Abstract
The enzyme-like activity of metal oxide nanoparticles is governed by a number of factors, including their size, shape, surface chemistry and substrate affinity. For CeO2 nanoparticles, one of the most prominent inorganic nanozymes that have diverse enzymatic activities, the size effect remains poorly understood. The low-temperature hydrothermal treatment of ceric ammonium nitrate aqueous solutions made it possible to obtain CeO2 aqueous sols with different particle sizes (2.5, 2.8, 3.9 and 5.1 nm). The peroxidase-like activity of ceria nanoparticles was assessed using the chemiluminescent method in different biologically relevant buffer solutions with an identical pH value (phosphate buffer and Tris-HCl buffer, pH of 7.4). In the phosphate buffer, doubling CeO2 nanoparticles’ size resulted in a two-fold increase in their peroxidase-like activity. The opposite effect was observed for the enzymatic activity of CeO2 nanoparticles in the phosphate-free Tris-HCl buffer. The possible reasons for the differences in CeO2 enzyme-like activity are discussed.
Keywords:
cerium dioxide; colloids; surface; hydroxyl species; enzyme-like activity; buffer; size effect 1. Introduction
Nanocrystalline cerium dioxide is well known as a multifunctional catalyst [1], a UV-protective material [2,3,4] and a component for highly sensitive gas sensors [5,6,7]. One of the main factors determining the functional characteristics of ceria-based materials is the size of CeO2 particles. For instance, Wu et al. found that the rate of photoinduced decomposition of the herbicide, N-(phosphonomethyl)-glycine, decreases by a factor of 6 with an increase in the particle size of cerium dioxide from 2 to 5 nm [8]. Torrente-Murciano et al. demonstrated that doubling the particle size (from 5 to 10 nm) leads to a decrease in the catalytic activity of CeO2 in the reaction of naphthalene oxidation to CO2 by a factor of 2.5 [9]. It is important to note that the size effect is typical not only for ultrasmall particles of cerium dioxide (less than 10 nm), but also for larger particles (up to 50 nm). Lin et al. found that the rate of conversion of carbon dioxide to methane (at 548 K) decreases by a factor of 3 with an increase in the size of CeO2 particles from 32 to 50 nm [10]. The dependence of the catalytic activity of cerium dioxide on particle size is generally associated with a number of factors, including changes in the surface-to-volume ratio, band gap energy, surface chemistry and electronic structure [11].
In recent years, it has been found that cerium dioxide demonstrates exceptional biological activity, exhibits antibacterial [12,13,14] and antiviral properties [15,16], is characterised by low cytotoxicity [17,18,19], and can act as a UV- and radioprotective agent [20,21]. One of the key mechanisms of the biological action of CeO2 is associated with its ability to mimic the activity of a number of enzymes and exhibit peroxidase- [22], catalase- [23], oxidase- [24], superoxide dismutase- [25], lipoperoxidase-, phospholipoperoxidase- [26], phosphatase- [27], phospholipase- [28], photolipase- [29], haloperoxidase- [30] and urease-like activity [31]. In particular, Lang et al. demonstrated a direct correlation between the antiviral activity of cerium dioxide against human coronavirus OC43 and its haloperoxidase-like activity [30].
Since cerium dioxide is able to catalyse reactions involving enzyme substrates, it might be expected that the size of CeO2 particles will affect the rate of such reactions. There is, however, a scarcity of data in the literature on the effect of particle size on the enzyme-like activity of CeO2. Shlapa et al. showed that an increase in the size of CeO2 particles by a factor of ~2 (from 7 to 15 nm) leads to a decrease in the oxidase-like activity of cerium dioxide by a factor of 1.2 [24]. Henych et al. found that the phospholipase-like activity of CeO2 with a particle size of 5 nm is more than 30 times higher than the activity of 10 nm CeO2 particles [28]. To the best of the authors’ knowledge, there are virtually no data on the size effect on the peroxidase-like activity of CeO2. At the same time, hydrogen peroxide is the most important reactive oxygen species (ROS) that causes oxidative stress in living systems, and the peroxidase activity of enzymes and their mimetics is of paramount importance in biological processes [32].
As a rule, spectrophotometric methods, based on determining the concentration of coloured products of catalytic reactions, are used to determine the ROS-scavenging ability of materials. For this purpose, TMB (3,3′,5,5′-tetramethylbenzidine) assays are the most commonly used. Nevertheless, this approach has several limitations due to the complex mechanism of TMB oxidation that can proceed via either one-electron or two-electron pathways. Moreover, a charge-transfer complex between TMB and its diimine final product can form. All of these products are characterised by different absorption wavelengths, extinction coefficients and formation rate constants [33,34]. For a deeper insight into the chemical interactions of cerium dioxide with biological systems, however, selective methods for determining its enzyme-like activity are of primary importance, especially those that are specific to particular reactive oxygen species (e.g., OH·, HO2· and O2·−). In this context, fluorescent [22] or chemiluminescent [35] methods are considered to be more accurate and informative.
Special attention should be paid to the correct choice of the medium used for the analysis of enzyme-like activity, e.g., the choice of a physiologically relevant buffer solution [36]. It is generally accepted that it is the pH of the medium used that determines the mechanism of CeO2 interaction with hydrogen peroxide [37], while the presence of phosphate ions affects the activity of cerium dioxide, although not in a completely unambiguous way [38,39,40,41].
In this regard, the accurate analysis of the size effect on the enzyme-like activity of cerium dioxide requires the use of a single synthetic technique that will allow the production of CeO2 with different particle sizes under the same, or similar, conditions, as well as an analysis of its enzyme-like activity within a single analytical approach, taking into account the chemical environment of the nanoparticles. In the present work, a quantitative analysis of the size effect on the peroxidase-like activity of nanocrystalline cerium dioxide was carried out using the chemiluminescent method with two different buffer solutions (namely phosphate and Tris-HCl buffer solutions). These buffer solutions had the same pH (7.4) but differed in the presence or absence of phosphate ions.
2. Results
2.1. Synthesis of Aqueous Cerium Dioxide Sols with a Given Particle Size
Since cerium dioxide is characterised by low solubility in aqueous media and there is a weak dependence of particle size on synthesis temperature, the preparation of CeO2 sols with different sizes of nanoparticles is a complex problem. The approach used in the present study, based on the thermohydrolysis (95 °C) of ceric ammonium nitrate, makes it possible to obtain ultrasmall (up to 5 nm) CeO2 particles possessing high surface activity [42]. In this work, solutions of (NH4)2[Ce(NO3)6] with concentrations of 0.046, 0.092, 0.185, 0.277 and 0.370 M were used to obtain cerium dioxide with a high yield (Table 1). In the course of the preliminary experiments, it was found that the use of a ceric ammonium nitrate solution with a lower concentration (0.046 M) did not produce a CeO2 sol. The resulting solution did not demonstrate the Tyndall effect, which indicated the absence of CeO2 nanoparticles in the solution. As a result of the thermal treatment of the (NH4)2[Ce(NO3)6] solutions with concentrations of 0.092–0.370 M, a series of CeO2 sols with different particle sizes were obtained.

Table 1.
Synthesis conditions and concentrations of aqueous CeO2 sols prepared by thermohydrolysis of ceric ammonium nitrate.
The XRD patterns of the CeO2 sols, which were dried at a low temperature (50 °C), are shown in Figure 1. According to the data obtained, the phase composition of the solid residues corresponds with nanocrystalline cubic cerium dioxide (sp. gr. Fmm, PDF2 00-034-0394). As can be seen from Figure 1a, the peak width decreases with an increase in (NH4)2[Ce(NO3)6] concentration. CeO2 crystallite size was evaluated using the XRD data based on the Scherrer formula. According to Figure 1b, the size of cerium dioxide crystallites increases consistently, in the range of 2–5 nm, with the changes in the concentration of (NH4)2[Ce(NO3)6].
