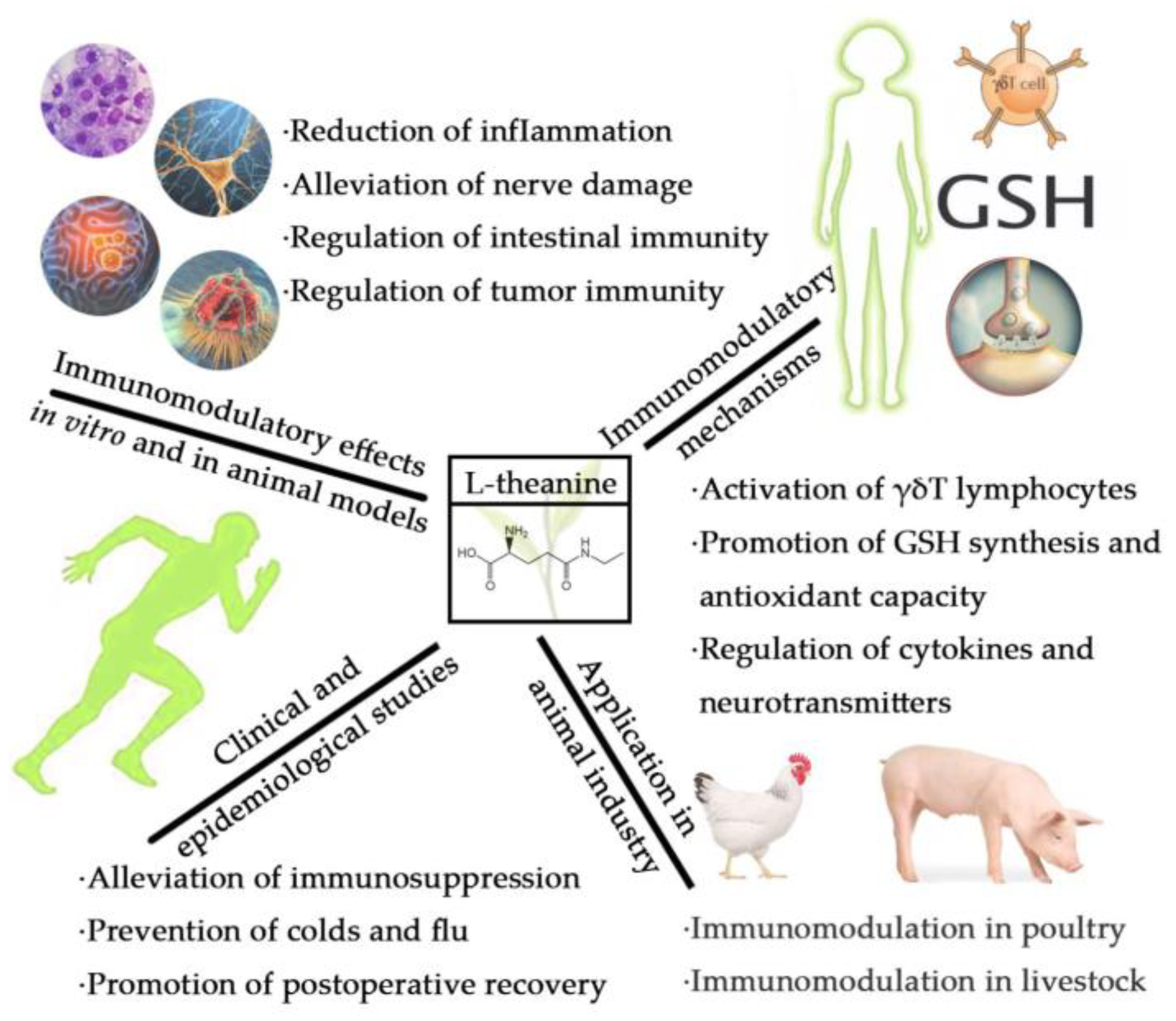
L-Theanine and Immunity: A Review
Abstract
1. Introduction
2. Clinical and Epidemiological Studies on Immune Regulation by L-Theanine
2.1. Alleviation of Immunosuppression Caused by Strenuous Exercise
2.2. Prevention of Colds and Flu
2.3. Promotion of Postoperative Recovery
3. Studies on Immunomodulatory Effects of L-Theanine In Vitro and in Animal Models
3.1. Reduction of Inflammation
3.2. Alleviation of Nerve Injury
3.3. Improvement of Intestinal Immunity
3.4. Regulation of Tumor Immunity
4. Immunomodulatory Mechanisms of L-Theanine
4.1. Activation of γδT Lymphocyte Function
4.2. Promotion of GSH Synthesis and Antioxidant Capacity
4.3. Regulation of Cytokines and Neurotransmitters
5. Application of L-Theanine as an Immunomodulator in the Animal Industry
5.1. Immunomodulation in Poultry
5.2. Immunomodulation in Livestock
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef]
- Zhongying, L.; Qiansong, R.; Ke, P.; Qin, L.; Ting, Y.; Yuqiao, D.; Shimao, F.; Wenjia, Z. Flavor characteristics of three amino acid monomers based on electronic tongue. Food Sci. Technol. 2022, 47, 296–302. [Google Scholar]
- van der Pijl, P.C.; Chen, L.; Mulder, T.P.J. Human disposition of L-theanine in tea or aqueous solution. J. Funct. Foods 2010, 2, 239–244. [Google Scholar] [CrossRef]
- Terashima, T.; Takido, J.; Yokogoshi, H. Time-dependent changes of amino acids in the serum, liver, brain and urine of rats administered with theanine. Biosci. Biotechnol. Biochem. 1999, 63, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Lin, Z.; Li, L. Toxicological studies on compound theanine preparation. Food Sci. 2011, 32, 262–267. [Google Scholar]
- Borzelleca, J.F.; Peters, D.; Hall, W. A 13-week dietary toxicity and toxicokinetic study with L-theanine in rats. Food Chem. Toxicol. 2006, 44, 1158–1166. [Google Scholar] [CrossRef]
- Fujii, S.; Inai, K. Tumorigenicity study of L-theanine administrated orally to mice. Food Chem. 2008, 110, 643–646. [Google Scholar] [CrossRef]
- Fiori, J.; Pasquini, B.; Caprini, C.; Orlandini, S.; Furlanetto, S.; Gotti, R. Chiral analysis of theanine and catechin in characterization of green tea by cyclodextrin-modified micellar electrokinetic chromatography and high performance liquid chromatography. J. Chromatogr. A 2018, 1562, 115–122. [Google Scholar] [CrossRef]
- Li, C.J.; Yan, Q.X.; Tang, S.X.; Xiao, W.J.; Tan, Z.L. L-theanine protects H9C2 cells from hydrogen peroxide-induced apoptosis by enhancing antioxidant capability. Med. Sci. Monitor 2018, 24, 2109–2118. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.M.; Li, Z.C.; Yu, Y.N.; Yang, L.Y.; Zhang, P.H.; Shen, W.J.; Wan, F.C.; He, J.H.; Xiao, W.J.; et al. Alterations of endotoxin distribution across different biofluids and relevant inflammatory responses by supplementing L-theanine in dairy cows during heat stress. Anim. Nutr. 2021, 7, 1253–1257. [Google Scholar] [CrossRef]
