Studies of Dopamine Oxidation Process by Atmospheric Pressure Glow Discharge Mass Spectrometry
Abstract
1. Introduction
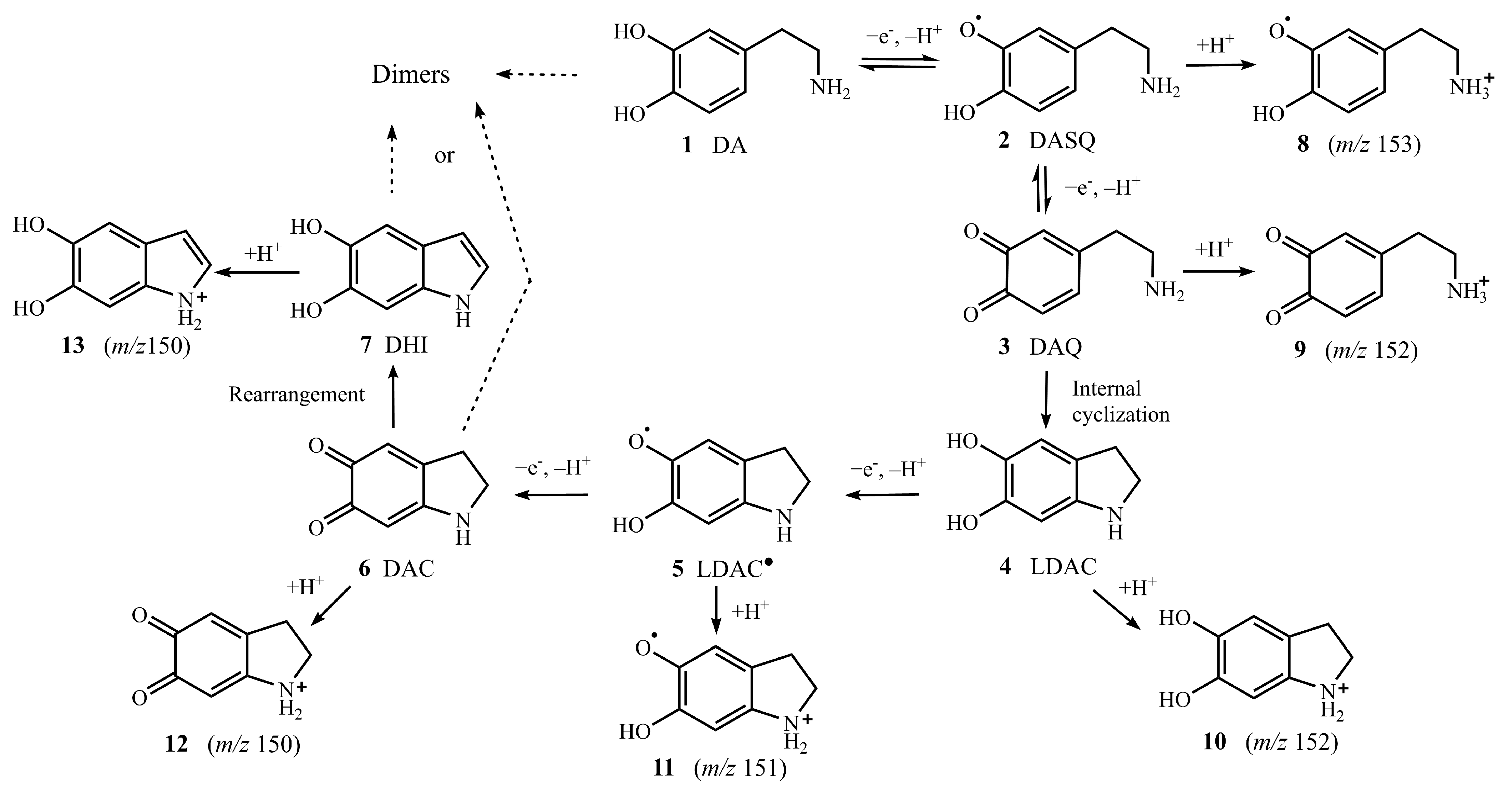
2. Results and Discussion
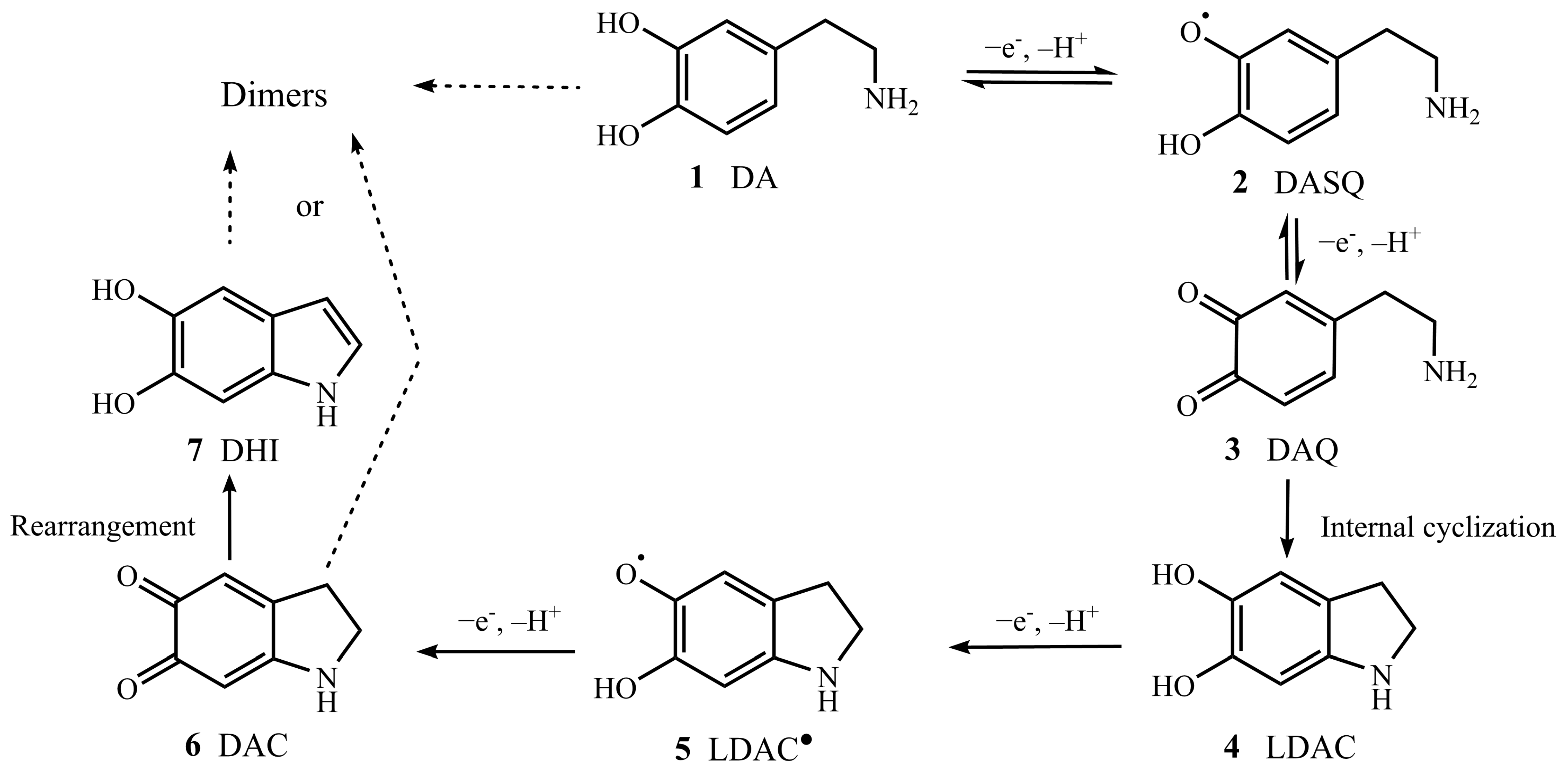
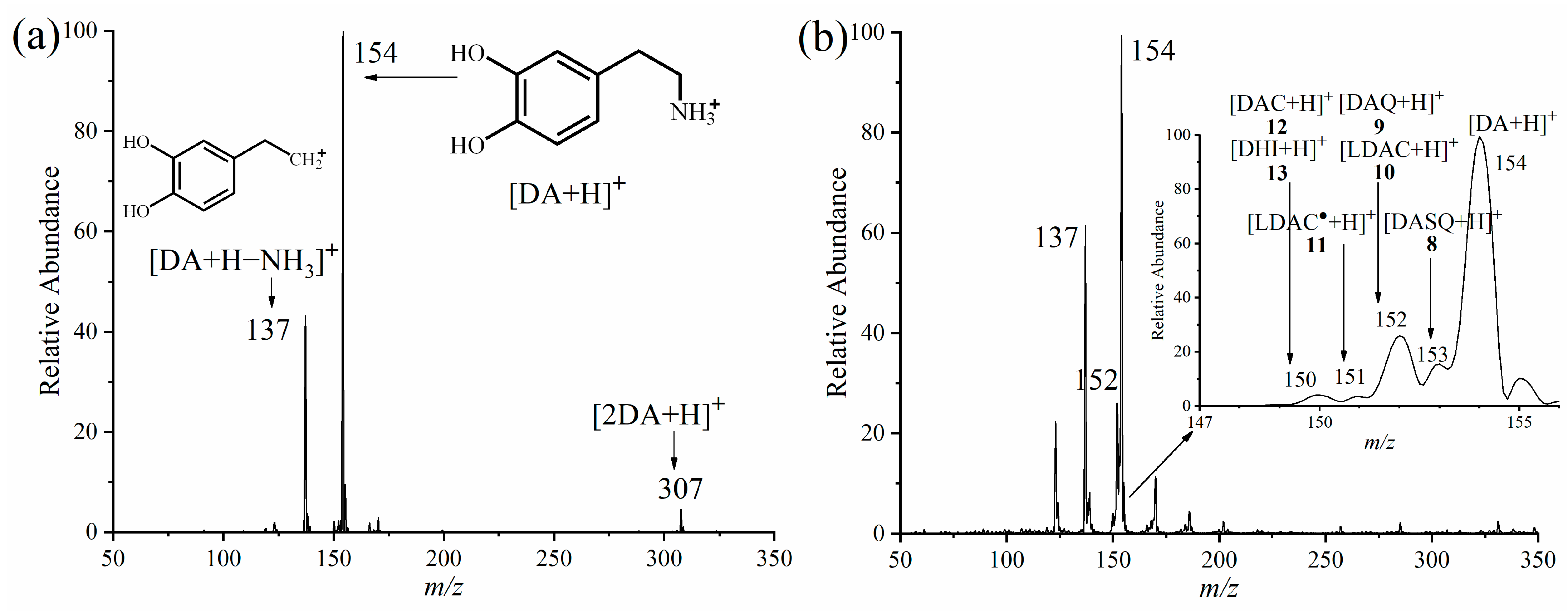
2.1. APGD-MS Studies of DA Oxidation Process Catalysed by PPO
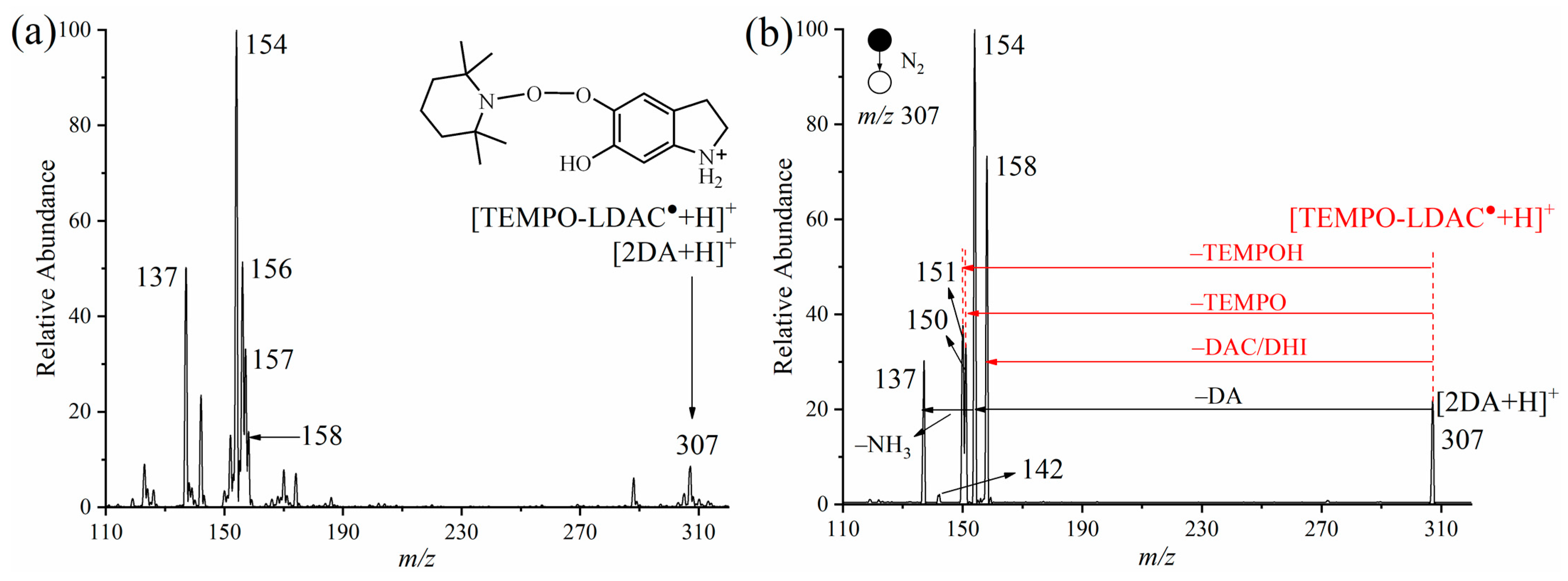
2.2. Verification of Protonated DASQ and LDAC Radical Cations with TEMPO by APGD-MS
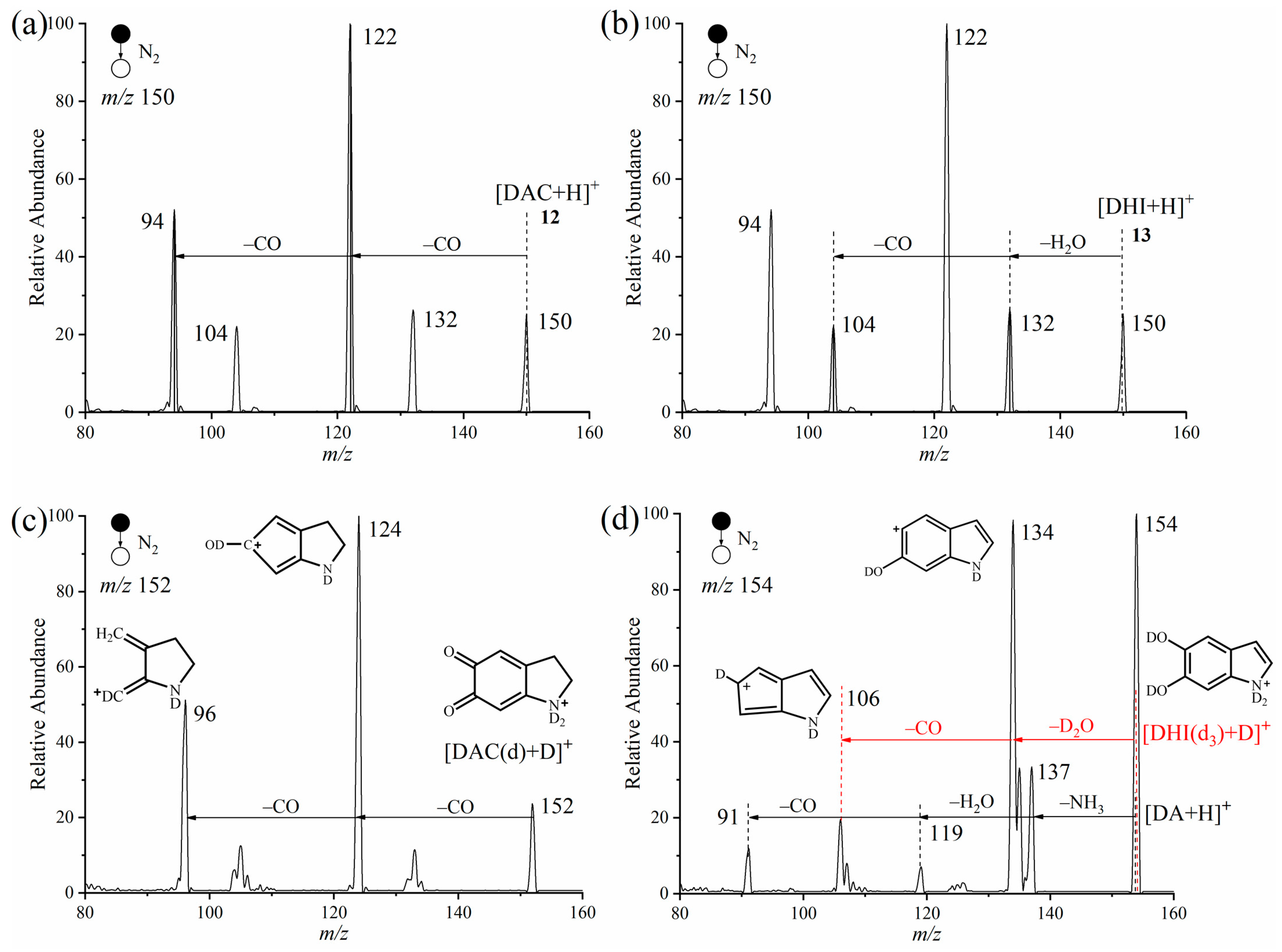
2.3. Verification of Isomers of Protonated DAC and DHI Cations with Deuterium Oxide by APGD-MS
2.4. Verification of PPO Activity during the DA Oxidation Process by UV–Vis Spectrophotometer
2.5. Possible Mechanism of DA Oxidation Reaction Catalysed by PPO
3. Materials and Methods
3.1. APGD-MS and UV–Vis Experiments
3.2. Materials and Reagents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J.; Khoshbouei, H. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, X.; Yang, C.S.; Zhang, J. An unrecognized fundamental relationship between neurotransmitters: Glutamate protects against catecholamine oxidation. Antioxidants 2021, 10, 1564. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Wang, G. Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, W.; Cao, S.; Jiang, W.; Peng, H.; Cai, S.; Chen, Z. NMR Spectroelectrochemistry in Studies of Dopamine Oxidation. Electrochemistry 2020, 88, 200–204. [Google Scholar] [CrossRef]