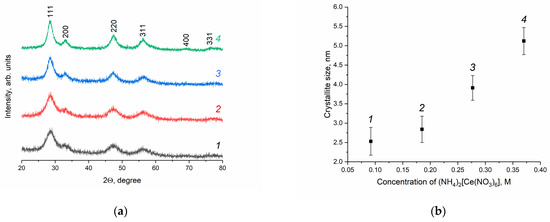
Figure 1.
(a) XRD patterns of CeO2 nanopowders prepared upon the drying (50 °C) of aqueous ceria sols. (b) Dependence of CeO2 crystallite size on the concentration of ceric ammonium nitrate in the reaction mixture. Ceria sols were obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M solutions of (NH4)2[Ce(NO3)6].
According to the generally accepted concepts of nucleation and crystal growth, the growth of solid-phase particles typically proceeds via the dissolution–crystallisation mechanism (Ostwald ripening). Conversely, for cerium dioxide and some other oxides, the mechanism of oriented attachment and growth of particles is usually observed [43,44], particularly under hydrothermal conditions [45]. Apparently, when taking into account the extremely low solubility of CeO2, the observed increase in the size of CeO2 particles with an increase in the initial concentration of (NH4)2[Ce(NO3)6] is due to the implementation of the nonclassical particle growth mechanism.
The high-resolution transmission electron microscopic (HRTEM) images confirm the results of the X-ray diffraction. As can be seen from Figure 2 (see also ESI, Figure S1 (Supplementary Materials)), the CeO2 particle size is approximately 3 nm. The HRTEM images display interplanar distances of about 1.9 Å, which can be attributed to the (220) planes in the crystal lattice of CeO2 [46,47,48].
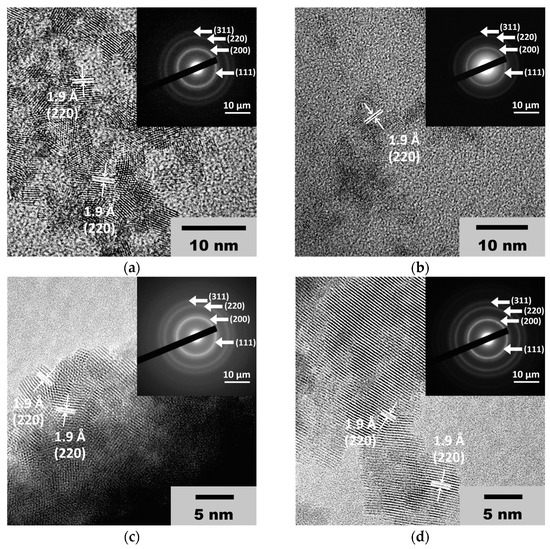
Figure 2.
TEM images and SAED patterns of the CeO2 sols obtained from (a) 0.092 M, (b) 0.185 M, (c) 0.277 M and (d) 0.370 M aqueous solutions of (NH4)2[Ce(NO3)6].
The dynamic light-scattering technique allowed the study of the size distribution of aggregates of individual ceria nanoparticles in the sols (Figure 3). The CeO2 sol obtained from 0.092 M ceric ammonium nitrate solution is characterised by bimodal distribution of aggregates. As the concentration of (NH4)2[Ce(NO3)6] in the starting solution increases, a transition to monomodal distribution of aggregates in the sols is observed. At the same time, with an increase in (NH4)2[Ce(NO3)6] concentration, the size of aggregates increases. It should be noted that during the 3 months of storage under ambient conditions, the size of aggregates in the sol obtained from the solution with the lowest concentration of (NH4)2[Ce(NO3)6] increases by 30% (from 10 to 13 nm), and the size of agglomerates increases by 10% (from 120 to 130 nm), while the aggregate size of the sols obtained from the solutions with higher concentrations of ceric ammonium nitrate (0.185–0.37 M) changes by no more than 15% (Figure 3b).
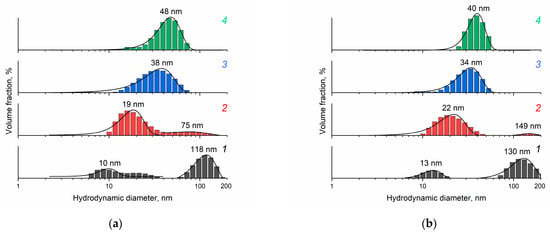
Figure 3.
Hydrodynamic diameter distribution for CeO2 particles in aqueous ceria sols obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M (NH4)2[Ce(NO3)6] aqueous solutions: (a) after synthesis and (b) after three months of storage under ambient conditions. The pH value of all the sols is approximately 2.4.
The results of the electrokinetic measurements are shown in Figure 4 and Figure S2. Thermohydrolysis of ceric ammonium nitrate yields an acidic environment; thus, CeO2 particles acquire a positive charge due to the protonation of surface hydroxyl groups [47]. The pH value of all of the sols obtained is approximately 2.4, so the values of the ζ-potentials are positive (Figure 4). As it follows from Figure 4, with an increase in the concentration of ceric ammonium nitrate in the starting solutions, the values of the ζ-potential of CeO2 nanoparticles increase from +29.9 to +38.2 mV. High ζ-potential values ensure long-term stability of CeO2 colloids, which demonstrate no signs of precipitation after three months of storage under ambient conditions (Figure 3).
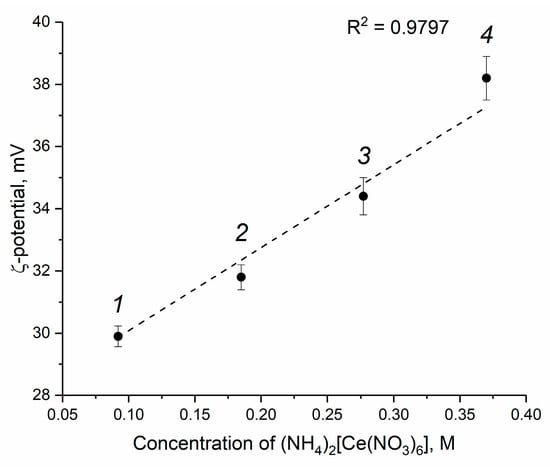
Figure 4.
Dependence of the ζ-potential of CeO2 aqueous sols on the concentration of initial ceric ammonium nitrate solution. The sols were obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.370 M and (4) 0.554 M solutions of (NH4)2[Ce(NO3)6]. The pH value of all the sols is approximately 2.4.
The IR spectroscopy (Figure 5) shows a broad absorption band in the 3400–3000 cm−1 region, which can be attributed to the stretching vibrations of physically adsorbed water [49]. The absorption band at 1625 cm−1 corresponds to the bending vibrations of molecular water [49]. The peaks at 1033 and 450 cm−1 can be attributed to the bending vibrations of the Ce–OH surface groups and to the stretching vibration of the Ce–O bond [50,51]. The absorption bands in the range of 1500–1300 cm−1 and at 1280 cm−1 correspond to the bending C–OH and stretching C–O vibrations of isopropanol traces [52]. The weak absorption band at 807 cm−1 can be attributed to the vibrations of nitrate species adsorbed on the oxide surface [53].
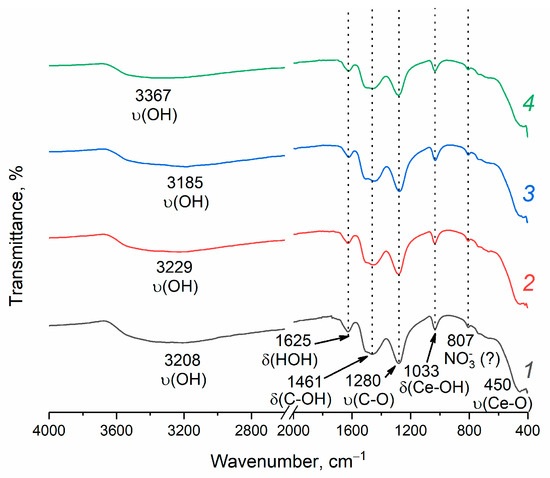
Figure 5.
