Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials
Abstract
1. Introduction
2. Fluorescent Polymer Functionalization Strategy
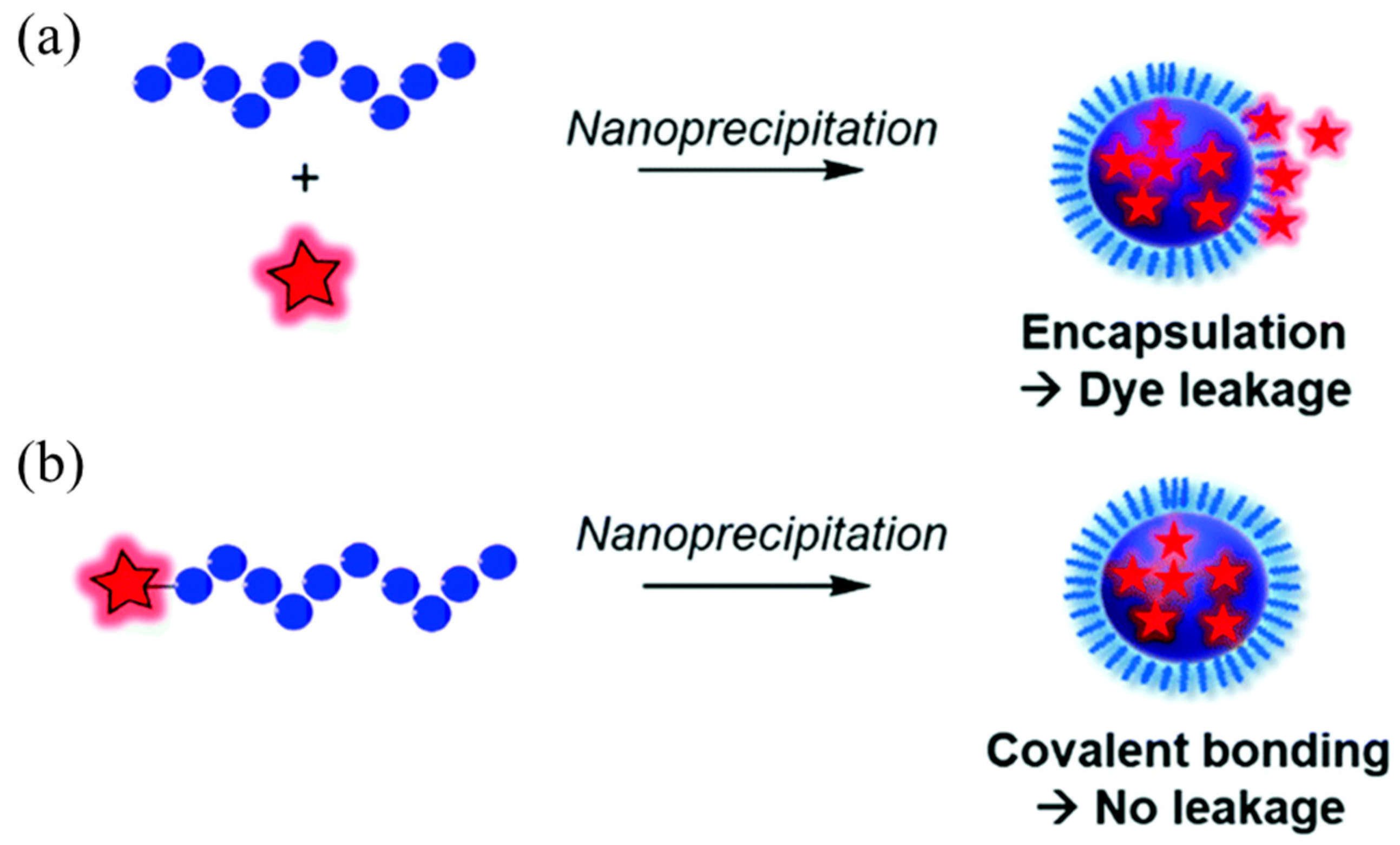
2.1. Physical Package
2.2. Covalent Linkage
3. Preparation Method of Fluorescent Polymer Nanomaterials
3.1. Active/Controllable Synthesis of Amphiphilic Block Polymers
3.1.1. ATRP
3.1.2. RAFT
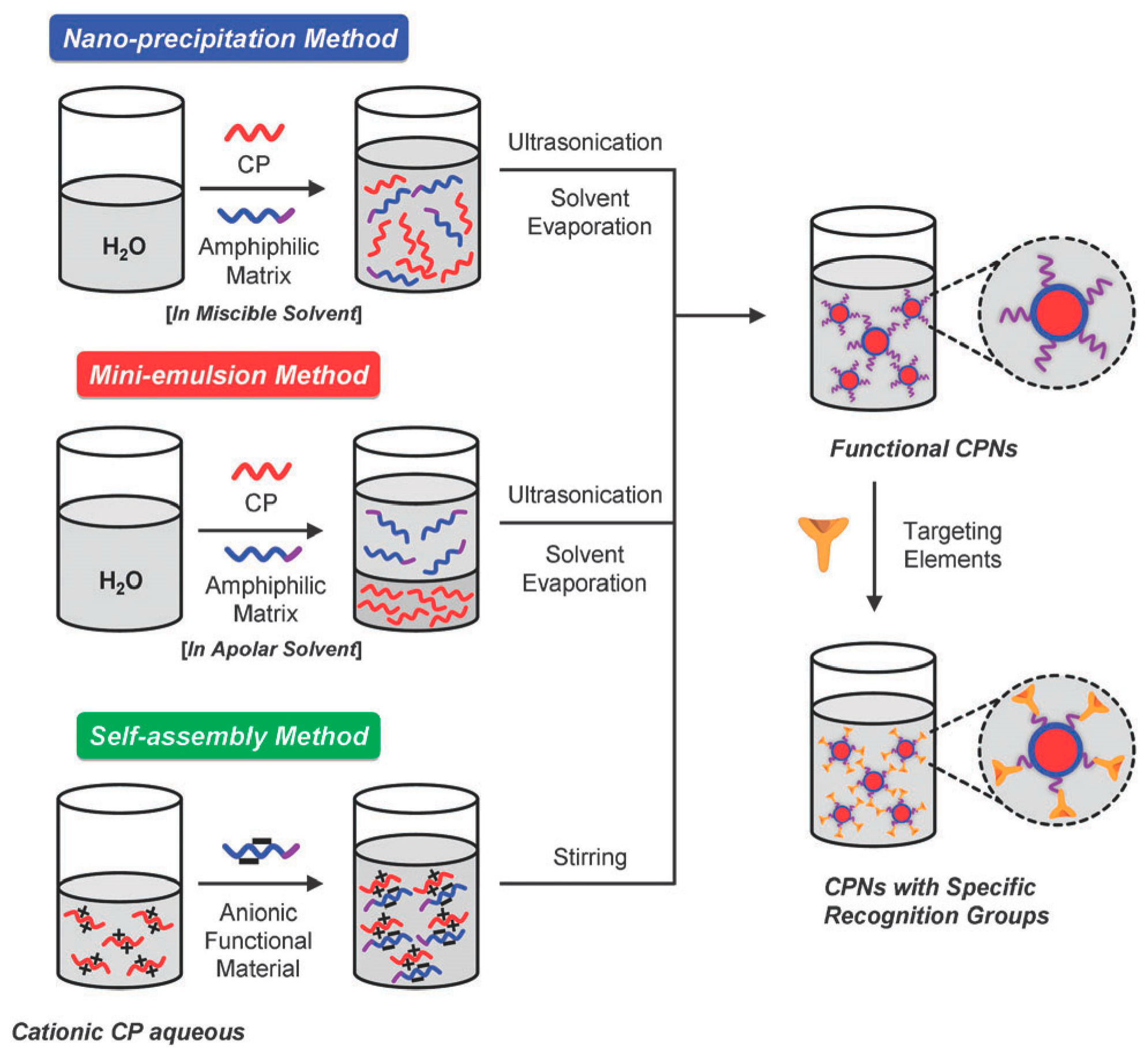
3.2. Preparation Method of Physically Encapsulating Fluorescent Polymer Nanoparticles
3.3. Preparation Method of Covalently Linked Fluorescent Polymer Nanoparticles
3.3.1. Preparation of Polymer Micelles Using Aggregation-Induced Self-Assembly (PISA)
3.3.2. Preparation of Nanogel Polymer Microspheres Using Precipitation Polymerization
4. Biotoxicity of Fluorescent Polymer Nanomaterials
5. Application of Fluorescent Polymer Nanomaterials in Bioimaging
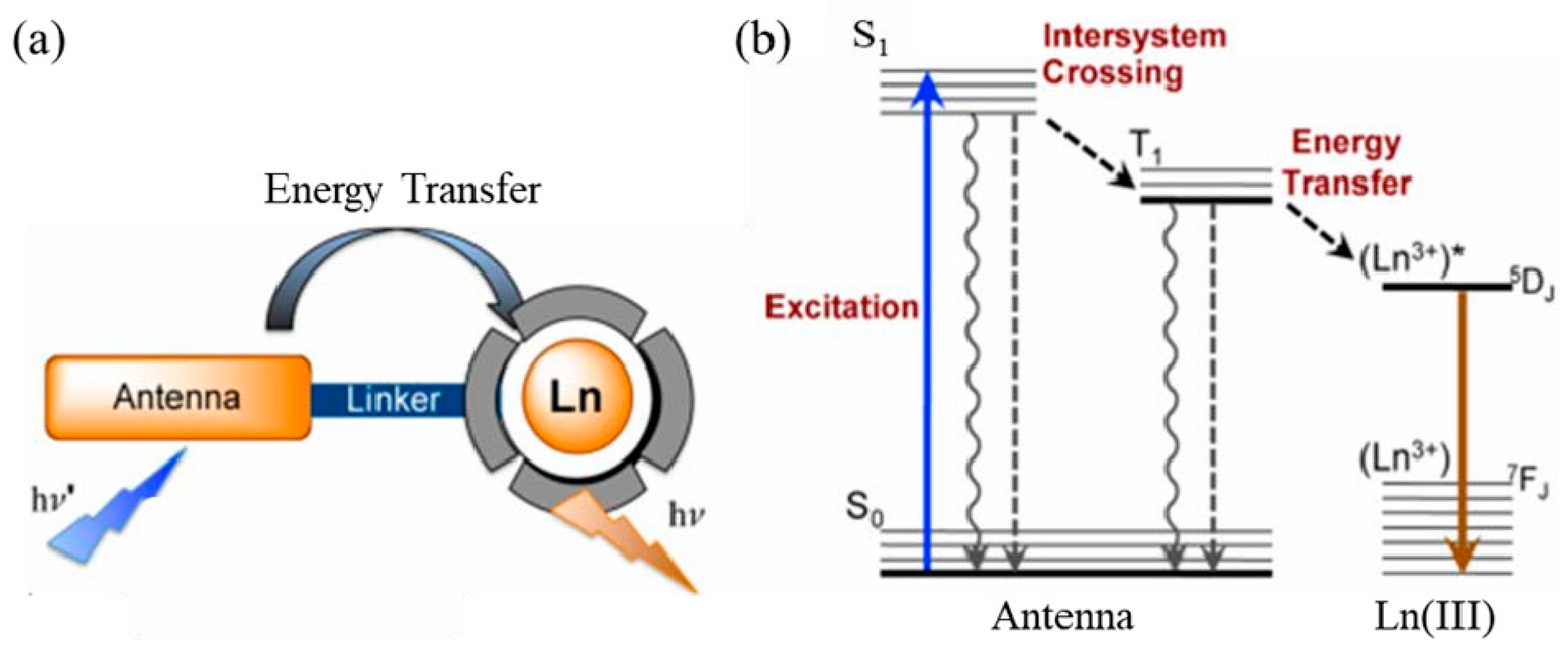
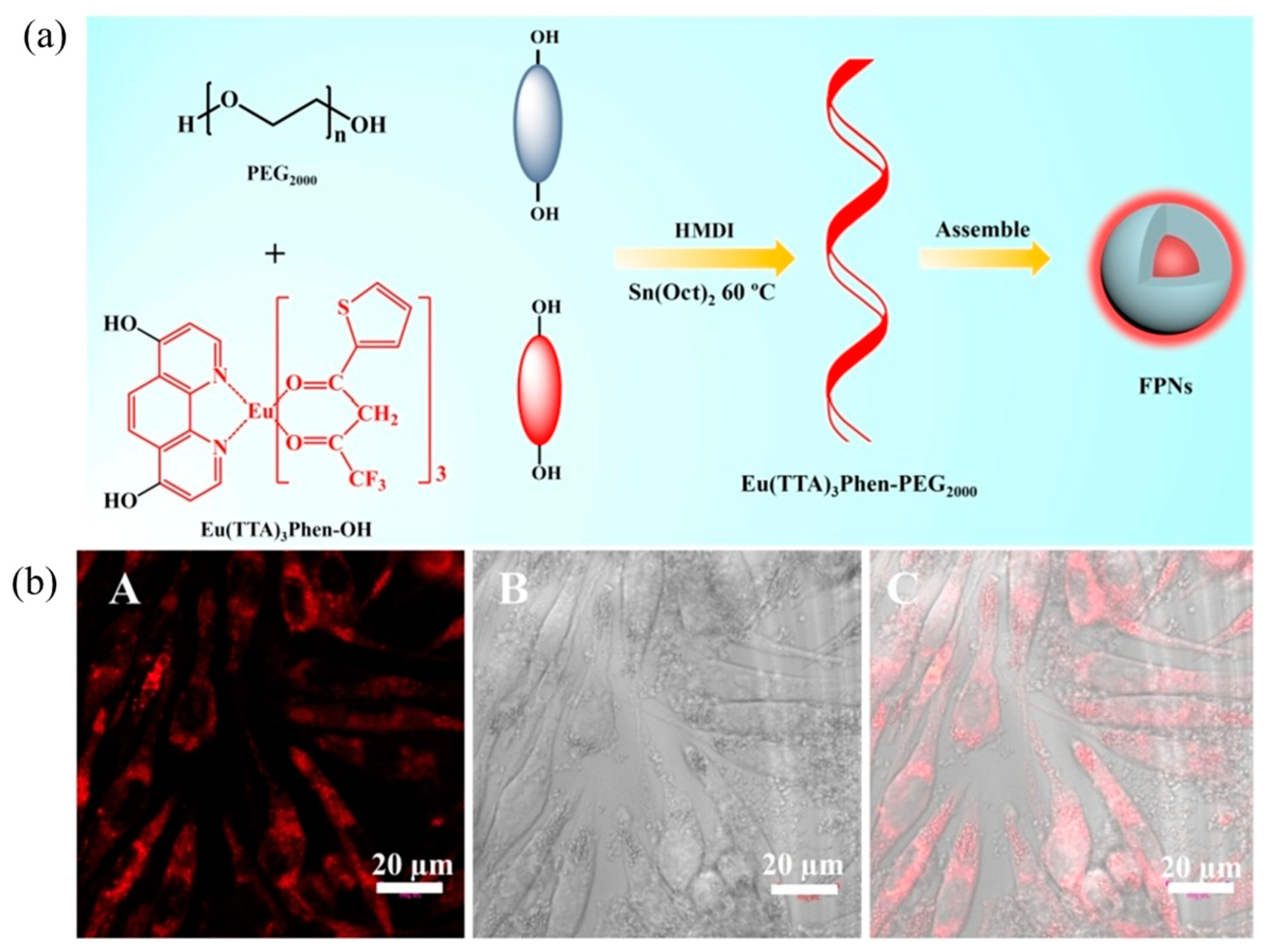
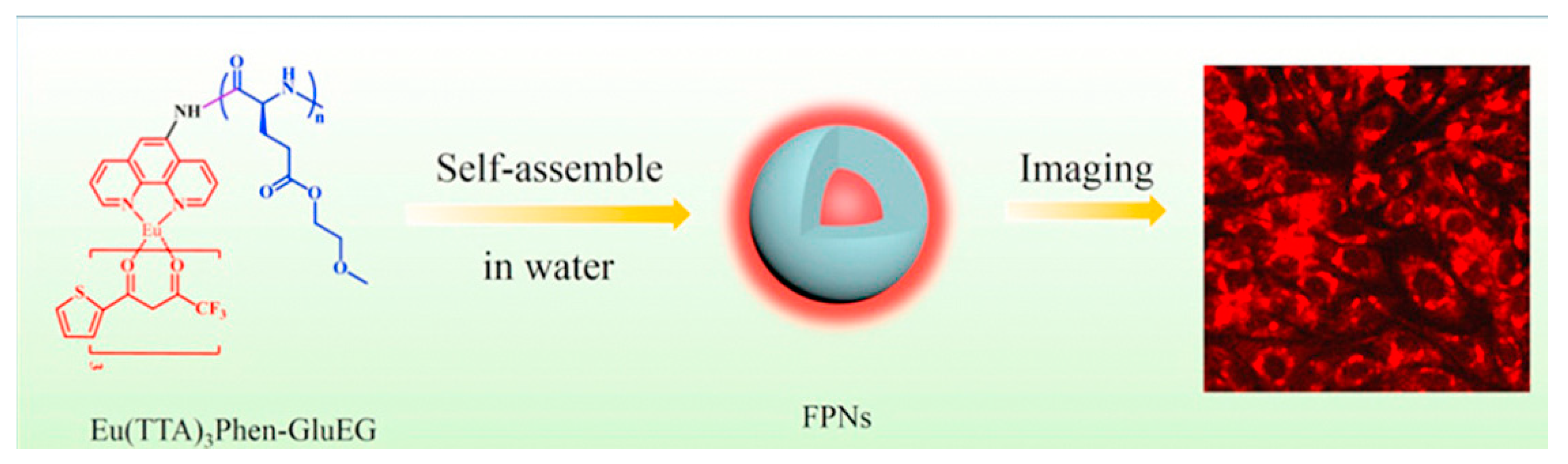
5.1. Polymer Nanomaterials Based on Rare Earth Luminescent Materials
5.1.1. Rare Earth Organic Complexes
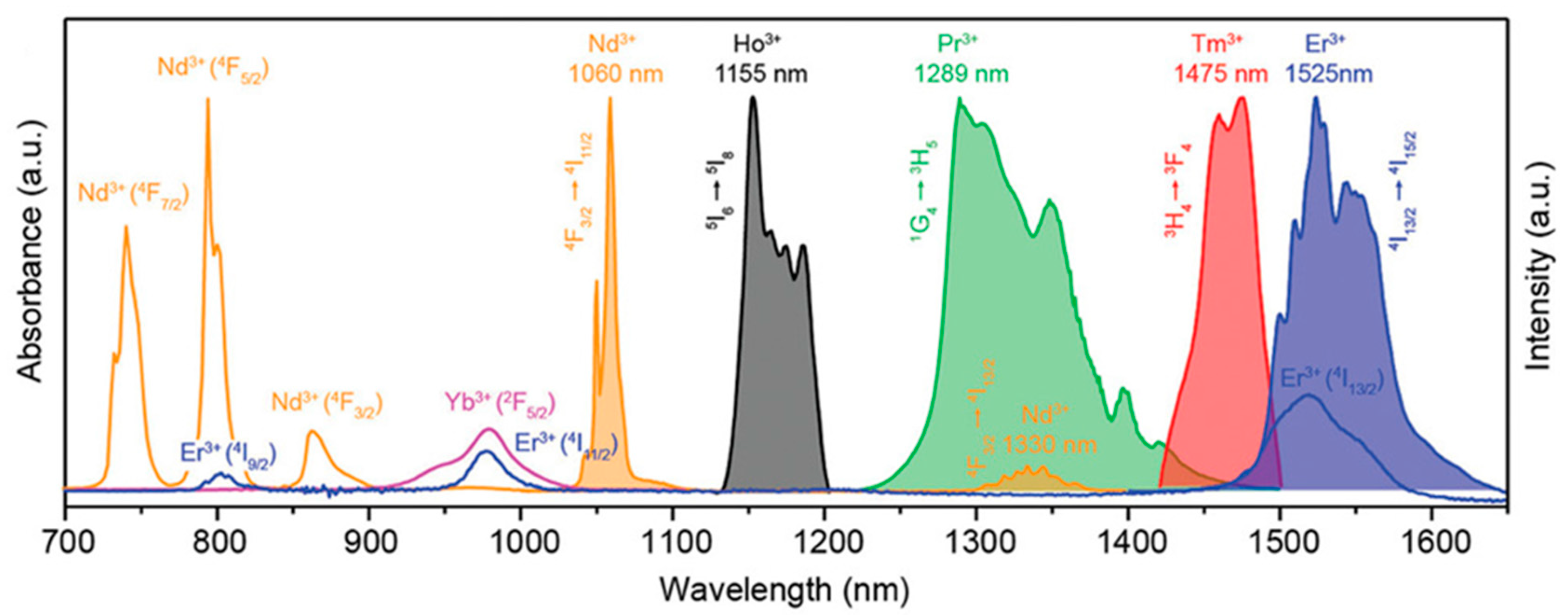
5.1.2. Rare Earth-Doped Upconversion Luminescent Materials
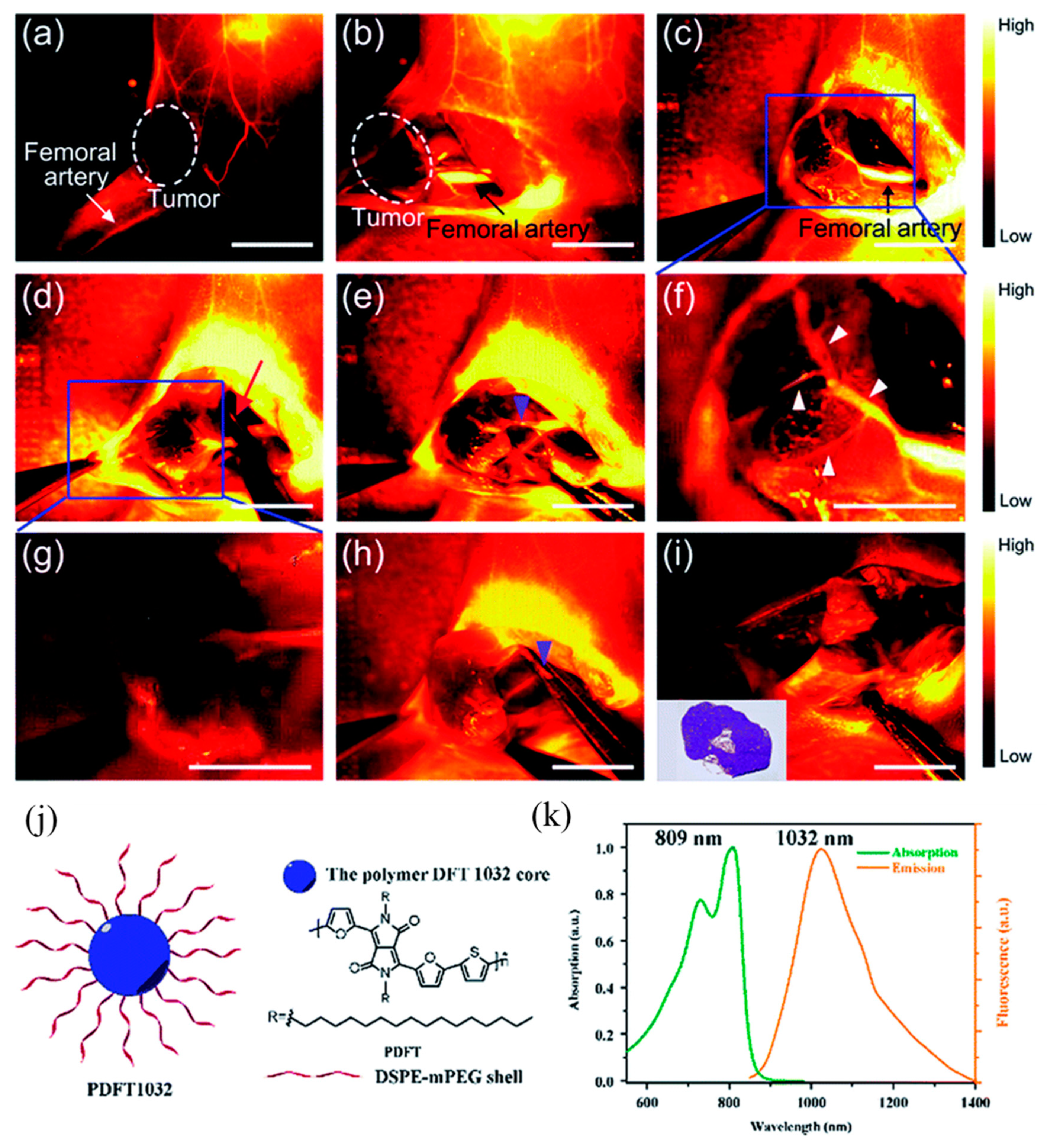
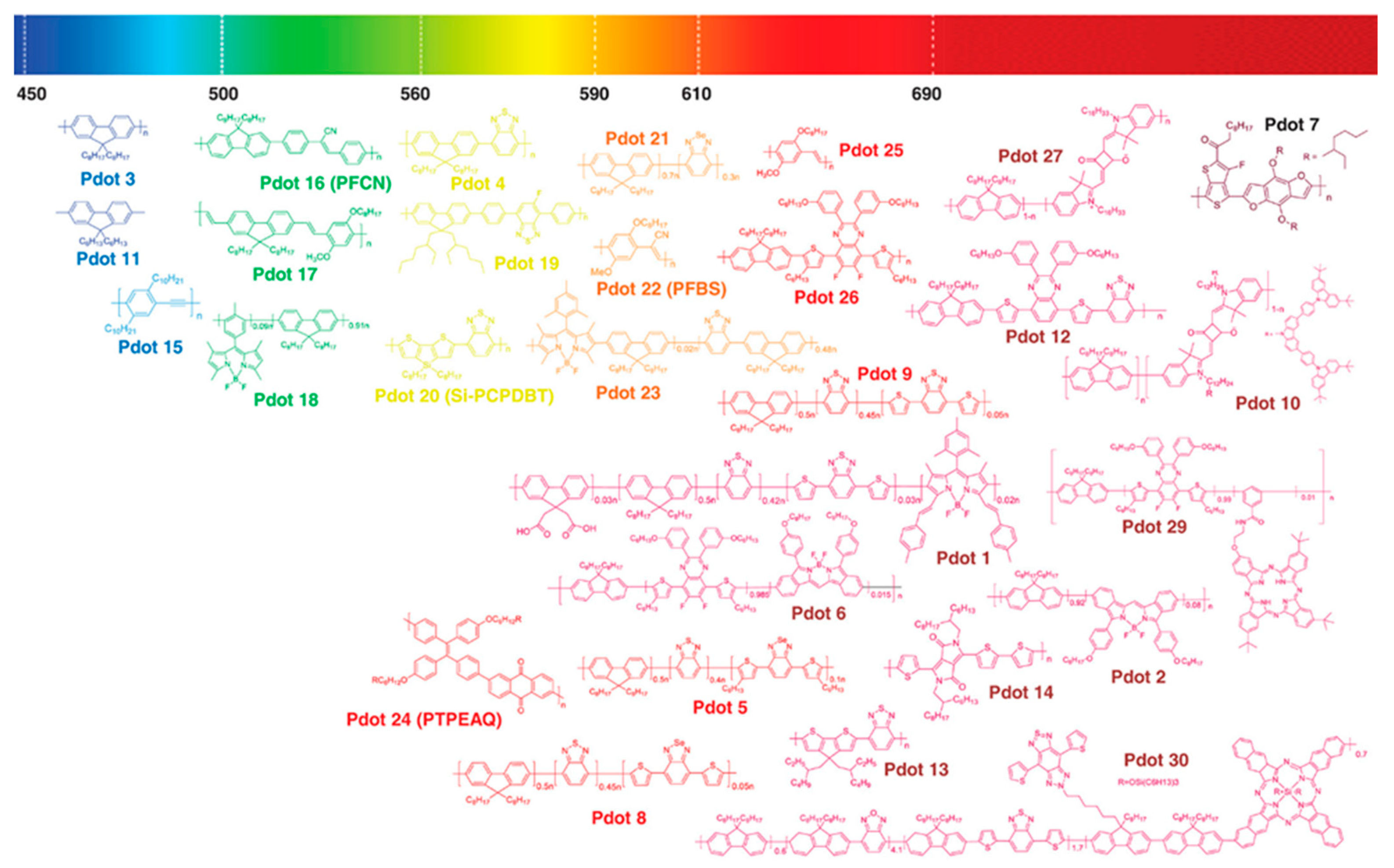
5.2. Polymer Nanomaterials Based on Semiconducting Polymers
5.3. Polymer Nanomaterials Based on Organic Fluorescent Small Molecules
6. Summary and Outlook
- (a)
