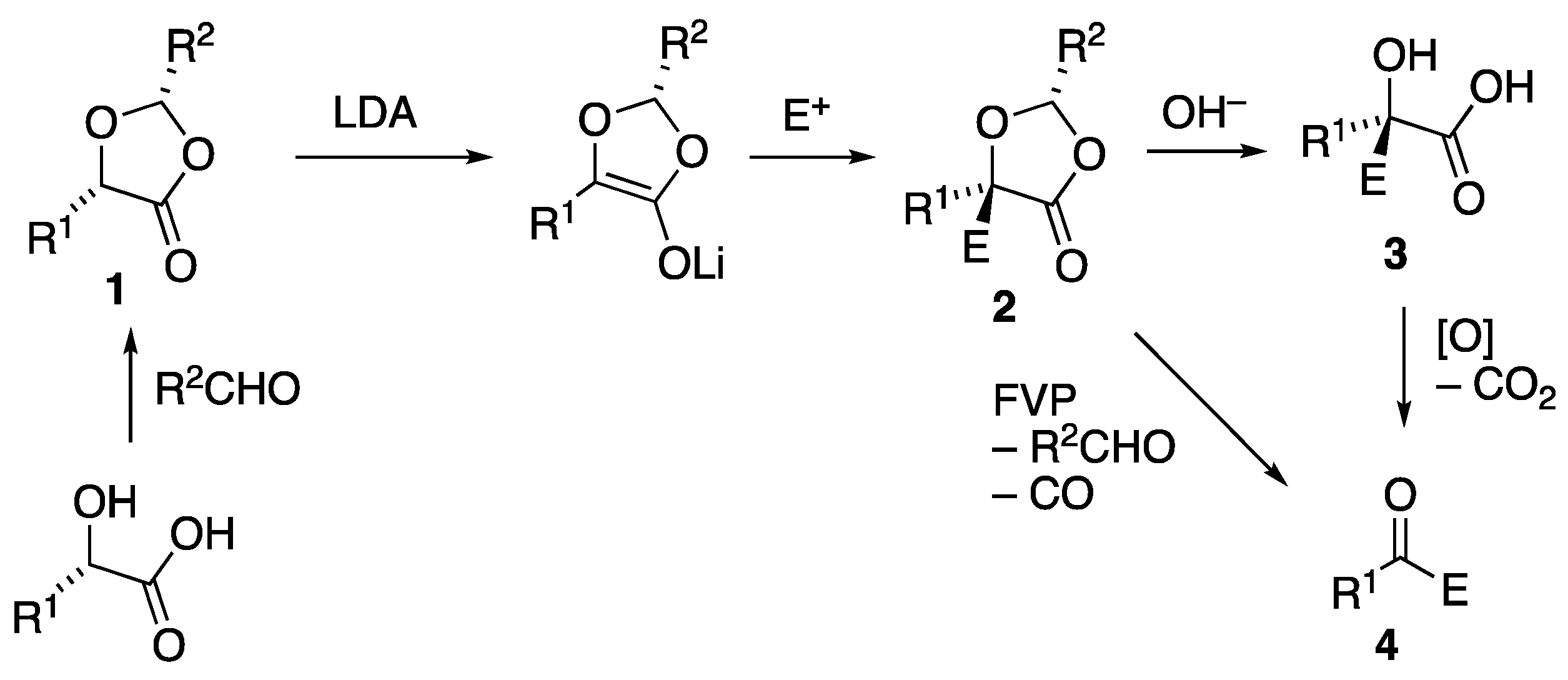
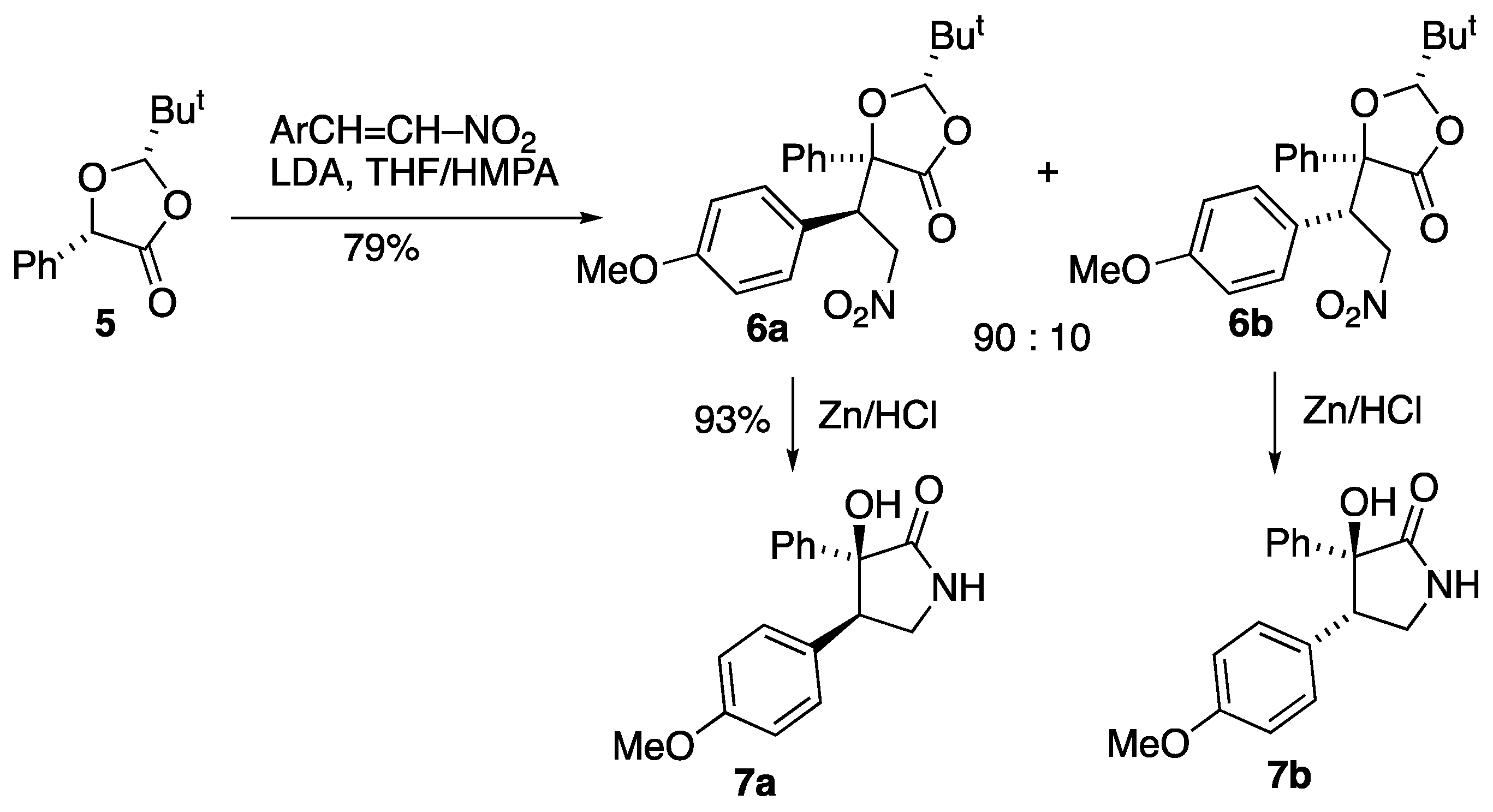
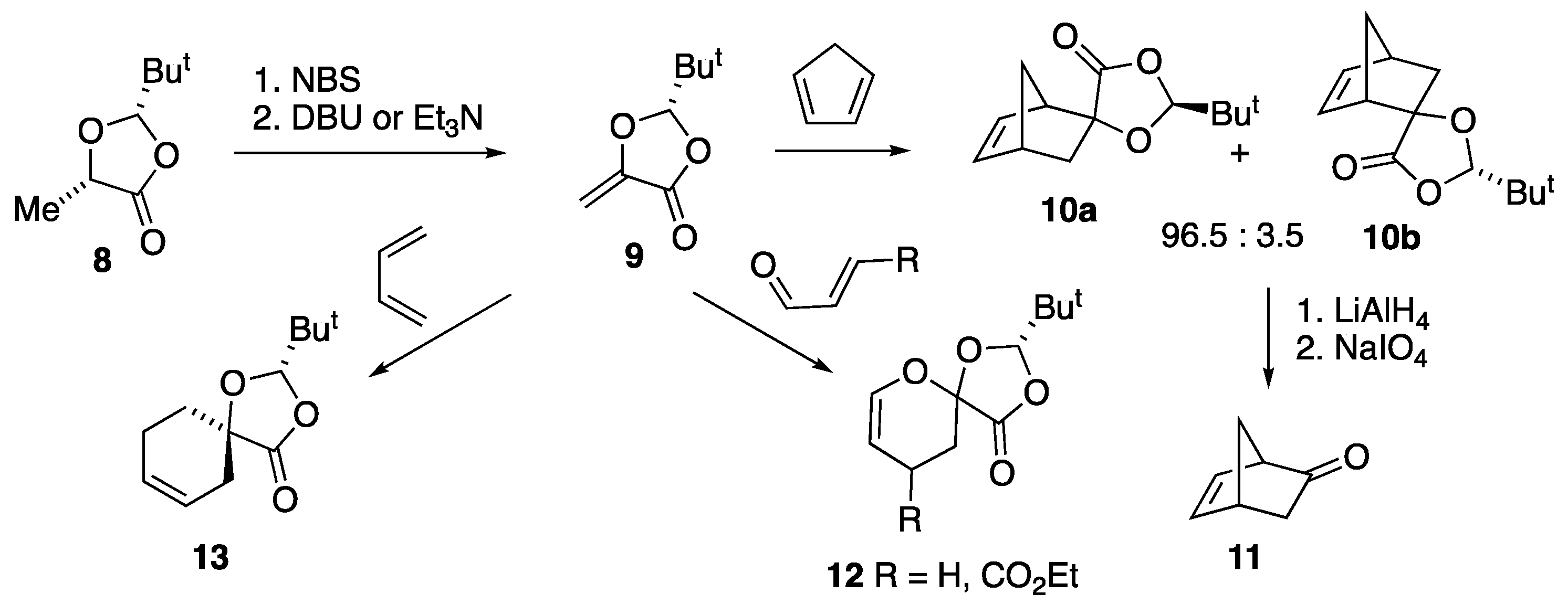
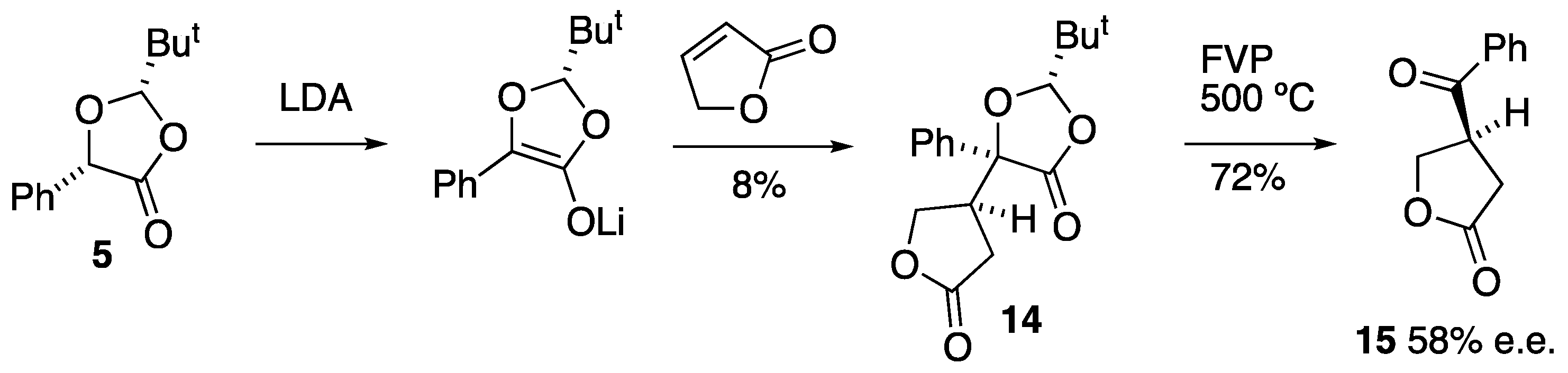
3.2. Preparation and Reactions of Dioxolanone Michael Addition Products
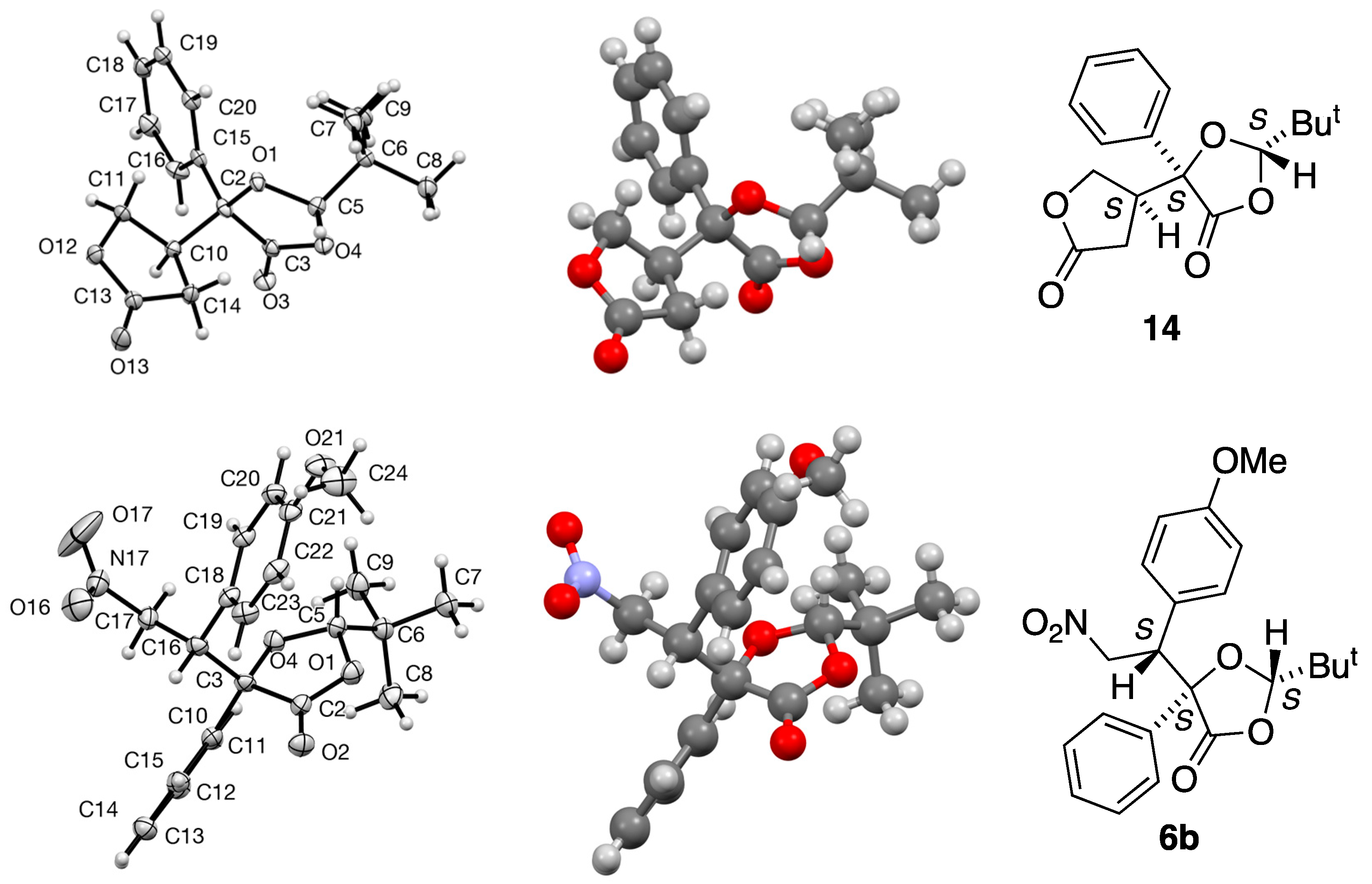
3.2.1. Preparation of (2S,5S,4′S)-2-t-Butyl-5-(2-oxo-tetrahydrofuran-4-yl)-5-phenyl-1,3-dioxolan-4-one 14
To a solution of LDA (4.4 mmol) in dry THF (24 cm3) at −78 °C, (2S,5S)-2-t-butyl-5-phenyl-1,3-dioxolan-4-one 5 (1.0 g, 4 mmol) dissolved in dry THF (8 cm3) was added slowly by syringe. The reaction mixture was stirred at −78 °C for 30 min and then warmed to −30 °C for 30 min. The reaction was then re-cooled to −78 °C and butenolide (0.41 g, 4.4 mmol) was added. The reaction mixture was kept at −78 °C for 2 h, then warmed to room temperature and allowed to stir overnight. Aqueous NH4Cl (25 cm3) was added and the mixture was extracted with ether (2 × 15 cm3). The combined organic layers were washed with water, dried and the solvent removed to yield a yellow liquid. The product was then purified by flash column chromatography (silica gel, Et2O/hexane, 1:2) to give the product as a colourless solid (79.1 mg, 8%), mp 175–178 °C; [α]D –24.8 (c = 0.592, CH2Cl2); νmax/cm−1 1789, 1457, 1369, 1200, 1027 and 694; 1H NMR (300 MHz) δH 0.92 (9 H, s, But), 2.55 (1 H, half AB pattern of d, J 17.4, 7.7, CH2-C=O), 2.69 (1 H, half AB pattern of d, J 17.4, 