One-Pot Synthesis of 1,3,4-Oxadiazines from Acylhydrazides and Allenoates
Abstract
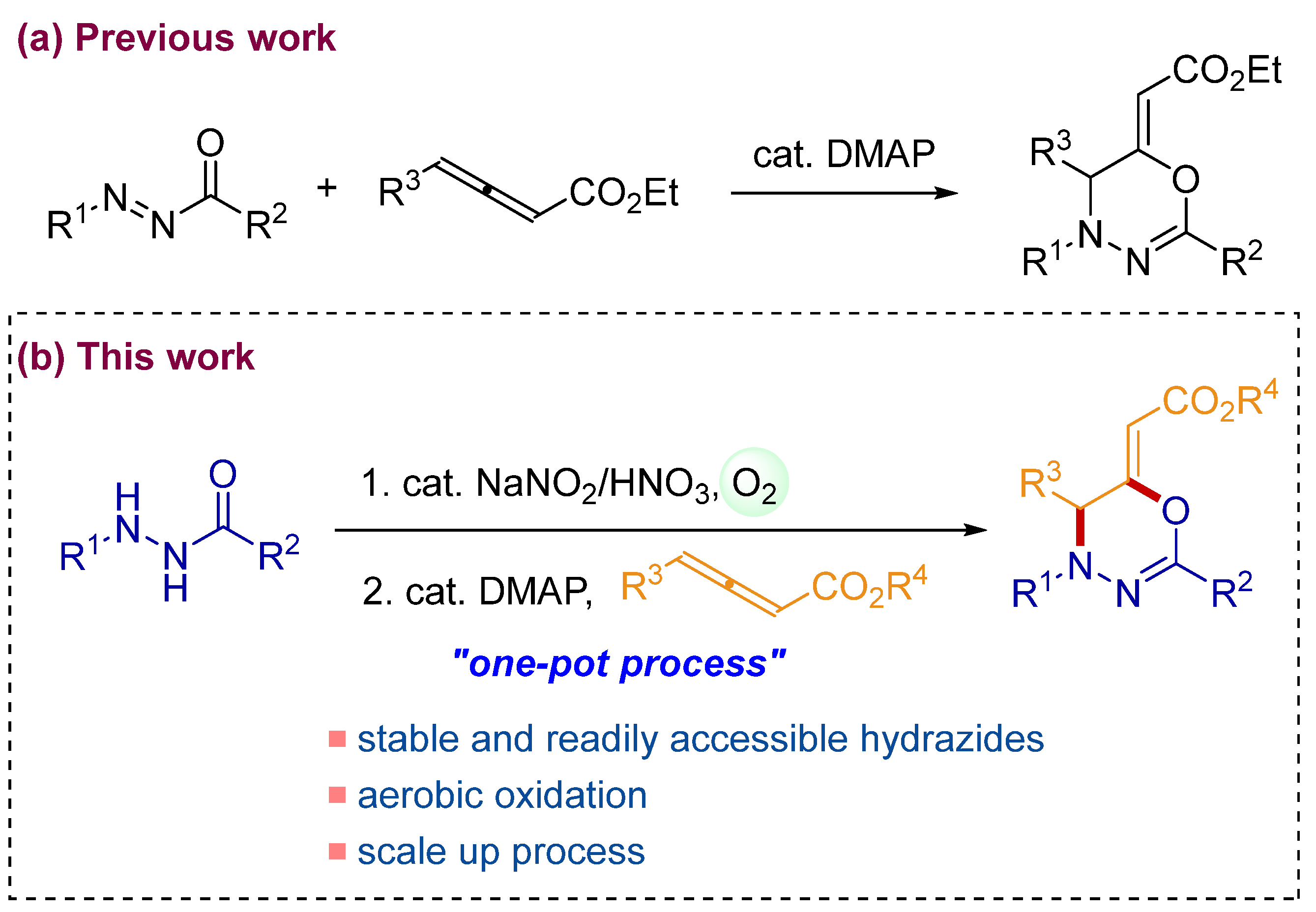
1. Introduction
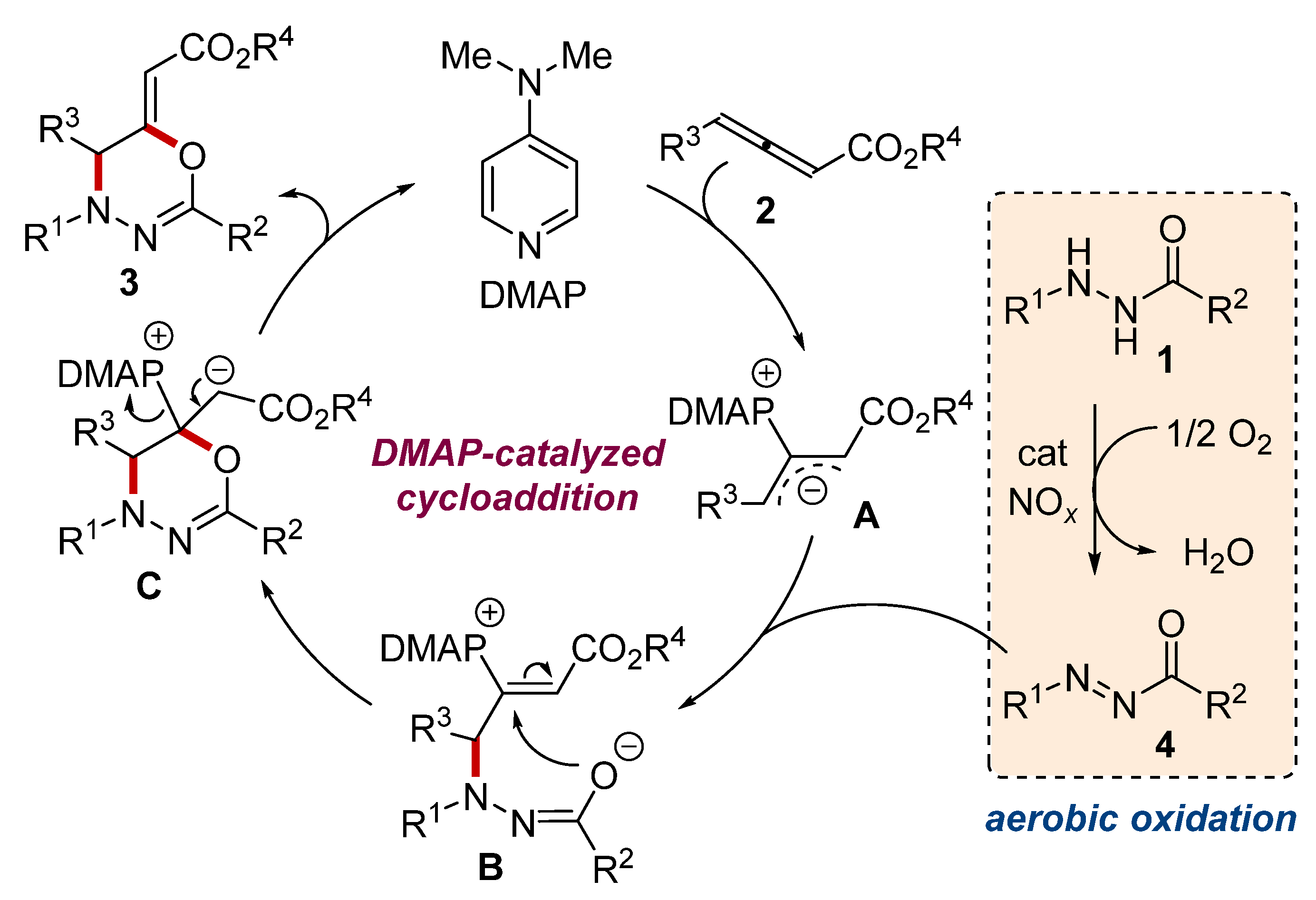
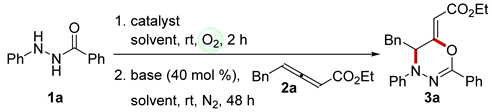
2. Results
3. Materials and Methods
3.1. General Information
3.2. Preparation of Acylhydrazides and Allenoates
3.2.1. Preparation of Acylhydrazides (1a–1j, 1l–1u, and 1w–1x) [49]
3.2.2. Preparation of N′-(tert-butyl)benzohydrazide (1k) [51]
3.2.3. Preparation of N′-phenylcyclohexanecarbohydrazide (1v)
3.2.4. Preparation of Allenoates [52]
3.3. General Procedure for One-Pot Synthesis of 1,3,4-Oxadiazines
3.4. Characterizations of the Newly Synthesized 1,3,4-Oxadiazines
3.5. 1H and 13C NMR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Merino, E. Synthesis of azobenzenes: The coloured pieces of molecular materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Moroz, E.; Castagner, B.; Leroux, J.-C. Activatable Cell Penetrating Peptide−Peptide Nucleic Acid Conjugate via Reduction of Azobenzene PEG Chains. J. Am. Chem. Soc. 2014, 136, 12868–12871. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical Molecular Switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Yamada, M.; Mukaiyama, T. Preparation of Esters of Phosphoric Acid by the Reaction of Trivalent Phosphorus Compounds with Diethyl Azodicarboxylate in the Presence of Alcohols. Bull. Chem. Soc. Jpn. 1967, 40, 935–939. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Yamada, M. Preparation of Esters of Carboxylic and Phosphoric Acid via Quaternary Phosphonium Salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- Mitsunobu, O. The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products. Synthesis 1981, 1981, 1–28. [Google Scholar] [CrossRef]
- But, T.Y.S.; Toy, P.H. The Mitsunobu Reaction: Origin, Mechanism, Improvements, and Applications. Chem. Asian J. 2007, 2, 1340–1355. [Google Scholar] [CrossRef]