- Fluorescent materials should be combined with current scientific theories and technologies. With advancements in science and technology, optical materials, as a traditional research field, can be further developed in the direction of diversification, high technology, and high performance when combined with advanced theory and technology. Thus, new subject areas are created [154,155].
- (b)
- Quantification of the structure–property relationship of multifunctional fluorescent polymer nanomaterials: Just as researchers have studied the “wetting” and “dewetting” of polymer-grafted core–shell particles in the matrix [156,157], essentially, the quantification of the effect of this structure on light transparency has developed from simple conclusions to a formalized theory. This method can be continued and expanded upon for other properties, forming quantitative structure–effect relationships for various properties.
- (c)
- The trade-off between key properties in the design of optical materials: The discussion in this article maintains a relatively consistent train of thought with most of the work. That is, starting from the material with a certain performance, the study on the impact of the structure on the improvement of this single performance, while ignoring the impact on other performances, or even the actual application environment. This is not conducive to the multifunctionalization of optical materials. Starting from key structural factors such as particle size, grafting, and loading in the structure of hybrid materials, the synergistic effects of a certain change in structure on various properties can be discussed to form an application-oriented material performance trade-off strategy.
- (d)
- Attention paid to the development of new optical functional materials: In addition to paying attention to the development of new photofunctional inorganic materials or polymer materials, we also need to pay attention to the development of new hybrid material systems. Carbonized polymer dots (CPDs) are a new type of nanofunctional elementary material that represent a new system of polymer nanohybridization [158,159]. In recent years, the advantages of this material have been reflected in its luminescent properties, and its synthesis process is considered to be a crosslinking carbonization process involving small molecules or polymer precursors. After several years of exploration, a family of CPDs with various luminescent properties such as full-color luminescence and narrow half-peak width emission has been obtained. Recently, Yang et al. further designed and applied this material to a material with both light transmission and mechanical properties, realizing the application of CPDs in the field of transparent optical films [160]. In addition, CPDs have also demonstrated their contributions in multiple fields such as imaging, sensing, and energy [161]. Their advantages such as low toxicity, environmental friendliness, and structural designability [162] lead us to believe that the introduction of new material systems such as CPDs will provide more excellent performance and broader application prospects for multifunctional fluorescent polymer nanomaterials.