- Takeshima, M.; Miyazaki, I.; Murakami, S.; Kita, T.; Asanuma, M. L-theanine protects against excess dopamine-induced neurotoxicity in the presence of astrocytes. J. Clin. Biochem. Nutr. 2016, 59, 93–99. [Google Scholar] [CrossRef]
- Wise, L.E.; Premaratne, I.D.; Gamage, T.F.; Lichtman, A.H.; Hughes, L.D.; Harris, L.S.; Aceto, M.D. L-theanine attenuates abstinence signs in morphine-dependent rhesus monkeys and elicits anxiolytic-like activity in mice. Pharmacol. Biochem. Behav. 2012, 103, 245–252. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; An, L.; Zhang, T.; Li, G. Chemoprotective effect of theanine in 1,2-dimethylhydrazine-induced colorectal cancer in rats via suppression of inflammatory parameters. J. Food Biochem. 2022, 46, e14073. [Google Scholar] [CrossRef]
- Peng, W.Q.; Xiao, G.; Li, B.Y.; Guo, Y.Y.; Guo, L.; Tang, Q.Q. L-theanine activates the browning of white adipose tissue through the AMPK/alpha-ketoglutarate/prdm16 axis and ameliorates diet-induced obesity in mice. Diabetes 2021, 70, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Bernardino, R.L.; Alves, M.G.; Silva, J.; Barros, A.; Sousa, M.; Casal, S.; Silva, B.M.; Oliveira, P.F. L-theanine promotes cultured human sertoli cells proliferation and modulates glucose metabolism. Eur. J. Nutr. 2019, 58, 2961–2970. [Google Scholar] [CrossRef]
- Ben, P.L.; Hu, M.N.; Wu, H.Z.; Zhang, Z.P.; Gao, Y.H.; Luo, L.; Yin, Z.M. L-theanine down-regulates the JAK/STAT3 pathway to attenuate the proliferation and migration of vascular smooth muscle cells induced by angiotensin II. Biol. Pharm. Bull. 2018, 41, 1678–1684. [Google Scholar] [CrossRef]
- Altinkaynak, Y.; Kural, B.; Akcan, B.A.; Bodur, A.; Ozer, S.; Yulug, E.; Mungan, S.; Kaya, C.; Orem, A. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2018, 108, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Tong, H.O.; Yan, Q.X.; Tang, S.X.; Han, X.F.; Xiao, W.J.; Tan, Z.L. L-theanine improves immunity by altering TH2/TH1 cytokine balance, brain neurotransmitters, and expression of phospholipase C in rat hearts. Med. Sci. Monitor 2016, 22, 8. [Google Scholar] [CrossRef]
- Tan, J.; Lin, Z.; Li, L. Immunity enhancing function of theanine compound preparation. J. Tea Sci. 2012, 32, 224–228. [Google Scholar]
- Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Kawada, S.; Kobayashi, K.; Ohtani, M.; Fukusaki, C. Cystine and theanine supplementation restores high-intensity resistance exercise-induced attenuation of natural killer cell activity in well-trained men. J. Strength Cond. Res. 2010, 24, 846–851. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Glapa, A.; Basta, P.; Petriczko, E.; Zolnowski, K.; Machalinski, B.; Trzeciak, J.; Luczkowska, K.; Skarpanska-Stejnborn, A. The effect of L-theanine supplementation on the immune system of athletes exposed to strenuous physical exercise. J. Int. Soc. Sport. Nutr. 2019, 16, 14. [Google Scholar] [CrossRef]