- Swamy, K.C.K.; Kumar, N.N.B.; Balaraman, E.; Kumar, K.V.P.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, F.; Suzuki, K.; Nitta, Y. A New Hydrogen-Abstracting Reaction with Diethyl Azodicarboxylate. J. Am. Chem. Soc. 1966, 88, 2328–2329. [Google Scholar] [CrossRef]
- Yoneda, F.; Suzuki, K.; Nitta, Y. A New Hydrogen-Abstracting Reaction with Diethyl Azodicarboxylate. J. Org. Chem. 1967, 32, 727–729. [Google Scholar] [CrossRef]
- Cao, H.T.; Grée, R. DEAD-(cat) ZnBr2 an efficient system for the oxidation of alcohols to carbonyl compounds. Tetrahedron Lett. 2009, 50, 1493–1494. [Google Scholar] [CrossRef]
- Stone, M.T. An Improved Larock Synthesis of Quinolines via a Heck Reaction of 2-Bromoanilines and Allylic Alcohols. Org. Lett. 2011, 13, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mourya, M.; Guin, D.; Joshi, Y.C.; Dobhal, M.P.; Basak, A.K. Diisopropyl azodicarboxylate mediated selective dehydrogenation of 2-amino-3-cyano 4H-chromenes. Tetrahedron Lett. 2017, 58, 1727–1732. [Google Scholar] [CrossRef]
- Bang, S.B.; Kim, J. Efficient dehydrogenation of 1,2,3,4-tetrahydroquinolines mediated by dialkyl azodicarboxylates. Synth. Commun. 2018, 48, 1291–1298. [Google Scholar] [CrossRef]
- Xu, X.; Li, X. Copper/Diethyl Azodicarboxylate Mediated Regioselective Alkynylation of Unactivated Aliphatic Tertiary Methylamine with Terminal Alkyne. Org. Lett. 2009, 11, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.N.; Singh, P.; Kaur, A.; Singh, P. C-1 Alkynylation of N-Methyltetrahydroisoquinolines through CDC: A Direct Access to Phenethylisoquinoline Alkaloids. Synlett 2012, 23, 760–764. [Google Scholar] [CrossRef]
- Huang, W.; Ni, C.; Zhao, Y.; Hu, J. DIAD-mediated metal-free cross dehydrogenative coupling between tertiary amines and α-fluorinated sulfones. New J. Chem. 2013, 37, 1684–1687. [Google Scholar] [CrossRef]