- (e)
- In order to promote the further application of fluorescent polymer nanomaterials in biomedicine, future research work can be optimized and expanded in the following aspects. At present, most light-emitting polymers have low light-emitting performance in NIR-II. The development of NIR-II light-emitting polymer materials with higher luminous intensity or photothermal efficiency using DA structure adjustment combined with theoretical calculations is still one of the important research directions for the future. Efficient enrichment of luminescent nanofunctional materials in tumor sites is another key to improving the efficiency of tumor diagnosis and treatment. Using rational molecular design, targeting groups can be effectively bonded to polymer chains to prepare light-emitting polymers with targeting functions, which is of great significance for the precise diagnosis and treatment of tumor sites. A single treatment for tumors is gradually being replaced by multimodal treatment. The therapeutic effect on tumors can be improved by constructing a multifunctional nanodiagnosis and treatment platform with properties such as chemotherapy, photodynamic therapy, or photothermal therapy.
- (f)
- Internal/external stimuli-responsive fluorescent polymer nanoparticles that can be used in theranostics and sensing applications cannot be ignored either [163]. In particular, they respond to internal stimuli, including redox, pH, and enzymes, and external stimuli, including temperature, light, and magnetic fields, for drug delivery and sensing applications [164,165]. In terms of generating stimulus-responsive signals, these signals allow for amplification and easy detection of biologically relevant events. More detailed modeling of the photophysical properties of existing materials and their properties will provide decisive input for designing better performing NPs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Abelha, T.F.; Dreiss, C.A.; Green, M.A.; Dailey, L.A. Conjugated polymers as nanoparticle probes for fluorescence and photoacoustic imaging. J. Mater. Chem. B 2020, 8, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Paulmurugan, R. Bioluminescent imaging systems for assay developments. Anal. Sci. 2021, 37, 233–247. [Google Scholar] [CrossRef]
- Kim, S.-B.; Furuta, T.; Ohmuro-Matsuyama, Y.; Kitada, N.; Nishihara, R.; Maki, S.A. Bioluminescent imaging systems boosting near-infrared signals in mammalian cells. Photochem. Photobiol. Sci. 2023, 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nishizono, A. In Vivo Bioluminescent Imaging of Rabies Virus Infection and Evaluation of Antiviral Drug. In Bioluminescence: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 347–352. [Google Scholar]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Liang, G. Peptide-based supramolecular hydrogels for bioimaging applications. Biomater. Sci. 2021, 9, 315–327. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.; Gambhir, S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Shen, M.; Kong, F.; Tong, L.; Luo, Y.; Yin, S.; Liu, C.; Zhang, P.; Wang, L.; Chu, P.K.; Ding, Y. Carbon capture and storage (CCS): Development path based on carbon neutrality and economic policy. Carbon Neutrality 2022, 1, 37. [Google Scholar] [CrossRef]
- Wang, F.; Tan, W.B.; Zhang, Y.; Fan, X.; Wang, M. Luminescent nanomaterials for biological labelling. Nanotechnology 2005, 17, R1. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2018, 119, 322–477. [Google Scholar] [CrossRef]
- Shen, M.; Ma, H. Metal-organic frameworks (MOFs) and their derivative as electrode materials for lithium-ion batteries. Coord. Chem. Rev. 2022, 470, 214715. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Wang, S. Conjugated polymer nanoparticles for imaging, cell activity regulation, and therapy. Adv. Funct. Mater. 2019, 29, 1806818. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Sun, X.-L.; Wan, W.-M.; Xiao, L.; Qian, Q. Boronic acid-containing polymeric nanomaterials via polymerization induced self-assembly as fructose sensor. Polymer 2022, 253, 125005. [Google Scholar] [CrossRef]
- Abraham, J.E.; Balachandran, M. Fluorescent Mechanism in Zero-Dimensional Carbon Nanomaterials: A Review. J. Fluoresc. 2022, 32, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Chen, H.; Zhang, Y.; Li, P.; Wu, W. Nanocellulose: A promising nanomaterial for fabricating fluorescent composites. Cellulose 2022, 29, 7011–7035. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Purif. Technol. 2022, 2022, 121734. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, J.; Jang, E.-S. Recent progress in multifunctional conjugated polymer nanomaterial-based synergistic combination phototherapy for microbial infection theranostics. Coord. Chem. Rev. 2022, 470, 214701. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Smart nanomaterials employed recently for drug delivery in cancer therapy: An intelligent approach. BioNanoScience 2022, 12, 1356–1365. [Google Scholar] [CrossRef]
- Shen, X.; Xu, W.; Ouyang, J.; Na, N. Fluorescence resonance energy transfer-based nanomaterials for the sensing in biological systems. Chin. Chem. Lett. 2022, 33, 4505–4516. [Google Scholar] [CrossRef]
- Yang, X.; Qin, X.; Ji, H.; Du, L.; Li, M. Constructing firefly luciferin bioluminescence probes for in vivo imaging. Org. Biomol. Chem. 2022, 20, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.J.; Li, C.; Zeng, J.; Cui, J.; Wu, S.; Gao, M. Self-illuminating NIR-II bioluminescence imaging probe based on silver sulfide quantum dots. ACS Nano 2022, 16, 16824–16832. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Cheon, S.Y.; Park, S.; Lee, D.; Lee, Y.; Han, S.; Kim, M.; Koo, H. Recent advances in optical imaging through deep tissue: Imaging probes and techniques. Biomater. Res. 2022, 26, 57. [Google Scholar] [CrossRef]
- Elsabahy, M.; Heo, G.S.; Lim, S.-M.; Sun, G.; Wooley, K.L. Polymeric nanostructures for imaging and therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, X.-Q.; Li, L.; Li, Y.-X.; Yu, L.-P.; Chen, Y. Ratiometric fluorescence sensor for point-of-care testing of bilirubin based on tetraphenylethylene functionalized polymer nanoaggregate and rhodamine B. Sens. Actuators B Chem. 2022, 369, 132392. [Google Scholar] [CrossRef]
- Ahumada, G.; Borkowska, M. Fluorescent polymers conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef]
- Bou, S.; Klymchenko, A.S.; Collot, M. Fluorescent labeling of biocompatible block copolymers: Synthetic strategies and applications in bioimaging. Mater. Adv. 2021, 2, 3213–3233. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, M.; Lin, Z.; Yang, H.; Zhang, C.; Cui, J.; Chen, J. Aptamer functionalization and high-contrast reversible dual-color photoswitching fluorescence of polymeric nanoparticles for latent fingerprints imaging. Sens. Actuators B Chem. 2022, 367, 132049. [Google Scholar] [CrossRef]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: A review. J. Pharm. Biomed. Anal. 2022, 212, 114628. [Google Scholar] [CrossRef]
- Wan, W.; Li, Z.; Wang, X.; Tian, F.; Yang, J. Surface-fabrication of fluorescent hydroxyapatite for cancer cell imaging and bio-printing applications. Biosensors 2022, 12, 419. [Google Scholar] [CrossRef]
