Fatty Acids and Bilirubin as Intrinsic Autofluorescence Serum Biomarkers of Drug Action in a Rat Model of Liver Ischemia and Reperfusion
Abstract
1. Introduction
2. Results
2.1. Fluorescing Fatty Acids in Serum
2.2. Bilirubin Fluorescence in the Serum
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Model
4.3. AF Spectrofluorometric Analysis
4.3.1. AF Spectral Fitting Procedure
4.3.2. Serum AF Spectrofluorometric Analysis for Fatty Acid Estimation
4.3.3. Serum AF Spectrofluorometric Analysis for Bilirubin
4.4. Serum Fatty Acid Quantification by GC/MS
4.5. Serum Bilirubin Biochemical Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | autofluorescence |
| FAME | methylated fatty acids |
| FWHM | full width at the half intensity maximum |
| FXR | Farnesoid-X Receptor |
| GC-MS | Gas-Chromatography-Mass Spectrometry |
| GMG | Half-Gaussian Modified Gaussian functions |
| HETE | hydroxyeicosatetraenoic acids |
| I/R | ischemia/reperfusion |
| MCD | methyl-choline deficient diet |
| NAD(P)H | nicotinamide adenine dinucleotide (phosphate) |
| OCA | obeticholic acid |
References
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-Based Optical Biopsy: An Effective Diagnostic Tool in Hepatology. Liver Int. 2018, 38, 1160–1174. [Google Scholar] [CrossRef]
- Croce, A.C. Light and Autofluorescence, Multitasking Features in Living Organisms. Photochem 2021, 1, 67–125. [Google Scholar] [CrossRef]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Vairetti, M.; Bertone, R.; Freitas, I.; Bottiroli, G. Autofluorescence Spectroscopy of Rat Liver during Experimental Transplantation Procedure. An Approach for Hepatic Metabolism Assessment. Photochem. Photobiol. Sci. 2005, 4, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Kawaguchi, Y.; Arita, J.; Akamatsu, N.; Kaneko, J.; Sakamoto, Y.; Hasegawa, K.; Harihara, Y.; Kokudo, N. Real-Time Confocal Laser Endomicroscopic Evaluation of Primary Liver Cancer Based on Human Liver Autofluorescence. J. Surg. Oncol. 2017, 115, 151–157. [Google Scholar] [CrossRef]
- Wang, H.; Liang, X.; Mohammed, Y.H.; Thomas, J.A.; Bridle, K.R.; Thorling, C.A.; Grice, J.E.; Xu, Z.P.; Liu, X.; Crawford, D.H.G.; et al. Real-Time Histology in Liver Disease Using Multiphoton Microscopy with Fluorescence Lifetime Imaging. Biomed. Opt. Express 2015, 6, 780. [Google Scholar] [CrossRef]