- Singh, K.N.; Kessar, S.V.; Singh, P.; Singh, P.; Kaur, M.; Batra, A. Transition-Metal-Free Arylation of N-Alkyl-tetrahydroisoquinolines under Oxidative Conditions: A Convenient Synthesis of C1-Arylated Tetrahydroisoquinoline Alkaloids. Synthesis 2014, 46, 2644–2650. [Google Scholar] [CrossRef]
- Suga, T.; Iizuka, S.; Akiyama, T. Versatile and highly efficient oxidative C(sp3)–H bond functionalization of tetrahydroisoquinoline promoted by bifunctional diethyl azodicarboxylate (DEAD): Scope and mechanistic insights. Org. Chem. Front. 2016, 3, 1259–1264. [Google Scholar] [CrossRef]
- Kim, Y.H.; Gil, M.G.; Kim, D.Y. Diethyl Azodicarboxylate-promoted Oxidative Coupling Reaction of N-Phenyl Tetrahydroisoquinoline with β-Keto Acids. Bull. Korean Chem. Soc. 2017, 38, 1499–1502. [Google Scholar] [CrossRef]
- Sharma, S.; Han, S.H.; Han, S.; Ji, W.; Oh, J.; Lee, S.-Y.; Oh, J.S.; Jung, Y.H.; Kim, I.S. Rh(III)-Catalyzed Direct Coupling of Azobenzenes with α-Diazo Esters: Facile Synthesis of Cinnolin-3(2H)-ones. Org. Lett. 2015, 17, 2852–2855. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Scheidt, K.A. Direct Amination of Homoenolates Catalyzed by N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2008, 130, 2740–2741. [Google Scholar] [CrossRef]
- Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. [4+2] Cycloaddition of Ketenes with N-Benzoyldiazenes Catalyzed by N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2009, 48, 192–195. [Google Scholar] [CrossRef]
- Morrill, L.C.; Lebl, T.; Slawin, A.M.Z.; Smith, A.D. Catalytic asymmetric α-amination of carboxylic acids using isothioureas. Chem. Sci. 2012, 3, 2088–2093. [Google Scholar] [CrossRef]
- Taylor, J.E.; Daniels, D.S.B.; Smith, A.D. Asymmetric NHC-Catalyzed Redox α-Amination of α-Aroyloxyaldehydes. Org. Lett. 2013, 15, 6058–6061. [Google Scholar] [CrossRef]
