Chemical Characterisation, Antidiabetic, Antibacterial, and In Silico Studies for Different Extracts of Haloxylon stocksii (Boiss.) Benth: A Promising Halophyte
Abstract
1. Introduction
2. Results
2.1. Phytochemical Composition
2.1.1. Total Phenolic Contents (TPC)
2.1.2. Total Flavonoid Contents (TFC)
2.1.3. GC-MS Analysis
2.1.4. Phytochemical Screening of Haloxylon stocksii by LC-ESI-MS2
2.2. Biological Activities of Aerial and Roots Extract of H. stocksii
2.2.1. Antioxidant Activities
2.2.2. Antibacterial Activity
2.2.3. Antidiabetic Activities
2.3. In silico Evaluation
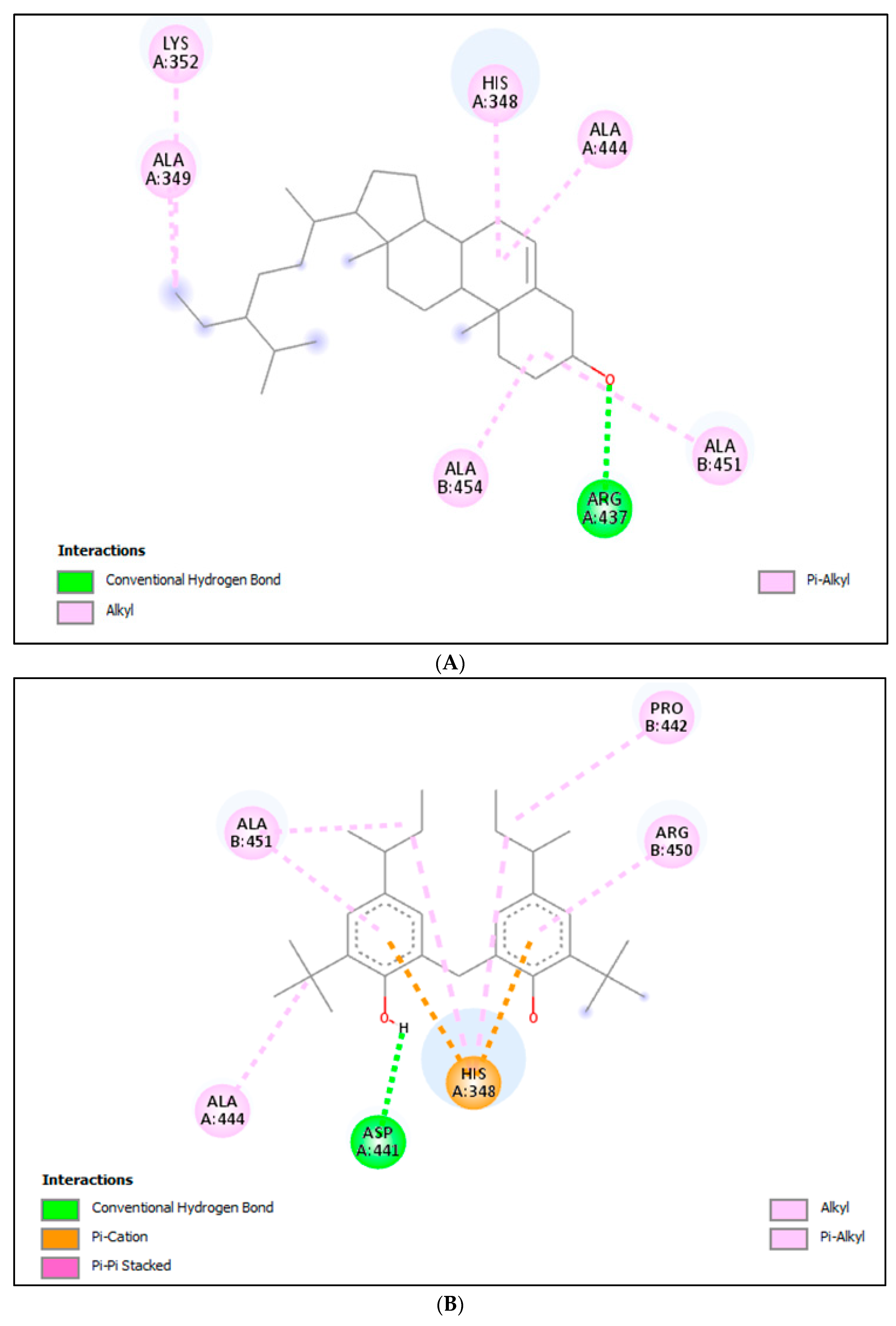
2.3.1. Molecular Docking (MD) for α-Amylase
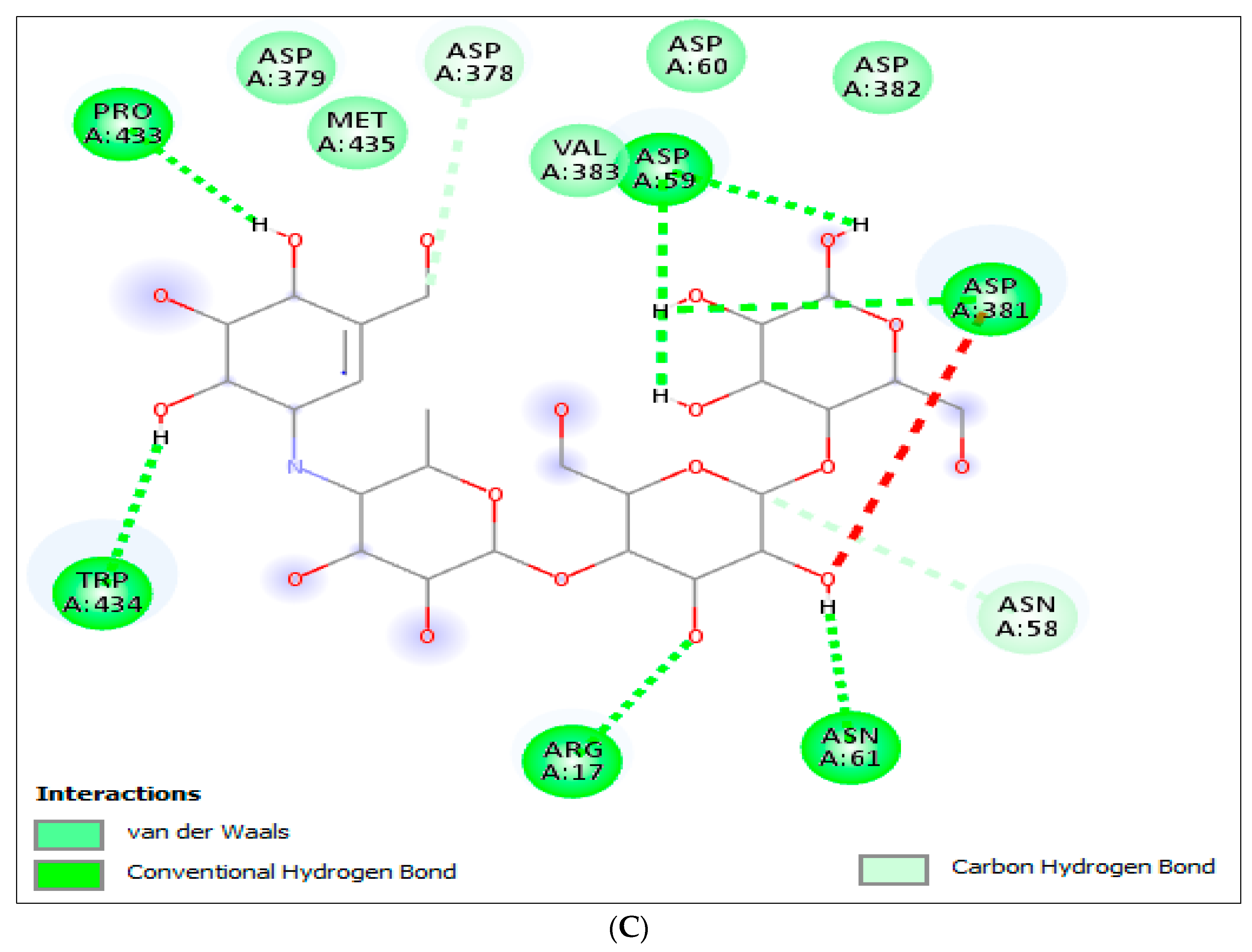
2.3.2. Molecular Docking (MD) for α-Glucosidase
2.3.3. ADMET Study
2.3.4. Lipinski Rule of Five
- The hydrogen bond donors should not be more than 5.
- The hydrogen bond acceptors should not be more than 10.
- The molecular mass should not be more than 500 daltons.
- Lipophilicity should not exceed the value of 5.
2.3.5. Toxicity Study
3. Discussion
4. Materials and Methods
4.1. Plant Identification and Sample Collection
4.2. Extract Preparation of the Plant Material
4.3. Phytochemical Composition
4.3.1. Estimation of Total Phenolic Content
4.3.2. GC-MS Analysis
4.3.3. Phytochemical Screening of Haloxylon stocksii by LC-ESI-MS2
4.4. Biological Profiling
4.4.1. Antioxidant Activities
Radical Scavenging Activity
- DPPH Assay
- 2.
- ABTS Assay
Reducing Power Assays
- CUPRAC Assay
- 2.
- FRAP Assay
4.4.2. Antibacterial Activities
Disc Diffusion Method
4.4.3. In vitro Antidiabetic Potential
- α-Amylase inhibitory assay:
- α-Glucosidase inhibitory assay:
4.5. In-Silico Evaluation
4.5.1. Molecular Docking Study
4.5.2. ADMET Analysis
4.5.3. Toxicity Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Verma, R.; Gupta, J. Challenges and future prospects of herbal medicine. Int. Res. Med. Heal Sci. 2018, 1, 12–15. [Google Scholar] [CrossRef]
- Robina, S.H.; Ahmad, J.; Javed, S.; Adnan, S.M.; Zahir, J.; Shagufa, M. Antimicrobial Activity of Ethyl Acetate, Chloroform and Deionized Water Extract of Leaves of Pteris Cretica. Ann. Romanian Soc. Cell Biol. 2021, 25, 1493–1501. [Google Scholar]
- Ahmed, M.; Khan, K.-U.-R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.-U.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R.; et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules 2022, 27, 4113. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res 2019, 10, 494–504. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A.J.M. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R.J.M. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Mroczek, A. Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Yousuf, M.; Khan, H.M.S.; Rasool, F.; Usman, F.; Ghalloo, B.A.; Umair, M.; Babalghith, A.O.; Kamran, M.; Aadil, R.M.; Al Jaouni, S.K. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules 2022, 27, 5990. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Vasudeva, N. Review on antioxidants and evaluation procedures. Chin. J. Integr. Med. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Atangwho, I.J.; Egbung, G.E.; Ahmad, M.; Yam, M.F.; Asmawi, M.Z. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013, 141, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Nisar, R.; Ahmad, S.; Khan, K.-U.-R.; Sherif, A.E.; Alasmari, F.; Almuqati, A.F.; Ovatlarnporn, C.; Khan, M.A.; Umair, M.; Rao, H. Metabolic Profiling by GC-MS, In Vitro Biological Potential, and In Silico Molecular Docking Studies of Verbena officinalis. Molecules 2022, 27, 6685. [Google Scholar] [CrossRef]