FTIR spectra of CeO2 nanopowders prepared by drying aqueous ceria sols obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M aqueous solutions of (NH4)2[Ce(NO3)6].
The Raman spectra of the dried ceria sols are typical for nanocrystalline CeO2 (Figure 6) and show an intense F2g mode peak at 453–448 cm−1, which is characteristic of a fluorite structure and is due to the symmetric stretching vibrations of the Ce-O bond [8]. A weak band at about 600 cm−1 can be attributed to the defect-induced (D) mode [54,55]. The band centred at ~270 cm−1 can be ascribed to the second-order transverse acoustic (2TA) mode [7]. The band at 330 cm−1 is characteristic of the 3TL mode of O-Ce-O vibrations [56].
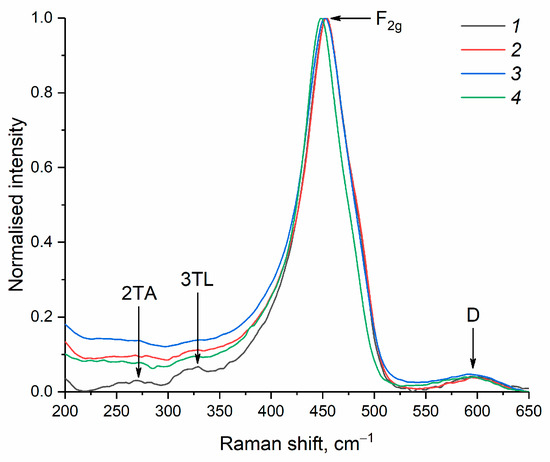
Figure 6.
Raman spectra of CeO2 nanopowders prepared by drying aqueous ceria sols obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M solutions of (NH4)2[Ce(NO3)6].
The main physicochemical characteristics (particle sizes according to the XRD and DLS data, and ζ-potential values) of cerium dioxide colloidal solutions are presented in Table 2.

Table 2.
The main parameters of CeO2 sols.
The specific surface area (SSA) of CeO2 was estimated from the particle sizes, determined according to the powder X-ray diffraction data and the literature data on the density of CeO2 (ρ = 7.215 g/cm3, PDF2 N°00-034-0394), according to Formula (1):
where 6000 is the shape factor, D is the diameter of a spherical CeO2 particle, and ρ is the density of CeO2. The proportion of surface cerium atoms in spherical CeO2 particles (Cesurf) was also evaluated, according to a previously proposed method [57].
2.2. Enzyme-like Activity of CeO2 Sols towards H2O2 Decomposition
The chemiluminescent method used for the analysis of peroxidase-like activity is based on the interaction of a chemiluminescent probe molecule (luminol) with reactive oxygen species through the formation of monoprotonated 3-aminophthalic acid in an electronically excited state [58]. The luminescence intensity of the luminol oxidation product is proportional to the concentration of free radicals, which depends on the activity of the enzyme-like inorganic material. This method of analysis makes it possible to determine the enzyme-like activity of nanomaterials with high analytical precision [59,60,61,62,63,64,65].
At the first stage, the analysis of the enzyme-like activity of cerium dioxide was carried out in a phosphate-buffered solution. The concentration of cerium dioxide in the reaction mixture was the same for all the sols and amounted to 250 μM. When the cerium dioxide sols were added to the reaction mixture containing a phosphate-buffered solution, luminol and hydrogen peroxide, an increase in the luminescence intensity of the luminol oxidation product was observed (Figure 7a). Thus, cerium dioxide catalysed the oxidation of luminol in the presence of H2O2; however, the appearance of the chemiluminescence curves differs from those obtained for horseradish peroxidase [58]. It is most likely that, in a phosphate-buffered solution, cerium dioxide exhibits prooxidant (more precisely, peroxidase-like) activity [66]. In this case, the chemiluminescence intensity increases with an increase in the size of CeO2 particles. A similar effect was observed for the different concentrations of the CeO2 sols at 500 μM and 6 mM.
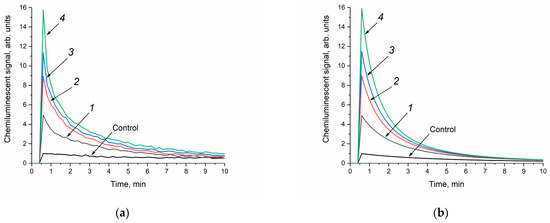
Figure 7.
Luminol (50 μM) chemiluminescence kinetic curves in the presence of H2O2 (10 mM) and CeO2 sols (250 μM) in a phosphate-buffered solution (100 mM, pH = 7.4): the experimental data were obtained via direct chemiluminescent measurements (a), and the calculated curves were obtained through kinetic modelling of the experimental data (b). The aqueous ceria sols were obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M solutions of (NH4)2[Ce(NO3)6]. Control: luminol and H2O2 in a phosphate-buffered solution without CeO2. The chemiluminescence signal was recorded at 36 °C.
The kinetic parameters of the luminol oxidation reactions with hydrogen peroxide in the presence of CeO2 were mathematically modelled (Figure 7b). At the first stage, the interaction of cerium dioxide with H2O2 with the formation of hydroxyl radicals was considered [67]. The chosen kinetic model of H2O2-induced luminol oxidation includes the decomposition of hydrogen peroxide (2), the interaction of luminol with hydroxyl radicals (3), and the final stage of chemiluminescence (4), where P is the reaction product:
As can be seen from Figure 7b, the theoretical kinetic curves fit the experimental data well. The rate constants of reactions (2)–(4) obtained as a result of the mathematical modelling are shown in Table 3.

Table 3.
Rate constants of H2O2-induced luminol oxidation reactions in the presence of aqueous sols of cerium dioxide in a phosphate-buffered solution (100 mM, pH = 7.4).
Since the chemiluminescent analysis technique is based on the registration of the luminescence of the reaction product (4), the constant k4 was chosen as the key kinetic parameter used to compare the peroxidase-like activity of cerium dioxide with different particle sizes. The rate of the chemiluminescence reaction of luminol (4) increased by a factor of 1.2 with a two-fold increase in the size of cerium dioxide particles (see Table 3).
It is known that cerium compounds have a high chemical affinity with phosphate ions, which leads to a change in the composition of CeO2 surface in phosphate-containing media [68,69,70]. The formation of surface complexes with phosphate species affects the enzyme-like activity of cerium dioxide. Thus, it was previously shown that ageing CeO2 in a phosphate-buffered solution reduces the superoxide dismutase activity of cerium dioxide (analysis in Tris-HCl, with pH of 7.4–7.5) [71,72]. Zhao et al. found that phosphate-containing compounds increase the oxidase-like activity of CeO2 by up to six times (acetate buffer, with pH of 4) [40].
In order to exclude the effect of phosphate species on the enzyme-like activity of cerium dioxide, an analysis was also performed on the peroxidase-like activity of CeO2 in Tris-HCl, which has a pH identical to the phosphate-buffered solution (pH = 7.4). Previously, it was shown that the use of Tris-HCl as a dispersion medium does not affect the antioxidant properties of the dispersed CeO2 phase [36]. The concentration of cerium dioxide in Tris-HCl was similar to that in the phosphate buffer and amounted to 250 μM. Upon the introduction of the ceria sols into the buffer solutions containing luminol and hydrogen peroxide, the resulting mixtures remained stable with no visible signs of sedimentation. Figure 8a shows that the addition of the CeO2 aqueous sols to a mixture of Tris-HCl, hydrogen peroxide and luminol leads to an increase in chemiluminescence intensity, compared with a control without cerium dioxide. At the same time, the luminescence intensity of the luminol oxidation product decreases with increasing CeO2 particle size.
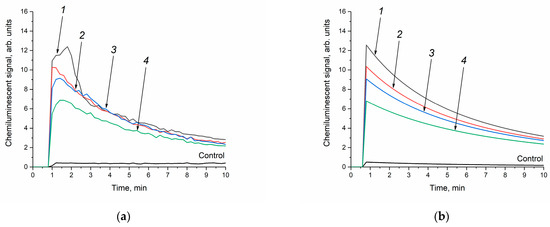
Figure 8.