- Kurihara, S.; Hiraoka, T.; Akutsu, M.; Sukegawa, E.; Bannai, M.; Shibahara, S. Effects of (L)-cystine and (L)-theanine supplementation on the common cold: A randomized, double-blind, and placebo-controlled trial. J. Amino Acids 2010, 2010, 307475. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.A.; Nantz, M.P.; Bukowski, J.F.; Percival, S.S. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma delta T cell function: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Nutr. 2007, 26, 445–452. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 7. [Google Scholar] [CrossRef]
- Miyagawa, K.; Hayashi, Y.; Kurihara, S.; Maeda, A. Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: Nutritional status-dependent immunogenicity. Geriatr. Gerontol. Int. 2008, 8, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, T.; Tsuchiya, T.; Oyama, A.; Tsuchiya, T.; Abe, N.; Sato, A.; Chiba, Y.; Kurihara, S.; Shibakusa, T.; Mikami, T. Perioperative oral administration of cystine and theanine enhances recovery after distal gastrectomy: A prospective randomized trial. J. Parenter. Enter. Nutr. 2013, 37, 384–391. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Tsuchiya, T.; Miyata, G.; Sato, T.; Takahashi, K.; Ariyoshi, K.; Oyamada, S.; Iwase, S. Efficacy of oral administration of cystine and theanine in patients with colorectal cancer undergoing capecitabine-based adjuvant chemotherapy after surgery: Study protocol for a multi-institutional, randomised, double-blinded, placebo-controlled, phase II trial. BMJ Open 2018, 8, 7. [Google Scholar]
- Tsuchiya, T.; Honda, H.; Oikawa, M.; Kakita, T.; Oyama, A.; Oishi, H.; Tochikubo, K.; Hashimoto, T.; Kurihara, S.; Shibakusa, T.; et al. Oral administration of the amino acids cystine and theanine attenuates the adverse events of S-1 adjuvant chemotherapy in gastrointestinal cancer patients. Int. J. Clin. Oncol. 2016, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sato, R.; Komura, T.; Ichikawa, H.; Hirashima, T.; Otake, S.; Akazawa, N.; Yazawa, T.; Abe, T.; Okada, T.; et al. Protective effect of the oral administration of cystine and theanine on oxaliplatin-induced peripheral neuropathy: A pilot randomized trial. Int. J. Clin. Oncol. 2020, 25, 1814–1821. [Google Scholar] [CrossRef]
- Shibakusa, T.; Mikami, T.; Kurihara, S.; Chiba, Y.; Tsuchiya, T.; Miyachi, T.; Oyama, A.; Tanaka, K.A.K.; Koyama, N. Enhancement of postoperative recovery by preoperative oral co-administration of the amino acids, cystine and theanine, in a mouse surgical model. Clin. Nutr. 2012, 31, 555–561. [Google Scholar] [CrossRef]
- Miyakuni, T.; Fukatsu, K.; Ri, M.; Murakoshi, S.; Inoue, Y.; Kurihara, S.; Takayama, T.; Yasuhara, H. Cystine and theanine improve survival after gut ischemia-reperfusion. Ann. Nutr. Metab. 2018, 73, 131–137. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, J.; Hu, J.Y.; Zou, Z.Q.; Zhao, Y.L.; Lai, L.H.; Xu, P.; Song, Y.J.; Cheng, H. L-theanine alleviates IMQ-induced psoriasis like skin inflammation by downregulating the production of IL-23 and chemokines. Front. Pharmacol. 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.J.; Tan, Z.; Lai, X.F.; Xu, Y.N.; Mai, C.L.; Zhang, J.; Lin, Z.J.; Liu, X.G.; Sun, S.L.; Zhou, L.J. Topical delivery of L-theanine ameliorates TPA-induced acute skin inflammation via downregulating endothelial PECAM-1 and neutrophil infiltration and activation. Chem.-Biol. Interact. 2018, 284, 69–79. [Google Scholar] [CrossRef]