- Popper, H.; Gyorgy, P.; Goldblatt, H. Fluorescent Material (Ceroid) in Experimental Nutritional Cirrhosis. Arch. Path. 1944, 37, 161–168. [Google Scholar]
- Palmer, D.N.; Husbands, D.R.; Winter, P.J.; Blunt, J.W.; Jolly, R.D. Ceroid Lipofuscinosis in Sheep. I. Bis(Monoacylglycero)Phosphate, Dolichol, Ubiquinone, Phospholipids, Fatty Acids, and Fluorescence in Liver Lipopigment Lipids. J. Biol. Chem. 1986, 261, 1766–1772. [Google Scholar] [CrossRef]
- Saif, M.; Kwanten, W.J.; Carr, J.A.; Chen, I.X.; Posada, J.M.; Srivastava, A.; Zhang, J.; Zheng, Y.; Pinter, M.; Chatterjee, S.; et al. Non-Invasive Monitoring of Chronic Liver Disease via near-Infrared and Shortwave-Infrared Imaging of Endogenous Lipofuscin. Nat. Biomed. Eng. 2020, 4, 801–813. [Google Scholar] [CrossRef]
- Ranjit, S.; Dvornikov, A.; Dobrinskikh, E.; Wang, X.; Luo, Y.; Levi, M.; Gratton, E. Measuring the Effect of a Western Diet on Liver Tissue Architecture by FLIM Autofluorescence and Harmonic Generation Microscopy. Biomed. Opt. Express 2017, 8, 3143. [Google Scholar] [CrossRef]
- Bloomer, J.R. Liver Metabolism of Porphyrins and Haem. J. Gastroenterol. Hepatol. 1998, 13, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sassa, S. Modern Diagnosis and Management of the Porphyrias. Br. J. Haematol. 2006, 135, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Okuno, H.; Shibatani, N.; Nakahashi, Y.; Seki, T.; Okazaki, K. Effect of Biliary Obstruction and Internal Biliary Drainage on Hepatic Cytochrome P450 Isozymes in Rats. World J. Gastroenterol. 2008, 14, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
- Chance, B. Spectra and Reaction Kinetics of Respiratory Pigments of Homogenized and Intact Cells. Nature 1952, 169, 215–221. [Google Scholar] [CrossRef]
- Huang, S.; Heikal, A.A.; Webb, W.W. Two-Photon Fluorescence Spectroscopy and Microscopy of NAD(P)H and Flavoprotein. Biophys. J. 2002, 82, 2811–2825. [Google Scholar] [CrossRef]
- Kunz, W.S. Spectral Properties of Fluorescent Flavoproteins of Isolated Rat Liver Mitochondria. FEBS Lett. 1986, 195, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; de Simone, U.; Freitas, I.; Boncompagni, E.; Neri, D.; Cillo, U.; Bottiroli, G. Human Liver Autofluorescence: An Intrinsic Tissue Parameter Discriminating Normal and Diseased Conditions. Lasers Surg. Med. 2010, 42, 371–378. [Google Scholar] [CrossRef] [PubMed]