- Adeloye, J.B.; Aluko, P.A.; Oluwajuyitan, T.D. In vitro α-amylase and α-glucosidase inhibitory activities, antioxidant activity, in vivo glycemic response and nutritional quality of dough meals from Dioscorea alata and Vernonia amygdalina. J. Food Meas. Charact. 2021, 15, 4083–4097. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Fofie, C.K.; Nguelefack-Mbuyo, E.P.; Wuyt, A.K. Multimodal α-glucosidase and α-amylase inhibition and antioxidant effect of the aqueous and methanol extracts from the trunk bark of Ceiba pentandra. BioMed Res. Int. 2020, 2020, 3063674. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Baber, A. Phytochemical and Biological Screening of Haloxylon stocksii. Ph.D. Thesis, The Islmia University of Bahawalpur, Bahawalpur, Pakistan, 2018. [Google Scholar]
- Abbas, S.; Rasheed, A.; Soriano, P.; Estrelles, E.; Gul, B.; Hameed, A. Moisture content and oxidative damage determine longevity of the seeds of potential cash crop halophyte Haloxylon stocksii. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2022, 156, 1478–1484. [Google Scholar] [CrossRef]
- Rathore, V.S.; Singh, J.P.; Roy, M.M. Haloxylon stocksii (Boiss.) Benth. et Hook. f., a promising halophyte: Distribution, cultivation and utilization. Genet. Resour. Crop Evol. 2012, 59, 1213–1221. [Google Scholar] [CrossRef]
- Babar, A.; Ahmad, S.; Rehman, T.; Sabah, N.U.; Arshad, M.A. A Review on Phytochemical Analysis and Ethnobotanical Uses of Haloxylon stocksii. RADS J. Pharm. Pharm. Sci. 2018, 6, 162–167. [Google Scholar]
- Natubhai, P.M.; Pandya, D.S.S.; Rabari, D.H.A. Anti-Inflammatory activity of leaf extracts of salvadora oleoides decne. Int. J. Pharm. Bio. Sci. 2013, 4, 985–993. [Google Scholar]
- Zafar, M.I.; Nazli, A.; Riaz, M.A.; Ashraf, M.R.; Iqbal, M.M.; Khan, A.; Iqbal, Z.; Javed, A.; Ahmed, S.; Haq, I.U. Toxic potential of alstonia scholaris and salvadora oleiodes leaves extract against subterranean termites, microtermes obesi (isoptera:termitidae). Int. J. Pharmacogn. 2020, 7, 109–115. [Google Scholar]
- Korejo, F.; Noreen, R.; Ali, S.A.; Humayun, F.; Rahman, A.; Sultana, V.; Ehteshamul-Haque, S. Evaluation of antibacterial and antifungal potential of endophytic fluorescent pseudomonas associated with salvadora persica L. and salvadora oleoides decne. Pak. J. Bot. 2017, 49, 1995–2004. [Google Scholar]
- Kumar, D.; Parcha, V.; Maithani, A. Combined effect on antihyperlipidemic activity of Bauhinia variegata (linn.) and Salvadora oleoides (decne.) in Triton WR-1339 induced hyperlipidemic rats. World J. Pharm. Sci. 2014, 2, 428–435. [Google Scholar]
- Yi, B.-H.; Kim, D.-H. Antioxidant activity of maltol, kojic acid, levulinic acid, furfural, 5-hydroxymethyl furfural, and pyrazine. Korean J. Food Sci. Technol. 1982, 14, 265–270. [Google Scholar]
- Jerković, I.; Kasum, A.; Marijanović, Z.; Tuberoso, C.I.G. Contribution to the characterisation of honey-based Sardinian product abbamele: Volatile aroma composition, honey marker compounds and antioxidant activity. Food Chem. 2011, 124, 401–410. [Google Scholar] [CrossRef]
- Teoh, Y.P.; Don, M.M. Effect of Temperature on Schizophyllum commune Growth and 4H-pyran-4-one, 2, 3-dihydro-3, 5-dihydroxy-6-methyl-Production using a Bubble Column Bioreactor. Chiang Mai J. Sci. 2015, 42, 539–548. [Google Scholar]