- Perez-Vargas, J.E.; Zarco, N.; Vergara, P.; Shibayama, M.; Segovia, J.; Tsutsumi, V.; Muriel, P. L-theanine prevents carbon tetrachloride-induced liver fibrosis via inhibition of nuclear factor kappa B and down-regulation of transforming growth factor beta and connective tissue growth factor. Hum. Exp. Toxicol. 2016, 35, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jin, S.W.; Choi, J.H.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Kim, Y.; Lee, K.J.; Chung, Y.C.; Jeong, H.G. Inhibitory effects of L-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017, 99, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhang, Z.H.; Li, Y.; Song, X.P.; Ma, T.W.; Liu, C.P.; Liu, L.; Yuan, R.; Wang, X.Y.; Gao, L. L-theanine reduced the development of knee osteoarthritis in rats via its anti-inflammation and anti-matrix degradation actions: In vivo and in vitro study. Nutrients 2020, 12, 14. [Google Scholar] [CrossRef]
- Yang, C.C.; Chang, K.C.; Wang, M.H.; Tseng, H.C.; Soung, H.S.; Fang, C.H.; Lin, Y.W.; Li, K.Y.; Tsai, C.C. L-theanine improves functional recovery after traumatic spinal cord injury in rats. J. Formos. Med. Assoc. 2020, 119, 1405–1414. [Google Scholar] [CrossRef]
- Sumathi, T.; Asha, D.; Nagarajan, G.; Sreenivas, A.; Nivedha, R. L-theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting upregulation of inflammatory cytokines and oxidative stress in rat brain. Environ. Toxicol. Pharmacol. 2016, 42, 99–117. [Google Scholar] [CrossRef]
- Ben, P.L.; Zhang, Z.P.; Zhu, Y.Y.; Xiong, A.Y.; Gao, Y.H.; Mu, J.Y.; Yin, Z.M.; Luo, L. L-theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology 2016, 57, 95–103. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Karig, S.; Kim, H.J. The combination of luteolin and L-theanine improved alzheimer disease-like symptoms by potentiating hippocampal insulin signaling and decreasing neuroinflammation and norepinephrine degradation in amyloid-beta-infused rats. Nutr. Res. 2018, 60, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Kong, J.Y.; Li, X.Y.; Yang, J.Y.; Xue, C.H.; Yanagita, T.; Wang, Y.M. Antarctic krill oil exhibited synergistic effects with nobiletin and theanine in ameliorating memory and cognitive deficiency in SAMP8 mice: Applying the perspective of the sea-land combination to retard brain aging. Front. Aging Neurosci. 2022, 14, 12. [Google Scholar] [CrossRef]
- Yoneda, J.; Nishikawa, S.; Kurihara, S. Oral administration of cystine and theanine attenuates 5-fluorouracil-induced intestinal mucositis and diarrhea by suppressing both glutathione level decrease and ROS production in the small intestine of mucositis mouse model. BMC Cancer 2021, 21, 12. [Google Scholar] [CrossRef]