- la Cour, M.F.; Mehrvar, S.; Kim, J.; Martin, A.; Zimmerman, M.A.; Hong, J.C.; Ranji, M. Optical Imaging for the Assessment of Hepatocyte Metabolic State in Ischemia and Reperfusion Injuries. Biomed. Opt. Express 2017, 8, 4419. [Google Scholar] [CrossRef]
- Rodimova, S.; Kuznetsova, D.; Bobrov, N.; Elagin, V.; Shcheslavskiy, V.; Zagainov, V.; Zagaynova, E. Mapping Metabolism of Liver Tissue Using Two-Photon FLIM. Biomed. Opt. Express 2020, 11, 4458. [Google Scholar] [CrossRef]
- Barkauskas, D.S.; Medley, G.; Liang, X.; Mohammed, Y.H.; Thorling, C.A.; Wang, H.; Roberts, M.S. Using in Vivo Multiphoton Fluorescence Lifetime Imaging to Unravel Disease-Specific Changes in the Liver Redox State. Methods Appl. Fluoresc. 2020, 8, 034003. [Google Scholar] [CrossRef]
- Barbiro, E.; Zurovsky, Y.; Mayevsky, A. Real Time Monitoring of Rat Liver Energy State during Ischemia. Microvasc. Res. 1998, 56, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sato, B.; Tanaka, A.; Mori, S.; Yanabu, N.; Kitai, T.; Tokuka, A.; Inomoto, T.; Iwata, S.; Yamaoka, Y.; Chance, B. Quantitative Analysis of Redox Gradient within the Rat Liver Acini by Fluorescence Images: Effects of Glucagon Perfusion. Biochim. Biophys. Acta 1995, 1268, 20–26. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Vairetti, M.; Bertone, R.; Freitas, I.; Bottiroli, G. Autofluorescence Properties of Isolated Rat Hepatocytes under Different Metabolic Conditions. Photochem. Photobiol. Sci. 2004, 3, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Mannucci, B.; Bottiroli, G.; Vairetti, M. Fluorescing Fatty Acids in Rat Fatty Liver Models. J. Biophotonics 2017, 10, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Berardo, C.; Bottiroli, G.; Vairetti, M.; di Pasqua, L.G. Spectrofluorometric Analysis of Autofluorescing Components of Crude Serum from a Rat Liver Model of Ischemia and Reperfusion. Molecules 2020, 25, 1327. [Google Scholar] [CrossRef]
- Lualdi, M.; Cavalleri, A.; Battaglia, L.; Colombo, A.; Garrone, G.; Morelli, D.; Pignoli, E.; Sottotetti, E.; Leo, E. Early Detection of Colorectal Adenocarcinoma: A Clinical Decision Support Tool Based on Plasma Porphyrin Accumulation and Risk Factors. BMC Cancer 2018, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Hennig, G.; Gruber, C.; Vogeser, M.; Stepp, H.; Dittmar, S.; Sroka, R.; Brittenham, G.M. Dual-Wavelength Excitation for Fluorescence-Based Quantification of Zinc Protoporphyrin IX and Protoporphyrin IX in Whole Blood. J. Biophotonics 2014, 7, 514–524. [Google Scholar] [CrossRef]
