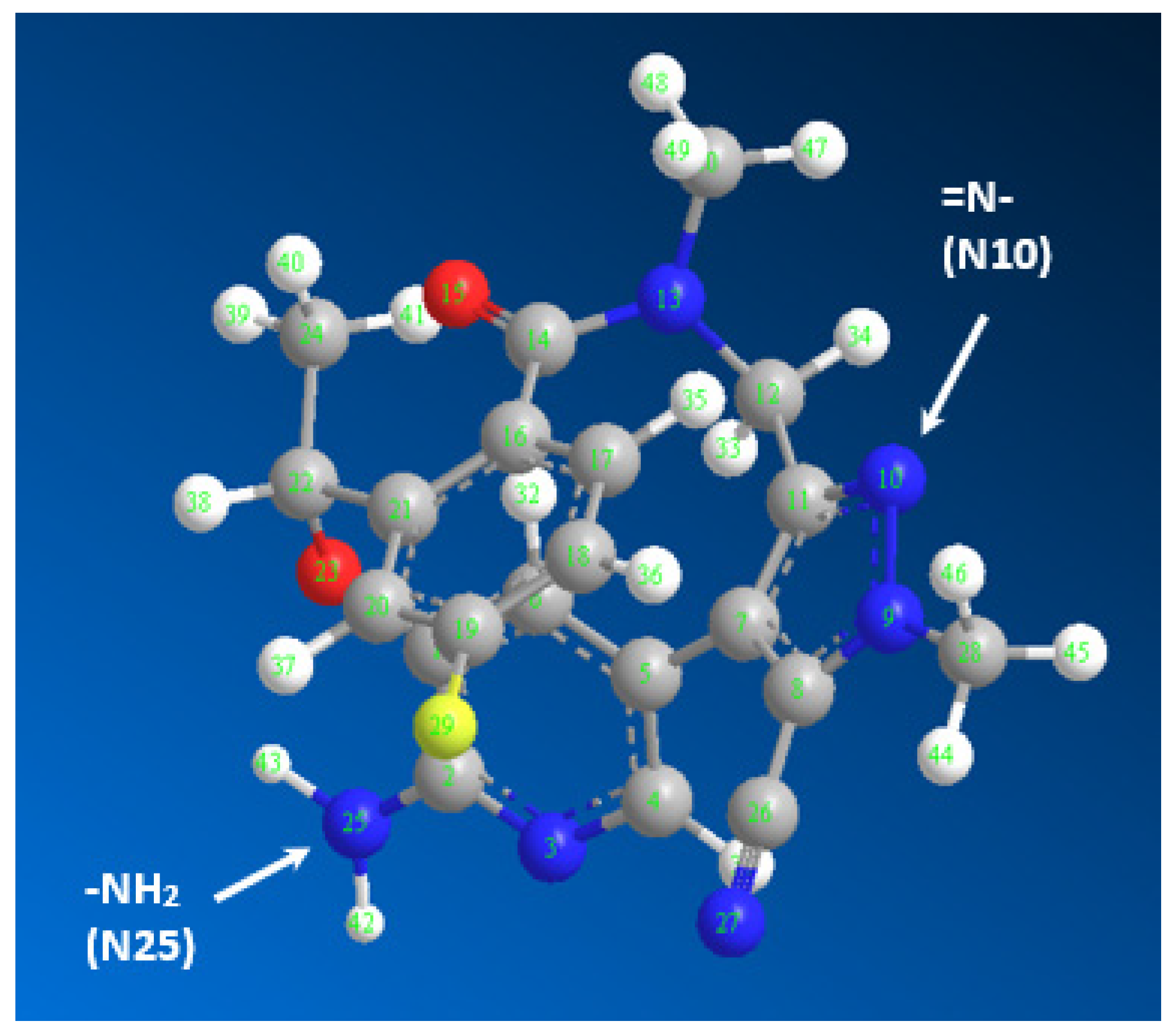
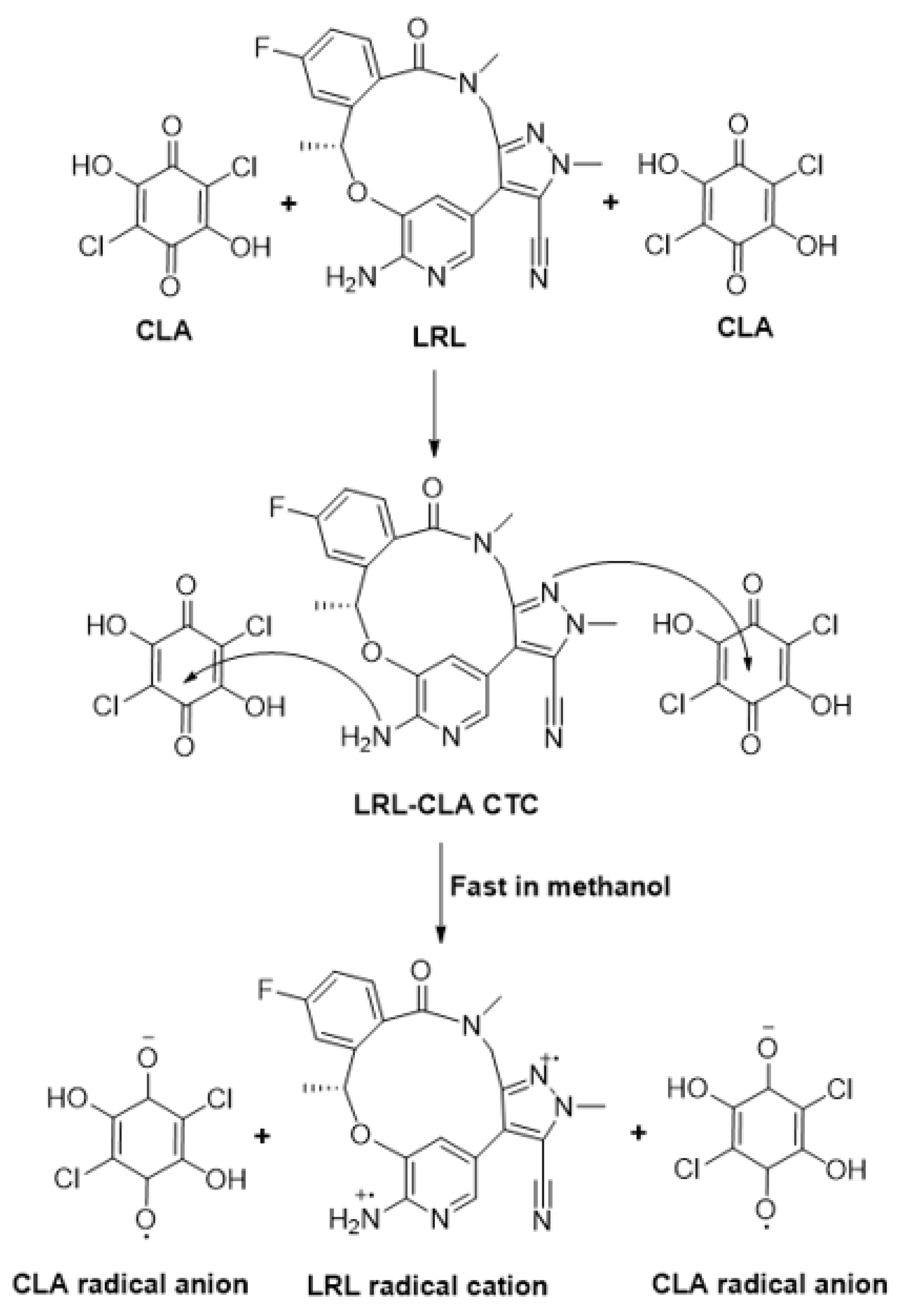
2.4. Molar Ratio and Computational Charge Calculation
2.5. Development of MW-SPA
2.5.1. Strategy and Design of the Assay
2.5.2. Optimization of MW-SPA Conditions
2.6. Validation of MW-SPA
2.6.1. Linear Range and Sensitivity
2.6.2. Precision and Accuracy
2.6.3. Specificity and Interference
2.7. Application of MW-SPA to the Quantitation of LRL in Lorbrena® Tablets
3. Materials and Methods
3.1. Apparatus
3.2. Chemicals and Materials
3.3. Preparation of LRL Standard and Tablet Solutions
3.4. Calculation of Association Constant and Molar Ratio
3.5. Calculation of Electron Densities on Atoms
3.6. MW-SPA Procedures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mulliken, R.S.; Pearson, W.B. Molecular Complexes; Wiley Publishers: New York, NY, USA, 1969. [Google Scholar]
- Foster, R. Organic Charge-Transfer Complexes; Academic Press: London, UK, 1969. [Google Scholar]
- Das, S.K.; Krishnamoorthy, G.; Dogra, S.K. Excited state intramolecular proton transfer in 2-(2′-hydroxyphenyl)-1 H-naphth-[2,3-d]-imidazole: Effects of solvents and pH. Can. J. Chem. 2000, 78, 191–205. [Google Scholar] [CrossRef]
- Datta, A.S.; Bagchi, S.; Chakrabortty, A.; Lahiri, S.C. Studies on the weak interactions and CT complex formations between chloranilic acid, 2,3-dichloro-5,6-dicyano-p-benzoquinone, tetracyanoethylene and papaverine in acetonitrile and their thermodynamic properties, theoretically, spectrophotometrically aided by FTIR. Spectrochim. Acta A 2015, 146, 119–128. [Google Scholar]
- Liu, H.; Liu, Z.; Jiang, W.; Fu, H. Tuning the charge transfer properties by optimized donor–acceptor cocrystal for FET applications: From P type to N type. J. Solid State Chem. 2019, 274, 47–51. [Google Scholar] [CrossRef]
- Murugesan, V.; Saravanabhavan, M.; Sekar, M. Synthesis, spectral, structural analysis and biological evaluation of a new hydrogen-bonded charge-transfer complex: 2,3-dimethylquinoxalinium-p-toluenesulfonate. J. Photochem. Photobiol. B 2014, 140, 20–27. [Google Scholar] [CrossRef]