- Otsuka, F.S.; Otaduy, M.C.G.; Nascimento, O.R.; Salmon, C.E.G. Quantification of paramagnetic ions in human brain tissue using EPR. Braz. J. Phys. 2022, 52, 94. [Google Scholar] [CrossRef]
- Gao, J.; Sheng, C.; Zhu, Y.; Dong, M.; Qian, R.; Zhuo, S. Inorganic Multi-Element Analysis of Foodstuff by Means of Low Power Total Reflection X-Ray Fluorescence Spectrometry Using Suspension Sampling. Spectrosc. Spect. Anal. 2020, 40, 945–949. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, B.; Zhuo, S.; Zhu, Y.; Huang, L.; Qian, R. Depth Profile Analysis of Molybdenum Disulfide Film by Glow Discharge Mass Spectrometry. At. Spectrosc. 2021, 42, 183–189. [Google Scholar] [CrossRef]
- Xu, L.; Qian, R.; Zhao, J.; Yang, W.; Gao, J.; Wang, Q.; Zhuo, S. Efficient identification of raw and ripe tung oil using headspace GC–MS. Rapid Commun. Mass Spectrom. 2021, 35, e9156. [Google Scholar] [CrossRef]
- Liu, X.; Kang, J.; Wang, Y.; Li, W.; Guo, H.; Xu, L.; Guo, X.; Zhou, F.; Jia, X. Amine-Triggered Dopamine Polymerization: From Aqueous Solution to Organic Solvents. Macromol. Rapid Commun. 2018, 39, e1800160. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Murase, T.; Zucca, F.A.; Zecca, L.; Ito, S. Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 2012, 25, 792–803. [Google Scholar] [CrossRef]