8.5, CH2-C=O), 3.21–3.32 (1 H, m, CH-CH2O), 4.02 (1 H, half AB pattern of d, J 9.7, 7.8, CH2-O), 4.17 (1 H, half AB pattern of d, J 9.7, 7.0, CH2-O), 5.46 (1 H, s, CH-But), 7.36–7.46 (3 H, m, Ph) and 7.64–7.70 (2 H, m, Ph); 13C NMR (75 MHz) δC 23.4 (But), 29.9 (CH2), 35.8 (C-Me3), 45.3 (CH), 67.8 (CH2), 80.7 (C), 111.4 (CH-But), 124.8 (2CH), 128.7 (2CH), 128.9 (CH), 135.5 (C), 171.7 (C=O) and 175.0 (C=O); HRMS (ES+): found 327.1200. C17H20NaO5 (M + Na) requires 327.1208.
3.2.2. Pyrolysis of (2S,5S,4′S)-2-t-Butyl-5-(2-oxo-tetrahydrofuran-4-yl)-5-phenyl-1,3-dioxolan-4-one 14
FVP of the reactant
14 (24.0 mg, 78.8 µmol) was performed at 500 °C and 3.8 × 10
−2 Torr. Preparative TLC (silica, diethyl ether) was used to separate the product from some unchanged starting material, giving the product (4
S)-4-benzoyltetrahydrofuran-2-one
15 as a yellow oil (10.8 mg, 72%). [α]
D −11.3 (c = 0.56, CHCl
3), [lit. [
21], –19.6 (c = 1.08, CHCl
3)], e.e.= 58%;
1H NMR (300 MHz) δ
H 2.81 (1 H, half AB pattern of d,
J 17.8, 9.3, CH
2-C=O), 3.03 (1 H, half AB pattern of d,
J 17.8, 7.4, CH
2-C=O), 4.34–4.44 (1 H, m, CH), 4.48 (1 H, half AB pattern of d,
J 9.0, 6.7, CH
2O), 4.63 (1 H, half AB pattern of d,
J 9.0, 8.5, CH
2O), 7.50–7.57 (2 H, m, Ph), 7.62–7.68 (1 H, m, Ph) and 7.91–7.96 (2 H, m, Ph);
13C NMR (75 MHz) δ
C 30.9 (CH
2-C=O), 42.1 (CH), 68.9 (CH
2O), 128.5 (2CH), 129.1 (2CH), 134.2 (CH), 134.8 (C), 175.4 (C=O) and 196.2 (C=O).