- Khan, I.M.; Gjuka, D.; Jiao, J.; Song, X.; Wang, Y.; Wang, J.; Wei, P.; El-Serag, H.B.; Marrero, J.A.; Beretta, L. A Novel Biomarker Panel for the Early Detection and Risk Assessment of Hepatocellular Carcinoma in Patients with Cirrhosis. Cancer Prev. Res. 2021, 14, 667–674. [Google Scholar] [CrossRef]
- Jiao, J.; Kwan, S.Y.; Sabotta, C.M.; Tanaka, H.; Veillon, L.; Warmoes, M.O.; Lorenzi, P.L.; Wang, Y.; Wei, P.; Hawk, E.T.; et al. Circulating Fatty Acids Associated with Advanced Liver Fibrosis and Hepatocellular Carcinoma in South Texas Hispanics. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1643–1651. [Google Scholar] [CrossRef]
- Ahn, S.B. Noninvasive Serum Biomarkers for Liver Steatosis in Nonalcoholic Fatty Liver Disease: Current and Future Developments. Clin. Mol. Hepatol. 2023, 29, S150–S156. [Google Scholar] [CrossRef]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma Fatty Acid Composition, Estimated Desaturase Activities, and Their Relation with the Metabolic Syndrome in a Population at High Risk of Cardiovascular Disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.C. Lipidomic Profiling of Bioactive Lipids by Mass Spectrometry during Microbial Infections. Semin. Immunol. 2013, 25, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Sancho, E.; Abelló, D.; Camps, J.; Joven, J. Novel Circulating Biomarkers for Non-Alcoholic Fatty Liver Disease: A Systematic Review. J. Cell Physiol. 2018, 233, 849–855. [Google Scholar] [CrossRef]
- Konishi, T.; Lentsch, A.B. Changes in Arachidonic Acid Metabolism during Liver Ischemia Triggers Induction of Inflammatory Injury. Hepatology 2018, 68, 1642–1643. [Google Scholar] [CrossRef]
- Elshenawy, O.H.; Shoieb, S.M.; Mohamed, A.; El-Kadi, A.O.S. Clinical Implications of 20-Hydroxyeicosatetraenoic Acid in the Kidney, Liver, Lung and Brain: An Emerging Therapeutic Target. Pharmaceutics 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Kupiec-Weglinski, J.W. Lipid Metabolites: The Alarm Signal to Trigger Liver Ischemia-Reperfusion Injury. Transplantation 2018, 102, 887–889. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R.; Weisz, J. Vitamin A: Recent Advances in the Biotransformation, Transport, and Metabolism of Retinoids. Curr. Opin. Gastroenterol. 2001, 17, 184–192. [Google Scholar] [CrossRef]