- Singh, N.; Khan, I.M.; Ahmad, A.; Javed, S. Preparation, spectral investigation and spectrophotometric studies of proton transfer complex of 2,2′-bipyridine with 3,5-dinitrobenzoic acid in various polar solvents. J. Mol. Struct. 2014, 1065, 74–85. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Alhadhrami, A.; Adam, A.M.A.; Grabchev, I.; Almeataq, M.; Al-Humaidi, J.Y.; Sharshar, T.; Refat, M.S. Preparation of elastic polymer slices have the semiconductors properties for use in solar cells as a source of new and renewable energy. J. Photochem. Photobiol. A 2018, 361, 76–85. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Alhadhrami, A.; Obaid, R.J.; Alsharif, M.A.; Adam, A.A.; Brabchev, I.; Refat, M.S. Preparation of some compounds and study their thermal stability for use in dye sensitized solar cells. J. Mol. Liq. 2018, 261, 565–582. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Arslan, M. Determination of electrical conduction mechanism and optical band gap of a new charge transfer complex: TCNQ-PANT. Solid State Commun. 2004, 132, 229–234. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Arslan, M. The fundamental absorption edge and optical constants of some charge transfer compounds. Opt. Mater. 2004, 27, 29–37. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Arslan, M.; Kucukislamoglu, M.; Zengin, M. Temperature dependence of the optical band gap and refractive index of poly (ethylene terepthalate) oligomer–DDQ complex thin film. Sol. Energy 2005, 79, 96–100. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mukherjee, A.S.; Seal, B.K. Charge-transfer complex formation between o-chloranil and a series of polynuclear aromatic hydrocarbons. Spectrochim. Acta A 2001, 57, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.M.; Costa, S.M.B.; Pansu, R. Structural changes in W/O Triton X-100/cyclohexane-hexanol/water microemulsions probed by a fluorescent drug Piroxicam. J. Colloid Interface Sci. 2000, 226, 260–268. [Google Scholar] [CrossRef]
- Dabestani, R.; Reszka, K.J.; Sigman, M.E. Surface catalyzed electron transfer from polycyclic aromatic hydrocarbons (PAH) to methyl viologen dication: Evidence for ground-state charge transfer complex formation on silica gel. J. Photochem. Photobiol. 1998, 117, 223–233. [Google Scholar] [CrossRef]
- Jakubiak, R.; Bao, Z.; Rothberg, L. Dendritic sidegroups as three-dimensional barriers to aggregation quenching of conjugated polymer fluorescence. Synth. Met. 2000, 114, 61–64. [Google Scholar] [CrossRef]