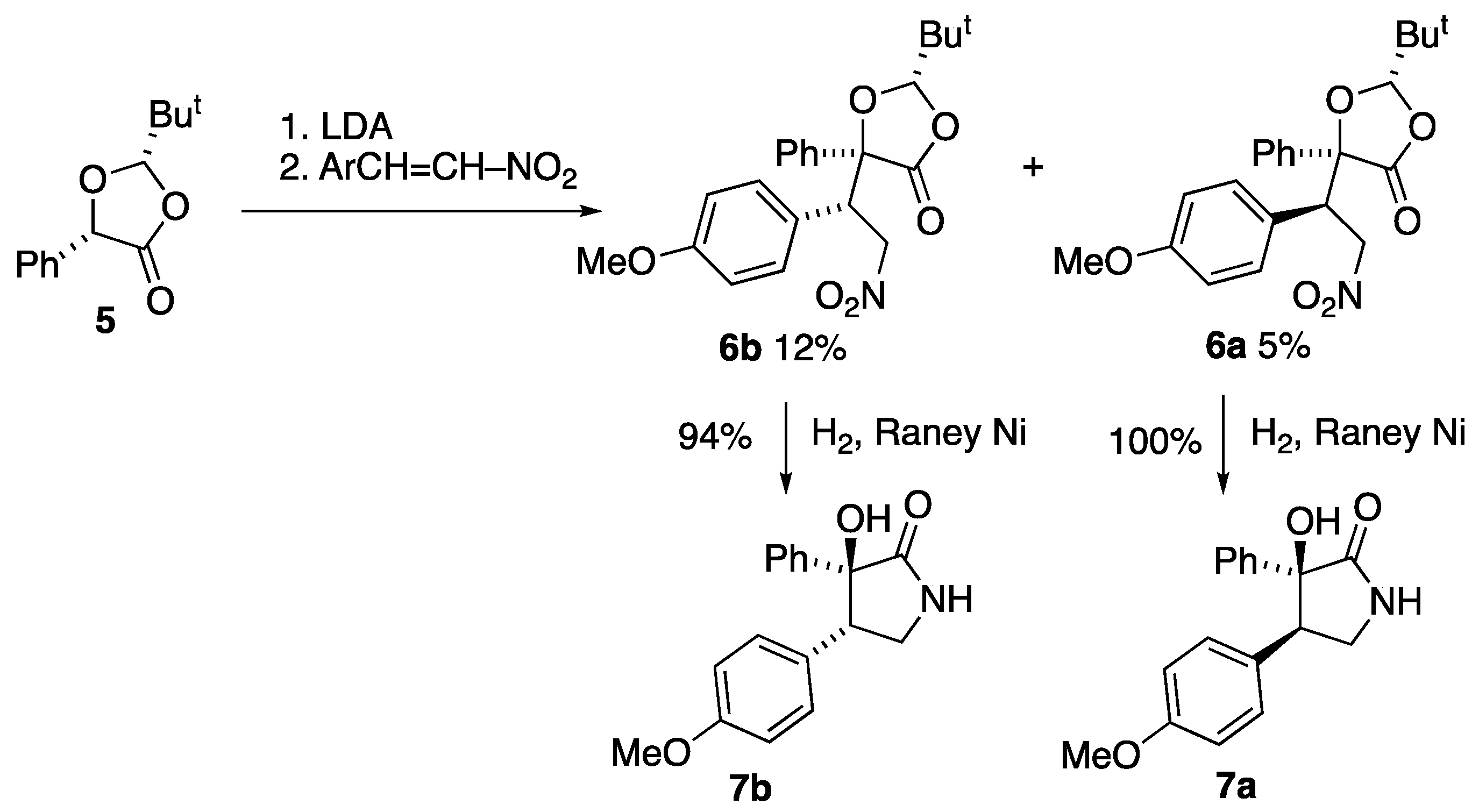
3.2.3. Preparation of (2S,5S)-2-t-Butyl-5-(1′-(4-methoxyphenyl)-2′-nitroethyl)-5-phenyl-1,3-dioxolan-4-one 6a/6b [11]
To a solution of LDA (22 mmol) in dry THF (100 cm3) at −78 °C, a solution of (2S,5S)-2-t-butyl-5-phenyl-1,3-dioxolan-4-one 5 (4.40 g, 20 mmol) in dry THF (30 cm3) was added slowly by syringe. The solution was stirred at −78 °C for 30 min, then warmed up to −20 °C for 30 min and then re-cooled to −78 °C. A solution of 1-methoxy-4-[(E)-2-nitrovinyl]benzene (3.94 g, 22 mmol) in dry THF (20 cm3) was then added slowly by syringe and the mixture was stirred at −78 °C for 2 h and then warmed up to room temperature and stirred overnight. Aqueous NH4Cl (100 cm3) was added and the mixture was extracted with ether (2 × 60 cm3). The combined organic layers were washed with water, dried and the solvent removed to yield a viscous brown liquid. 1H NMR spectroscopic analysis showed that the diastereomers were present in a 3:4 ratio along with a number of impurities. Separation was achieved by flash column chromatography (silica gel, Et2O/hexane, 1:5). This gave first the minor product 6a (0.41 g, 5%) and on removal of solvent this was recrystallised to give a pure product (0.24 g, 3%) as colourless prism-like crystals. This was followed by the major product 6b as colourless crystals (1.01 g, 12%).
Minor isomer, (2
S,5
S,1′
R)-2-
t-butyl-5-(1′-(4-methoxyphenyl)-2′-nitroethyl)-5-phenyl-1,3-dioxolan-4-one
6a: mp 98–102 °C, (lit. [
11], 96–98 °C); [α]
D –61.07, (c = 1.5, CHCl
3) [lit. [
11], −54.2, (c = 1.5, CHCl
3)]; (Found: C, 66.4; H, 6.4; N, 3.5. C
22H
25NO
6 requires C, 66.2; H, 6.3; N, 3.5%); ν
max/cm
−1 1778, 1612, 1559, 1513, 1424, 1404 and 1350;
1H NMR (300 MHz) δ
H 0.71 (9 H, s, Bu
t), 3.81 (3 H, s, OMe), 4.10 (1 H, dd,
J 11.1, 4.7, CH
2), 4.43 (1H, s,
HC-Bu
t), 4.48 (1 H, dd,
J 13.5, 4.7, CH
2), 5.07 (1 H, dd,
J 13.5, 11.1, C
H-C
6H
4-OMe), 6.86 (2 H, d,
J 8.7, Ar H-3, H-5), 7.20 (2 H, d,
J 8.7, Ar H-2, H-6), 7.36–7.42 (3 H, m, Ph) and 7.59–7.64 (2 H, m, Ph);
13C NMR (75 MHz) δ
C 23.1 (Bu
t), 35.0 (C-Me
3), 52.3 (
CH-CH
2-NO
2), 55.2 (MeO), 75.0 (CH
2), 83.6 (C), 110.6 (C-Bu
t), 114.2 (2CH), 125.1 (C), 125.5 (2CH), 128.7 (2CH), 129.0 (2CH), 130.2 (CH), 136.1 (C), 159.8 (C), and 171.3 (C=O);
m/
z 422.11 (M
+ + Na
+, 100%).
Major isomer, (2S,5S,1′S)-2-t-butyl-5-(1′-(4-methoxyphenyl)-2′-nitroethyl)-5-phenyl-1,3-dioxolan-4-one 6b: mp 139–140 °C; [α]D +37.6 (c = 1, CH2Cl2) (Found: C, 66.5; H, 6.2; N, 3.6. C22H25NO6 requires C, 66.2; H, 6.3; N, 3.5%); νmax/cm−1 1786, 1611, 1585, 1555, 1514, 1459, 1450, 1436, 1380, 1350, 1301, 1252, 1200 and 1184; 1H NMR (300 MHz) δH 0.84 (9 H, s, But), 3.82 (3 H, s, OMe), 4.19 (1 H, dd, J 14.2, 4.4, CH2), 4.20 (1H, dd, J 12.8, 4.4, CH2), 4.65 (1 H, s, HC-But), 4.87 (1 H, dd, J 14.2, 12.7, CH-C6H4-OMe), 6.91 (2 H, d, J 8.8, Ar H-3, H-5), 7.35 (2 H, d, J 8.8, Ar H-2, H-6), 7.38–7.50 (3 H, m, Ph) and 7.78–7.83 (2 H, m, Ph); 13C NMR (75 MHz) δC 23.5 (But), 35.4 (C-Me3), 51.8 (CH-CH2-NO2), 55.3 (MeO), 74.7 (CH2), 84.1 (C), 110.9 (C-But), 114.5 (2CH), 124.5 (C), 125.0 (2CH), 128.9 (2CH), 129.0 (2CH), 130.4 (CH), 135.9 (C), 160.1 (C) and 171.4 (C=O); HRMS (ES+): found 422.1584. C22H25NaNO6 (M + Na) requires 422.1580.