- Jiang, M.-C.; Liu, H.-B.; Wang, J.-Q.; Li, S.; Zheng, Z.; Wang, D.; Wei, H.; Yu, C.-Y. Optimized aptamer functionalization for enhanced anticancer efficiency in vivo. Int. J. Pharm. 2022, 628, 122330. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, M.; Michel, A.; Petit, L.; Garnier, M.; Ménager, C.; Griffete, N. Biotinylated magnetic molecularly imprinted polymer nanoparticles for cancer cell targeting and controlled drug delivery. Chem. Commun. 2022, 58, 5642–5645. [Google Scholar] [CrossRef] [PubMed]
- Shou, K.; Tang, Y.; Chen, H.; Chen, S.; Zhang, L.; Zhang, A.; Fan, Q.; Yu, A.; Cheng, Z. Diketopyrrolopyrrole-based semiconducting polymer nanoparticles for in vivo second near-infrared window imaging and image-guided tumor surgery. Chem. Sci. 2018, 9, 3105–3110. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Cong, Y.; Kang, Y.; Wu, Z.; Li, L. Conjugated polymer-functionalized stretchable supramolecular hydrogels to monitor and control cellular behavior. ACS Appl. Mater. Interfaces 2022, 14, 12674–12683. [Google Scholar] [CrossRef]
- Hamadani, C.M.; Chandrasiri, I.; Yaddehige, M.L.; Dasanayake, G.S.; Owolabi, I.; Flynt, A.; Hossain, M.; Liberman, L.; Lodge, T.P.; Werfel, T.A. Improved nanoformulation and bio-functionalization of linear-dendritic block copolymers with biocompatible ionic liquids. Nanoscale 2022, 14, 6021–6036. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Schanze, K.S. Functionalization of water-soluble conjugated polymers for bioapplications. ACS Appl. Mater. Interfaces 2022, 14, 20506–20519. [Google Scholar] [CrossRef]
- Nothling, M.D.; Bailey, C.G.; Fillbrook, L.L.; Wang, G.; Gao, Y.; McCamey, D.R.; Monfared, M.; Wong, S.; Beves, J.E.; Stenzel, M.H. Polymer grafting to polydopamine free radicals for universal surface functionalization. J. Am. Chem. Soc. 2022, 144, 6992–7000. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F.; Yang, Y.-W. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Shahi, S.; Roghani-Mamaqani, H.; Talebi, S.; Mardani, H. Stimuli-responsive destructible polymeric hydrogels based on irreversible covalent bond dissociation. Polym. Chem. 2022, 13, 161–192. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Besprozvannykh, V.; Shlyakhtin, A.; Tavtorkin, A.; Legkov, S.; Chinova, M.; Arutyunyan, I.; Soboleva, A.; Fatkhudinov, T.; Ivchenko, P. Chain-End Functionalization of Poly (ε-caprolactone) for Chemical Binding with Gelatin: Binary Electrospun Scaffolds with Improved Physico-Mechanical Characteristics and Cell Adhesive Properties. Polymers 2022, 14, 4203. [Google Scholar] [CrossRef]
- Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: A Mechanistic Perspective. J. Am. Chem. Soc. 2022, 144, 15413–15430. [Google Scholar] [CrossRef] [PubMed]
- Dworakowska, S.; Lorandi, F.; Gorczyński, A.; Matyjaszewski, K. Toward green atom transfer radical polymerization: Current status and future challenges. Adv. Sci. 2022, 9, 2106076. [Google Scholar] [CrossRef]
- Kim, J.; Cattoz, B.; Leung, A.H.; Parish, J.D.; Becer, C.R. Enabling Reversible Addition-Fragmentation Chain-Transfer Polymerization for Brush Copolymers with a Poly (2-oxazoline) Backbone. Macromolecules 2022, 55, 4411–4419. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methylaluminum bis (2, 6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Coessens, V.; Pintauer, T.; Matyjaszewski, K. Functional polymers by atom transfer radical polymerization. Prog. Polym. Sci. 2001, 26, 337–377. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Cui, X.; Zhu, W.; Tang, H. Development of environmentally friendly atom transfer radical polymerization. Polymers 2020, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Chakma, P.; Zeitler, S.M.; Baum, F.; Yu, J.; Shindy, W.; Pozzo, L.D.; Golder, M.R. Mechanoredox Catalysis Enables a Sustainable and Versatile Reversible Addition-Fragmentation Chain Transfer Polymerization Process. Angew. Chem. Int. Ed. 2023, 62, e202215733. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.G.; Petit, L.M.; Whitfield, R.; Anastasaki, A.; Barner-Kowollik, C.; Konkolewicz, D. Ubiquitous Nature of Rate Retardation in Reversible Addition–Fragmentation Chain Transfer Polymerization. J. Am. Chem. Soc. 2021, 143, 17769–17777. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.; Mayadunne, R.T.; Meijs, G.F.; Moad, C.L.; Moad, G. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559. [Google Scholar] [CrossRef]
- Moad, G.; Chiefari, J.; Chong, Y.K.; Krstina, J.; Mayadunne, R.T.A.; Postma, A.; Rizzardo, E.; Thang, S.H. Living free radical polymerization with reversible addition–fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49, 993–1001. [Google Scholar] [CrossRef]
- Li, M.; Fromel, M.; Ranaweera, D.; Rocha, S.; Boyer, C.; Pester, C.W. SI-PET-RAFT: Surface-initiated photoinduced electron transfer-reversible addition–fragmentation chain transfer polymerization. ACS Macro Lett. 2019, 8, 374–380. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Jiang, Y.; Liao, S. Organocatalytic, stereoselective, cationic reversible addition–fragmentation chain-transfer polymerization of vinyl ethers. J. Am. Chem. Soc. 2021, 144, 679–684. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, D.; Lu, Z.; Liu, K. Chiral self-assembly of nanoparticles induced by polymers synthesized via reversible addition–fragmentation chain transfer polymerization. ACS Nano 2019, 13, 1479–1489. [Google Scholar] [CrossRef]
- Strover, L.T.; Postma, A.; Horne, M.D.; Moad, G. Anthraquinone-mediated reduction of a trithiocarbonate chain-transfer agent to initiate electrochemical reversible addition–fragmentation chain transfer polymerization. Macromolecules 2020, 53, 10315–10322. [Google Scholar] [CrossRef]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Kurokawa, N.; Yoshikawa, H.; Hirota, N.; Hyodo, K.; Masuhara, H. Size-dependent spectroscopic properties and thermochromic behavior in poly (substituted thiophene) nanoparticles. ChemPhysChem 2004, 5, 1609–1615. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; McNeill, J. Preparation and encapsulation of highly fluorescent conjugated polymer nanoparticles. Langmuir 2006, 22, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem. 2011, 123, 3492–3496. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric nanoparticles–multifunctional materials of the future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, T.W.; Kim, G.-Y.; Kang, B. Review of Conjugated Polymer Nanoparticles: From Formulation to Applications. ACS Appl. Nano Mater. 2022, 5, 17436–17460. [Google Scholar] [CrossRef]
- Wang, Q.-B.; Zhang, C.-J.; Yu, H.; Zhang, X.; Lu, Q.; Yao, J.-S.; Zhao, H. The sensitive “Turn-on” fluorescence platform of ascorbic acid based on conjugated polymer nanoparticles. Anal. Chim. Acta 2020, 1097, 153–160. [Google Scholar] [CrossRef]