- Shirakami, Y.; Lee, S.A.; Clugston, R.D.; Blaner, W.S. Hepatic Metabolism of Retinoids and Disease Associations. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 124–136. [Google Scholar] [CrossRef]
- Blaner, W.S.; Li, Y.; Brun, P.J.; Yuen, J.J.; Lee, S.A.; Clugston, R.D. Vitamin A Absorption, Storage and Mobilization. Subcell. Biochem. 2016, 81, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L.; Ostrow, J.D. Bilirubin Chemistry and Metabolism; Harmful and Protective Aspects. Curr. Pharm. Des. 2009, 15, 2869–2883. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Santin, G.; Vairetti, M.; Bottiroli, G. Bilirubin: An Autofluorescence Bile Biomarker for Liver Functionality Monitoring. J. Biophotonics 2014, 7, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Lamola, A.A. Newborn Jaundice Technologies: Unbound Bilirubin and Bilirubin Binding Capacity in Neonates. Semin. Perinatol. 2011, 35, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A. Bilirubin as a Therapeutic Molecule: Challenges and Opportunities. Antioxidants 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ashino, T.; Kobayashi, Y. Chemical-Induced Coordinated and Reciprocal Changes in Heme Metabolism, Cytochrome P450 Synthesis and Others in the Liver of Humans and Rodents. J. Toxicol. Sci. 2016, 41, SP89–SP103. [Google Scholar] [CrossRef]
- Kurtin, W.E.; Heo, R.; Breimeir, D.J.; Tran, N.T.V.; Elizondo, E.; Salas, R.E.; Morales, M.; Huang, L.; Frank, B. Effects of PH on the Absorption, Emission and Light Scattering Spectroscopy of Bilirubin and Xanthobilirubic Acid in Sodium Taurocholate Solution. J. Chem. Society. Perkin Trans. 1998, 2, 1677–1682. [Google Scholar] [CrossRef]
- Cu, A.; Bellah, G.G.; Lightner, D.A. On the Fluorescence of Bilirubin. J. Am. Chem. Soc. 1975, 97, 2579–2580. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mostovnikov, V.A.; Mostovnikova, G.R.; Tret’yakova, A.I. Spectral Fluorescence and Polarization Characteristics of Z,Z-Bilirubin IXα. J. Appl. Spectrosc. 2007, 74, 120–132. [Google Scholar] [CrossRef]