3.2.4. Reduction of 6b Using Raney Ni under Hydrogen to Give the γ-Lactam (3S,4S)-3-Hydroxy-4-(4-methoxyphenyl)-3-phenylpyrrolidin-2-one 7b
Raney Ni (6.00 g) and 6b (0.51 g, 1.27 mmol) were stirred in methanol (20 cm3) in a hydrogenation flask under hydrogen gas until the required amount of gas (85.3 cm3) was consumed. The mixture was filtered through a layer of Celite to remove the nickel. The filtrate was concentrated to yield the product 7b (0.34 g, 94%) as a white powder, mp 226–227 °C; [α]D −161.2 (c = 0.5, MeOH); (Found: C, 71.7; H, 6.0; N, 5.0. C17H17NO3 requires C, 72.1; H, 6.1; N, 4.9%); νmax/cm−1 3301, 1772, 1612, 1516, 1464 and 1377; 1H NMR (300 MHz) δH 3.53 (1 H, m, CH2), 3.60 (1 H, dd, J 10.3, 7.8, CH2), 3.72 (3 H, s, OMe), 3.89 (1 H, dd, J 10.3, 7.8, CH), 6.49 (1 H, br s, NH or OH), 6.61–6.62 (2 H, m, Ar), 6.69–6.74 (2 H, m, Ar), 6.99–7.04 (2 H, m, Ph) and 7.14–7.20 (3 H, m, Ph); 13C NMR (75 MHz) δC 42.8 (CH2), 53.6 (CH), 55.2 (OMe), 81.3 (C-OH), 113.3 (2CH), 126.1 (2CH), 127.5 (C), 127.9 (CH), 128.0 (2CH), 129.6 (2CH), 137.9 (C), 158.7 (C) and 178.3 (C=O); m/z (ES) 306.14 (M + Na+, 100%).
3.2.5. Reduction of 6a Using Raney Ni under Hydrogen to Give the γ-Lactam, (3S,4R)-3-Hydroxy-4-(4-methoxyphenyl)-3-phenylpyrrolidin-2-one 7a
Raney Ni (6.00 g) and
6a (0.45 g, 1.13 mmol) were stirred in methanol (20 cm
3) as for the formation of
7b. The filtrate was concentrated to yield the product
7a (0.32 g, 100%) as a white powder, mp 220–222 °C (lit. [
11], 220–222 °C); [α]
D −38.4 (c = 0.5, MeOH) [lit. [
11], –37.9 (c = 0.5 MeOH)]; (Found: C, 72.2; H, 5.7; N, 5.1. C
17H
17NO
3 requires C, 72.1; H, 6.1; N, 4.9%); ν
max/cm
−1 3327, 1700, 1517, 1378, 1248, 1178 and 1028;
1H NMR (300 MHz) δ
H (DMSO-d
6) 3.45 (2 H, m, CH
2), 3.60 (1 H, t,
J 6.76, CH), 3.69 (3 H, s, OMe), 5.98 (1 H, s, NH), 6.75 (2 H, d,
J 8.7, Ar), 6.97 (2 H, d,
J 8.7, Ar), 7.17–7.31 (5 H, m, Ph) and 8.21 (1 H, s, OH);
13C NMR (75 MHz) δ
C (DMSO-d
6) 44.0 (CH
2), 53.5 (CH), 54.9 (Ome), 78.7 (C-OH), 112.9 (2CH), 126.4 (2CH), 126.7 (CH), 127.3 (2CH), 127.9 (C), 130.5 (2CH), 142.4 (C), 158.1 (C) and 176.1 (C=O);
m/
z (ES) 306.09 (M + Na
+, 100%).
3.3. Preparation and Reactions of Diels–Alder Adducts of (2R)-2-t-Butyl-5-methylene-1,3-dioxolan-4-one 9
3.3.1. Preparation of (2S,5R,1′R,2′R,4′R,5′R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-oxatricyclo[3.2.1.02,4]octane] 16
The alkene 10a (0.78 g, 3.5 mmol) was stirred in CH2Cl2 (25 cm3) for 5 min. Sodium carbonate (5 g) was then added followed by addition of peracetic acid (40%, 0.532 g, 7.0 mmol) in acetic acid. The reaction proceeded immediately with vigorous bubbling and the mixture was allowed to stir for 3 days. The mixture was filtered to remove the sodium acetate and the solvent was then removed to yield the product as colourless oil (0.67 g).1H NMR spectroscopic analysis indicated that the product had been formed in a 2:1 ratio with the starting material. The crude product was separated by flash column chromatography (silica gel, Et2O:hexane, 1:3) giving the product (0.22 g, 26%) as a colourless liquid. νmax/cm−1 1787, 1639, 1485, 1407, 1348, 1289, 1245, 1208 and 1175; 1H NMR (300 MHz) δH 0.95 (9 H, s, But), 1.39–1.42 (1 H, dd, J 3.7, 1.8, H2C-8′), 1.45–1.47 (1 H, m, H2C-8′), 1.53 (1 H, dd, J 13.4, 3.8, H2C-7′), 2.21 (1 H, dd, J 13.4, 4.1, H2C-7′), 2.60 (1 H, dd, J 3.7, 1.6, HC-1′), 2.83 (1H, q, J 1.3, HC-5′), 3.35 (1 H, half AB pattern of d, J 3.7, 1.0, HC-4′), 3.38 (1 H, half AB pattern of d, J 3.6, 1.1, HC-2′) and 5.17 (1 H, s, HC-But); 13C NMR (75 MHz) δC 23.2 (But), 24.0 (CH2), 34.4 (C-Me3), 36.2 (CH), 37.1 (CH2), 42.9 (CH), 48.2 (CH), 51.2 (CH), 85.1 (C), 108.0 (But-C) and 175.1 (C=O); m/z (ES) 261.20 (M + Na+, 3%), M+ 100%, 281.15; M+ 23%, 195.07.
3.3.2. Preparation of (2S,5R,1′R,2′R,4′R,5′R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-ethoxycarbonyl-3′-azatricyclo[3.2.1.02,4]octane] 17
A mixture of 10a (0.55 g, 2.4 mmol) and ethyl azidoformate (0.828 g, 7.2 mmol) was placed in a quartz tube and positioned 15 cm from a 400 W medium pressure mercury lamp. After 10 min irradiation the solution changed colour from colourless to pale yellow and a stream of N2 gas was released. The reaction was left on for approximately 8 h until the starting material was judged to be consumed (TLC). The crude product was purified by flash column chromatography (silica gel, dry loaded, Et2O:hexane, 1:1), to furnish the product (0.6 g, 81%) as pale yellow crystals mp 90–91 °C [α]D +61.76 (c = 0.102, CH2Cl2); (Found C, 62.0; H, 7.3; N, 4.2. C16H23NO5 requires C, 62.1; H, 7.5; N, 4.5%); νmax/cm−1 1789, 1720, 1406, 1378, 1298, 1278, 1265, 1228, 1187 and 1173; δH 0.95 (9 H, s, But), 1.28 (3 H, t, J 7.0, CH3), 1.45–1.60 (3 H, m, H2C-7′, H2C-8′), 2.17 (1 H, dd, J 12.9, 4.0, H2C-7′), 2.62–2.67 (1 H, m, HC-1′), 2.86–2.87 (1 H, m, HC-5′), 2.88 (1 H, m, HC-2′), 2.96–3.00 (1 H, m, HC-4′), 4.15 (2 H, q, J 7.0, CH2CH3) and 5.16 (1 H, s, CH-But); δC 14.3 (CH3 of OEt), 23.2 (But), 25.7 (C-8′), 34.4 (C-Me3), 35.0 (HC-2′), 35.4 (C-1′), 37.5 (C-7′), 38.7 (HC-4′), 42.1 (C-5′), 62.5 (CH2 in OEt), 84.6 (C-6′/C-5), 108.0 (CH-But), 162.1 (N-C=O) and 174.9 (C=O); HRMS (ES+): found 332.1474. C16H23NaNO5 (M + Na) requires 332.1474.
3.3.3. Preparation of Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,2′-bicyclo[2.2.2]oct-5′-ene] 18
A solution of 9 (1.51 g, 9.7 mmol) and 1,3-cyclohexadiene (0.77 g, 9.6 mmol) in toluene (15 cm3) was heated in a 50 cm3 autoclave at 150 °C for 84 h. Once the reaction mixture had cooled the contents of the flask were washed into an evaporating flask using CH2Cl2 and the solvent was then removed to yield a brown liquid (2.26 g). The 1H NMR spectrum showed that the product had formed but further purification was required. This was attempted by flash column chromatography (silica gel, Et2O/hexane, 1:4) to give the product (0.98 g, 43%) as a yellow oil with a strong sweet smell; νmax/cm−1 1789, 1407, 1367, 1342, 1289, 1252, 1178 and 1113; δH 0.92 (9 H, s, But), 1.11–1.21 (1 H, m), 1.24–1.31 (1H, m), 1.54–1.58 (1 H, m), 1.58–1.61 (1 H, m), 1.86–1.96 (1 H, m), 2.07 (1 H, dd, J 13.8, 2.2), 2.68–2.76 (1 H, m), 2.90–2.96 (1 H, m), 6.18–6.24 (1 H, m) and 6.4–6.46 (1 H, m); additional peaks present at 1.0, 1.23, 2.85–2.91 (m) and 5.18 indicated presence of other diastereomers; δC 19.0 (CH2), 23.3 (But), 23.5 (CH2), 29.8 (CH), 33.6 (CH), 34.6 (C-Me3), 39.2 (CH2), 82.1 (C), 107.4 (C-But), 130.7 (=CH), 135.2 (=CH), C=O not apparent. m/z (CI) 237.15, (M + H+, 100%); HRMS (CI): found 237.1494. C14H21NO3 (M + H) requires 237.1491.