- Fang, F.; Li, M.; Zhang, J.; Lee, C.-S. Different strategies for organic nanoparticle preparation in biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Indoria, S.; Singh, V.; Hsieh, M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. Double-hydrophilic block copolymers: Synthesis and application as novel surfactants and crystal growth modifiers. Macromol. Rapid Commun. 2001, 22, 219–252. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Zhao, Q.L.; Lu, H.C.; Huang, J.; Cao, S.K.; Ma, Z. Polymethylene-b-polystyrene diblock copolymer: Synthesis, property, and application. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1894–1900. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Wang, Y.; Li, L.; Zhou, N.; Cai, Y.; Zhang, Z.; Zhu, X. Azoreductase-triggered fluorescent nanoprobe synthesized by RAFT-mediated polymerization-induced self-assembly for drug release. Polym. Chem. 2020, 11, 5619–5629. [Google Scholar] [CrossRef]
- Sun, H.; Cao, W.; Zang, N.; Clemons, T.D.; Scheutz, G.M.; Hu, Z.; Thompson, M.P.; Liang, Y.; Vratsanos, M.; Zhou, X. Proapoptotic Peptide Brush Polymer Nanoparticles via Photoinitiated Polymerization-Induced Self-Assembly. Angew. Chem. 2020, 132, 19298–19304. [Google Scholar] [CrossRef]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling nanomaterial size and shape for biomedical applications via polymerization-induced self-assembly. Macromol. Rapid Commun. 2019, 40, 1800438. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Nguyen, D.; Pham, B.T.; Gilbert, R.G.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Ab initio emulsion polymerization by RAFT-controlled self-assembly. Macromolecules 2005, 38, 2191–2204. [Google Scholar] [CrossRef]
- Wan, W.-M.; Hong, C.-Y.; Pan, C.-Y. One-pot synthesis of nanomaterials via RAFT polymerization induced self-assembly and morphology transition. Chem. Commun. 2009, 39, 5883–5885. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-M.; Sun, X.-L.; Pan, C.-Y. Morphology transition in RAFT polymerization for formation of vesicular morphologies in one pot. Macromolecules 2009, 14, 4950–4952. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2021, 253, 117213. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. Synthesis and characterization of pH responsive alginate based-hydrogels as oral drug delivery carrier. J. Polym. Res. 2020, 27, 251. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Minato, H.; Inui, T.; Uchihashi, T.; Suzuki, D. Nanostructures, thermoresponsiveness, and assembly mechanism of hydrogel microspheres during aqueous free-radical precipitation polymerization. Langmuir 2020, 37, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Yang, X.; Huang, W. Synthesis of narrow or monodisperse poly (divinylbenzene) microspheres by distillation− precipitation polymerization. Macromolecules 2004, 37, 9746–9752. [Google Scholar] [CrossRef]
- Fan, M.; Wang, F.; Wang, C. Reflux precipitation polymerization: A new platform for the preparation of uniform polymeric nanogels for biomedical applications. Macromol. Biosci. 2018, 18, 1800077. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef]
- Rama Narsimha Reddy, A.; Narsimha Reddy, Y.; Himabindu, V.; Rama Krishna, D. Induction of oxidative stress and cytotoxicity by carbon nanomaterials is dependent on physical properties. Toxicol. Ind. Health 2011, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Shokrgozar, M.A.; Sardari, S.; Moghadam, M.K.; Vali, H.; Laurent, S.; Stroeve, P. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 2011, 3, 1127–1138. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zhao, L.-Y.; Wang, Y.-Y.; Li, D.-Y.; Tao, D.; Li, L.-Y.; Tang, J.-T. Magnetic stent hyperthermia for esophageal cancer: An in vitro investigation in the ECA-109 cell line. Oncol. Rep. 2012, 27, 791–797. [Google Scholar]
- Lanone, S.; Boczkowski, J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr. Mol. Med. 2006, 6, 651–663. [Google Scholar] [CrossRef]
- Rahman, I. Regulation of nuclear factor-κB, activator protein-1, and glutathione levels by tumor necrosis factor-α and dexamethasone in alveolar epithelial cells. Biochem. Pharmacol. 2000, 60, 1041–1049. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Jimenez, L.A.; Torres, M.; Forman, H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox Signal. 2005, 7, 42–59. [Google Scholar] [CrossRef]
- Guanming, Q.; Xikum, L.; Tai, Q.; Haitao, Z.; Honghao, Y.; Ruiting, M. Application of rare earths in advanced ceramic materials. J. Rare Earths 2007, 25, 281–286. [Google Scholar] [CrossRef]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Zhang, Z.; He, T.; Mi, X.; Kong, T.; Fu, Z.; Zheng, H.; Xu, H. Self-suspended rare-earth doped up-conversion luminescent waveguide: Propagating and directional radiation. Opto-Electron. Adv. 2020, 3, 190045. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent progress of rare-earth doped upconversion nanoparticles: Synthesis, optimization, and applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Werts, M.H. Making sense of lanthanide luminescence. Sci. Prog. 2005, 88, 101–131. [Google Scholar] [CrossRef]
- Montgomery, C.P.; Murray, B.S.; New, E.J.; Pal, R.; Parker, D. Cell-penetrating metal complex optical probes: Targeted and responsive systems based on lanthanide luminescence. Acc. Chem. Res. 2009, 42, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.G.; Samuel, A.P.; Raymond, K.N. From antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Ding, Z.; He, Y.; Rao, H.; Zhang, L.; Nguyen, W.; Wang, J.; Wu, Y.; Han, C.; Xing, C.; Yan, C. Novel Fluorescent Probe Based on Rare-Earth Doped Upconversion Nanomaterials and Its Applications in Early Cancer Detection. Nanomaterials 2022, 12, 1787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, R.; Yan, G.-H.; Sun, J.; Li, C.; Wang, H.; Shi, L.; Capobianco, J.A.; Sun, L. Smart self-assembled nanosystem based on water-soluble pillararene and rare-earth-doped upconversion nanoparticles for ph-responsive drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 4910–4920. [Google Scholar] [CrossRef]
- Sun, L.-D.; Wang, Y.-F.; Yan, C.-H. Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles: Small size and tunable emission/excitation spectra. Acc. Chem. Res. 2014, 47, 1001–1009. [Google Scholar] [CrossRef]
- Cardoso Dos Santos, M.; Runser, A.; Bartenlian, H.; Nonat, A.M.; Charbonnière, L.J.; Klymchenko, A.S.; Hildebrandt, N.; Reisch, A. Lanthanide-complex-loaded polymer nanoparticles for background-free single-particle and live-cell imaging. Chem. Mater. 2019, 31, 4034–4041. [Google Scholar] [CrossRef]
- Ai, K.; Zhang, B.; Lu, L. Europium-based fluorescence nanoparticle sensor for rapid and ultrasensitive detection of an anthrax biomarker. Angew. Chem. 2009, 121, 310–314. [Google Scholar] [CrossRef]
- Tan, H.; Liu, B.; Chen, Y. Lanthanide coordination polymer nanoparticles for sensing of mercury (II) by photoinduced electron transfer. ACS Nano 2012, 6, 10505–10511. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, X.; Huang, Q.; Tian, J.; Huang, H.; Wan, Q.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. Facile fabrication of biodegradable lanthanide ions containing fluorescent polymeric nanoparticles: Characterization, optical properties and biological imaging. Mater. Chem. Phys. 2018, 207, 226–232. [Google Scholar] [CrossRef]