- Takahasi, K.; Horino, K.; Komura, T.; Murata, K. Photovoltaic properties of porphyrin thin films mixed with o-chloranil. Bull. Chem. Soc. Jpn. 1993, 66, 733–738. [Google Scholar] [CrossRef]
- Eychmüller, A.; Rogach, A.L. Chemistry and photophysics of thiol-stabilized II-VI semiconductor nanocrystals. Pure Appl. Chem. 2000, 72, 179–188. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; Wani, T.A.; Al-Majed, A.R.A. Microwell spectrophotometric method with high-throughput for determination of the macrolide antibiotics in their pharmaceutical formulations. Latin Am. J. Pharm. 2014, 33, 928–934. [Google Scholar]
- Saleh, G.A.; Askal, H.F.; Darwish, I.A.; El-Shorbagi, A.N.A. Spectroscopic analytical study for the charge-transfer complexation of certain cephalosporins with chloranilic acid. Anal. Sci. 2003, 19, 281–287. [Google Scholar] [CrossRef]
- Darwish, I.A.; Alzoman, N.Z.; Alshehri, J.M.; Khalil, N.Y.; Abdel-Rahman, H.M. Charge-transfer reaction of 1,4-benzoquinone with crizotinib: Spectrophotometric study, computational molecular modeling and use in development of microwell assay for crizotinib. Spectrochim. Acta A 2014, 131, 347–354. [Google Scholar] [CrossRef]
- Darwish, I.A.; Alzoman, N.Z.; Alshehri, J.M.; Khalil, N.Y.; Abdel-Rahman, H.M. Charge-transfer reaction of chloranilic acid with crizotinib: Spectrophotometric study, computational modeling and use in development of microwell assay for crizotinib. J. Solut. Chem. 2014, 43, 1282–1295. [Google Scholar] [CrossRef]
- Darwish, I.A.; Wani, T.A.; Khalil, N.Y.; Abdel-Rahman, H.M. High throughput microwell spec-trophotometric assay for olmesartan medoxomil in tablets based on its charge-transfer reaction with DDQ. Acta Pharm. 2014, 64, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Mahmoud, A.M.; Al-Majed, A.R.A. Novel analytical approach for reducing the consumption of organic solvents in the charge transfer-based spectrophotometric analysis of losartan potassium. Int. J. Res. Pharm. Sci. 2010, 1, 391–395. [Google Scholar]
- Khalil, N.Y.; Wani, T.A.; Darwish, I.A.; Assiri, I.S. Charge-transfer reaction of cediranib with 2,3-dichloro-3,5-dicyano-1,4-benzoquinone: Spectrophotometric investigation and use in development of microwell assay for cediranib. Trop. J. Pharm. Res. 2015, 14, 1667–1672. [Google Scholar] [CrossRef]