Luminol (50 μM) chemiluminescence kinetic curves in the presence of H2O2 (10 mM) and CeO2 sols (250 μM) in a Tris-HCl buffer solution (100 mM, pH = 7.4): the experimental data were obtained via direct chemiluminescent measurements (a), and the calculated curves were obtained through kinetic modelling of the experimental data (b). The aqueous ceria sols were obtained from (1) 0.092 M, (2) 0.185 M, (3) 0.277 M and (4) 0.370 M solutions of (NH4)2[Ce(NO3)6]. Control: luminol and H2O2 in a Tris-HCl buffer solution without CeO2. The chemiluminescence signal was recorded at 36 °C.
The appearance of the chemiluminescence curves in the Tris-HCl solution is almost similar to the shape of the curves recorded in the phosphate-buffered solution (Figure 7a and Figure 8a). However, in the Tris-HCl solution, the chemiluminescence intensity decreases more slowly, indicating a decrease in the rate of reactions (3) and (4). As it can be seen from Table 4, the k4 values for the experiments conducted in the Tris-HCl solution are lower than in the phosphate-buffered solution. At the same time, in the Tris-HCl solution, the k2 values are three orders of magnitude higher than in the phosphate buffer. The latter observation supports the abovementioned crucial influence of phosphate species on the reactivity of CeO2 nanoparticles. In the absence of phosphate species, the rate of CeO2 interaction with hydrogen peroxide is significantly higher.

Table 4.
Rate constants of H2O2-induced luminol oxidation in the presence of aqueous cerium dioxide sols in a Tris-HCl solution (100 mM, pH = 7.4).
It is common knowledge that the treatment of cerium dioxide sol with hydrogen peroxide can lead to the formation of peroxide and hydroperoxide species on the surface of CeO2 [73,74]. When H2O2 is added to the CeO2 sol in the Tris-HCl buffer solution (Figure 9, curve 3), a red shift of the Ce4+ absorption band edge (curve 1) is observed, which can be attributed to the formation of peroxo complexes on the CeO2 surface [75,76]. The appearance of a shoulder in the absorption spectrum at 400 nm is consistent with the fact that the colloidal solution acquires a yellow colour, which is characteristic of cerium peroxo or hydroperoxo complexes [77,78]. Conversely, the solution of CeO2 in the phosphate buffer remains colourless after hydrogen peroxide has been added (curve 2). In this case, the red shift of the absorption band upon treatment with H2O2 is less pronounced than for the CeO2 solution in Tris-HCl, and no shoulder is observed for the absorption spectrum at ~400 nm. This result clearly indicates the difference in the manifestation of the enzyme-like activity of cerium dioxide in a decomposition reaction of hydrogen peroxide in a phosphate-buffered solution and in Tris-HCl.
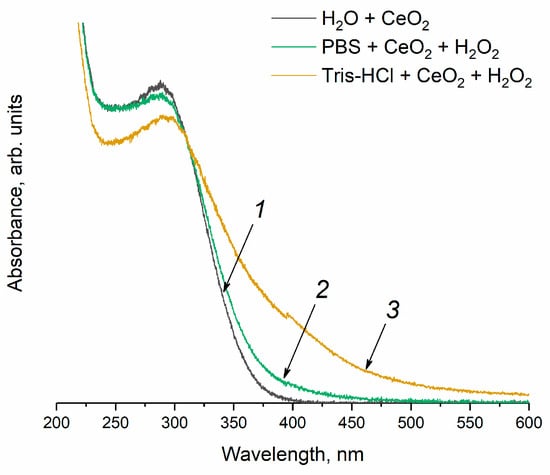
Figure 9.
UV-vis absorption spectra of (1) CeO2 sol (250 μM, particle size is 2.8 nm) in (2) phosphate-buffered solution and (3) Tris-HCl buffer solution after the addition of hydrogen peroxide (10 mM).
For the quantitative comparison of the enzyme-like activity of the cerium dioxide sols in the phosphate-buffered solution and Tris-HCl buffer solution, the value of the integrated intensity (light sum) for a selected period of time (10 min) was used. Figure 10 shows the light sum as a function of (a) CeO2 particle size and (b) the calculated specific surface area of cerium dioxide. In the phosphate-buffered solution, the peroxidase-like activity of CeO2 almost doubles as the particle size doubles, while in the Tris-HCl solution, it decreases by a factor of 1.4.
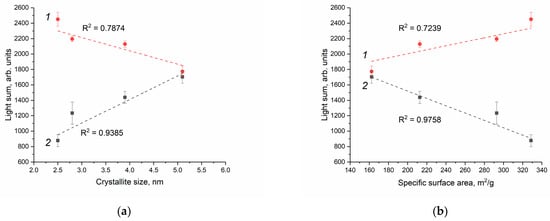
Figure 10.
Dependence of light sums for the reaction of luminol oxidation in the presence of cerium dioxide, obtained by integrating the chemiluminescence kinetic curves, on (a) particle size and (b) CeO2 specific surface area in the Tris-HCl buffer solution (1) and phosphate-buffered solution (2). The pH value of both buffer solutions is 7.4.
As follows from Figure 10, the quantitative dependence obtained for the enzyme-like activity of cerium dioxide towards H2O2 is opposite. In the phosphate-buffered solution, the peroxidase-like activity of CeO2 sols increases with an increase in the particle size of the dispersed phase. This result is quite unexpected, since, with a decrease in particle size, the specific surface area available for interaction with the components of the reaction mixture, including reactive oxygen species, increases. Liu et al., however, previously reported a similar result for citrate-stabilised CeO2 sols. The peroxidase-like activity of cerium dioxide in an acetate buffer solution (pH 4.5) increased by a factor of ~2.3 with an increase in the particle size by a factor of ~1.5 (from 3 to 4 nm) [79]. It is important to note that the redox activity of cerium dioxide depends on pH and that, according to some data, the prooxidant activity of CeO2 increases notably in an acidic environment [37,80].
The interaction of cerium dioxide with the components of the reaction mixture, including phosphate ions, is expected to change the chemical composition of the surface of CeO2 particles. The main functional groups on the surface of cerium dioxide are hydroxyl groups, the amount and reactivity of which directly depend on particle size [81,82]. Previously, Wang et al. showed that surface hydroxylation of ceria nanoparticles is a key factor in determining the adsorption kinetics of phosphate species from KH2PO4 solution. With an increase in the concentration of hydroxyl groups by 1.3 times (from 60 to 76%), the number of adsorbed phosphate species increases by 2.5 times, and the rate of their adsorption increases by 620 times [83]. Thus, phosphate ions can inhibit the interaction of surface-active centres of CeO2 with substrates (luminol and hydrogen peroxide). This effect is apparently the main reason for the decrease in the peroxidase-like activity of CeO2 with a decrease in particle size from 5.1 to 2.8 nm (Figure 10).
It should be noted that the enzyme-like activity of cerium dioxide with a particle size of 5.1 nm is virtually the same in both the phosphate-buffered solution and the Tris-HCl solution (Figure 10). Conversely, the peroxidase-like activity of CeO2 with a particle size of 2.5–3.9 nm in the Tris-HCl solution noticeably exceeds the activity of cerium dioxide in the phosphate-buffered solution. Most probably, this difference can be attributed to the different chemical composition of the Tris-HCl solution and the phosphate-buffered solution. McCormack et al. found that the surface of cerium dioxide particles acquires a negative charge due to the interaction with phosphate species [84]. The surface charge significantly affects the rate of reactions involving charged substrates [34,85]. In the process of free-radical oxidation of luminol, negatively charged intermediates (luminol hydroperoxide anion) can also be formed [58,86]. It is likely that in the phosphate-buffered solution, the interaction of cerium dioxide with negatively charged luminol radical is hindered, resulting in reduced peroxidase-like activity (Figure 10). In addition, the chemiluminescence intensity of luminol depends on the chemical composition of the buffer solution and increases in the presence of halide ions, including Cl− [87].