- Xu, D.; Liu, M.; Huang, Q.; Chen, J.; Huang, H.; Deng, F.; Wen, Y.; Tian, J.; Zhang, X.; Wei, Y. One-step synthesis of europium complexes containing polyamino acids through ring-opening polymerization and their potential for biological imaging applications. Talanta 2018, 188, 1–6. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F. A new generation of NIR-II probes: Lanthanide-based nanocrystals for bioimaging and biosensing. Adv. Opt. Mater. 2019, 7, 1801417. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef]
- Tao, Z.; Dang, X.; Huang, X.; Muzumdar, M.D.; Xu, E.S.; Bardhan, N.M.; Song, H.; Qi, R.; Yu, Y.; Li, T. Early tumor detection afforded by in vivo imaging of near-infrared II fluorescence. Biomaterials 2017, 134, 202–215. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting polymers for neural applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low bandgap semiconducting polymers for polymeric photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low band gap conjugated semiconducting polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Yan, X.; Xiong, M.; Deng, X.-Y.; Liu, K.-K.; Li, J.-T.; Wang, X.-Q.; Zhang, S.; Prine, N.; Zhang, Z.; Huang, W. Approaching disorder-tolerant semiconducting polymers. Nat. Commun. 2021, 12, 5723. [Google Scholar] [CrossRef]
- Tsai, W.K.; Chan, Y.H. Semiconducting polymer dots as near-infrared fluorescent probes for bioimaging and sensing. J. Chin. Chem. Soc. 2019, 66, 9–20. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Wang, C.; Peng, R.; Liu, Z. Conjugated polymers for photothermal therapy of cancer. Polym. Chem. 2014, 5, 1573–1580. [Google Scholar] [CrossRef]
- Chen, P.; Ma, Y.; Zheng, Z.; Wu, C.; Wang, Y.; Liang, G. Facile syntheses of conjugated polymers for photothermal tumour therapy. Nat. Commun. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Hu, R.; Chen, H.; Li, M.; Wang, J.; Wang, Y.; Liu, L.; Lv, F.; Liang, X.-J. Near-infrared (NIR)-absorbing conjugated polymer dots as highly effective photothermal materials for in vivo cancer therapy. Chem. Mater. 2016, 28, 8669–8675. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Nie, C.; Zhu, C.; Yang, Q.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles for light-activated anticancer and antibacterial activity with imaging capability. Langmuir 2012, 28, 2091–2098. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Q.; Shi, F.; Liu, C.; Tang, Y. NIR-mediated nanohybrids of upconversion nanophosphors and fluorescent conjugated polymers for high-efficiency antibacterial performance based on fluorescence resonance energy transfer. Adv. Healthc. Mater. 2016, 5, 2967–2971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, T.; Li, J.; Sun, P.; Wang, Y.; Shi, W.; Han, W.; Wang, W.; Fan, Q.; Huang, W. Facial control intramolecular charge transfer of quinoid conjugated polymers for efficient in vivo NIR-II imaging. ACS Appl. Mater. Interfaces 2019, 11, 16311–16319. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 4206. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Wang, B.W.; Jiang, K.; Li, J.X.; Luo, S.H.; Wang, Z.Y.; Jiang, H.F. 1, 1-Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation-Caused-Quenching Molecule 1, 1-Diphenylethene through Simple Thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X.; Liu, Z.; Liu, H.; Chen, D.; He, S.; Zheng, J.; Yang, B.; Qin, W.; Zhang, X. Semiconducting Polymer Dots with Dual-Enhanced NIR-IIa Fluorescence for Through-Skull Mouse-Brain Imaging. Angew. Chem. Int. Ed. 2020, 59, 3691–3698. [Google Scholar] [CrossRef]
- Wu, C.; Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Ed. 2013, 52, 3086–3109. [Google Scholar] [CrossRef]
- Wu, C.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Tsai, W.-K.; Wang, C.-I.; Liao, C.-H.; Yao, C.-N.; Kuo, T.-J.; Liu, M.-H.; Hsu, C.-P.; Lin, S.-Y.; Wu, C.-Y.; Pyle, J.R. Molecular design of near-infrared fluorescent Pdots for tumor targeting: Aggregation-induced emission versus anti-aggregation-caused quenching. Chem. Sci. 2019, 10, 198–207. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Sun, C.; Zebibula, A.; Zhang, H.; Kwok, R.T.; Zhao, X.; Xi, W.; Lam, J.W.; Qian, J.; Tang, B.Z. Real-time and high-resolution bioimaging with bright aggregation-induced emission dots in short-wave infrared region. Adv. Mater. 2018, 30, 1706856. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mater. 2018, 30, 1800766. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, D.; Sheng, Z.; Min, T.; Zha, M.; Ni, J.-S.; Zheng, H.; Li, K. Self-assembled AIEgen nanoparticles for multiscale NIR-II vascular imaging. Biomaterials 2021, 264, 120365. [Google Scholar] [CrossRef]
- Fang, Y.; Shang, J.; Liu, D.; Shi, W.; Li, X.; Ma, H. Design, synthesis, and application of a small molecular NIR-II fluorophore with maximal emission beyond 1200 nm. J. Am. Chem. Soc. 2020, 142, 15271–15275. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Huang, W.; Li, C.; Li, G.; Yang, W.C.; Liu, S.H.; Yin, J.; Sun, Y.; Yang, G.F. Near-Infrared Fluorescence/Photoacoustic Agent with an Intensifying Optical Performance for Imaging-Guided Effective Photothermal Therapy. Adv. Ther. 2020, 3, 2000170. [Google Scholar] [CrossRef]
- Rao, R.S.; Singh, S.P. Near-Infrared (>1000 nm) Light-Harvesters: Design, Synthesis and Applications. Chem. Eur. J. 2020, 26, 16582–16593. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xia, G.; Yang, Y.; Si, L.; Wang, H.; Wang, H. Maximal Emission beyond 1200 nm Dicyanovinyl-Functionalized Squaraine for in vivo Vascular Imaging. Chem. Commun. 2023, 59, 3598–3601. [Google Scholar] [CrossRef]
- Tu, L.; Xu, Y.; Ouyang, Q.; Li, X.; Sun, Y. Recent advances on small-molecule fluorophores with emission beyond 1000 nm for better molecular imaging in vivo. Chin. Chem. Lett. 2019, 30, 1731–1737. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wang, H.; Antaris, A.L.; Li, L.; Diao, S.; Ma, R.; Nguyen, A.; Hong, G.; Ma, Z.; Wang, J. Traumatic brain injury imaging in the second near-infrared window with a molecular fluorophore. Adv. Mater. 2016, 28, 6872–6879. [Google Scholar] [CrossRef]
- Antaris, A.L.; Chen, H.; Diao, S.; Ma, Z.; Zhang, Z.; Zhu, S.; Wang, J.; Lozano, A.X.; Fan, Q.; Chew, L. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 2017, 8, 15269. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Antaris, A.L.; Chen, H.; Xiao, Y.; Lu, X.; Jiang, L.; Diao, S.; Yu, K.; Wang, Y. Live imaging of follicle stimulating hormone receptors in gonads and bones using near infrared II fluorophore. Chem. Sci. 2017, 8, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Kalay, S.; Stetsyshyn, Y.; Donchak, V.; Harhay, K.; Lishchynskyi, O.; Ohar, H.; Panchenko, Y.; Voronov, S.; Çulha, M. pH-Controlled fluorescence switching in water-dispersed polymer brushes grafted to modified boron nitride nanotubes for cellular imaging. Beilstein J. Nanotechnol. 2019, 10, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Nese, A.; Lebedeva, N.V.; Sherwood, G.; Averick, S.; Li, Y.; Gao, H.; Peteanu, L.; Sheiko, S.S.; Matyjaszewski, K. pH-responsive fluorescent molecular bottlebrushes prepared by Atom Transfer Radical polymerization. Macromolecules 2011, 44, 5905–5910. [Google Scholar] [CrossRef]