- Greene, B.I.; Lamola, A.A.; Shank, C.V. Picosecond Primary Photoprocesses of Bilirubin Bound to Human Serum Albumin. Proc. Natl. Acad. Sci. USA 1981, 78, 2008–2012. [Google Scholar] [CrossRef]
- Granucci, G.; Mazzoni, M.; Persico, M.; Toniolo, A. A Computational Study of the Excited States of Bilirubin IX. Phys. Chem. Chem. Phys. 2005, 7, 2594–2598. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; di Pasqua, L.G.; Berardo, C.; Vairetti, M. Fluorescence Excitation Properties of Bilirubin in Solution and in Serum. J. Photochem. Photobiol. B 2021, 215, 112121. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, R.; Fiorucci, S.; Camaioni, E.; Clerici, C.; Costantino, G.; Maloney, P.R.; Morelli, A.; Parks, D.J.; Willson, T.M. 6α-Ethyl-Chenodeoxycholic Acid (6-ECDCA), a Potent and Selective FXR Agonist Endowed with Anticholestatic Activity. J. Med. Chem. 2002, 45, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Palladini, G.; Pasqua, L.G.D.; Berardo, C.; Richelmi, P.; Cadamuro, M.; Fabris, L.; Perlini, S.; Adorini, L.; Vairetti, M. Obeticholic Acid Reduces Biliary and Hepatic Matrix Metalloproteinases Activity in Rat Hepatic Ischemia/Reperfusion Injury. PLoS ONE 2020, 15, e0238543. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Rizzo, V.; Adorini, L.; Richelmi, P.; Vairetti, M. The Farnesoid X Receptor Agonist Obeticholic Acid Upregulates Biliary Excretion of Asymmetric Dimethylarginine via MATE-1 during Hepatic Ischemia/Reperfusion Injury. PLoS ONE 2018, 13, e0191430. [Google Scholar] [CrossRef] [PubMed]
- Udenfriend, S. 6—Proteins and Peptides. In Fluorescence Assay in Biology and Medicine; Academic Press: London, UK, 1969; pp. 248–283. ISBN 9780127058023. [Google Scholar]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-Time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Chorvat, D.; Kirchnerova, J.; Cagalinec, M.; Smolka, J.; Mateasik, A.; Chorvatova, A. Spectral Unmixing of Flavin Autofluorescence Components in Cardiac Myocytes. Biophys. J. 2005, 89, L55–L57. [Google Scholar] [CrossRef]
- Brown, S.B. (Ed.) An Introduction to Spectroscopy for Biochemists; Academic Press: London, UK, 1980; pp. 14–65. [Google Scholar]
- Tamura, Y.; Inoue, H.; Takemoto, S.; Hirano, K.; Miyaura, K. A Rapid Method to Measure Serum Retinol Concentrations in Japanese Black Cattle Using Multidimensional Fluorescence. J. Fluoresc. 2021, 31, 91–96. [Google Scholar] [CrossRef]