3.3.4. Pyrolysis of (2S,5S,1′R,5′R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-oxatricyclo[3.2.1.02,4]octane] 16 to give 3-oxatricyclo[3.2.1.02,4]octan-6-one 19
FVP of
16 (0.207g, 0.86 mmol) was performed at 550 °C and 3.8 × 10
−2 Torr. Purification of the material in the cold trap using preparative TLC (SiO
2, Et
2O/hexane, 1:3) to give the product
19 (0.015 g, 14%) as a white solid at R
f ~0.25–0.4; [α]
D +310 (c = 0.22, CHCl
3), [lit. [
24], +321 (c = 2.1, CHCl
3) for 86% e.e.], e.e. 83%; δ
H 1.21–1.27 (1 H, br m, H
2C-8), 1.67–1.75 (1 H, m, H
2C-8), 1.92 (1 H, dd,
J 17.7, 4.7, H
2C-7), 2.11 (1 H, dd,
J 17.7, 3.9, H
2C-7), 2.80–2.83 (1 H, m, HC-1), 2.85–2.88 (1 H, m, HC-5), 3.27–3.30 (1 H, m, HC-2) and 3.42–3.46 (1 H, m, HC-4); δ
C 25.0 (CH
2), 35.1 (CH), 39.6 (CH
2), 46.9 (CH), 50.8 (CH–O), 51.8 (CH–O) and 211.8 (C=O).
3.3.5. Pyrolysis of (2S,5R,1′R,2′R,4′R,5′R)-Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,6′-3′-ethoxycarbonyl-3′-azatricyclo[3.2.1.02,4]octane 17 giving 20
FVP of 17 (0.1959 g, 0.6 mmol) was performed at 525 °C and 3.5 × 10−2 Torr. 1H NMR spectroscopic analysis indicated a 1:1 ratio of unchanged starting material and a new product. Purification was achieved using preparative TLC (SiO2, Et2O/hexane, 1:1). The product (Rf ~0.55–0.65) proved to be (2R,5R,1′R,2′R,4′R,5′R)-spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-ethoxycarbonyl-3′-azatricyclo[3.2.1.02,4]octane] 20 (0.1 g, 51%) obtained as a colourless solid, mp 122–123 °C; [α]D –18.86 (c = 0.082, CH2Cl2); νmax/cm−1 1789, 1720; δH 0.97 (9 H, s, But), 1.29 (3 H, t, J 7.1, CH3), 1.46–1.63 (2 H, m), 1.63–1.72 (1 H, m), 2.00 (1 H, dd, J 12.6, 4.2, H2C-7′), 2.61–2.68 (1 H, m), 2.76 (1 H, m), 2.82–2.89 (1 H, m), 3.10 (1 H, m), 4.16 (2 H, q, J 7.1, CH2-CH3) and 5.10 (1 H, s, CH-But); δC 14.3 (CH3 ofEt), 23.3 (But), 25.7 (CH2), 34.6 (C-Me3), 34.8 (CH), 35.8 (CH), 37.7 (CH2), 38.2 (CH), 44.7 (CH), 62.5 (CH2 of Et), 84.7 (C-5/C-6′), 108.7 (CH-But), 162.1 (N-C=O) and 174.9 (C=O); m/z (CI) 310.17 (M + H, 10%), 224.09 (100) and 196 (65); HRMS (CI): found 310.1653. C16H24NO5 (M + H) requires 310.1654.
3.3.6. Preparation of Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,2′-1′,4′,5′,6′-tetraphenyl-7′-oxobicyclo[2.2.1]hept-5′-ene] 21
A solution of
9 (3.50 g, 22.4 mmol) and tetraphenylcyclopentadienone (8.61 g, 22.4 mmol) in dry toluene (25 cm
3) was heated under reflux for 24 h. Insoluble material (0.69 g) was removed by filtration and evaporation; the filtrate gave a purple solid.
1H NMR spectroscopic analysis showed a large amount of the starting diene remained. Attempted purification by flash column chromatography (dry loaded; SiO
2, Et
2O/hexane, 1:2) gave a fraction from which pale purple crystals (0.53 g) precipitated, mp 156–158 °C, which were identified as the partially reduced starting material 2,3,4,5-tetraphenylcyclopent-2-enone (lit. [
25], 160–163 °C) which was the major product of the reaction; ν
max/cm
−1 1692, 1550 and 1463; δ
H 3.75 (1 H, d,
J 2.5, 4-H), 4.56 (1 H, d,
J 2.5, 5-H) and 6.89–7.34 (20 H, m, Ph); δ
C 57.6 (C-4) 63.0 (C-5), 127.0 (Ph), 127.1 (Ph), 127.5 (Ph), 127.7 (Ph), 128.1 (Ph), 128.2 (Ph), 128.4 (Ph), 128.9 (Ph), 129.0 (Ph), 129.4 (Ph), 129.8 (Ph), 131.7 (C1-Ph), 134.4 (C1-Ph), 139.3 (C1-Ph), 140.0 (C1-Ph), 141.4 (C-CO), 168.9 (C-3) and 205.9 (C=O);
m/
z (ES) 409.13 (M+Na
+, 100%); HRMS (ES): found 409.1586. C
29H
22NaO (M + Na) requires 409.1568.
The filtrate from these fractions was concentrated to yield a purple solid (0.45 g), which was recrystallised from toluene to yield the desired product (0.06 g, 5%) as purple crystals mp 178–180 °C; [α]D –292.5 (c =0.04, CH2Cl2); νmax/cm−1 1795, 1782, 1377, 1242 and 1139; δH 0.74 (9 H, s, But), 3.10 and 3.21 (2 H, AB pattern, J 12.8, CH2), 3.61 (1 H, s, CH-But), 6.66–6.72 (2 H, m, Ph), 6.86–6.97 (6 H, m, Ph), 7.06–7.16 (3 H, m, Ph), 7.16–7.31 (7 H, m, Ph) and 7.52–7.57 (2 H, m, Ph); δC 23.0 (But), 34.5 (CMe3), 42.4 (C-3′), 62.0 (C-4′), 87.0 (C-2′/C-5), 109.7 (C-But), 127.1 (Ph), 127.2 (Ph), 127.3 (Ph), 127.5 (Ph), 127.6 (Ph), 127.8 (Ph), 127.9 (Ph), 128.0 (Ph), 128.2 (Ph), 128.5 (Ph), 129.1 (Ph), 129.3 (Ph), 130.1 (Ph), 130.9 (Ph), 132.1 (Ph), 133.4 (Ph), 145.2 (C-ipso), 174.0 (C=O) and 197.5 (C-7′) [C-2′/C-5, C-4′ C-ipso, C=O and C-7′ were assigned by HMBC]; m/z (ES) 563.22 (M+ Na+, 10%) and 407.13 (M+–CO and dioxolanone, 100%); HRMS (ES): found 563.2197. C37H32NaO4 (M + Na) requires 563.2198.