- Praveena, P.E.; Periasamy, S.; Kumar, A.; Singh, N. Cytokine profiles, apoptosis and pathology of experimental Pasteurella multocida serotype A1 infection in mice. Res. Vet. Sci. 2010, 89, 332–339. [Google Scholar] [CrossRef]
- Nascimento, M.A.D.; Vargas, J.P.C.; Rodrigues, J.G.A.; Leão, R.A.C.; De Moura, P.H.B.; Leal, I.C.R.; Bassut, J.; De Souza, R.O.M.A.; Wojcieszak, R.; Itabaiana, I. Lipase-catalyzed acylation of levoglucosan in continuous flow: Antibacterial and biosurfactant studies. RSC Adv. 2022, 12, 3027–3035. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Jitendra, R.; Sonu, R.; Narayan, S.R. Chemical study of fatty acids in mimusops elengiseeds by GC-MS and comparison of its antibacterial activity with trimethoprim. World J. Pharm. Res. 2015, 4, 1022–1026. [Google Scholar]
- Mohamad, O.A.; Li, L.; Ma, J.-B.; Hatab, S.; Xu, L.; Guo, J.-W.; Rasulov, B.A.; Liu, Y.-H.; Hedlund, B.P.; Li, W.-J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Meechaona, R.; Sengpracha, W.; Banditpuritat, J.; Kawaree, R.; Phutdhawong, W. Fatty acid content and antioxidant activity of Thai bananas. Maejo Int. J. Sci. Technol. 2007, 1, 222–228. [Google Scholar]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Suseem, S.; Saral, A.M. Analysis on essential fatty acid esters of mushroom pleurotus eous and its antibacterial activity. Asian J. Pharm. Clin. Res. 2013, 6, 188–191. [Google Scholar]
- An, K.; Fu, M.; Zhang, H.; Tang, D.; Xu, Y.; Xiao, G. Effect of ethyl oleate pretreatment on blueberry (Vaccinium corymbosum L.): Drying kinetics, antioxidant activity, and structure of wax layer. J. Food Sci. Technol. 2019, 56, 783–791. [Google Scholar] [CrossRef]
- Khan, N.; Ali, A.; Qadir, A.; Ali, A.; Warsi, M.H.; Tahir, A.; Ali, A. GC-MS Analysis and Antioxidant Activity of Wrightia tinctoria R. Br. Leaf Extract. J. AOAC Int. 2021, 104, 1415–1419. [Google Scholar] [CrossRef]
- Yatsuda, R.; Rosalen, P.; Cury, J.; Murata, R.; Rehder, V.; Melo, L.; Koo, H. Effects of Mikania genus plants on growth and cell adherence of mutans streptococci. J. Ethnopharmacol. 2005, 97, 183–189. [Google Scholar] [CrossRef]
- Krishnaveni, M.; Dhanalakshmi, R.; Nandhini, N. GC-MS analysis of phytochemicals, fatty acid profile, antimicrobial activity of Gossypium seeds. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 273–276. [Google Scholar]
- Husein, A.I.; Ali-Shtayeh, M.S.; Jamous, R.M.; Jondi, W.J.; Zatar, N.A.-A. Phthalate derivatives are naturally occurring in Arum Palaestinum. Int. J. Curr. Res. Aca. Rev 2014, 2, 195–203. [Google Scholar]
- Sivasubramanian, R.; Brindha, P. In-vitro cytotoxic, antioxidant and GC-MS studies on Centratherum punctatum Cass. Int. J. Pharm. Pharm. Sci 2013, 4, e8. [Google Scholar]
- Mohammad, A.K.; Mohammad, R.D.; Masumeh, K.; Somayeh, N. The GC-MS analyses of the n-hexane extract of Nitraria schoberi L., its total phenolics and in vitro antioxidant activity. J. Med. Plants Res. 2012, 6, 4874–4878. [Google Scholar]
- Izu, G.O.; Adeyi, A.O.; Erukainure, O.L.; Islam, M.S. Gamma-sitosterol–rich fraction from the methanolic extract of Ficus exasperata restores diabetes associated pathophysiological alterations in an alloxan-induced diabetic rats. Biokemistri 2022, 33. Available online: https://ojs.klobexjournals.com/index.php/bkr/article/view/1351/0 (accessed on 25 March 2023).