- Matsuu-Matsuyama, M.; Shichijo, K.; Tsuchiya, T.; Kondo, H.; Miura, S.; Matsuda, K.; Sekine, I.; Nakashima, M. Protective effects of a cystine and theanine mixture against acute radiation injury in rats. Environ. Toxicol. Pharmacol. 2020, 78, 8. [Google Scholar] [CrossRef] [PubMed]
- Qing-Yun, Q.; Xian-Ying, S.; Ling, L.; Zhi-Hua, G.; Wei, X.; Wen-Jun, X. L-theanine modulates intestine-specific immunity by regulating the differentiation of CD4+ T cells in ovalbumin-sensitized mice. J. Agric. Food Chem. 2022, 70, 14851–14863. [Google Scholar]
- Saeed, M.; Xu, Y.T.; Zhang, T.T.; Qian, R.; Chao, S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult. Sci. 2019, 98, 842–854. [Google Scholar] [CrossRef]
- Lei, M.S.; Zuo, J.H.; Li, M.; Gu, Q.H.; Hu, C.P. Theanine improves the function of dendritic cells via the downregulation of cyclooxygenase-2 expression. Chin. Med. J. 2014, 127, 1545–1549. [Google Scholar]
- Ji, D.X.; Wang, Y.S.; Zhang, H.R.; Chen, L.L.; Liu, X.; Sun, F.J.; Liu, K.; Yao, J.W.; Zhang, G.Y. Suppression of proliferation and migration in highly-metastatic lung cancer cells as well as tumor growth by a new synthesized compound TBrC and its molecular mechanisms of action. Cytotechnology 2014, 66, 899–911. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Ye, X.S.; Ji, D.X.; Zhang, H.R.; Sun, F.J.; Shang, C.Q.; Zhang, Y.; Wu, E.X.; Wang, F.F.; Wu, F.; et al. Inhibition of lung tumor growth by targeting EGFR/VEGFR-Akt/NF-kappa B pathways with novel theanine derivatives. Oncotarget 2014, 5, 8528–8543. [Google Scholar] [CrossRef]
- Liu, J.N.; Sun, Y.P.; Zhang, H.R.; Ji, D.X.; Wu, F.; Tian, H.H.; Liu, K.; Zhang, Y.; Wu, B.H.; Zhang, G.Y. Theanine from tea and its semi-synthetic derivative TBrC suppress human cervical cancer growth and migration by inhibiting EGFR/Met-Aktil\IF-kappa B signaling. Eur. J. Pharmacol. 2016, 791, 297–307. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Li, Z.; Wan, X.C.; Zhang, Y.; Zhu, R.Q.; Liu, Z.Z.; Ji, D.X.; Zhang, H.R.; Wu, F.; Tian, H.H.; et al. Repression of human hepatocellular carcinoma growth by regulating Met/EGFR/VEGFR-Akt/NF-kappa B pathways with theanine and its derivative, (R)-2-(6,8-Dibromo-2-oxo-2H-chromene-3-carboxamido)-5-(ethylamino)-5-oxo pentanoic ethyl ester (DTBrC). J. Agric. Food Chem. 2016, 64, 7002–7013. [Google Scholar] [CrossRef]
- Sugiyama, T.; Sadzuka, Y.; Nagasawa, K.; Ohnishi, N.; Yokoyama, T.; Sonobe, T. Membrane transport and antitumor activity of pirarubicin, and comparison with those of doxorubicin. Jpn. J. Cancer Res. 1999, 90, 775–780. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Yamashita, Y.; Kishimoto, S.; Fukushima, S.; Takeuchi, Y.; Sonobe, T. Glutamate transporter mediated increase of antitumor activity by theanine, an amino acid in green tea. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2002, 122, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.X.; Cao, W.W.; Xing, S.S.; Li, L.; He, Y.Q.; Hao, Z.N.; Wang, S.; He, H.Y.; Li, C.H.; Zhao, Q.Q.; et al. Enhancing effects of theanine liposomes as chemotherapeutic agents for tumor therapy. Acs Biomater. Sci. Eng. 2019, 5, 3373–3379. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, H.Y.; Luan, J.L.; Yagasaki, K.; Zhang, G.Y. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology 2009, 59, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.Q.; Ben, P.L.; Wang, Q.; Zhu, Y.Y.; Yin, Z.M.; Luo, L. Theanine, an antitumor promoter, induces apoptosis of tumor cells via the mitochondrial pathway. Mol. Med. Rep. 2018, 18, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Shojaei-Zarghani, S.; Khosroushahi, A.Y.; Rafraf, M. Oncopreventive effects of theanine and theobromine on dimethylhydrazine-induced colon cancer model. Biomed. Pharmacother. 2021, 134, 111140. [Google Scholar] [CrossRef]
- Fan, X.R.; Zhou, J.Y.; Bi, X.W.; Liang, J.J.; Lu, S.; Yan, X.T.; Luo, L.; Yin, Z.M. L-theanine suppresses the metastasis of prostate cancer by downregulating MMP9 and Snail. J. Nutr. Biochem. 2021, 89, 108556. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.B.; Wang, L.S.; Das, H.; Li, L.; Reinhold, V.N.; Bukowski, J.F. Antigens in tea-beverage prime human V gamma 2V delta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl. Acad. Sci. USA 2003, 100, 6009–6014. [Google Scholar] [CrossRef]
- Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: Implications for innate immunity. Immunity 1999, 11, 57–65. [Google Scholar] [CrossRef]
- Bukowski, J.F.; Percival, S.S. L-theanine intervention enhances human gamma delta T lymphocyte function. Nutr. Rev. 2008, 66, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Yan, Q.X.; Tang, S.X.; Xiao, W.J.; Tan, Z.L. Alteration of mevalonate pathway in rat splenic lymphocytes: Possible role in cytokines secretion regulated by L-theanine. Biomed Res. Int. 2018, 2018, 8. [Google Scholar] [CrossRef]
- Rimaniol, A.C.; Mialocq, P.; Clayette, P.; Dormont, D.; Gras, G. Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am. J. Physiol.-Cell Physiol. 2001, 281, C1964–C1970. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Shibahara, S.; Arisaka, H.; Akiyama, Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. J. Vet. Med. Sci. 2007, 69, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kurihara, S.; Higashi, N.; Morikawa, S.; Kase, T.; Maeda, A.; Arisaka, H.; Shibahara, S.; Akiyama, Y. Combined administration of L-cystine and L-theanine enhances immune functions and protects against influenza virus infection in aged mice. J. Vet. Med. Sci. 2010, 72, 157–165. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, L.; Peng, Y.Q.; Yuan, D.Y.; Zhang, S.; Gong, Z.H.; Xiao, W.J. L-Theanine attenuates liver aging by inhibiting advanced glycation end products in D-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp. Gerontol. 2020, 131, 110823. [Google Scholar] [CrossRef]
- Li, G.L.; Ye, Y.; Kang, J.J.; Yao, X.Y.; Zhang, Y.Z.; Jiang, W.; Gao, M.; Dai, Y.D.; Xin, Y.Q.; Wang, Q.; et al. L-theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012, 50, 363–372. [Google Scholar] [CrossRef]