However, in the Tris-HCl solution, the enzyme-like activity of CeO2 towards hydrogen peroxide naturally decreases with increasing particle size (Figure 10). Similarly, Baldim et al. showed that, in a buffer solution of Tris-HCl (pH 7.5), the superoxide dismutase-like activity of colloidal solutions of CeO2 decreased by 5 times with an increase in the particle size of cerium dioxide from 5 to 23 nm [76]. Lee et al. found that the antioxidant activity of CeO2 stabilised with polyacrylic acid and octylamine also depends on the particle size and decreases by 6 times with the increase in particle size from 4 to 8 nm [88]. In both cases, the decrease in the enzyme-like activity of CeO2 was attributed to a decrease in the ratio of Ce3+ ions; however, the actual oxidation state of cerium in nanocrystalline CeO2 is currently the subject of extensive debates [89]. Recently, the presence of trivalent cerium in nanoscale CeO2 has been questioned [81,89].
Since peroxidases and nanozymes that mimic their activity use hydrogen peroxide to oxidise the substrate (including luminol) [90], the sorption of hydrogen peroxide on the CeO2 surface can be considered the most important stage of the catalytic reaction. Using computational methods, Wang et al. proposed the following mechanism for the peroxidase-like activity of cerium dioxide: adsorption of hydrogen peroxide, dissociation of H2O2 with the formation of hydroxyl radicals, and ·OH reduction [91]. This mechanism is fully consistent with the abovementioned reactions (2)–(4) used to describe the kinetics of H2O2-induced luminol oxidation in the presence of cerium dioxide in a Tris-HCl buffer solution. Therefore, with an increase in the specific surface of CeO2 (Table 2) in the absence of phosphate ions, the sorption of hydrogen peroxide increases, and the process of luminol oxidation proceeds more intensively (see Table 4, constant k4), which was observed in the Tris-HCl solution (Figure 10b).
Thus, the data obtained in the present study indicate that the determination of the enzyme-like activity of CeO2 is a complex task that requires a detailed analysis of a number of factors. The peroxidase-like activity of cerium dioxide is affected by the pH of the medium [37] and the composition of the particle surface [88]. The reactivity of the luminol chemiluminescent probe directly depends on the pH of the buffer solution and, presumably, can be enhanced by the components of the analysed mixture containing halide species (including the buffer). It is also necessary to take into account the presence of species in the reaction mixture that can specifically interact with the enzyme-like material and significantly change its activity. The correct choice of the chemical environment is fundamentally important for obtaining reliable data on the enzyme-like activity of CeO2, including its activity under conditions close to the intracellular environment. An analysis of the interaction of cerium dioxide with reactive oxygen species requires further thorough investigation, and this work gives impetus to the development of appropriate approaches.
3. Materials and Methods
3.1. Materials
Ammonium cerium(IV) nitrate (99.9%, Lanhit (Moscow, Russia)), isopropyl alcohol (99.9%, Chimmed (Moscow, Russia)), hydrogen peroxide (99.9%, Chimmed), luminol (Sigma-Aldrich, St. Louis, MO, USA, 123072), potassium dihydrogen phosphate (Sigma-Aldrich, P0662), potassium hydrogen phosphate (Sigma-Aldrich, P5655), tris-hydrochloride (Merck, Lowe, NJ, USA, 10812846001), and Milli-Ω Water (18.2 MΩ/cm).
3.2. Synthesis of CeO2 Sols
Four samples of nanoceria were prepared via thermohydrolysis of ammonium cerium(IV) nitrate without using additives. (NH4)2[Ce(NO3)6] solutions with different concentrations (0.092, 0.185, 0.277, 0.370 and 0.554 M) were placed in a 100 mL SynthwareTM autoclave (filling degree of 25%) and heated at 95 °C for 24 h. The resulting yellow precipitates were separated by centrifugation (relative centrifugal force of 20,000× g), washed three or four times with isopropyl alcohol, re-dispersed in an appropriate amount of distilled water, and boiled for 3 h to remove the remaining isopropanol. The concentration of CeO2 in the resulting sols was determined gravimetrically. Before the analysis of enzyme-like activity, the resulting sols were diluted to the same concentration, c(CeO2) = 0.05 M.
3.3. Characterisation Methods
The X-ray powder diffraction pattern analysis (XRD) of the sols (dried at 50 °C) was performed using a Bruker (Billerica, MA, USA) D8 Advance diffractometer (CuKα radiation) in the angle range of 20–80° 2θ, with a step of 0.02° 2θ and a signal acquisition time of 0.4 s per step. The full-profile analysis of diffraction patterns was performed using the Bruker (Billerica, MA, USA) TOPAS v.4.2 software, and the diffraction maxima were fitted to Voigt pseudo-functions.
CeO2 nanoparticles were investigated using a JEOL JEM 2100 UHR (Akisima, Tokyo, Japan) transmission electron microscope (TEM) at an acceleration voltage of 200 kV. A drop of the aqueous sol was placed on the formvar/carbon Cu grid (Ted Pella Inc., Redding, CA, USA) and left to evaporate. The samples were treated in an HPT-100 plasma cleaner (Henniker Plasma, Runcorn, UK) prior to being inserted into the microscope chamber in order to remove organic residues from the grid surface. The acquisition of micrographs was performed using a Quemesa 11 MegaPixel CCD (Olympus, Shinjuku/Tokyo, Japan) camera in the bright field mode.
The dynamic light scattering (DLS) and ζ-potential measurements were carried out using a Photocor Compact-Z analyser (Photocor, Moscow, Russia) equipped with a 636.65 nm laser. The sample preparation was carried out using Milli-Ω Water (18.2 MΩ/cm), and a temperature equilibrium was ensured between the sample cell and the cuvette holder. The correlation function for each of the samples was gained by averaging 10 curves, each being acquired for 20 s. Then, the data were filtered by adjusting the permissible deviation of the scattering intensity from the average value (no more than 10%), taking into account the shift of the baseline.
The optical absorption spectra were recorded using quartz cuvettes (10.0 mm optical path length) in a 200–600 nm range, at 0.1 nm steps, on an SF-2000 spectrophotometer (OKB Spectr, Saint-Petersburg, Russia) with a deuterium-halogen light source.
The IR spectra of the samples were recorded in attenuated total reflection geometry using a Spectrum 65 FT IR spectrometer (Perkin Elmer, Waltham, MA, USA) with a spectral resolution of 2 cm−1, in the wavenumber range of 400–4000 cm–1.
The Raman spectra were recorded using a Confotec NR500 spectrometer (SOL Instruments, Minsk, Belarus) with a 514 nm laser, using a ×40 (NA = 0.75) lens at ~30 mW laser power. The spectral resolution was 0.8 cm−1.
The enzyme-like activity (peroxidase/catalase-like) of the CeO2 sols was investigated using the chemiluminescent method with the reaction of luminol oxidation in the presence of hydrogen peroxide in a phosphate (100 mM, pH of 7.4, K2HPO4) or a Tris-HCl (100 mM, pH of 7.4) buffer solution, at 36 °C. The background luminescence was recorded for 60 s after mixing the solutions of hydrogen peroxide (10 mM) and luminol (50 μM). Then, an aliquot (5 μL) of the CeO2 sol was added (250 μM), and a chemiluminescent signal was recorded for 10 min. Light sums were calculated via numerical integration of the chemiluminescence curves using the PowerGraph software v.3.3 (Moscow, Russia).