- Umek, N. The effects of biologically important divalent and trivalent metal cations on the cyclization step of dopamine autoxidation reaction: A quantum chemical study. Arab. J. Chem. 2022, 15, 104153. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Lim, T.M. Glutathione conjugates with dopamine-derived quinones to form reactive or non-reactive glutathione-conjugates. Neurochem. Res. 2010, 35, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lanekoff, I.T.; Laskin, J.; Dewald, H.D.; Chen, H. Study of electrochemical reactions using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 2012, 84, 5737–5743. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.; El-Nour, K.M.A.; Brajter-Toth, A. Detection of transient dopamine antioxidant radicals using electrochemistry in electrospray ionization mass spectrometry. Electrochim. Acta 2017, 249, 145–154. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, N.; Zhang, P.K.; Chen, Y.; Xia, X.H.; Chen, H.Y.; Xu, J.J. Coupling a Wireless Bipolar Ultramicroelectrode with Nano-electrospray Ionization Mass Spectrometry: Insights into the Ultrafast Initial Step of Electrochemical Reactions. Angew. Chem. Int. Ed. Engl. 2020, 59, 18244–18248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cao, Y.; Liu, J.; Zhang, H.; Kan, G.; Yu, K. Mass spectrometric observation on free radicals during electrooxidation of dopamine. Anal. Chim. Acta 2022, 1193, 339403. [Google Scholar] [CrossRef]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient ionization mass spectrometry: Recent developments and applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; Li, N.; He, J.; You, H.; Jiang, J. Intermediate detection in real time using reactive surface desorption dielectric-barrier discharge ionization mass spectrometry. J. Mass Spectrom. 2018, 53, 511–517. [Google Scholar] [CrossRef]
- Alves, M.R.; Sauer, J.S.; Prather, K.A.; Grassian, V.H.; Wilkins, C.L. Liquid sampling-atmospheric pressure glow discharge ionization as a technique for the characterization of salt-containing organic samples. Anal. Chem. 2020, 92, 8845–8851. [Google Scholar] [CrossRef]
- Fandino, J.; Orejas, J.; Chauvet, L.; Blanco, D.; Guillot, P.; Pisonero, J.; Bordel, N. Evaluation of a modified halo flowing atmospheric pressure afterglow ion source for the analysis of directly injected volatile organic compounds. J. Anal. At. Spectrom. 2020, 35, 2002–2010. [Google Scholar] [CrossRef]