- Alzoman, N.Z.; Sultan, M.A.; Maher, H.M.; Alshehri, M.M.; Wani, T.A.; Darwish, I.A. Analytical study for the charge-transfer complexes of rosuvastatin calcium with π-acceptors. Molecules 2013, 18, 7711–7725. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Wani, T.A.; Khalil, N.Y.; Al-Shaikh, A.A.; Al-Morshadi, N. Development of a novel 96-microwell assay with high throughput for determination of olmesartan medoxomil in its tablets. Chem. Cent. J. 2012, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Ahmad, A.; Zargar, S.; Khalil, N.Y.; Darwish, I.A. Use of response surface methodolo-gy for development of new microwell-based spectrophotometric method for determination of atrovastatin calcium in tablets. Chem. Cent. J. 2012, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Khalil, N.Y.; Darwish, H.W.; Sayed, A.Y.A. Experimental and computational evaluation of chloranilic acid as an universal chromogenic reagent for the development of a novel 96-microwell spectrophotometric assay for tyrosine kinase inhibitors. Molecules 2021, 26, 744–751. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; Darwish, H.W.; Alzoman, N.Z.; Al-Hossaini, A.M. Spectrophotometric and computational investigations of charge transfer complexes of chloranilic acid with tyrosine kinase inhibitors and application to development of novel universal 96-microwell assay for their determination in pharmaceutical formulations. Spectrochim. Acta A 2021, 252, 119482–119489. [Google Scholar] [CrossRef]
- Basit, S.; Ashraf, Z.; Lee, K.; Latif, M. First macrocyclic 3rd-generation ALK inhibitor for treatment of ALK/ROS1 cancer: Clinical and designing strategy update of lorlatinib. Eur. J. Med. Chem. 2017, 134, 348–356. [Google Scholar] [CrossRef]
- Awad, M.M.; Shaw, A.T. ALK inhibitors in non-small cell lung cancer: Crizotinib and beyond. Clin. Adv. Hematol. Oncol. 2014, 12, 429–439. [Google Scholar]
- U.S. Food & Drug Administration. FDA Approves lorlatinib for metastatic ALK-Positive NSCLC. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lorlatinib-metastatic-alk-positive-nsclc (accessed on 29 January 2023).