4. Conclusions
Thermohydrolysis of solutions of ceric ammonium nitrate with concentrations in the range of 0.092–0.370 M produced ceria colloidal solutions with different particle sizes in the range of 2–5 nm. All the obtained sols showed good colloid stability over three months of storage under ambient conditions. For the quantitative analysis of the enzyme-like activity of the CeO2 sols with respect to hydrogen peroxide, a chemiluminescent method was chosen to determine reactive oxygen species based on the interaction of the probe (luminol) with hydroxyl radicals. To address the effect of chemical environment on the CeO2 nanozyme property, the analysis was conducted using two different buffers (Tris-HCl and PBS) at an identical pH (7.4). The peroxidase-like activity of cerium dioxide in the Tris-HCl buffer solution decreased with an increase in particle size. The opposite dependence was registered in the phosphate-buffered solution, while the rate of luminol oxidation in the phosphate-rich medium was significantly lower than in the Tris-HCl medium. According to the kinetic modelling, in the phosphate-rich medium, the rate of CeO2 interaction with H2O2 was more than three orders of magnitude lower than in the Tris-HCl medium. The mechanisms of the enzyme-like activity of CeO2 nanoparticles in different media are discussed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28093811/s1. Figure S1: TEM image of CeO2 nanoparticles in the ceria sol obtained from 0.185 M aqueous solution of (NH4)2[Ce(NO3)6]; Figure S2: ζ-potential values of the CeO2 aqueous sols prepared from (NH4)2[Ce(NO3)6] solutions after three months of storage under ambient conditions.
Author Contributions
Conceptualisation, A.E.B. and V.K.I.; methodology, A.D.F., M.M.S. and A.E.B.; validation, A.D.F. and M.M.S.; formal analysis, A.D.F.; investigation, A.D.F., S.Y.K. and K.A.C.; resources, A.E.B. and V.K.I.; data curation, A.D.F.; writing—original draft preparation, A.D.F.; writing—review and editing, A.E.B. and V.K.I.; visualisation, A.D.F. and S.Y.K.; supervision, V.K.I.; project administration, V.K.I.; funding acquisition, V.K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 19-13-00416.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The analysis of the composition, structure and properties of the obtained materials was carried out using the equipment of the JRC PMR IGIC RAS.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zahir, M.H.; Helal, A.; Suleiman, R.K.; Haq, B.; Kumar, A.M. UV-Protected Polyurethane/f-Oil Fly Ash-CeO2 Coating: Effect of Pre-Mixing f-Oil Fly Ash-CeO2 with Monomers. Polymers 2021, 13, 3232. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Jiang, S.; Bai, H.; Zhang, S. Use of CeO2 Nanoparticles to Enhance UV-Shielding of Transparent Regenerated Cellulose Films. Polymers 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Aklalouch, M.; Calleja, A.; Granados, X.; Ricart, S.; Boffa, V.; Ricci, F.; Puig, T.; Obradors, X. Hybrid sol–gel layers containing CeO2 nanoparticles as UV-protection of plastic lenses for concentrated photovoltaics. Sol. Energy Mater. Sol. Cells 2014, 120, 175–182. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensing properties of nanostructured CeO2-xZrO2 thin films obtained by the sol-gel method. J. Alloys Compd. 2019, 773, 1023–1032. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Chen, J.; Wang, G.-Y.; Wang, D.; Zeng, X.-F.; Wang, J.-X. Citric acid-assisted ultrasmall CeO2 nanoparticles for efficient photocatalytic degradation of glyphosate. Chem. Eng. J. 2021, 425, 130640. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Gilbank, A.; Puertolas, B.; Garcia, T.; Solsona, B.; Chadwick, D. Shape-dependency activity of nanostructured CeO2 in the total oxidation of polycyclic aromatic hydrocarbons. Appl. Catal. B Environ. 2013, 132, 116–122. [Google Scholar] [CrossRef]
- Lin, S.; Li, Z.; Li, M. Tailoring metal-support interactions via tuning CeO2 particle size for enhancing CO2 methanation activity over Ni/CeO2 catalysts. Fuel 2023, 333, 126369. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N.; Kang, Z.C. (Eds.) Binary Rare Earth Oxides; Springer: Dordrecht, The Netherlands, 2004; ISBN 9781402025686. [Google Scholar]
- Zhuo, M.; Ma, J.; Quan, X. Cytotoxicity of functionalized CeO2 nanoparticles towards Escherichia coli and adaptive response of membrane properties. Chemosphere 2021, 281, 130865. [Google Scholar] [CrossRef]
- Leung, Y.H.; Yung, M.M.N.; Ng, A.M.C.; Ma, A.P.Y.; Wong, S.W.Y.; Chan, C.M.N.; Ng, Y.H.; Djurišić, A.B.; Guo, M.; Wong, M.T.; et al. Toxicity of CeO2 nanoparticles—The effect of nanoparticle properties. J. Photochem. Photobiol. B Biol. 2015, 145, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Estes, L.M.; Singha, P.; Singh, S.; Sakthivel, T.S.; Garren, M.; Devine, R.; Brisbois, E.J.; Seal, S.; Handa, H. Characterization of a nitric oxide (NO) donor molecule and cerium oxide nanoparticle (CNP) interactions and their synergistic antimicrobial potential for biomedical applications. J. Colloid Interface Sci. 2021, 586, 163–177. [Google Scholar] [CrossRef]
- Derevianko, S.; Vasylchenko, A.; Kaplunenko, V.; Kharchuk, M.; Demchenko, O.; Spivak, M. Antiviral Properties of Cerium Nanoparticles. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 187–204. [Google Scholar] [CrossRef]
- Nefedova, A.; Rausalu, K.; Zusinaite, E.; Vanetsev, A.; Rosenberg, M.; Koppel, K.; Lilla, S.; Visnapuu, M.; Smits, K.; Kisand, V.; et al. Antiviral efficacy of cerium oxide nanoparticles. Sci. Rep. 2022, 12, 18746. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, X.; Zhang, H.; Lin, S.; Meng, H.; Sun, B.; George, S.; Xia, T.; Nel, A.E.; Zink, J.I. Designed Synthesis of CeO2 Nanorods and Nanowires for Studying Toxicological Effects of High Aspect Ratio Nanomaterials. ACS Nano 2012, 6, 5366–5380. [Google Scholar] [CrossRef]
- Wang, L.; Ai, W.; Zhai, Y.; Li, H.; Zhou, K.; Chen, H. Effects of Nano-CeO2 with Different Nanocrystal Morphologies on Cytotoxicity in HepG2 Cells. Int. J. Environ. Res. Public Health 2015, 12, 10806–10819. [Google Scholar] [CrossRef]
- Lazić, V.; Živković, L.S.; Sredojević, D.; Fernandes, M.M.; Lanceros-Mendez, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Tuning Properties of Cerium Dioxide Nanoparticles by Surface Modification with Catecholate-type of Ligands. Langmuir 2020, 36, 9738–9746. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; de Oliveira, M.M.; Singh, S.; Sakthivel, T.S.; Neal, C.J.; Seal, S.; Ueda-Nakamura, T.; de Lautenschlager, S.O.S.; Nakamura, C.V. Ceria Nanoparticles Decrease UVA-Induced Fibroblast Death through Cell Redox Regulation Leading to Cell Survival, Migration and Proliferation. Front. Bioeng. Biotechnol. 2020, 8, 577557. [Google Scholar] [CrossRef]
- Wang, C.; Blough, E.; Dai, X.; Olajide, O.; Driscoll, H.; Leidy, J.W.; July, M.; Triest, W.E.; Wu, M. Protective Effects of Cerium Oxide Nanoparticles on MC3T3-E1 Osteoblastic Cells Exposed to X-Ray Irradiation. Cell. Physiol. Biochem. 2016, 38, 1510–1519. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, L.; Li, Q.; Jiao, L.; Yu, X.; Gao, X.; Qiu, H.; Zhang, Z.; Bing, W. Will the Bacteria Survive in the CeO2 Nanozyme-H2O2 System? Molecules 2021, 26, 3747. [Google Scholar] [CrossRef] [PubMed]