- Dilshad, R.; Ahmad, S.; Aati, H.; Al-qahtani, J.H.; Sherif, A.E.; Hussain, M.; Ghalloo, B.A.; Tahir, H.; Basit, A.; Ahmed, M. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Molecular Docking Studies of Typha domingensis. Arab. J. Chem. 2022, 15, 104133. [Google Scholar] [CrossRef]
- Dores, R.G.; Guimarães, S.F.; Braga, T.V.; Fonseca, M.; Martins, P.M.; Ferreira, T.C. Phenolic compounds, flavonoids and antioxidant activity of leaves, flowers and roots of goat weed. Hortic. Bras. 2014, 32, 486–490. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I.J.A. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L. ) roots, woods, canes, stems, and leaves. 2020, 9, 398. [Google Scholar]
- Grzegorczyk-Karolak, I.; Kuźma, L.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crop. Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Peerzada, S.; Khan, M.T.; Akhtar, M.F.; Saleem, A.; Hamid, I.; Akhtar, B.; Ali, S.; Ahmed, S.; Raza, M. Phytochemical, anti-inflammatory, anti-nociceptive and cytotoxic basis for the use of Haloxylon stocksii. Pak. J. Pharm. Sci. 2020, 33, 887–894. [Google Scholar]
- He, L.; Gao, Y.; Zhao, L. Online coupling of bubbling extraction with gas chromatography-mass spectrometry for rapid quantitative analysis of volatiles in beer. J. Chromatogr. A 2022, 1665, 462800. [Google Scholar] [CrossRef]
- Meena, B.R.; Meena, S.; Chittora, D.; Sharma, K. Antifungal efficacy of Thevetia peruviana leaf extract against Alternaria solani and characterization of novel inhibitory compounds by Gas Chromatography-Mass Spectrometry analysis. Biochem. Biophys. Rep. 2021, 25, 100914. [Google Scholar] [CrossRef]
- Vander Pyl, C.; Feeney, W.; Arroyo, L.; Trejos, T. Capabilities and Limitations of GC-MS and LC-MS/MS for Trace Detection of Organic Gunshot Residues from Skin Specimens. Forensic Chem. 2023, 51, 100471. [Google Scholar] [CrossRef]
- Ghalloo, B.A.; Khan, K.-U.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical Profiling, In Vitro Biological Activities, and In Silico Molecular Docking Studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Ahmad, S.; Sherif, A.E.; Aati, H.Y.; Ovatlarnporn, C.; Khan, M.A.; Rao, H.; Ahmad, I.; Shahzad, M.N.; Ghalloo, B.A. New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC–MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation. Arab. J. Chem. 2022, 15, 104135. [Google Scholar] [CrossRef]
- Khursheed, A.; Ahmad, S.; Khan, K.-U.-R.; Tousif, M.I.; Aati, H.Y.; Ovatlarnporn, C.; Rao, H.; Khurshid, U.; Ghalloo, B.A.; Tabassum, S. Efficacy of Phytochemicals Derived from Roots of Rondeletia odorata as Antioxidant, Antiulcer, Diuretic, Skin Brightening and Hemolytic Agents—A Comprehensive Biochemical and In Silico Study. Molecules 2022, 27, 4204. [Google Scholar] [CrossRef]
- Al-Qahtani, J.; Abbasi, A.; Aati, H.Y.; Al-Taweel, A.; Al-Abdali, A.; Aati, S.; Yanbawi, A.N.; Khan, M.A.; Ghalloo, B.A.; Anwar, M. Phytochemical, Antimicrobial, Antidiabetic, Thrombolytic, anticancer Activities, and in silico studies of Ficus palmata Forssk. Arab. J. Chem. 2023, 16, 104455. [Google Scholar] [CrossRef]
- Yasin, G.; Noor, S.; Irshad, S.; Haq, I.; Fatima, S.; Anwer, I.; Khan, A. Solvents Based Extraction of Antioxidants and their Activity from Some Plants of Cholistan Desert, Pakistan. Int. J. Pharm. Phytopharm. Res. 2020, 10, 70–76. [Google Scholar]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mahmoud, A.Z.; Farshori, N.N.; Alfaraj, R.; Al-Sheddi, E.S.; Alsarra, I.A. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J. Biol. Sci. 2019, 26, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, A.K.; Dobhal, M.; Sharma, M.; Gupta, R. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Balaha, M.; Ahmed, N.; Geddawy, A.; Kandeel, S. Fraxetin prevented sodium fluoride-induced chronic pancreatitis in rats: Role of anti-inflammatory, antioxidant, antifibrotic and anti-apoptotic activities. Int. Immunopharmacol. 2021, 93, 107372. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Benbrahim, K.F.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- Kumar, A.; Aswal, S.; Semwal, R.B.; Chauhan, A.; Joshi, S.K.; Semwal, D.K. Role of plant-derived alkaloids against diabetes and diabetes-related complications: A mechanism-based approach. Phytochem. Rev. 2019, 18, 1277–1298. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Tran, H.