- European Medicines Agency. Lorviqua: Lorlatinib. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lorviqua (accessed on 29 January 2023).
- Darwish, I.A. Analytical study for the charge transfer complexes of losartan potassium. Anal. Chim. Acta 2005, 549, 212–220. [Google Scholar] [CrossRef]
- Gohar, G.A.; Habeeb, M.M. Proton transfer equilibria, temperature and substituent effects on hydrogen bonded complexes between chloranilic acid and anilines. Spectroscopy 2000, 14, 99–107. [Google Scholar] [CrossRef]
- Karipcin, F.; Dede, B.; Caglar, Y.; Hür, D.; Ilican, S.; Caglar, M.; Şahin, Y. A new dioxime ligand and its trinuclear copper (II) complex: Synthesis, characterization and optical properties. Opt. Commun. 2007, 272, 131–137. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I.; Tatchell, A.R.; Furnis, B.S.; Hannaford, A.J.; Smith, P.G. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Group UK Ltd.: London, UK, 1989. [Google Scholar]
- Polarity Index. Available online: http://macro.lsu.edu/howto/solvents/polarity%20index.htm (accessed on 29 January 2023).
- Benesi, H.A.; Hildebrand, J. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Pandeeswaran, M.; Elango, K. Solvent effect on the charge transfer complex of oxatomide with 2, 3-dichloro-5, 6-dicyanobenzoquinone. Spectrochim. Acta A 2006, 65, 1148–1153. [Google Scholar] [CrossRef]
- Aloisi, G.; Pignataro, S. Molecular complexes of substituted thiophens with σ and π acceptors. Charge transfer spectra and ionization potentials of the donors. J. Chem. Soc. Faraday Trans. 1972, 69, 534–539. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1985; p. 161. [Google Scholar]
- Tsubumora, H.; Lang, R. Molecular complexes and their spectra. XIII. complexes of iodine with am-ides, diethyl sulfide and diethyl disulfide. J. Am. Chem. Soc. 1961, 83, 2085–2092. [Google Scholar] [CrossRef]
- Briegleb, G.; Czekalla, J. Intensity of electron transition bands in electron donator–acceptor complexes. Z. Physik. Chem. 1960, 24, 37. [Google Scholar] [CrossRef]
- Chakravarthi, K.K.; Himabindu, K. RP-HPLC method development and validation for the determination of lorlatinib in bulk and its pharmaceutical formulation. Intern. J. Res. Pub. Rev. 2021, 9, 958–963. [Google Scholar]
- Spatari, C.; Li, W.; Schinkel, A.H.; Ragno, G.; Schellens, J.H.; Beijnen, J.H.; Sparidans, R.W. Bioanalytical assay for the quantification of the ALK inhibitor lorlatinib in mouse plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2018, 1083, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, Y.; Qi, S.; Zhou, H.; Li, C.; Jin, D.; Li, G. Pharmacokinetic study and tissue distribution of lorlatinib in mouse serum and tissue samples by liquid chromatography-mass spectrometry. J. Anal. Methods Chem. 2019, 2019, 7574369. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Li, W.; Schinkel, A.H.; Schellens, J.H.M.; Beijnen, J.H. Bioanalytical liquid chromatography-tandem mass spectrometric assay for the quantification of the ALK inhibitors alec-tinib, brigatinib and lorlatinib in plasma and mouse tissue homogenates. J. Pharm. Biomed. Anal. 2018, 161, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, D.; Simon, P.; Bruijn, P.D.; Stijn, L.W.; Koolen, A.M. Quantitation of osimertinib, alectinib and lorlatinib in human cerebrospinal fluid by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2023, 225, 115233–115240. [Google Scholar] [CrossRef]