- Shlapa, Y.; Solopan, S.; Sarnatskaya, V.; Siposova, K.; Garcarova, I.; Veltruska, K.; Timashkov, I.; Lykhova, O.; Kolesnik, D.; Musatov, A.; et al. Cerium dioxide nanoparticles synthesized via precipitation at constant pH: Synthesis, physical-chemical and antioxidant properties. Colloids Surf. B Biointerfaces 2022, 220, 112960. [Google Scholar] [CrossRef] [PubMed]
- Shlapa, Y.; Timashkov, I.; Veltruska, K.; Siposova, K.; Garcarova, I.; Musatov, A.; Solopan, S.; Kubovcikova, M.; Belous, A. Structural and physical-chemical characterization of redox active CeO2 nanoparticles synthesized by precipitation in water-alcohol solutions. Nanotechnology 2021, 32, 315706. [Google Scholar] [CrossRef]
- Gupta, A.; Das, S.; Neal, C.J.; Seal, S. Controlling the surface chemistry of cerium oxide nanoparticles for biological applications. J. Mater. Chem. B 2016, 4, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Sozarukova, M.M.; Proskurnina, E.V.; Popov, A.L.; Kalinkin, A.L.; Ivanov, V.K. New facets of nanozyme activity of ceria: Lipo- and phospholipoperoxidase-like behaviour of CeO2 nanoparticles. RSC Adv. 2021, 11, 35351–35360. [Google Scholar] [CrossRef]
- Wu, X.; Wei, J.; Wu, C.; Lv, G.; Wu, L. ZrO2/CeO2/polyacrylic acid nanocomposites with alkaline phosphatase-like activity for sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120165. [Google Scholar] [CrossRef]
- Henych, J.; Šťastný, M.; Ederer, J.; Němečková, Z.; Pogorzelska, A.; Tolasz, J.; Kormunda, M.; Ryšánek, P.; Bażanów, B.; Stygar, D.; et al. How the surface chemical properties of nanoceria are related to its enzyme-like, antiviral and degradation activity. Environ. Sci. Nano 2022, 9, 3485–3501. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, T.; Qu, C.; Zhang, S.; Li, X.; Qu, Y. Photolyase-Like Catalytic Behavior of CeO2. Nano Lett. 2019, 19, 8270–8277. [Google Scholar] [CrossRef]
- Lang, J.; Ma, X.; Chen, P.; Serota, M.D.; Andre, N.M.; Whittaker, G.R.; Yang, R. Haloperoxidase-mimicking CeO2−x nanorods for the deactivation of human coronavirus OC43. Nanoscale 2022, 14, 3731–3737. [Google Scholar] [CrossRef]
- Korschelt, K.; Schwidetzky, R.; Pfitzner, F.; Strugatchi, J.; Schilling, C.; von der Au, M.; Kirchhoff, K.; Panthöfer, M.; Lieberwirth, I.; Tahir, M.N.; et al. CeO2−x nanorods with intrinsic urease-like activity. Nanoscale 2018, 10, 13074–13082. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Mechanism of the Oxidation of 3,5,3′,5′-Tetramethylbenzidine by Myeloperoxidase Determined by Transient- and Steady-State Kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Pütz, E.; Smales, G.J.; Jegel, O.; Emmerling, F.; Tremel, W. Tuning ceria catalysts in aqueous media at the nanoscale: How do surface charge and surface defects determine peroxidase- and haloperoxidase-like reactivity. Nanoscale 2022, 14, 13639–13650. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C. Haloperoxidase-Catalyzed Luminol Luminescence. Antioxidants 2022, 11, 518. [Google Scholar] [CrossRef]
- Xue, Y.; Zhai, Y.; Zhou, K.; Wang, L.; Tan, H.; Luan, Q.; Yao, X. The Vital Role of Buffer Anions in the Antioxidant Activity of CeO2 Nanoparticles. Chem. A Eur. J. 2012, 18, 11115–11122. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, Z.; Koshy, P.; Sorrell, C.C.; Hart, J.N. Density Functional Theory Investigation of the Biocatalytic Mechanisms of pH-Driven Biomimetic Behavior in CeO2. ACS Appl. Mater. Interfaces 2022, 14, 11937–11949. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef]
- Wang, X.; Lopez, A.; Liu, J. Adsorption of Phosphate and Polyphosphate on Nanoceria Probed by DNA Oligonucleotides. Langmuir 2018, 34, 7899–7905. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Lopez, A.; Su, H.; Liu, J. Promotion and Inhibition of the Oxidase-Mimicking Activity of Nanoceria by Phosphate, Polyphosphate, and DNA. ChemBioChem 2020, 21, 2178–2186. [Google Scholar] [CrossRef]
- Molinari, M.; Symington, A.R.; Sayle, D.C.; Sakthivel, T.S.; Seal, S.; Parker, S.C. Computer-Aided Design of Nanoceria Structures as Enzyme Mimetic Agents: The Role of Bodily Electrolytes on Maximizing Their Activity. ACS Appl. Bio Mater. 2019, 2, 1098–1106. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Teplonogova, M.A.; Ivanova, O.S.; Shekunova, T.O.; Ivonin, I.V.; Baranchikov, A.Y.; Ivanov, V.K. Facile method for fabrication of surfactant-free concentrated CeO2 sols. Mater. Res. Express 2017, 4, 055008. [Google Scholar] [CrossRef]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal Synthesis of CeO2 Nanocrystals: Ostwald Ripening or Oriented Attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Ivanov, V.K.K.; Fedorov, P.P.P.; Baranchikov, A.Y.Y.; Osiko, V.V.V. Oriented attachment of particles: 100 years of investigations of non-classical crystal growth. Russ. Chem. Rev. 2014, 83, 1204–1222. [Google Scholar] [CrossRef]
- Tret’yakov, Y.D.; Baranchikov, A.E.; Kopitsa, G.P.; Ivanov, V.K.; Polezhaeva, O.S. Oxygen nonstoichiometry of nanocrystalline ceria. Russ. J. Inorg. Chem. 2010, 55, 325–327. [Google Scholar] [CrossRef]
- Grabchenko, M.; Mikheeva, N.; Mamontov, G.; Salaev, M.; Liotta, L.; Vodyankina, O. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zhang, X.; He, Y.; Feng, J.; Li, D. Shape/Crystal Facet of Ceria Induced Well-Dispersed and Stable Au Nanoparticles for the Selective Hydrogenation of Phenylacetylene. Catal. Lett. 2019, 149, 361–372. [Google Scholar] [CrossRef]
- Sreeremya, T.S.; Krishnan, A.; Remani, K.C.; Patil, K.R.; Brougham, D.F.; Ghosh, S. Shape-Selective Oriented Cerium Oxide Nanocrystals Permit Assessment of the Effect of the Exposed Facets on Catalytic Activity and Oxygen Storage Capacity. ACS Appl. Mater. Interfaces 2015, 7, 8545–8555. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1977; ISBN 0471629790. [Google Scholar]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size Dependence of Lattice Parameter and Electronic Structure in CeO2 Nanoparticles. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef]
- Deus, R.C.; Cilense, M.; Foschini, C.R.; Ramirez, M.A.; Longo, E.; Simões, A.Z. Influence of mineralizer agents on the growth of crystalline CeO2 nanospheres by the microwave-hydrothermal method. J. Alloys Compd. 2013, 550, 245–251. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Springer Netherlands: Dordrecht, The Netherlands, 1975; ISBN 9789401160193. [Google Scholar]
- Little, L.H. Infrared Spectra of Adsorbed Species; Academic Press: Cambridge, MA, USA, 1966. [Google Scholar]
- Kang, D.; Yu, X.; Ge, M. Morphology-dependent properties and adsorption performance of CeO2 for fluoride removal. Chem. Eng. J. 2017, 330, 36–43. [Google Scholar] [CrossRef]