- de Pee, S.; Dary, O. Biochemical Indicators of Vitamin A Deficiency: Serum Retinol and Serum Retinol Binding Protein. J. Nutr. 2002, 132, 2895S–2901S. [Google Scholar] [CrossRef]
- Rao, J.; Qian, X.; Wang, P.; Pu, L.; Zhai, Y.; Wang, X.; Zhang, F.; Lu, L. All-Trans Retinoic Acid Preconditioning Protects against Liver Ischemia/Reperfusion Injury by Inhibiting the Nuclear Factor Kappa B Signaling Pathway. J. Surg. Res. 2013, 180, e99–e106. [Google Scholar] [CrossRef]
- Chaves, G.V.; Peres, W.A.F.; Gonçalves, J.C.; Ramalho, A. Vitamin A and Retinol-Binding Protein Deficiency among Chronic Liver Disease Patients. Nutrition 2015, 31, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D. Bilirubin as a Metabolic Hormone: The Physiological Relevance of Low Levels. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef]
- Gordon, D.M.; Hong, S.H.; Kipp, Z.A.; Hinds, T.D. Identification of Binding Regions of Bilirubin in the Ligand-Binding Pocket of the Peroxisome Proliferator-Activated Receptor-A (PPARalpha). Molecules 2021, 26, 2975. [Google Scholar] [CrossRef]
- Athar, H.; Ahmad, N.; Tayyab, S.; Qasim, M.A. Use of Fluorescence Enhancement Technique to Study Bilirubin-Albumin Interaction. Int. J. Biol. Macromol. 1999, 25, 353–358. [Google Scholar] [CrossRef]
- Park, H.K.; Kang, S.W.; Park, M.S. Hesperidin Ameliorates Hepatic Ischemia-Reperfusion Injury in Sprague-Dawley Rats. Transplant. Proc. 2019, 51, 2828–2832. [Google Scholar] [CrossRef] [PubMed]
- Lasheen, N.N.; Elayat, W.M.; Elrefai, M.F.M.; Zaki, W.S.; Ahmed, E.H.; El Sheikh, R.M.N.; Abo Raya, D.S.A.; Gad, F.R.S. Possible Role of Garlic Oil in Ameliorating Renal Injury after Liver Ischemia/Reperfusion in Rats. J. Physiol. Pharmacol. 2019, 70, 765–778. [Google Scholar] [CrossRef]
- Güler, S.; Aslaner, A.; Ellidağ, H.Y.; Yildirim, Ş.; Çakir, T. The Protective Effect of Boric Acid on Cholestatic Rat Liver Ischemia Reperfusion Injury. Turk. J. Med. Sci. 2021, 51, 2716–2726. [Google Scholar] [CrossRef]
- Mazzoni, M.; Agati, G.; Troup, G.J.; Pratesi, R. Analysis of Wavelength-Dependent Photoisomerization Quantum Yields in Bilirubins by Fitting Two Exciton Absorption Bands. J. Opt. A Pure Appl. Opt. 2003, 5, S374. [Google Scholar] [CrossRef]