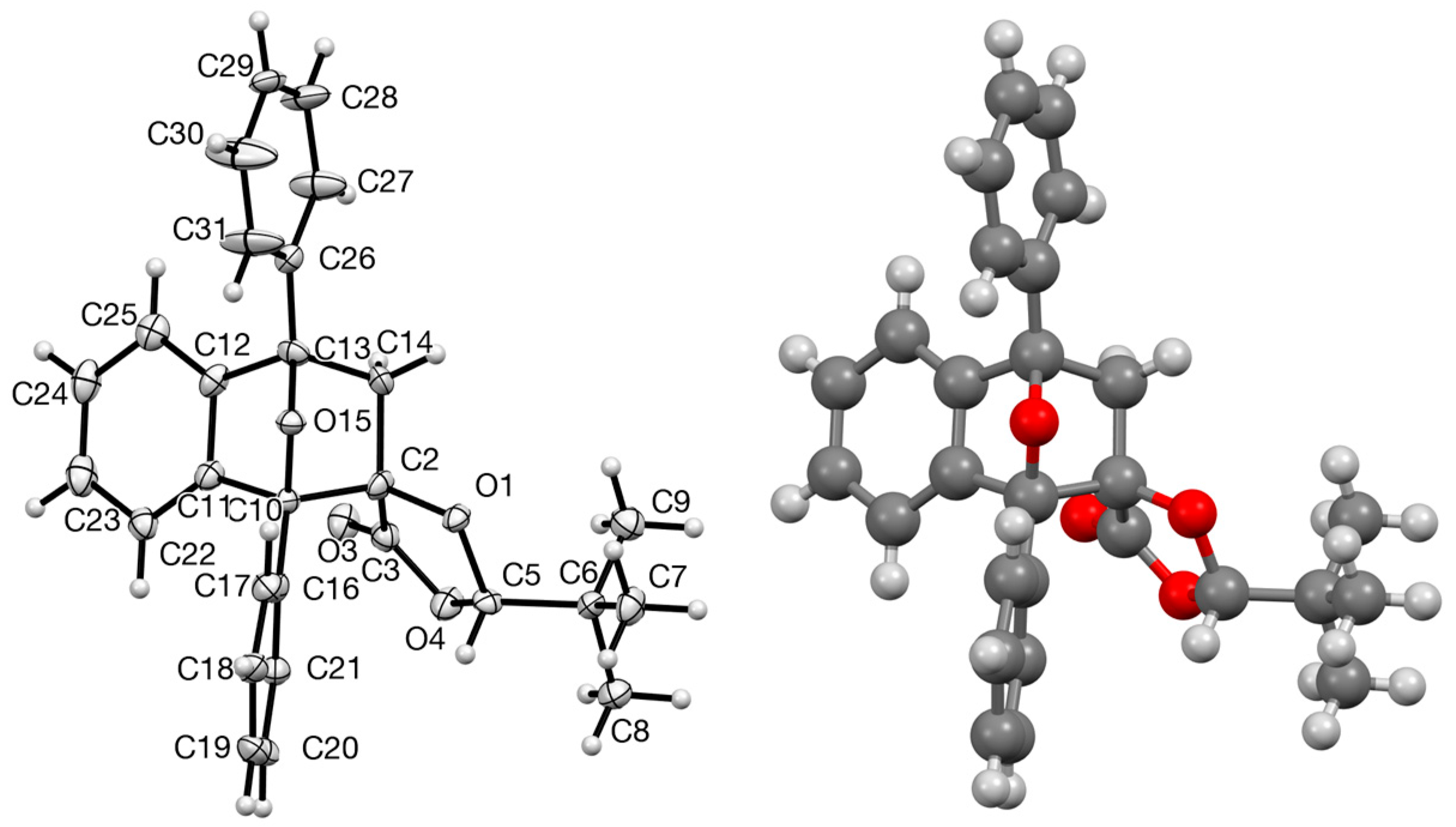
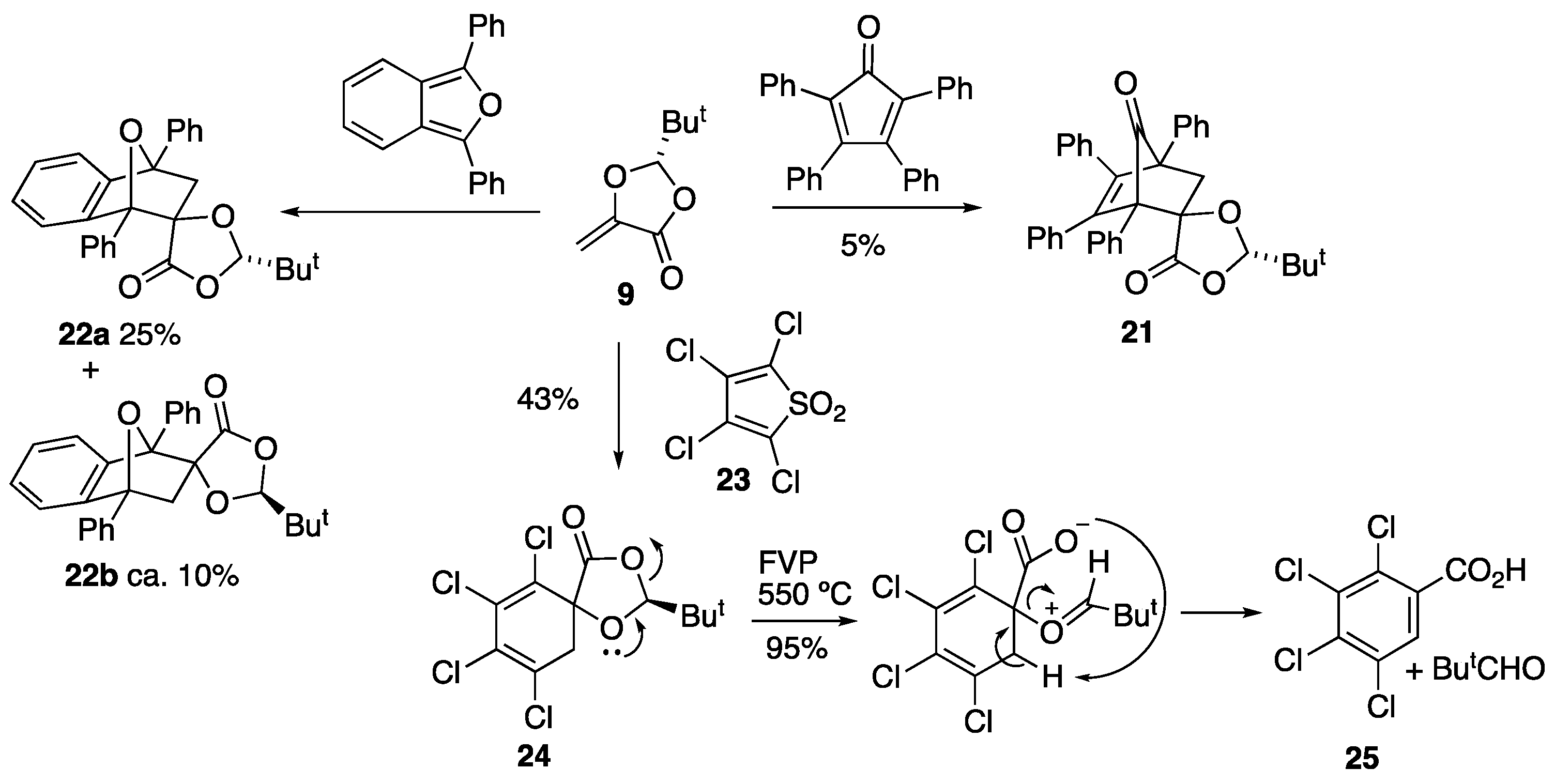
3.3.7. Preparation of Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 22a and 22b
A solution of 9 (3.11 g, 19.9 mmol) and 1,3-diphenylisobenzofuran (4.85 g, 17.9 mmol) in toluene (30 cm3) was heated under reflux for 36 h, then cooled and the solvent was removed to yield a bright yellow solid (7.86 g). 1H NMR spectroscopic analysis indicated that the product had formed; however, there was still a large amount of diene remaining. The material was subjected to flash column chromatography (dry loaded; SiO2, Et2O/hexane, 1:2), followed by preparative TLC (Et2O/hexane, 1:3) to give the major product, (2S,5R,1′R,8′S)-spiro[2-t-butyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 22a (1.58 g, 25%) as pale yellow crystals, mp 155–156 °C; [α]D –16.4 (c = 1, CH2Cl2); (Found: C, 79.45; H, 6.45. C28H26O4 requires C 78.9; H, 6.2%); νmax/cm−1 1795, 1345, 1311, 1285, 1243 and 1143; δH 0.76 (9 H, s, But), 2.77 and 2.86 (2 H, AB pattern, J 12.3, H2C-10′), 4.26 (1 H, s, CH-But), 6.90–6.96 (1 H, m, Ph), 7.14–7.29 (4 H, m, Ph), 7.42–7.55 (5 H, m, Ph), 7.60–7.67 (2 H, m, Ph) and 7.74–7.80 (2 H, m, Ph); δC 23.0 (Me3), 34.8 (C-Me3), 49.4 (CH2), 88.0 (C-1′ or C-8′), 88.2 (C-1′ or C-8′), 93.8 (C-9′/C-5), 109.4 (CH-But), 118.7 (CH), 122.2 (CH), 125.5 (2CH), 126.3 (2CH), 126.7 (CH), 128.0 (CH), 128.3 (2CH), 128.5 (2CH), 128.6 (2CH), 134.7 (C), 137.2 (C), 141.3 (C), 149.3 (C) and 171.9 (C=O).
The minor diastereomer, (2S,5R,1′R,8′R)-spiro[2-t-butyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 22b (probable stereochemistry shown), was obtained in a mixed fraction with the major diastereomer. It could not be isolated in pure form but the following data was obtained: δH 0.84 (9 H, s, But), 2.37 (1 H, d, J 11.9, HC-10′), 3.14 (1 H, d, J 11.9, HC-10′), 5.39 (1 H, s, CH-But), 7.00 (1 H, m, Ph), 7.20–7.26 (4 H, m, Ph), 7.40–7.55 (5 H, m, Ph), 7.59–7.65 (2 H, m, Ph) and 7.72–7.80 (2 H, m, Ph); δC 23.2 (Me3), 35.8 (C-Me3), 49.1 (CH2), 87.0 (Ph-C-O (x2)), 91.0 (C-C=O), 109.6 (C-But), 119.1 (CH), 122.3 (CH), 125.2 (2CH), 126.4 (2CH), 126.8 (CH), 128.3 (CH), 128.4 (2CH), 128.5 (2CH), 128.6 (2CH), 134.6 (C), 137.4 (C), 141.3 (C), 149.1 (C) and 172.5 (C=O).
Apart from some recovered diene starting material (0.84 g), a large amount of the oxidised starting diene, 1,2-dibenzoylbenzene (2.39 g, 55%) was isolated, mp 142–146 °C (lit. [
26], 146–148 °C); δ
H 7.26 (2 H, s), 7.38 (4 H, m), 7.52 (2 H, m), 7.62 (2 H, s) and 7.70 (4 H, m); δ
C 128.3 (4CH), 129.6 (2CH), 129.8 (4CH), 130.3 (2CH), 133.0 (2CH), 137.1 (2C), 140.0 (2C) and 196.6 (2C=O) (good agreement with lit. [
27]);
m/
z (ES) 309.04 (M + Na
+, 100%).
3.3.8. Preparation of Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,5′-1′,2′,3′,4′-tetrachlorocyclohexa-1′,3′-diene] 24
A solution of 9 (1.53 g, 0.9 mmol) and tetrachlorothiophene 1,1-dioxide 23 (2.49 g, 9 mmol) in CH2Cl2 (25 cm3) was stirred for 11 days. The solvent was then removed and the residual yellow solid recrystallised from CH2Cl2/hexane. The pure product (1.33 g, 43%) was isolated as pale yellow crystals, mp 86–87 °C; [α]D +8.6 (c = 0.5, CH2Cl2); (Found: C, 41.9; H, 3.4, C12H24Cl4O3 requires C 41.7; H, 3.5%); νmax/cm−1 1797, 1611, 1481, 1466, 1260, 1205, 1113, 1110 and 1058; δH 0.98 (9 H, s, But), 2.93 (1 H, d, J 18.8, CH2), 3.58 (1 H, d, J 18.8, CH2) and 5.48 (1 H, s, CH-But); δC 23.2 (But), 34.6 (C-Me3), 42.3 (CH2), 110.8 (CH-But), 127.4 (2 × C-Cl), 131.2 (2 × C-Cl) and 177.6 (C=O); m/z (CI+) 345.95 (M+, 12.5%) and 260.89 (M+–CO–C4H10, 100).
3.3.9. Pyrolysis of Spiro[2-t-Butyl-1,3-dioxolan-4-one-5,5′-1′,2′,3′,4′-tetrachlorocyclohexa-1′,3′-diene] 24
FVP of the reactant 24 (99.7 mg, 0.28 mmol) was performed at 550 °C and 2.3 × 10−2 Torr. The cold trap contained pivalaldehyde and no trace of the expected product tetrachlorophenol was seen. The solid brown/orange product obtained at the furnace exit was 2,3,4,5-tetrachlorobenzoic acid 25 in a quantitative yield (determined by dissolution in acetone and solvent removal). The melting point was unobtainable due to decomposition; νmax/cm−1 1797; δH (CD3SOCD3) 8.09 (s, CH); δC (CD3SOCD3) 129.2 (CH), 130.0 (C), 131.7 (C), 132.6 (C), 133.2 (C), 134.0 (C) and 164.8 (C=O).