- Liu, K.H.; Liu, E.S.; Lin, L.; Hu, Y.; Yuan, Y.; Xiao, W.J. l-Theanine mediates the p38MAPK signaling pathway to alleviate heat-induced oxidative stress and inflammation in mice. Food Funct. 2022, 13, 2120–2130. [Google Scholar] [CrossRef]
- Sugiyama, T.; Sadzuka, Y. Theanine, a specific glutamate derivative in green tea, reduces the adverse reactions of doxorubicin by changing the glutathione level. Cancer Lett. 2004, 212, 177–184. [Google Scholar] [CrossRef]
- Di, X.; Yan, J.; Zhao, Y.; Zhang, J.; Shi, Z.; Chang, Y.; Zhao, B. L-theanine protects the app (Swedish mutant) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 2010, 168, 778–786. [Google Scholar] [CrossRef]
- Wang, D.X.; Gao, Q.; Zhao, G.S.; Kan, Z.P.; Wang, X.X.; Wang, H.S.; Huang, J.B.; Wang, T.T.; Qian, F.; Ho, C.T.; et al. Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice. J. Agric. Food Chem. 2018, 66, 7674–7683. [Google Scholar] [CrossRef]
- Liu, A.; Lin, L.; Xu, W.; Gong, Z.H.; Liu, Z.H.; Xiao, W.J. L-theanine regulates glutamine metabolism and immune function by binding to cannabinoid receptor 1. Food Funct. 2021, 12, 5755–5769. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhao, X.H.; Chen, K.K.; Geng, Z.Y. Effect of L-theanine on meat quality, muscle amino acid profiles, and antioxidant status of broilers. Anim. Sci. J. 2020, 91, 8. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, Z.Y.; Chen, K.K.; Zhao, X.H.; Wang, C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult. Sci. 2019, 98, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, Z.H.; Zhao, J.F.; Huo, D.X.; Fan, Z.Y.; Hou, D.X.; He, X. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim. Nutr. 2018, 4, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jelveh, K.; Rasouli, B.; Kadim, I.T.; Slozhenkina, M.I.; Gorlov, I.F.; Seidavi, A.; Phillips, C.J.C. The effects of green tea in the diet of broilers challenged with coccidiosis on their performance, carcass characteristics, intestinal mucosal morphology, blood constituents and ceca microflora. Vet. Med. Sci. 2022, 8, 2511–2520. [Google Scholar] [CrossRef]
- Saeed, M.; Xu, Y.T.; Faiz-ul, H.; Arain, M.A.; Abd El-Hack, M.E.; Noreldin, A.E.; Sun, C. Influence of graded levels of L-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int. J. Mol. Sci. 2018, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Tang, Y.F.; Long, L.A.; Zhang, H.H. Effects of dietary L-theanine on growth performance, antioxidation, meat quality, and intestinal microflora in white feather broilers with acute oxidative stress. Front. Vet. Sci. 2022, 9, 9. [Google Scholar] [CrossRef]
- Wen, H.; Wei, S.; Zhang, S.; Hou, D.; Xiao, W.; He, X. Effects of L-theanine on performance and immune function of yellow-feathered broilers. Chin. J. Anim. Nutr. 2012, 24, 1946–1954. [Google Scholar]