-D.; Van, T.M.; Andriana, Y.; Khanh, T.D.; Quan, N.V.; Ahmad, A. Isolation and purification of bioactive compounds from the stem bark of Jatropha podagrica. Molecules 2019, 24, 889. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Kashangura, C.; Chidewe, C.; Benhura, M.A.; Stray-Pedersen, B.; Mduluza, T. Characterization of Anti− Salmonella typhi compounds from medicinal mushroom extracts from Zimbabwe. Int. J. Med. Mushrooms 2019, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Aisiah, S.; Rini, R.K.; Tanod, W.A.; Fatmawati, F.; Fauzana, N.A.; Olga, O.; Riyadi, P.H. Metabolomic profiling of Jeruju (Acanthus ilicifolius) leaf extract with antioxidant and antibacterial activity on Aeromonas hydrophila growth. J. Appl. Pharm. Sci. 2022, 12, 057–069. [Google Scholar] [CrossRef]
- Wahab, A.; Ahmed, E.; Nawaz, S.A.; Sharif, A.; Haq, R.U.; Malik, A.; Choudhary, M.I.; Raza, M. A pharmacological and toxicological evaluation of Haloxylon recurvum. Nat. Prod. Res. 2008, 22, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Senhaji, S.; Lamchouri, F.; Toufik, H. Phytochemical content, antibacterial and antioxidant potential of endemic plant anabasis aretioïdes coss. & moq.(Chenopodiaceae). BioMed Res. Int. 2020, 2020, 6152932. [Google Scholar]
- Sekhon-Loodu, S.; Rupasinghe, H.V. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Su, S.; Cao, M.; Wu, G.; Long, Z.; Cheng, X.; Fan, J.; Xu, Z.; Su, H.; Hao, Y.; Li, G. Hordenine protects against hyperglycemia-associated renal complications in streptozotocin-induced diabetic mice. Biomed. Pharmacother. 2018, 104, 315–324. [Google Scholar] [CrossRef]
- Ozer, M.S.; Sarikurkcu, C.; Tepe, B. Phenolic composition, antioxidant and enzyme inhibitory activities of ethanol and water extracts of Chenopodium botrys. RSC Adv. 2016, 6, 64986–64992. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Dib, M.E.A.; Medjdoub, H.; Tabti, B. Antidiabetic activity of aqueous leaf extract of Atriplex halimus L. (Chenopodiaceae) in streptozotocin–induced diabetic rats. Asian Pac. J. Trop. Dis. 2014, 4, 181–184. [Google Scholar] [CrossRef]
- Aati, H.Y.; Anwar, M.; Al-Qahtani, J.; Al-Taweel, A.; Khan, K.-U.-R.; Aati, S.; Usman, F.; Ghalloo, B.A.; Asif, H.M.; Shirazi, J.H.; et al. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant. Antibiotics 2022, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ahmad, S.; Aati, H.Y.; Sherif, A.E.; Ashkan, M.F.; Alrahimi, J.; Motwali, E.A.; Tousif, M.I.; Khan, M.A.; Hussain, M. Phytochemical, Antioxidant, Enzyme Inhibitory, Thrombolytic, Antibacterial, Antiviral and In Silico Studies of Acacia jacquemontii Leaves. Arab. J. Chem. 2022, 15, 104345. [Google Scholar] [CrossRef]
- Dilshad, R.; Khan, K.-U.-R.; Saeed, L.; Sherif, A.E.; Ahmad, S.; Ovatlarnporn, C.; Nasim, J.; Hussain, M.; Ghalloo, B.A.; Basit, A.; et al. Chemical Composition and Biological Evaluation of Typha domingensis Pers. to Ameliorate Health Pathologies: In Vitro and In Silico Approaches. BioMed Res. Int. 2022, 2022, 8010395. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.U.; Haq, I.-U.; Bungau, S. An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alshahrani, M.A.; Nahari, M.H.; Hassan, S.S.U.; Jan, M.S.; Ayaz, M.; Ullah, F.; Alshehri, O.M.; Alshehri, M.A.; Rashid, U. In-Vitro, In-Vivo, Molecular Docking and ADMET Studies of 2-Substituted 3, 7-Dihydroxy-4H-chromen-4-one for Oxidative Stress, Inflammation and Alzheimer’s Disease. Metabolites 2022, 12, 1055. [Google Scholar] [CrossRef]




| Extract | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| AMHS | 119.58 ± 2.45 a | 99.19 ± 1.14 a |
| ADHS | 102.65 ± 1.79 b | 87.54 ± 0.73 b |
| RMHS | 91.54 ± 2.65 c | 65.65 ± 0.65 c |
| RDHS | 77.65 ± 1.91 d | 54.65 ± 0.84 d |
| Sr. No. | Compound Name | Plant Part | Molecular Formula | M.W. (g/moL) | Rt (min) | Percentage Area (%) | Chemical Class | Biological Activity |
|---|---|---|---|---|---|---|---|---|
| 1 | Furfural | A, R | C5H4O2 | 96.08 | 2.73 | 2.43 | Furan aldehyde | Antimicrobial andantioxidant [25] |
| 2 | 2-Furancarboxaldehyde, 5-methyl- | A, R | C6H6O2 | 110.1 | 3.65 | 2.38 | Furans and aldehyde | Antimicrobial and antioxidant [26] |
| 3 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | A, R | C5H6O4 | 130.10 | 5.91 | 3.80 | Pyrones | Antimicrobial and antioxidant [27] |
| 4 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | A, R | C6H6O3 | 126.1 | 6.64 | 24.05 | Aryl-aldehude | Antioxidant and antibacterial [28] |