- Zhang, D.; Latif, M.; Gamez, G. Instantaneous differentiation of functional isomers via reactive flowing atmospheric pressure afterglow mass spectrometry. Anal. Chem. 2021, 93, 9986–9994. [Google Scholar] [CrossRef]
- Guć, M.; Cegłowski, M.; Pawlaczyk, M.; Kurczewska, J.; Reszke, E.; Schroeder, G. Application of FAPA mass spectrometry for analysis of fragrance ingredients used in cosmetics. Measurement 2021, 168, 108326. [Google Scholar] [CrossRef]
- Shi, L.; Habib, A.; Bi, L.; Hong, H.; Begum, R.; Wen, L. Ambient Ionization Mass Spectrometry: Application and Prospective. Crit. Rev. Anal. Chem. 2022, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Innocent, C.; Allien, L.; Poitry, S.; Tsacopoulos, M. An electrochemical method for making enzyme microsensors. Application to the detection of dopamine and glutamate. Anal. Chem. 1997, 69, 968–971. [Google Scholar] [CrossRef]
- Gonzalez-Sepulveda, M.; Laguna, A.; Carballo-Carbajal, I.; Galiano-Landeira, J.; Romero-Gimenez, J.; Cuadros, T.; Parent, A.; Peñuelas, N.; Compte, J.; Nicolau, A.; et al. Validation of a reversed phase UPLC-MS/MS method to determine dopamine metabolites and oxidation intermediates in neuronal differentiated SH-SY5Y cells and brain tissue. ACS Chem. Neurosci. 2020, 11, 2679–2687. [Google Scholar] [CrossRef]