- Zhang, C.; Chen, K.K.; Zhao, X.H.; Wang, C.; Geng, Z.Y. Effect of l-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal 2019, 13, 1145–1153. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, L.L.; Qin, Y.N.; Mao, Z.Y.; Jia, G.; Zhao, H.; Liu, G.M.; Huang, Z.Q. Effect of dietary L-theanine supplementation on skeletal muscle fiber type transformation in weaning piglets. Anim. Biotechnol. 2022, 33, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Chen, L.L.; Qin, Y.N.; Mao, Z.Y.; Huang, Z.Q.; Jia, G.; Zhao, H.; Liu, G.M. Dietary L-theanine supplementation improves lipid metabolism and antioxidant capacity in weaning piglets. Anim. Biotechnol. 2022, 33, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, B.K.; Lim, J.H.; Kim, M.S.; Song, I.B.; Park, S.C.; Jung, H.K.; Hong, J.H.; Yun, H.I. Effects of beta-Glucan from Paenibacillus polymyxa and L-theanine on Growth Performance and Immunomodulation in Weanling Piglets. Asian Australas. J. Anim. Sci. 2008, 21, 1753–1759. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, Z.Q.; Zheng, Z.; Li, N.; Mu, S.Q.; Ma, Y.; Zhou, Z.J.; Yan, J.; Lu, C.L.; Wang, W.J.; et al. Effects of L-theanine on intestinal morphology, barrier function, and MAPK signaling pathways in diquat-challenged piglets. Anim. Biotechnol. 2021, 1–8, Online ahead of print. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, L.L.; Jia, G.; Zhao, H.; Liu, G.M.; Huang, Z.Q. L-theanine improves intestinal barrier functions by increasing tight junction protein expression and attenuating inflammatory reaction in weaned piglets. J. Funct. Foods 2023, 100, 10. [Google Scholar] [CrossRef]
- Yang, L.Y.; Zhang, L.M.; Zhang, P.H.; Zhou, Y.L.; Huang, X.G.; Yan, Q.X.; Tan, Z.L.; Tang, S.X.; Wan, F.C. Alterations in nutrient digestibility and performance of heat-stressed dairy cows by dietary L-theanine supplementation. Anim. Nutr. 2022, 11, 350–358. [Google Scholar] [CrossRef] [PubMed]
| Physicochemical Property | Description |
|---|---|
| Molecular formula | C7H14N2O3 |
| Molecular weight | 174.198 g/mol |
| Melting point | 207 °C |
| Density | 1.2 ± 0.1 g/cm3 |
| Appearance | Crystalline solid |
| Solubility | Soluble in water and insoluble in ether, alcohol |
| Taste | Odorless, umami, and sweet taste |
| Stability | Stable in acidic and unstable in alkaline conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. https://doi.org/10.3390/molecules28093846
Chen S, Kang J, Zhu H, Wang K, Han Z, Wang L, Liu J, Wu Y, He P, Tu Y, et al. L-Theanine and Immunity: A Review. Molecules. 2023; 28(9):3846. https://doi.org/10.3390/molecules28093846
Chicago/Turabian StyleChen, Shuna, Jiaxin Kang, Huanqing Zhu, Kaixi Wang, Ziyi Han, Leyu Wang, Junsheng Liu, Yuanyuan Wu, Puming He, Youying Tu, and et al. 2023. "L-Theanine and Immunity: A Review" Molecules 28, no. 9: 3846. https://doi.org/10.3390/molecules28093846
APA StyleChen, S., Kang, J., Zhu, H., Wang, K., Han, Z., Wang, L., Liu, J., Wu, Y., He, P., Tu, Y., & Li, B. (2023). L-Theanine and Immunity: A Review. Molecules, 28(9), 3846. https://doi.org/10.3390/molecules28093846