- Palladini, G.; Ferrigno, A.; Rizzo, V.; Boncompagni, E.; Richelmi, P.; Freitas, I.; Perlini, S.; Vairetti, M. Lobe-Specific Heterogeneity and Matrix Metalloproteinase Activation after Ischemia/Reperfusion Injury in Rat Livers. Toxicol. Pathol. 2012, 40, 722–730. [Google Scholar] [CrossRef]
- Turato, C.; Vairetti, M.; Cagna, M.; Biasiolo, A.; Ferrigno, A.; Quarta, S.; Ruvoletto, M.; De Siervi, S.; Pontisso, P.; Di Pasqua, L.G. SerpinB3 Administration Protects Liver against Ischemia-Reperfusion Injury. Eur. J. Histochem. 2022, 66, 3561. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]

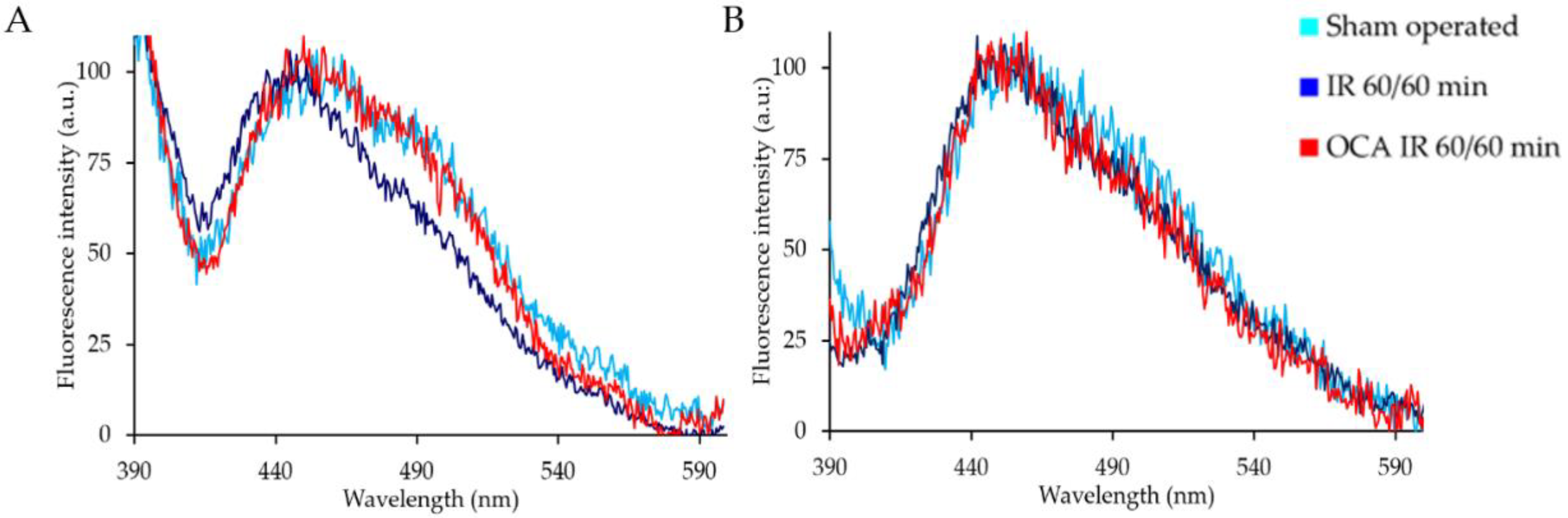

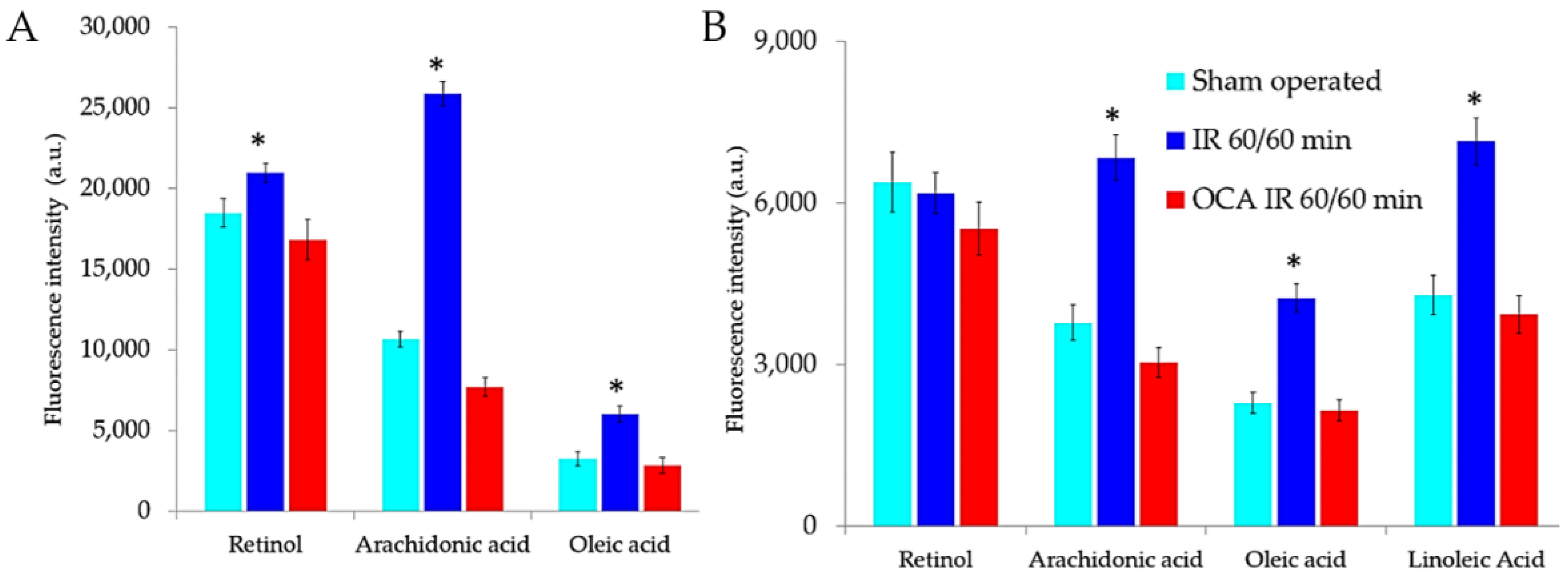

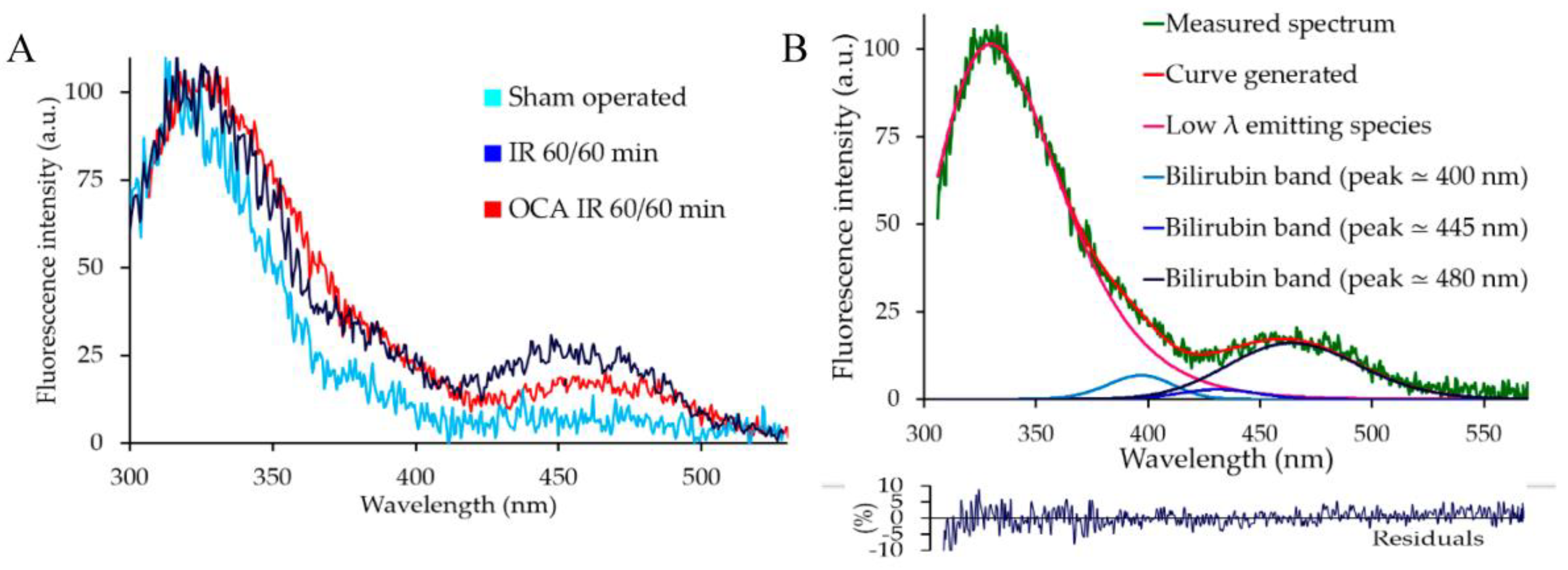

| Liver Treatment | Integrated Emission Intensity (a.u.) | |
|---|---|---|
| 310 nm Excitation | 366 nm Excitation | |
| Sham-operated | 42,454.25 ± 2029.32 * | 18,039.26 ± 1567.21 * |
| Ischemia Reperfusion—60/60 min | 66,351.5 ± 1919.70 * | 25,702.316 ± 1575.70 * |
| OCA—Ischemia Reperfusion—60/60 min | 35,123.24 ± 2644.97 * | 15,815.66 ± 1424.97 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.C.; Ferrigno, A.; Palladini, G.; Mannucci, B.; Vairetti, M.; Di Pasqua, L.G. Fatty Acids and Bilirubin as Intrinsic Autofluorescence Serum Biomarkers of Drug Action in a Rat Model of Liver Ischemia and Reperfusion. Molecules 2023, 28, 3818. https://doi.org/10.3390/molecules28093818
Croce AC, Ferrigno A, Palladini G, Mannucci B, Vairetti M, Di Pasqua LG. Fatty Acids and Bilirubin as Intrinsic Autofluorescence Serum Biomarkers of Drug Action in a Rat Model of Liver Ischemia and Reperfusion. Molecules. 2023; 28(9):3818. https://doi.org/10.3390/molecules28093818
Chicago/Turabian StyleCroce, Anna C., Andrea Ferrigno, Giuseppina Palladini, Barbara Mannucci, Mariapia Vairetti, and Laura G. Di Pasqua. 2023. "Fatty Acids and Bilirubin as Intrinsic Autofluorescence Serum Biomarkers of Drug Action in a Rat Model of Liver Ischemia and Reperfusion" Molecules 28, no. 9: 3818. https://doi.org/10.3390/molecules28093818
APA StyleCroce, A. C., Ferrigno, A., Palladini, G., Mannucci, B., Vairetti, M., & Di Pasqua, L. G. (2023). Fatty Acids and Bilirubin as Intrinsic Autofluorescence Serum Biomarkers of Drug Action in a Rat Model of Liver Ischemia and Reperfusion. Molecules, 28(9), 3818. https://doi.org/10.3390/molecules28093818