- Bauer, N.A.; Hoque, E.; Wolf, M.; Kleigrewe, K.; Hofmann, T. Detection of the formyl radical by EPR spin-trapping and mass spectrometry. Free Radic. Biol. Med. 2018, 116, 129–133. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Z.; Bai, R.; Wang, D.; Cui, F.; Zhang, J.; Strathmann, T.J. Role of TEMPO in enhancing permanganate oxidation toward organic contaminants. Environ. Sci. Technol. 2021, 55, 7681–7689. [Google Scholar] [CrossRef]
- Lemos-Amado, F.; Domingues, P.; Ferrer-Correia, A.; Remião, F.; Milhazes, N.; Borges, F.; Carvalho, F.D.; Bastos, M.L. Electrospray tandem mass spectrometry of aminochromes. Rapid Commun. Mass Spectrom. 2001, 15, 2466–2471. [Google Scholar] [CrossRef]
- Shi, C.; Jia, H.; Chen, S.; Huang, J.; Peng, Y.E.; Guo, W. Hydrogen/deuterium exchange aiding metabolite identification in single-cell nanospray high-resolution mass spectrometry analysis. Anal. Chem. 2022, 94, 650–657. [Google Scholar] [CrossRef]
- Bisaglia, M.; Mammi, S.; Bubacco, L. Kinetic and structural analysis of the early oxidation products of dopamine: Analysis of the interactions with α-synuclein. J. Biol. Chem. 2007, 282, 15597–15605. [Google Scholar] [CrossRef] [PubMed]