| 5 | 1,6-Anhydro-β-ᴅ-glucopyranose | R | C6H10O5 | 162.14 | 9.22 | 5.95 | Anhydrohexose | Antibacterial [29] |
| 6 | Hexadecanoic acid, methyl ester | A, R | C17H34O2 | 270.5 | 10.52 | 1.13 | Fatty acid ester | Antibacterial and antioxidant [30] |
| 7 | Pentadecanoic acid, 14-methyl-, methyl ester | R | C17H34O2 | 270.5 | 10.53 | 1.70 | Fatty acid methyl ester | Antioxidant and antimicrobial [31] |
| 8 | 9-Octadecenoic acid, methyl ester | A, R | C19H36O2 | 296.5 | 11.98 | 1.75 | Fatty acid ester | Antibacterial, antioxidant, and anti-inflammatory [32] |
| 9 | Octadecanoic acid, methyl ester | R | C19H38O2 | 298.5 | 12.16 | 0.29 | Fatty acid methyl ester | Cytotoxicity, antioxidant activity, and anti-inflammatory [33,34] |
| 10 | Heptadecanoic acid, 16-methyl-methyl ester | A | C19H38O2 | 298.5 | 12.15 | 0.08 | Fatty acid ester | Antibacterial and antioxidant [35] |
| 11 | Ethyl Oleate | A, R | C20H38O2 | 310.5 | 12.51 | 0.31 | Fatty acid ethyl ester | Antioxidant and antimicrobial [36] |
| 12 | Eicosanoic acid, methyl ester | A, R | C21H42O2 | 326.6 | 13.83 | 0.03 | Fatty acid ester | Antimicrobial and antioxidant [37] |
| 13 | 1-Octadecene | R | C18H36 | 252.5 | 13.49 | 0.23 | Octadecene | Antimicrobial [38] |
| 14 | Phenol, 2,2′-methylenebis[6-(1, 1-dimethylethyl)-4-(1-methylpropyl) | A | C29H44O2 | 424.7 | 14.89 | 0.05 | Phenol | |
| 15 | Docosanoic acid, methyl ester | A | C23H46O2 | 354.6 | 15.87 | 0.04 | Fatty acid methyl ester | Antioxidant [39] |
| 16 | Di-n-octyl phthalate | A, R | C24H38O4 | 390.6 | 16.16 | 0.21 | Phthalate ester | Anticancer and antioxidant [40] |
| 17 | Phthalic acid, bis(7-methyloctyl) ester | A | C26H42O4 | 418.6 | 19.77 | 0.18 | Phthalate ester | Antioxidant and antimicrobial [41] |
| 18 | γ-Sitosterol | A | C29H50O | 414.7 | 21.14 | 0.25 | Phytosterols | Analgesic, antioxidant, antidiabetic, andantibacterial [42,43,44] |
| Sr. No. | Retention Time (Minutes) | M/Z | Compound Name | Molecular Formula | Molecular Mass | Chemical Class |
|---|---|---|---|---|---|---|
| 1 | 3.504 | 152.107 | N-Methyltyramine | C9H13NO | 151.099 | Amines |
| 2 | 3.505 | 121.065 | Acetophenone | C8H8O | 120.057 | Aromatic ketones |
| 3 | 4.004 | 166.122 | Hordenine | C10H15NO | 165.115 | Phenyl amines |
| 4 | 10.477 | 420.144 | Methyl 2-(4-oxo-3-((4-(p-tolyl)thiazol-2-yl)methyl)-3,4-dihydrophthalazin-1-yl)acetate | C23H21N3O3 S | 419.137 | Acetamides |
| 5 | 11.821 | 209.044 | Fraxetin | C10H8O5 | 208.037 | Flavonoids |
| 6 | 15.267 | 223.059 | 6,8-Dihydroxy-7-methoxy-3-methyl-1H-isochromen-1-one | C11H10O5 | 222.053 | Flavonoids |
| 7 | 16.846 | 300.122 | Unknown | C17H17NO4 | 299.115 | |
| 8 | 17.395 | 247.179 | nor-3-Methylfentanyl | C15H22N2O | 246.173 | Opioids |
| 9 | 19.106 | 314.138 | Moupinamide | C18H19NO4 | 313.131 | Amides |
| 10 | 27.591 | 343.117 | 2-(2,6-Dimethoxyphenyl)-5,6-dimethoxy-4H-chromen-4-one | C19H18O6 | 342.109 | |
| 11 | 29.578 | 286.143 | Piperine | C17H19NO3 | 285.136 | Alkaloids |
| 12 | 38.689 | 399.251 | Tris(2-butoxyethyl) phosphate | C18H39O7P | 398.243 | Phosphate esters |
| 13 | 43.688 | 403.232 | Acetyl tributyl citrate | C20H34O8 | 402.225 | Organophophate esters |
| 14 | 45.134 | 352.321 | Unknown | C22H41NO2 | 351.314 | |
| 15 | 45.755 | 309.242 | Unknown | C19H32O3 | 308.235 | |
| 16 | 46.749 | 256.263 | Hexadecanamide | C16H33NO | 255.256 | Fatty acid amides |
| 17 | 48.076 | 354.337 | Unknown | C22H43NO2 | 353.329 | |
| 18 | 48.144 | 331.284 | 1-Palmitoylglycerol | C19H38O4 | 330.277 | Glycerolipid |
| 19 | 49.561 | 191.143 | (1S)-Tricyclo[7.3.1.0~2,7~]tridec-2(7)- en-13-one | C13H18O | 190.136 | Cyclic ketone |
| 20 | 52..221 | 284.294 | Stearamide | C18H37NO | 283.287 | Long-chain fatty acid |
| 21 | 55.801 | 338.342 | Erucamide | C22H43NO | 337.334 | Long-chain fatty acid |
| 22 | 55.152 | 394.347 | Unkown | C28H43N | 393.339 | |
| 23 | 56.318 | 122.096 | N,N-Dimethylaniline | C8H11N | 121.089 | Aromatic amines |
| Extract | DPPH (mg TE/g Extract) | ABTS (mg TE/g Extract) | FRAP (mg TE/g Extract) | CUPRAC (mg TE/g Extract) |
|---|---|---|---|---|
| AMHS | 145.45 ± 2.94 a | 98.07 ± 3.47 a | 231.76 ± 7.69 a | 410.08 ± 10.51 a |
| ADHS | 121.65 ± 3.4 b | 66.65 ± 2.93 b | 192.27 ± 5.7 b | 367.42 ± 8.35 b |
| RMHS | 93.41 ± 1.99 c | 68.07 ± 1.15 b | 168.67 ± 3.81 c | 332.65 ± 7.91 c |