3.4. Preparation and Reactions of Diels–Alder Adducts of 5-Methylene-2,2-dimethyl-1,3-dioxolan-4-one 26
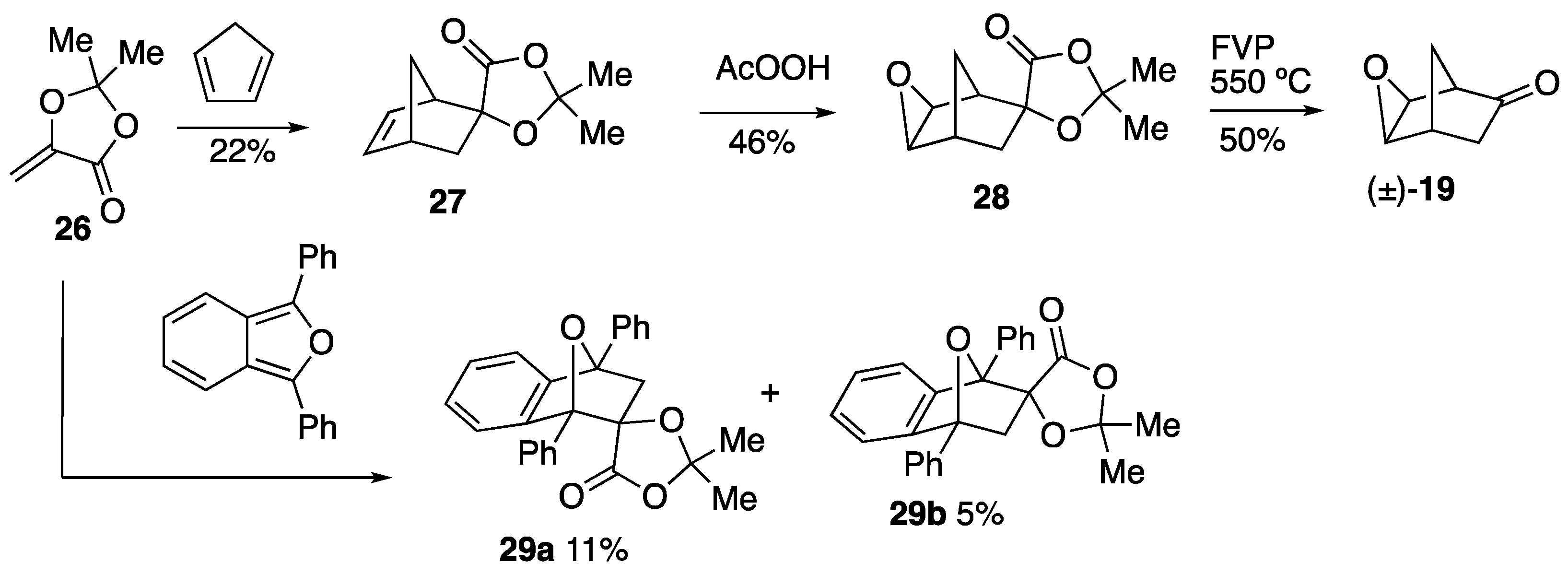
3.4.1. Preparation of Spiro[2,2-Dimethyl-1,3-dioxolan-4-one-5,2′-bicyclo[2.2.1]hept-5′-ene] 27
A solution of 26 (2.47 g, 19 mmol) and freshly distilled cyclopentadiene (1.85 g, 29 mmol) in toluene (75 cm3) was heated under reflux for 18 h. The solvent was removed and the crude product was purified by flash column chromatography (SiO2, dry loaded, Et2O/hexane, 1:4). The product was obtained as a yellow liquid (0.81 g, 22%) which contained some impurities of both starting materials which were probably produced by a retro-Diels–Alder reaction on the column. νmax/cm−1 1844, 1785, 1671, 1281, 1174, 1134, 1034, 1080 and 1007; δH 1.37 (1 H, dd, J 12.3, 4.0, H2C-7′), 1.46–1.52 (1 H, m, H2C-7′), 1.51 (3 H, q, J 0.6, CH3), 1.59 (3 H, q, J 0.6, CH3), 2.04–2.08 (1 H, m, H2C-3′), 2.32 (1 H, dd, J 12.3, 3.6, H2C-3′), 2.95–3.00 (1 H, m, HC-1′ or HC-4′), 3.05–3.09 (1 H, m, HC-1′ or HC-4′), 6.17 (1 H, dd, J 5.6, 3.0, =CH) and 6.48 (1 H, dd, J 5.6, 3.1, =CH); δC 27.5 (Me) 27.7 (Me), 42.0 (CH), 42.3 (CH2), 47.3 (CH2), 52.4 (CH), 84.9 (C-5/C-2′), 108.9 (C-2), 133.0 (=CH), 140.3 (=CH) and 176.6 (C=O). m/z (ES) 217.03 (M + Na+, 100%).
3.4.2. Preparation of Spiro[2,2-Dimethyl-1,3-dioxolan-4-one-5,6′-3′-oxatricyclo[3.2.1.02,4]octane] 28
The alkene 27 (0.80 g, 4.0 mmol) was stirred in CH2Cl2 (20 cm3) for 5 min. Sodium carbonate (5 g) was then added, followed by the addition of peracetic acid (40% in acetic acid, 0.60 g, 8.0 mmol). The reaction proceeded immediately with vigorous bubbling. The mixture was allowed to stir for 6 days and then filtered to remove the sodium salts. The filtrate was evaporated to yield the product as a colourless oil (0.86 g). 1H NMR spectroscopic analysis indicated that the product had been formed; however, some starting material and other impurities remained. The crude product was purified by Kugelrohr distillation to furnish the product as a brown oil (0.40 g) in which a small amount of impurities remained including a minor diastereomer (indicated by 13C NMR); δH 1.33–1.40 (1 H, m, H2C-8′), 1.48–1.55 (1 H, m, H2C-8′ or H2C-7′), 1.54 (3 H, s, Me), 1.61 (3 H, s, Me), 1.57–1.60 (1 H, m, H2C-8′ or H2C-7′), 2.19 (1 H, dd, J 13.2, 4.1, H2C-7′), 2.56–2.60 (1 H, m, HC-1′ or HC-5′), 2.72–2.74 (1 H, m, HC-1′ or HC-5′), 3.30 and 3.44 (2 H, AB pattern of d, J 13.6, 3.6, HC-2′ and HC-4′); δC 23.9 (C-8′ or C-7′), 27.3 (Me), 27.5 (Me), 36.3 (C-1′ or C-5′), 39.3 (C-8′ or C-7′), 46.4 (C-1′ or C-5′), 47.9 (C-2′ or C-4′), 50.9 (C-2′ or C-4′), 84.3 (C-5/C-6′), 109.4 (C-2) and 174.9 (C=O); m/z (ES) 233.06 (M + Na+, 100%); HRMS (CI): found 211.0968. C11H15O4 (M + H) requires 211.0970.
3.4.3. Preparation of Spiro[2,2-Dimethyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 29a and 29b
A solution of 26 (1.02 g, 7.9 mmol) and 1,3-diphenylisobenzofuran (2.14 g, 7.9 mmol) in toluene (25 cm3) was heated under reflux for 48 h. The reaction mixture was cooled and the solvent removed to yield an orange liquid. The crude mixture was purified by flash column chromatography (SiO2, dry loaded, Et2O/hexane, 1:2) to give the major product 29a as pale green crystals (0.13 g, 4%) mp 189–190 °C. A further quantity of this product (0.20 g, 7%) was isolated in further recrystallisations. Later fractions contained the minor product 29b which was recrystallised to give off-white crystals (0.15 g, 5%) mp 180–181 °C. An amount of mixed product (0.73 g) remained that could potentially be recrystallised to yield further product.
Major product; (5R,1′R,8′S)-spiro[2,2-dimethyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 29a (Found: C, 77.9; H, 5.4. C26H22O4 requires C 78.4; H, 5.6%); νmax/cm−1 1783, 1460, 1314, 1294, 1252, 1127, 1008 and 984; δH 0.98 (3 H, s, Me), 1.46 (3 H, s, Me), 2.79 and 2.91 (2 H, AB pattern, J 11.6, H2C-10′), 6.92–6.99 (1 H, m), 7.21–7.27 (2 H, m), 7.38–7.55 (7 H, m) and 7.62–7.69 (4 H, m); δC 25.7 (Me), 28.3 (Me), 51.0 (C-10′), 87.2 (C-1′ or C-8′), 88.7 (C-1′ or C-8′), 94.1 (C-5/C-9′), 109.9 (C-2), 118.3 (CH), 122.9 (CH), 125.6 (2CH), 126.2 (2CH), 126.6 (CH), 128.0 (CH), 128.1 (CH), 128.2 (CH), 128.5 (4CH), 135.0 (C), 137.2 (C) 141.2 (C), 149.2 (C) and 171.8 (C=O); m/z (ES) 421.20 (M + Na+, 100%).
Minor product; (5R,1′S,8′R)-spiro[2,2-dimethyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 29b: νmax/cm−1 1787, 1661, 1377, 1276 and 938; δH 1.59 (3 H, s, CH3), 1.69 (3 H, s, CH3), 2.41 (1 H, d, J 11.7, H2C-10′), 3.13 (1 H, d, J 11.7, H2C-10′) and 7.00–7.86 (14 H, m, Ph); δC 26.5 (CH3), 27.6 (CH3), 49.3 (C-10′), 86.5 (C-1′ or C-8′), 88.5 (C-1′ or C-8′), 89.8 (C-5/C-9′), 109.5 (C-2), 119.0 (CH), 121.7(CH), 125.3 (2CH), 126.2 (2CH), 127.0 (CH), 127.7 (CH), 128.3 (4CH), 128.5 (2CH), 134.8 (C), 137.1 (C), 143.0 (C), 149.0 (C) and 172.4 (C=O); m/z (CI) 421.20 (M + Na+, 100%).