- Salomaki, M.; Marttila, L.; Kivela, H.; Ouvinen, T.; Lukkari, J. Effects of ph and oxidants on the first steps of polydopamine formation: A thermodynamic approach. J. Phys. Chem. B 2018, 122, 6314–6327. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Waite, T.D. Cu (II)-catalyzed oxidation of dopamine in aqueous solutions: Mechanism and kinetics. J. Inorg. Biochem. 2014, 137, 74–84. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.; Zhu, Y.; Zhu, Z.; Qian, R.; Zhuo, S.; Liu, A.; Li, X.; Li, W.; Chen, Q. Studies of Dopamine Oxidation Process by Atmospheric Pressure Glow Discharge Mass Spectrometry. Molecules 2023, 28, 3844. https://doi.org/10.3390/molecules28093844
Dai D, Zhu Y, Zhu Z, Qian R, Zhuo S, Liu A, Li X, Li W, Chen Q. Studies of Dopamine Oxidation Process by Atmospheric Pressure Glow Discharge Mass Spectrometry. Molecules. 2023; 28(9):3844. https://doi.org/10.3390/molecules28093844
Chicago/Turabian StyleDai, Dongli, Yueqin Zhu, Zhenli Zhu, Rong Qian, Shangjun Zhuo, Anqi Liu, Xian Li, Wei Li, and Qiao Chen. 2023. "Studies of Dopamine Oxidation Process by Atmospheric Pressure Glow Discharge Mass Spectrometry" Molecules 28, no. 9: 3844. https://doi.org/10.3390/molecules28093844
APA StyleDai, D., Zhu, Y., Zhu, Z., Qian, R., Zhuo, S., Liu, A., Li, X., Li, W., & Chen, Q. (2023). Studies of Dopamine Oxidation Process by Atmospheric Pressure Glow Discharge Mass Spectrometry. Molecules, 28(9), 3844. https://doi.org/10.3390/molecules28093844