| RDHS | 74.98 ± 1.28 d | 52.98 ± 0.82 c | 145.64 ± 2.6 d | 296.12 ± 5.84 d |
| Strain Name | ZI of Std | ZI of AMHS | ZI of ADHS | ZI of RMHS | ZI of RDHS |
|---|---|---|---|---|---|
| Bacillus subtilis | 23 ± 1.55 | 20 ± 1.33 | 18 ± 0.71 | 17 ± 1.39 | 19 ± 1.62 |
| Bacillus pumilus | 25 ± 0.77 | 22 ± 1.51 | 16 ± 1.66 | 15 ± 0.57 | 14 ± 1.26 |
| Micrococcus luteus | 23 ± 0.95 | 16 ± 0.65 | 19 ± 1.51 | 14 ± 1.19 | 14 ± 0.87 |
| Staphylococcus epidermidis | 24 ± 1.13 | 18 ± 0.83 | 17 ± 1.15 | 17 ± 1.28 | 16 ± 0.63 |
| Escherichia coli | 22 ± 0.47 | 17 ± 1.20 | 16 ± 1.25 | 19 ± 1.42 | 18 ± 0.96 |
| Bordetella bronchispetica | 25 ± 1.31 | 12 ± 0.33 | 10 ± 0.43 | 15 ± 1.09 | 14 ± 0.75 |
| Pseudomonas aeruginosa | NA | 9 ± 0.35 | 8 ± 0.21 | 17 ± 1.37 | 14 ± 1.16 |
| Sr. No. | Ligands | Binding Affinity | Amino Acid Interactions | |
|---|---|---|---|---|
| Hydrogen Bonding | Pi Alkyl | |||
| 1 | Octadecanoic acid, methyl ester | −5 | Gln63 | Trp58, Trp59, Tyr62, Leu162, Leu165, His101, His305 |
| 2 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | −5.1 | Gly309, Asp317, Arg346 | |
| 3 | 9-Octadecenoic acid, methyl ester | −5.2 | Gln63 | Trp59, Tyr62, Leu162, Leu165 |
| 4 | Eicosanoic acid, methyl ester | −5.3 | Trp59, Tyr62, Ala198, Leu162, Leu165, His201 | |
| 5 | 1,6-Anhydro-β-ᴅ-glucopyranose | −5.8 | Asn301, Gln302, Gly304, Ile312, Arg346 | Unfavorable: Arg267 |
| 6 | Phthalic acid, bis(7-methyloctyl) ester | −5.9 | His101, Glu233, Asp300 | Pi-sigma: Trp59, Leu162 Pi-Alkyl: Trp58, Tyr62, His305 |
| 7 | Di-n-octyl phthalate | −6 | Asp197 | Pi-sigma: Trp59, Ile235 Alkyl/Pi-Alkyl: Trp58, Tyr62, Leu162, Leu165, Ala198, His305 |
| 8 | Phenol, 2,2′-methylenebis[6-(1, 1-dimethylethyl)-4-(1-methylpropyl) | −8.5 | His305 | Pi-Anion: Asp300 Pi-sigma: Tyr62 Pi-Pi Stacked: Trp58, Trp59 Alkyl/Pi-Alkyl: Leu165, His299 |
| 9 | γ-Sitosterol | −8.7 | Alkyl/Pi-Alkyl: Trp59, Leu165, Ala198 | |
| Acarbose | −6.9 | Asp197, Glu233, His305, Lys352, Asp356 | Pro54, Trp58, Trp59, His101, Leu162, Ser163, Leu165, Arg195, Ala198, His299, Asp300, Trp357 | |
| Sr. No. | Ligands | Binding Affinity | Amino Acid Interactions | |
|---|---|---|---|---|
| Hydrogen Bonding | Pi Alkyl | |||
| 1 | Pentadecanoic acid, 14-methyl-, methyl ester | −5 | Val380, Asp401, Gly402 | Val334, Val335, Lys398, Phe397 |
| 2 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | −5.2 | His515 | Pi-Alkyl: Lys352, Ala514 Amide-Pi Stacked: Phe516 |
| 3 | Ethyl Oleate | −5.4 | Arg457 | Pi-sigma: Phe463 Alkyl/Pi-Alkyl: Leu95, Arg456 |
| 4 | Eicosanoic acid, methyl ester | −5.4 | Arg347, Gly432 | His348, Ala349, Lys352, Ala444, Arg450, Ala454 |
| 5 | 1,6-Anhydro-β-ᴅ-glucopyranose | −5.7 | Arg437, Asp441, Ala451 | |
| 6 | Di-n-octyl phthalate | −5.6 | Gly439, Gly581, His515 | Pi-sigma: Ala349 Alkyl/Pi-Alkyl: Ala43, Leu93 |
| 7 | Phthalic acid, bis(7-methyloctyl) ester | −6.2 | Ser44, Asn443, Arg450 | Pi-sigma: Phe516 Alkyl/Pi-Alkyl: Tyr41, Lys352, Val435 |
| 8 | Phenol, 2,2′-methylenebis[6-(1, 1-dimethylethyl)-4-(1-methylpropyl) | −8.1 | Asp441 | Pi-Cation: His348 Alkyl/Pi-Alkyl: Pro442, Ala444, Arg450, Ala451 |
| 9 | γ-Sitosterol | −8.9 | Arg437 | His348, Ala349, Lys352, Ala444, Ala451, Ala454 |
| Acarbose | −6.8 | Arg17, Asp59, Asn61, Asp381, Pro433, Trp434 | Asn58, Asp60, Asp378, Asp379, Asp382, Val383, Met435 | |
| Sr. No. | Compound Name | Gastrointestinal Absorption | Blood–brain Barrier Permeant | Pgp Inhibitor | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Octadecanoic acid, methyl ester | High | No | No | Yes | No | No | No | No | −2.19 |
| 2. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6- methyl- | High | No | No | No | No | No | No | No | −7.44 |
| 3. | Ethyl Oleate | High | No | No | Yes | No | No | No | No | −2.82 |
| 4. | Eicosanoic acid, methyl ester | Low | No | No | Yes | No | No | No | No | −1.69 |
| 5. | 1,6-Anhydro-β-ᴅ-glucopyranose | High | No | Yes | No | No | No | No | No | −8.82 |
| 6. | Phthalic acid, bis(7-methyloctyl) ester | Low | No | No | No | No | No | No | Yes | −3.61 |
| 7. | Di-n-octyl phthalate | High | No | No | No | No | No | No | Yes | −2.93 |
| 8. | Phenol,2,2′-methylenebis[6-(1,1-dimethylethyl)-4-(1- methylpropyl) | Low | No | Yes | No | No | No | Yes | No | −2.60 |
| 9. | γ-Sitosterol | Low | No | No | No | No | No | No | No | −2.20 |
| 10. | Acarbose | Low | No | Yes | No | No | No | No | No | −16.29 |