- Liu, S.; de Beer, S.; Batenburg, K.M.; Gojzewski, H.; Duvigneau, J.; Vancso, G.J. Designer Core–Shell Nanoparticles as Polymer Foam Cell Nucleating Agents: The Impact of Molecularly Engineered Interfaces. ACS Appl. Mater. Interfaces 2021, 13, 17034–17045. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, H.; Ma, Y.; Liu, J.; Zhang, L. Diffusion of polymer-grafted nanoparticles with dynamical fluctuations in unentangled polymer melts. Phys. Chem. Chem. Phys. 2022, 24, 11322–11335. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Ru, Y.; Ai, L.; Jia, T.; Liu, X.; Lu, S.; Tang, Z.; Yang, B. Recent advances in chiral carbonized polymer dots: From synthesis and properties to applications. Nano Today 2020, 34, 100953. [Google Scholar] [CrossRef]
- Pan, K.; Liu, C.; Zhu, Z.; Feng, T.; Tao, S.; Yang, B. Soft–Hard Segment Combined Carbonized Polymer Dots for Flexible Optical Film with Superhigh Surface Hardness. ACS Appl. Mater. Interfaces 2022, 14, 14504–14512. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Zeng, Q.; Feng, T.; Tao, S.; Zhu, S.; Yang, B. Precursor-dependent structural diversity in luminescent carbonized polymer dots (CPDs): The nomenclature. Light Sci. Appl. 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.; Hu, Z.; Zhang, H.; Li, Q.; Ji, X. Information Storage Based on Stimuli-Responsive Fluorescent 3D Code Materials. Adv. Funct. Mater. 2022, 32, 2113274. [Google Scholar] [CrossRef]
- Battistelli, G.; Cantelli, A.; Guidetti, G.; Manzi, J.; Montalti, M. Ultra-bright and stimuli-responsive fluorescent nanoparticles for bioimaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2016, 8, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Mazrad, Z.A.I.; Lee, K.; Chae, A.; In, I.; Lee, H.; Park, S.Y. Progress in internal/external stimuli responsive fluorescent carbon nanoparticles for theranostic and sensing applications. J. Mater. Chem. B 2018, 6, 1149–1178. [Google Scholar] [CrossRef] [PubMed]





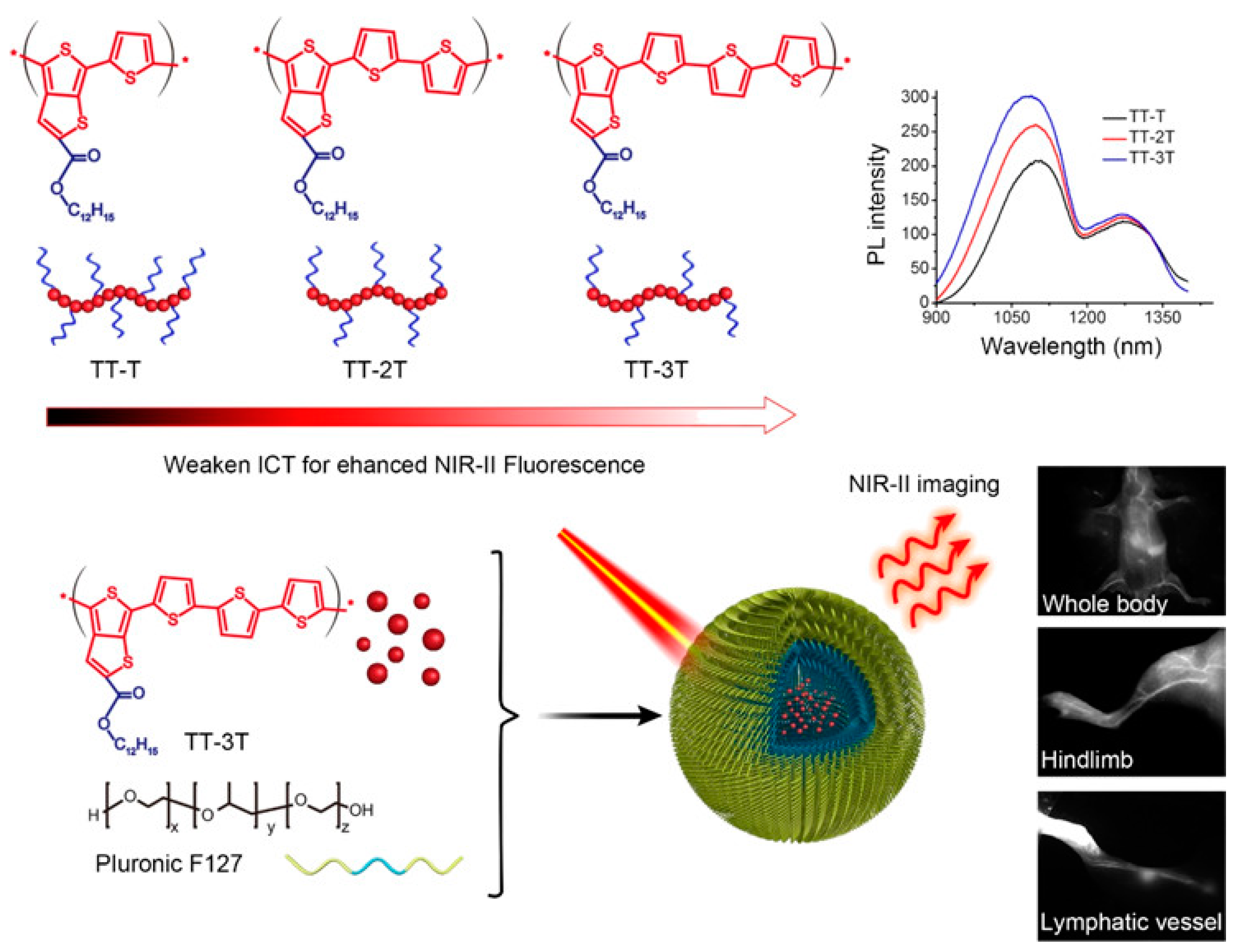
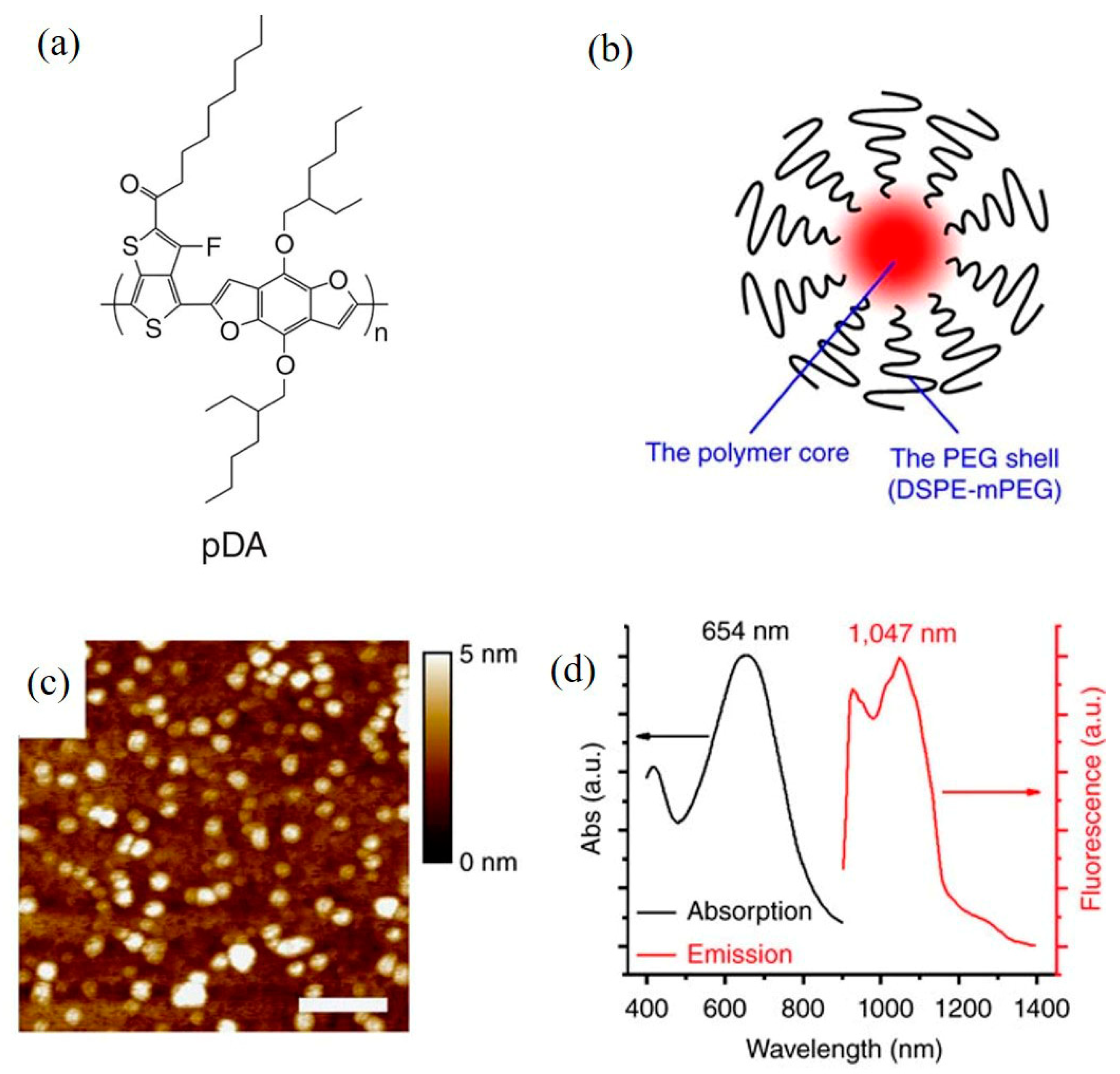
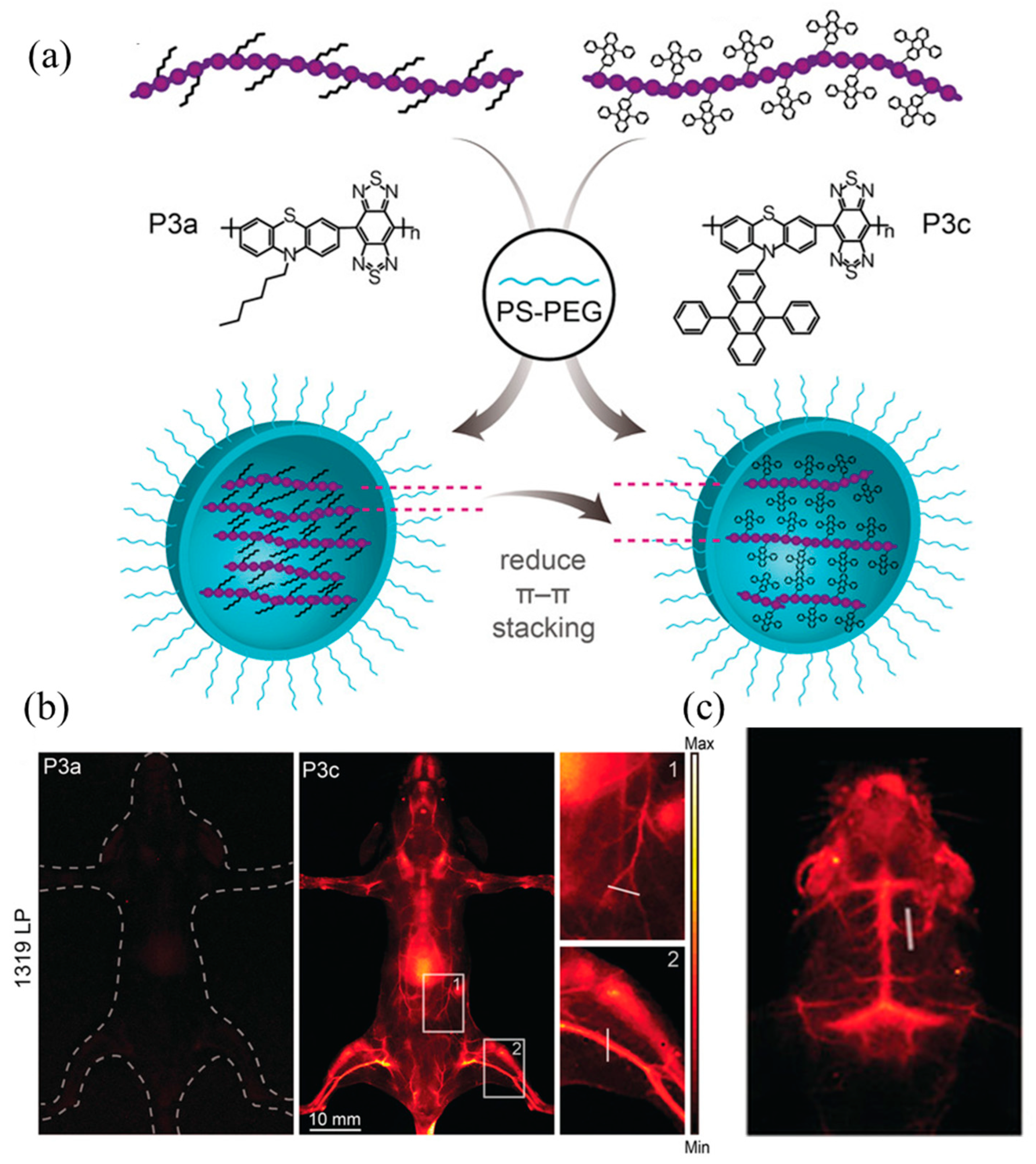

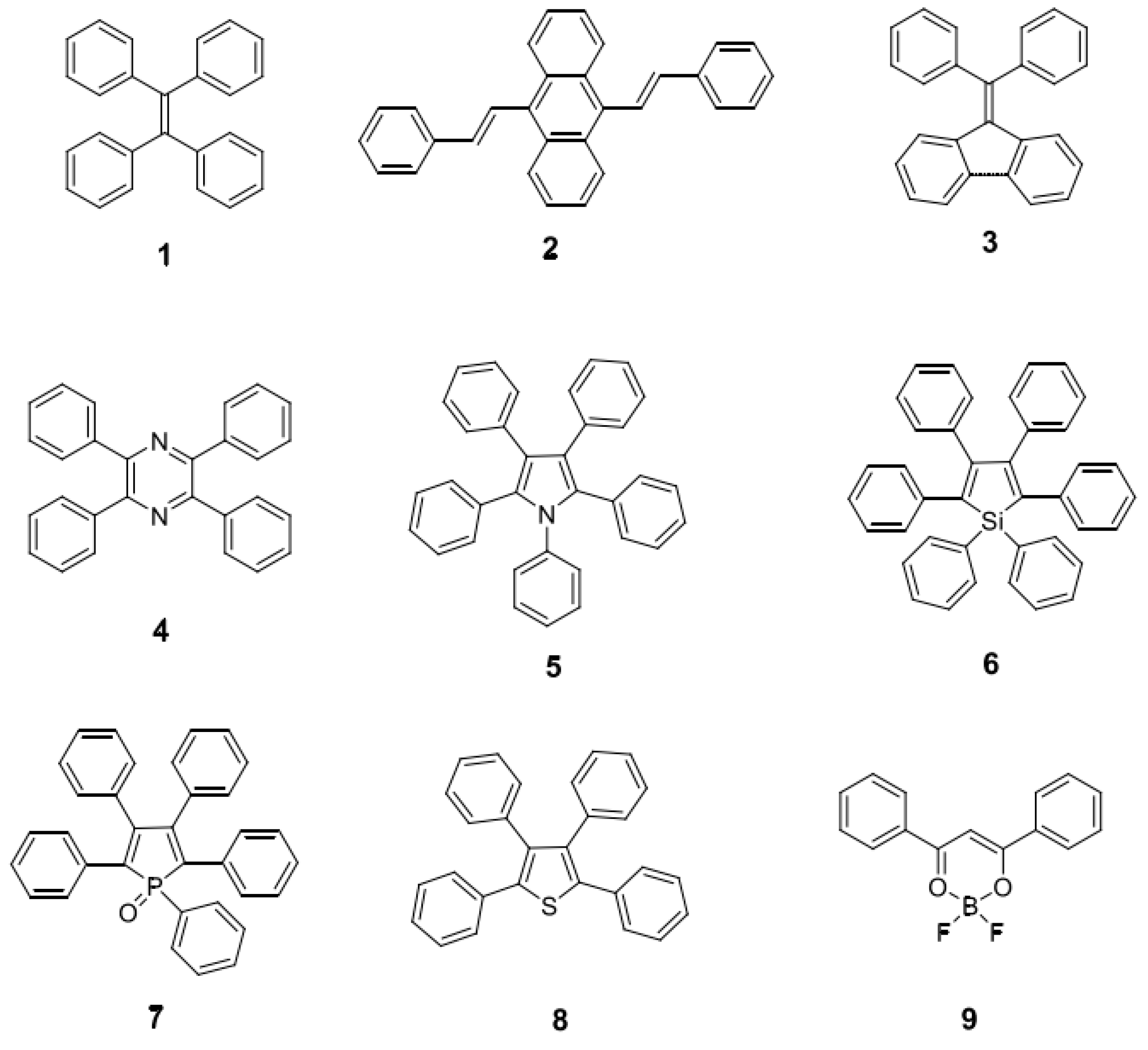
| Synthesis Technology | Advantages | Disadvantages | |
|---|---|---|---|
| Living/controllable synthesis of amphiphilic block polymers | ATRP | The reaction temperature is mild, the operation is simple, and it is easy to industrialize. | The intermediate process is completely uncontrolled; the amount of transition metal complex is large; the aging of the polymer. |
| RAFT | It has a wide range of applications, good polymerization ability, and the molecular weight of the obtained polymer is uniform. | Few applicable monomers, limited scope of molecular design, expensive. | |
| Physical package | Nanoprecipitation | Simple operation, fast, high reproducibility, good dispersion of colloidal nanoparticles and easy functionalization. | There are fewer types of polymers, and the process of particle growth is not easy to control. |
| Microemulsion method | Narrow particle size distribution, controllable, good stability. | Surfactants are difficult to remove and have large particle sizes. | |
| Self-assembly method | It is very convenient to prepare various exotic three-dimensional structures; it is also possible to prepare porous materials that inherit the original morphology and structure | Unstable under physiological conditions. | |
| Covalent linkage | PISA | The process is simple, the price adjustment is gentle, and nano-medicine can be prepared in one step. | The operation is complicated, the reaction takes a long time, and the concentration of the prepared nanoparticles is low (≤1 mg/mL), which makes it impossible to achieve large-scale mass production. |
| Precipitation polymerization | The particle size of the polymer is uniform and clean, the viscosity of the polymerization system is low, and no surfactant and stabilizer are needed. | Low microsphere yield and high solvent toxicity. | |
| Fluorescent Nanomaterial Type | Intrinsic Material Toxicity | Materials | Biological System |
|---|---|---|---|
| Carbon dots | Low | C-dots, PEG stabilized | Mice |
| Carbon nanotubes | Low–medium | Many types of CNTs | Various in vitro/in vivo |
| Dendrimers | High | Various dendrimer types | Various in vitro/in vivo |
| Doped graphene QDs | Medium | N-doped graphene quantum dots | Red blood cells |
| Fluorescent beads | Medium (polymer) | Polystyrene nanoparticles | Endothelial cells |
| Low (silica) | Silica nanoparticles | Epithelial cells and fibroblasts | |
| Fluorescent proteins | Medium | Red fluorescent protein | HeLa cells |
| Graphene oxide | Medium | Graphene oxide | Various in vitro/in vivo |
| Graphene oxide | Red blood cells | ||
| Organic dyes | Medium | Various organic fluorophores | Various in vitro/in vivo |
| Metal clusters | Medium | MPA or GSH stabilized Au clusters | Colonic epithelial cells |
| GSH and BSA stabilized Au25 clusters | Mice | ||
| Nanodiamonds | Low | Detonation nanodiamond | Various in vitro/in vivo |
| Various diamond types | Human liver cancer and HeLa cells in vitro | ||
| Detonation nanodiamond | Human embryonic kidney cells and Xenopus laevis embryos | ||
| P-dots | Medium | Quinoxaline based polymer, STV conjugated | Zebrafish embryo |
| Polybutylcyanoacrylate | HeLa and human embryonic kidney cells/rats | ||
| Quantum dots | High | CdSe–ZnS; PEG, BSA or polymer stabilized | Rats |
| CdTe | Mice | ||
| Several types | Various in vitro/in vivo | ||
| Several types | Various in vitro/in vivo | ||
| Rare earth nanoparticles | Medium-high | UCNPs, NaYF4:Yb,Tm, polyacrylic acid coated | Mice |
| UCNPs, NaYF4:Yb,Tm | HeLa cells, caenorhabditis elegans | ||
| DCNPs, Gd2O2S:Tb3+ | Human peripheral blood mononuclear cells, human-derived macrophages, HeLa cells |
| Fluorescent Substance | Excitation Wavelength | Emission Wavelength | |
|---|---|---|---|
| EX nm | EX (sub) | EM nm | |
| Alexa Fluor 532 | 532 | 554 | |
| Cy3 | 550 | 570 | |
| DsRed | 557 | 579 | |
| EtBr | 300 | 518 | 605 |
| FITC | 490 | 525 | |
| Gel Green | 250 | 500 | 530 |
| GFP | 488 | 507 | |
| mCherry | 580 | 610 | |
| SYBR Gold | 495 | 540 | |
| SYBR Green I | 498 | 522 | |
| SYPRO Red | 550 | 300 | 630 |
| SYPRO Ruby | 280 | 450 | 620 |
| TagRFP | 555 | 583 | |
| Gel Red | 270 | 510 | 600 |
| Probes Size | PFBT ~10 nm | IgG-Alexa 488 ~1 nm | Qdot 565 ~15 nm |
|---|---|---|---|
| Abs/FL λmax (nm) | 460/540 | 496/519 | UV/565 |
| ɛ (M−1cm−1) λ = 488 nm | 1.0 × 107 | 5.3 × 104 | 2.9 × 105 |
| Quantum yield (%) | 30 | 90 | 30~50 |
| Fluorescence lifetime (ns) | 0.6 | 4.2 | ~20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Q.; Li, P.; Xia, Y.; Hu, D.; Li, W.; Shi, D.; Song, K. Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials. Molecules 2023, 28, 3819. https://doi.org/10.3390/molecules28093819
Bu Q, Li P, Xia Y, Hu D, Li W, Shi D, Song K. Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials. Molecules. 2023; 28(9):3819. https://doi.org/10.3390/molecules28093819
Chicago/Turabian StyleBu, Qingpan, Ping Li, Yunfei Xia, Die Hu, Wenjing Li, Dongfang Shi, and Kai Song. 2023. "Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials" Molecules 28, no. 9: 3819. https://doi.org/10.3390/molecules28093819
APA StyleBu, Q., Li, P., Xia, Y., Hu, D., Li, W., Shi, D., & Song, K. (2023). Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials. Molecules, 28(9), 3819. https://doi.org/10.3390/molecules28093819