- Chen, C.; Zhan, Y.; Zhou, J.; Li, D.; Zhang, Y.; Lin, X.; Jiang, L.; Zheng, Q. Cu/CeO2 Catalyst for Water-Gas Shift Reaction: Effect of CeO2 Pretreatment. ChemPhysChem 2018, 19, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Agarwal, R.G.; Kim, H.-J.; Mayer, J.M. Nanoparticle O–H Bond Dissociation Free Energies from Equilibrium Measurements of Cerium Oxide Colloids. J. Am. Chem. Soc. 2021, 143, 2896–2907. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, Y.A.; Proskurnina, E.V. Free radicals and cell chemiluminescence. Biochemistry 2009, 74, 1545–1566. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Shen, Q.; Guo, C.; Hou, Z.; Zhang, J. Hot Topics and Challenges of Regenerative Nanoceria in Application of Antioxidant Therapy. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Giussani, A.; Farahani, P.; Martínez-Muñoz, D.; Lundberg, M.; Lindh, R.; Roca-Sanjuán, D. Molecular Basis of the Chemiluminescence Mechanism of Luminol. Chem. Eur. J. 2019, 25, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Heindl, D.; Josel, H.-P. Chemiluminescent Detection with Horseradish Peroxidase and Luminol. In Nonradioactive Analysis of Biomolecules; Springer: Berlin/Heidelberg, Germany, 2000; pp. 258–261. [Google Scholar]
- Deng, M.; Xu, S.; Chen, F. Enhanced chemiluminescence of the luminol-hydrogen peroxide system by BSA-stabilized Au nanoclusters as a peroxidase mimic and its application. Anal. Methods 2014, 6, 3117–3123. [Google Scholar] [CrossRef]
- Guan, G.; Yang, L.; Mei, Q.; Zhang, K.; Zhang, Z.; Han, M.-Y. Chemiluminescence Switching on Peroxidase-Like Fe3O4 Nanoparticles for Selective Detection and Simultaneous Determination of Various Pesticides. Anal. Chem. 2012, 84, 9492–9497. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 173, 112817. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, X.; Li, J.; Lan, Q.; Min, L.; Ren, C.; Hu, X.; Torrente-Rodríguez, R.M.; Gao, W.; Yang, Z. A nanozyme tag enabled chemiluminescence imaging immunoassay for multiplexed cytokine monitoring. Chem. Commun. 2018, 54, 13813–13816. [Google Scholar] [CrossRef]
- Vlasova, I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef]
- Sozarukova, M.M.; Proskurnina, E.V.; Ivanov, V.K. Prooxidant potential of CeO2 nanoparticles towards hydrogen peroxide. Nanosyst. Phys. Chem. Math. 2021, 12, 283–290. [Google Scholar] [CrossRef]
- Römer, I.; Briffa, S.M.; Arroyo Rojas Dasilva, Y.; Hapiuk, D.; Trouillet, V.; Palmer, R.E.; Valsami-Jones, E. Impact of particle size, oxidation state and capping agent of different cerium dioxide nanoparticles on the phosphate-induced transformations at different pH and concentration. PLoS ONE 2019, 14, e0217483. [Google Scholar] [CrossRef]
- Vlasova, N.; Markitan, O. Phosphate–nucleotide–nucleic acid: Adsorption onto nanocrystalline ceria surface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129214. [Google Scholar] [CrossRef]
- Dahle, J.T.; Livi, K.; Arai, Y. Effects of pH and phosphate on CeO2 nanoparticle dissolution. Chemosphere 2015, 119, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T. Tunable phosphate-mediated stability of Ce3+ ions in cerium oxide nanoparticles for enhanced switching efficiency of their anti/pro-oxidant activities. Biomater. Sci. 2021, 9, 1345. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S. Role of phosphate on stability and catalase mimetic activity of cerium oxide nanoparticles. Colloids Surf. B Biointerfaces 2015, 132, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Damatov, D.; Mayer, J.M. (Hydro)peroxide ligands on colloidal cerium oxide nanoparticles. Chem. Commun. 2016, 52, 10281–10284. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411. [Google Scholar] [CrossRef]
- Bashir, S.M.; Idriss, H. The reaction of propylene to propylene-oxide on CeO2: An FTIR spectroscopy and temperature programmed desorption study. J. Chem. Phys. 2020, 152, 044712. [Google Scholar] [CrossRef] [PubMed]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Baer, D.R.; Samudrala, S.; Engelhard, M.H.; Amonette, J.E.; Thevuthasan, S.; Seal, S. Influence of Aging and Environment on Nanoparticle Chemistry: Implication to Confinement Effects in Nanoceria. J. Phys. Chem. C 2012, 116, 14108–14114. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.J.; Sakthivel, T.S.; Fu, Y.; Seal, S. Aging of Nanoscale Cerium Oxide in a Peroxide Environment: Its Influence on the Redox, Surface, and Dispersion Character. J. Phys. Chem. C 2021, 125, 27323–27334. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Liu, Q.; Lin, A.; Li, S.; Zhang, Y.; Wang, Q.; Li, T.; An, X.; Zhou, Z.; et al. Synthesis-temperature-regulated multi-enzyme-mimicking activities of ceria nanozymes. J. Mater. Chem. B 2021, 9, 7238–7245. [Google Scholar] [CrossRef]
- Seminko, V.V.; Maksimchuk, P.O.; Grygorova, G.V.; Hubenko, K.O.; Yefimova, S.L. Features of ROS generation during hydrogen peroxide decomposition by nanoceria at different pH values. Funct. Mater. 2021, 28, 420. [Google Scholar] [CrossRef]
- Ghosalya, M.K.; Li, X.; Beck, A.; van Bokhoven, J.A.; Artiglia, L. Size of Ceria Particles Influences Surface Hydroxylation and Hydroxyl Stability. J. Phys. Chem. C 2021, 125, 9303–9309. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Butorin, S.M.; Konyukhova, A.D.; Egorov, A.V.; Shiryaev, A.A.; Baranchikov, A.E.; Dorovatovskii, P.V.; Huthwelker, T.; Gerber, E.; et al. Towards the surface hydroxyl species in CeO2 nanoparticles. Nanoscale 2019, 11, 18142–18149. [Google Scholar] [CrossRef]
- Wu, B.; Lo, I.M.C. Surface Functional Group Engineering of CeO2 Particles for Enhanced Phosphate Adsorption. Environ. Sci. Technol. 2020, 54, 4601–4608. [Google Scholar] [CrossRef]
- McCormack, R.N.; Mendez, P.; Barkam, S.; Neal, C.J.; Das, S.; Seal, S. Inhibition of Nanoceria’s Catalytic Activity due to Ce3+ Site-Specific Interaction with Phosphate Ions. J. Phys. Chem. C 2014, 118, 18992–19006. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Chen, K.; Zhu, W.; Liu, X.; Wang, R.; Zhang, X.; Hu, N.; Suo, Y.; Wang, J. Facet-selective response of trigger molecule to CeO2 {1 1 0} for up-regulating oxidase-like activity. Chem. Eng. J. 2017, 330, 746–752. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, R.; Chai, Y.; Wang, C.; Wu, X. Cerium oxide–graphene as the matrix for cholesterol sensor. Anal. Biochem. 2013, 436, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bause, D.E.; Patterson, H.H. Enhancement of luminol chemiluminescence with halide ions. Anal. Chem. 1979, 51, 2288–2289. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V.L. Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Cafun, J.-D.; Kvashnina, K.O.; Casals, E.; Puntes, V.F.; Glatzel, P. Absence of Ce3+ Sites in Chemically Active Colloidal Ceria Nanoparticles. ACS Nano 2013, 7, 10726–10732. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Hubalek Kalbacova, M.; Šmíd, B.; Johánek, V.; Janata, M.; Dinhová, T.N.; Bělinová, T.; Mazur, M.; Vorokhta, M.; Strnad, L. Poly(acrylic acid)-mediated synthesis of cerium oxide nanoparticles with variable oxidation states and their effect on regulating the intracellular ROS level. J. Mater. Chem. B 2021, 9, 7386–7400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, X.; Gao, X. Density Functional Theory Mechanistic Insight into the Peroxidase- and Oxidase-like Activities of Nanoceria. J. Phys. Chem. C 2021, 125, 23098–23104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).