| Sr. No. | Compounds Name | Lipinski’s rule | Solubility | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBD | HBA | MWT (g/moL) | Lipophilicity | MR | LR | ESOL Class | Ali Class | Silicos-IT Class | ||
| 1. | Octadecanoic acid, methyl ester | 0 | 2 | 298.50 | 4.81 | 94.73 | 1 | Moderately soluble | Poorly soluble | Poorly soluble |
| 2. | 4H-Pyran-4-one, 2,3- dihydro-3,5-dihydroxy-6- methyl- | 2 | 4 | 144.13 | 1.19 | 32.39 | 0 | Very soluble | Very soluble | Soluble |
| 3. | Ethyl Oleate | 0 | 2 | 296.49 | 4.75 | 94.26 | 1 | Moderately soluble | Poorly soluble | Poorly soluble |
| 4. | Eicosanoic acid, methyl ester | 0 | 2 | 326.56 | 5.35 | 104.35 | 1 | Poorly soluble | Poorly soluble | Poorly soluble |
| 5. | 1,6-Anhydro-β-ᴅ-glucopyranose | 3 | 5 | 162.14 | 1.27 | 32.38 | 0 | Highly soluble | Highly soluble | Soluble |
| 6. | Phthalic acid, bis(7- methyloctyl) ester | 0 | 4 | 418.61 | 5.41 | 125.91 | 1 | Poorly soluble | Poorly soluble | Poorly soluble |
| 7. | Di-n-octyl phthalate | 0 | 4 | 390.56 | 4.14 | 116.30 | 1 | Poorly soluble | Poorly soluble | Poorly soluble |
| 8. | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-(1- methylpropyl) | 2 | 2 | 424.66 | 4.95 | 137.25 | 1 | Poorly soluble | Poorly soluble | Poorly soluble |
| 9. | γ-Sitosterol | 1 | 1 | 414.71 | 4.79 | 133.23 | 1 | Poorly soluble | Poorly soluble | Poorly soluble |
| 10. | Acarbose | 14 | 19 | 645.60 | 0.63 | 136.69 | 3 | Highly soluble | Highly soluble | Soluble |
| Sr. No. | Compound Name | Predicted LD50 (mg/kg) | Predicted Toxicity Class | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity |
|---|---|---|---|---|---|---|---|---|
| 1. | Octadecanoic acid, methyl ester | 5000 | 5 | Not active | Not active | Not active | Not active | Not active |
| 2. | 4H-Pyran-4-one, 2,3- dihydro-3,5-dihydroxy-6- methyl- | 595 | 4 | Not active | Not active | Not active | Active | Not active |
| 3. | Ethyl oleate | 3000 | 5 | Not active | Not active | Not active | Not active | Not active |
| 4. | Eicosanoic acid, methyl ester | 5000 | 5 | Not active | Not active | Not active | Not active | Not active |
| 5. | 1,6-Anhydro-β-ᴅ-glucopyranose | 23,000 | 6 | Not active | Not active | Not active | Not active | Not active |
| 6. | Phthalic acid, bis(7- methyloctyl) ester | 1340 | 4 | Not active | Active | Inactive | Not active | Not active |
| 7. | Di-n-octyl phthalate | 1340 | 4 | Not active | Active | Not active | Not active | Not active |
| 8. | Phenol, 2,2′-methylenebis[6- (1, 1-dimethylethyl)-4-(1- methylpropyl) | 3430 | 5 | Not active | Not active | Not active | Not active | Not active |
| 9. | γ-Sitosterol | 890 | 4 | Not active | Not active | Active | Not active | Not active |
| 10. | Acarbose | 24,000 | 6 | Active | Not active | Active | Not active | Not active |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, S.N.R.; Afzal, S.; Khan, K.-u.-R.; Aati, H.Y.; Rao, H.; Ghalloo, B.A.; Shahzad, M.N.; Khan, D.A.; Esatbeyoglu, T.; Korma, S.A. Chemical Characterisation, Antidiabetic, Antibacterial, and In Silico Studies for Different Extracts of Haloxylon stocksii (Boiss.) Benth: A Promising Halophyte. Molecules 2023, 28, 3847. https://doi.org/10.3390/molecules28093847
Rizvi SNR, Afzal S, Khan K-u-R, Aati HY, Rao H, Ghalloo BA, Shahzad MN, Khan DA, Esatbeyoglu T, Korma SA. Chemical Characterisation, Antidiabetic, Antibacterial, and In Silico Studies for Different Extracts of Haloxylon stocksii (Boiss.) Benth: A Promising Halophyte. Molecules. 2023; 28(9):3847. https://doi.org/10.3390/molecules28093847
Chicago/Turabian StyleRizvi, Syed Nabil Raza, Samina Afzal, Kashif-ur-Rehman Khan, Hanan Y. Aati, Huma Rao, Bilal Ahmad Ghalloo, Muhammad Nadeem Shahzad, Duraiz Ahmed Khan, Tuba Esatbeyoglu, and Sameh A. Korma. 2023. "Chemical Characterisation, Antidiabetic, Antibacterial, and In Silico Studies for Different Extracts of Haloxylon stocksii (Boiss.) Benth: A Promising Halophyte" Molecules 28, no. 9: 3847. https://doi.org/10.3390/molecules28093847
APA StyleRizvi, S. N. R., Afzal, S., Khan, K.-u.-R., Aati, H. Y., Rao, H., Ghalloo, B. A., Shahzad, M. N., Khan, D. A., Esatbeyoglu, T., & Korma, S. A. (2023). Chemical Characterisation, Antidiabetic, Antibacterial, and In Silico Studies for Different Extracts of Haloxylon stocksii (Boiss.) Benth: A Promising Halophyte. Molecules, 28(9), 3847. https://doi.org/10.3390/molecules28093847