3.4.4. FVP of 28 to Give 3-Oxatricyclo[3.2.1.02,4]octan-6-one, 19
FVP of the reactant 28 (0.1412 g, 0.67 mmol) was performed at 550 °C and 2.4 × 10−2 Torr. Purification using preparative TLC (SiO2, Et2O/hexane, 1:3) gave a brown oil which proved to be a 1:1 mixture of the starting material and 19; δH 1.21–1.27 (1 H, br m, H2C-8), 1.63–1.74 (1 H, m, H2C-8), 1.92 (1 H, dd, J 17.7, 4.7, H2C-7), 2.11 (1 H, dd, J 17.7, 3.9, H2C-7), 2.79–2.84 (1 H, m, HC-1), 2.85–2.88 (1 H, m, HC-5), 3.27–3.30 (1 H, m, HC-2) and 3.42–3.46 (1 H, m, HC-4).
3.5. X-ray Structure Determination of Adducts
Data were collected on a Bruker SMART diffractometer using graphite monochromated Mo Kα radiation
λ = 0.71073 Å. The data were deposited at the Cambridge Crystallographic Data Centre and can be obtained free of charge via
http://www.ccdc.cam.ac.uk/getstructures (accessed on 28 March 2023). The structure was solved by direct methods and refined by full-matrix least-squares against F
2 (SHELXL, Version 2018/3 [
32]).
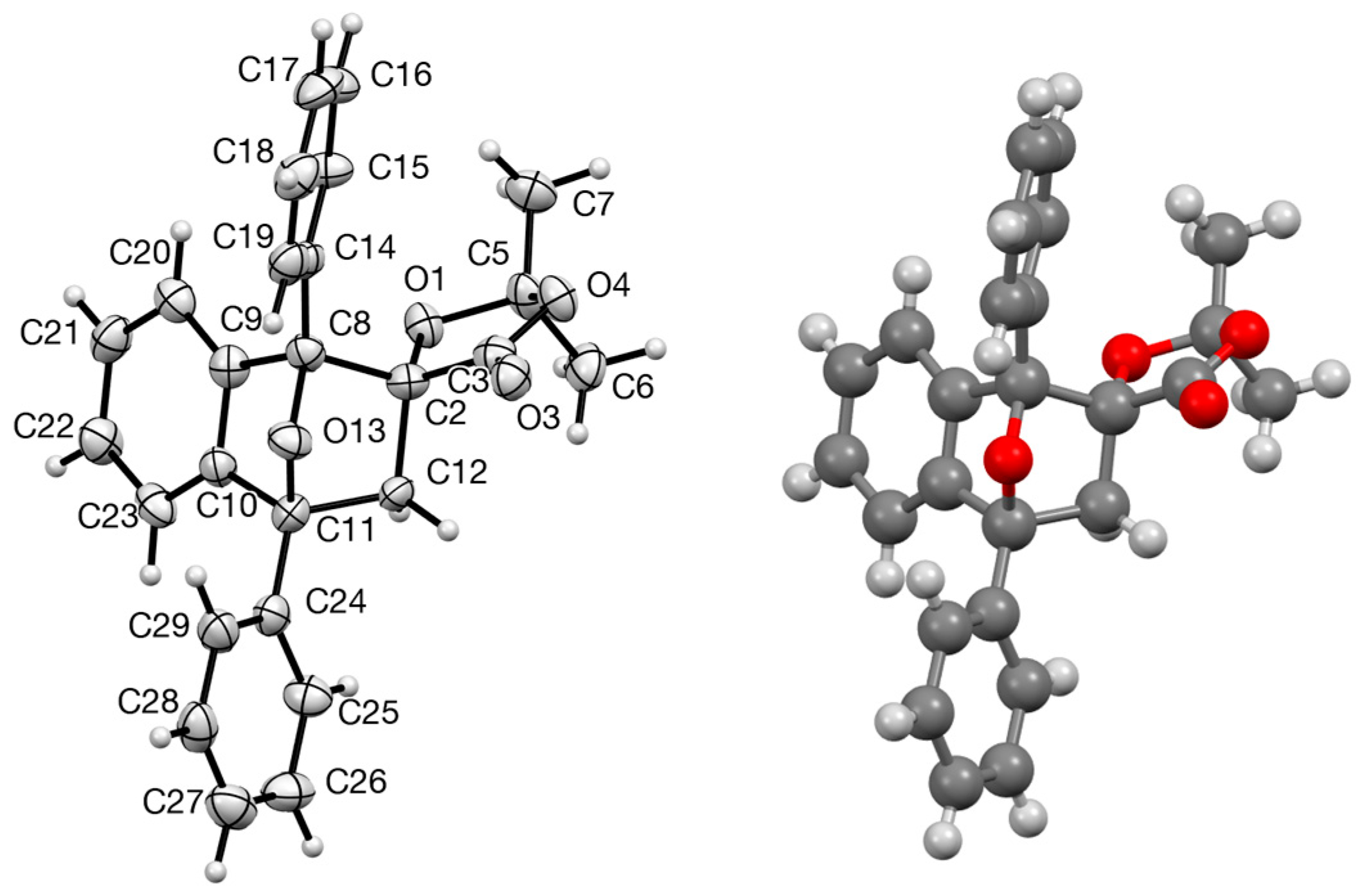
3.5.1. (2S,5S,1′S)-2-t-butyl-5-(1′-(4-methoxyphenyl)-2′-nitroethyl)-5-phenyl-1,3-dioxolan-4-one 6b
Crystal data for C23.75H25NO6, M = 420.46, colourless prism, crystal dimensions 0.20 × 0.15 × 0.05 mm, orthorhombic, space group P21212 (No. 18), a = 18.4427(17), b = 19.4772(17), c = 6.5275(6) Å, V = 2344.8(4) Å3, Z = 4, Dc = 1.191 g cm−3, T = 93(2) K, R1 = 0.0695, Rw2 = 0.1475 for 3851 reflections with I > 2σ(I) and 299 variables. CCDC 2240754.
3.5.2. (2S,5S,4′S)-2-t-butyl-5-(2-oxo-tetrahydrofuran-4-yl)-5-phenyl-1,3-dioxolan-4-one 14
Crystal data for C17H20O5, M = 304.33, colourless prism, crystal dimensions 0.12 × 0.06 × 0.02 mm, monoclinic, space group P21 (No. 4), a = 10.375(2), b = 6.2534(14), c = 11.802(3) Å, β = 91.135(9)°, V = 765.6(3) Å3, Z = 2, Dc = 1.320 g cm−3, T = 93(2) K, R1 = 0.0328, Rw2 = 0.0800 for 2396 reflections with I > 2σ(I) and 200 variables. CCDC 2240564.
3.5.3. (2S,5R,1′R,2′R,4′R,5′R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-ethoxycarbonyl-3′-azatricyclo[3.2.1.02,4]octane 17
Crystal data for C16H23NO5, M = 309.35, colourless platelet, crystal dimensions 0.15 × 0.10 × 0.02 mm, orthorhombic, space group P212121 (No. 19), a = 8.0456(18), b = 11.070(2), c = 17.625(4) Å, V = 1569.7(6) Å3, Z = 4, Dc = 1.309 g cm−3, T = 93(2) K, R1 = 0.0362, Rw2 = 0.0912 for 1534 reflections with I > 2σ(I) and 201 variables. CCDC 2240565.
3.5.4. (2R,5R,1′R,2′R,4′R,5′R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,6′-3′-ethoxycarbonyl-3′-azatricyclo[3.2.1.02,4]octane 20
Crystal data for C16H23NO5, M = 309.35, colourless prism, crystal dimensions 0.30 × 0.30 × 0.15 mm, orthorhombic, space group P212121 (No. 19), a = 6.2494(3), b = 14.2020(7), c = 18.2439(8) Å, V = 1619.22(13) Å3, Z = 4, Dc = 1.269 g cm−3, T = 125(2) K, R1 = 0.0530, Rw2 = 0.1270 for 1629 reflections with I > 2σ (I) and 200 variables. CCDC 2240563.
3.5.5. (2S,5R,1′R,8′S)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 22a
Crystal data for C30H31O4.50 (C28H26O4 • 0.5 Et2O), M = 463.55, colourless prism, crystal dimensions 0.15 × 0.15 × 0.15 mm, monoclinic, space group C2 (No. 5), a = 16.893(5), b = 8.986(4), c = 16.665(6) Å, β = 93.015(9)°, V = 2526.2(16) Å3, Z = 4, Dc = 1.219 g cm−3, T = 93(2) K, R1 = 0.0709, Rw2 = 0.1840, for 2141 reflections with I > 2σ (I) and 332 variables. CCDC 2240562.
3.5.6. (5R,1′S,8′R)-Spiro[2,2-dimethyl-1,3-dioxolan-4-one-5,9′-1′,8′-diphenyl-11′-oxatricyclo[6.2.1.02,7]undeca-2′,4′,6′-triene] 29b
Crystal data for C26H22O4, M = 398.44, colourless prism, crystal dimensions 0.25 × 0.20 × 0.03 mm, monoclinic, space group P21/c (No. 14), a = 10.850(5), b = 10.546(4), c = 17.931(8) Å, β = 102.202(11)°, V = 2005.5(15) Å3, Z = 4, Dc = 1.320 g cm−3, T = 93(2) K, R1 = 0.1799, Rw2 = 0.5039 for 2788 reflections with I > 2σ (I) and 273 variables. CCDC 2240561.