- Wenlong, L.; Perpinioti, N.; Schinkel, A.H.; Beijnen, J.H.; Sparidans, R.W. Bioanalytical assay for the new-generation ROS1/TRK/ALK inhibitor repotrectinib in mouse plasma and tissue homogenate using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2020, 1144, 122098–122104. [Google Scholar]
- Görög, S. Ultraviolet-Visible Spectrophotometry in Pharmaceutical Analysis; CRC Press: New York, NY, USA, 2018. [Google Scholar]
- Kroll, P.; Sagmeister, P.; Reichelt, W.; Neutsch, L.; Klein, T.; Herwig, C. Ex situ online monitoring: Application, challenges and opportunities for biopharmaceuticals processes. Pharm. Bioprocess. 2014, 2, 285–300. [Google Scholar] [CrossRef]
- Gouda, A. Charge transfer spectrophotometric methods for the determination of two antihistaminic drugs in pharmaceutical formulations. Int. J. Pharm. Pharm. Sci. 2014, 6, 334–341. [Google Scholar]
- Darwish, I.A.; Refaat, I.H. Spectrophotometric analysis of selective serotonin reuptake inhibitors based on formation of charge-transfer complexes with tetracyanoquinondimethane and chloranilic acid. J. AOAC Int. 2006, 89, 326–333. [Google Scholar] [CrossRef]
- Wennborg, H.; Bonde, J.P.; Stenbeck, M.; Olsen, J. Adverse reproduction outcomes among employee in biomedical research laboratories. Scand. J. Work Environ. Health 2002, 28, 5–11. [Google Scholar] [CrossRef]
- Kristensen, P.; Hilt, B.; Svendsen, K.; Grimsrud, T.K. Incidence of lymphohaematopoietic cancer at a university laboratory: A cluster investigation. Eur. J. Epidemiol. 2008, 23, 11–15. [Google Scholar] [CrossRef]
- ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; ICH Harmonised Guideline; Validation of Analytical Procedure: Q2(R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principle of Instrumental Analysis, 7th ed.; Saunder: Philadelphia, PA, USA, 2014. [Google Scholar]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chem. 1928, 9, 113–203. [Google Scholar]



| Constant/Property | Value |
|---|---|
| Molar absorptivity, ε (L mol−1 cm−1) | 0.55 × 103 |
| Association constant, K (L mol−1) | 0.40 × 103 |
| Ionization potential, Ip (eV) | 0.92 × 102 |
| Energy, hν (eV) | 2.3465 |
| Resonance energy, RN (eV) | 0.6688 |
| Dissociation energy, W (eV) | 3.2523 |
| Oscillator strength, f | 0.4331 |
| Standard free energy change, △G0 (J mol−1) | −0.15 × 102 |
| Transition dipole moment, µ(Debye) | 0.12 × 102 |
| Atom Number | Atom Type | Charge | Atom Number | Atom Type | Charge |
|---|---|---|---|---|---|
| C(1) | Aromatic C, in benzene | 0.0825 | C(20) | Aromatic C, in benzene | −0.15 |
| C(2) | Aromatic C, in benzene | 0.41 | C(21) | Aromatic C, in benzene | −0.1435 |
| N(3) | Aromatic N, with s lone pair | −0.62 | C(22) | Alkyl C, SP3 | 0.4235 |
| C(4) | Aromatic C, in benzene | 0.16 | O(23) | Alcohol, ether O | −0.3625 |
| C(5) | Aromatic C, in benzene | 0 | C(24) | Alkyl C, SP3 | 0 |
| C(6) | Aromatic C, in benzene | −0.15 | N(25) | Enamine, aniline N | −0.9 |
| C(7) | Aromatic C, in 5-ring | 0 | C(26) | Cyano C | 0.5371 |
| C(8) | Aromatic C, in 5-ring | −0.1316 | N(27) | Triple bond N | −0.5571 |
| N(9) | Aromatic N, in 5-ring | 0.314 | C(28) | Alkyl carbon, SP3 | 0.2556 |
| N(10) | Aromatic N, in 5-ring | −0.7068 | F(29) | Fluorine | −0.19 |
| C(11) | Aromatic C, in 5-ring | 0.1078 | C(30) | Alkyl carbon, SP3 | 0.3001 |
| C(12) | Alkyl C, SP3 | 0.4811 | H(31)–H(32) | H attached to C | 0.15 |
| N(13 | Amide N | −0.6602 | H(33)–H(34) | H attached to C | 0 |
| C(14) | Amide carbonyl C | 0.5438 | H(35)–H(37) | H attached to C | 0.15 |
| O(15) | Carbonyl O, in amide | −0.57 | H(38)–H(41) | H attached to C | 0 |
| C(16) | Aromatic C, in benzene | 0.0862 | H(42)–H(43) | H of enamine N | 0.4 |
| C(17)–C(18) | Aromatic C, in benzene | −0.15 | H(44)–H(49) | H attached to C | 0 |
| C(19) | Aromatic C, in benzene | 0.19 |
| Condition | Studied Range | Optimum Value |
|---|---|---|
| CLA conc. (%, w/v) | 0.1–8 | 2 |
| Solvent | Different a | Methanol |
| Reaction time (min) | 0–30 | Instantaneous b |
| λmax (nm) | 400–800 | 530 c |
| Parameter | Value |
|---|---|
| Linear range (µg/well) | 5–200 |
| Intercept (a) | 0.008 |
| Standard deviation of intercept (SDa) | 3.9 × 10−3 |
| Slope (b) | 0.006 |
| Standard deviation of slope (SDb) | 2.8 × 10−3 |
| Correlation coefficient (r) | 0.9996 |
| Limit of detection (LOD, µg/well) | 2.1 |
| Limit of quantitation (LOQ, µg/well) | 6.5 |
| Taken Concentration (µg/well) | Precision: Relative Standard Deviation (%) | Accuracy: Recovery (% ± SD) a | |
|---|---|---|---|
| Intra-Assay, n = 3 | Inter-Assay, n = 6 | ||
| 12.5 | 2.15 | 2.45 | 99.6 ± 2.3 |
| 25 | 1.81 | 2.36 | 98.2 ± 1.8 |
| 50 | 1.53 | 2.14 | 99.1 ± 1.2 |
| 100 | 1.62 | 1.80 | 100.2 ± 2.4 |
| 200 | 1.42 | 1.82 | 99.1 ± 2.2 |
| Nominal Concentration (μg/mL) | Found Concentration a (μg/mL) | Recovery a (%) | |
|---|---|---|---|
| 125 | 122.75 | 98.2 ± 2.3 | |
| 500 | 497 | 99.4 ± 1.2 | |
| 1000 | 991 | 99.1 ± 2.4 | |
| 2000 | 1994 | 99.7 ± 2.2 | |
| Mean | 99.1 | ||
| SD | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, H.W.; Darwish, I.A.; Ali, A.M.; Almutairi, H.S. Charge Transfer Complex of Lorlatinib with Chloranilic Acid: Characterization and Application to the Development of a Novel 96-Microwell Spectrophotometric Assay with High Throughput. Molecules 2023, 28, 3852. https://doi.org/10.3390/molecules28093852
Darwish HW, Darwish IA, Ali AM, Almutairi HS. Charge Transfer Complex of Lorlatinib with Chloranilic Acid: Characterization and Application to the Development of a Novel 96-Microwell Spectrophotometric Assay with High Throughput. Molecules. 2023; 28(9):3852. https://doi.org/10.3390/molecules28093852
Chicago/Turabian StyleDarwish, Hany W., Ibrahim A. Darwish, Awadh M. Ali, and Halah S. Almutairi. 2023. "Charge Transfer Complex of Lorlatinib with Chloranilic Acid: Characterization and Application to the Development of a Novel 96-Microwell Spectrophotometric Assay with High Throughput" Molecules 28, no. 9: 3852. https://doi.org/10.3390/molecules28093852
APA StyleDarwish, H. W., Darwish, I. A., Ali, A. M., & Almutairi, H. S. (2023). Charge Transfer Complex of Lorlatinib with Chloranilic Acid: Characterization and Application to the Development of a Novel 96-Microwell Spectrophotometric Assay with High Throughput. Molecules, 28(9), 3852. https://doi.org/10.3390/molecules28093852