- Morrill, L.C.; Smith, S.M.; Slawin, A.M.Z.; Smith, A.D. Isothiourea-Mediated Asymmetric Functionalization of 3-Alkenoic Acids. J. Org. Chem. 2014, 79, 1640–1655. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Lee, R.; Lv, Y.; Huang, K.-W.; Zhong, G. Asymmetric NHC-Catalyzed Aza-Diels–Alder Reactions: Highly Enantioselective Route to α-Amino Acid Derivatives and DFT Calculations. Org. Lett. 2014, 16, 3872–3875. [Google Scholar] [CrossRef]
- Savva, A.C.; Mirallai, S.I.; Zissimou, G.A.; Berezin, A.A.; Demetriades, M.; Kourtellaris, A.; Constantinides, C.P.; Nicolaides, C.; Trypiniotis, T.; Koutentis, P.A. Preparation of Blatter Radicals via Aza-Wittig Chemistry: The Reaction of N-Aryliminophosphoranes with 1-(Het)aroyl-2-aryldiazenes. J. Org. Chem. 2017, 82, 7564–7575. [Google Scholar] [CrossRef]
- Bigotto, A.; Forchiassin, M.; Fisaliti, A.; Russo, C. Reactions of cyclohexanone enamines with asymmeric diimides. Tetrahedron Lett. 1979, 20, 4761–4764. [Google Scholar] [CrossRef]
- Forchiassin, M.; Risaliti, A.; Russo, C. 1,3,4-Oxadiazine derivatives from cyclohexanone enamines and asymmetric diimides: Possibility of ring-chain tautomerism in such heterocyclic system. Tetrahedron 1981, 37, 2921–2928. [Google Scholar] [CrossRef]
- Zhou, R.; Han, L.; Zhang, H.; Liu, R.; Li, R. A Deoxygenative [4+1] Annulation Involving N-Acyldiazenes for an Efficient Synthesis of 2,2,5-Trisubstituted 1,3,4-Oxadiazole Derivatives. Adv. Synth. Catal. 2017, 359, 3977–3982. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, J.-Y.; Zhang, Y.-Z.; Mei, G.-J.; Shi, F. Catalytic Asymmetric [2+3] Cyclizations of Azlactones with Azonaphthalenes. Angew. Chem. Int. Ed. 2018, 57, 5398–5402. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Meng, L.-G.; Peng, T.; Zhu, L.; Wang, L. 4-Dimethylaminopyridine-Catalyzed Regioselective [3+2] Cycloaddition of Isatin-Derived Morita−Baylis−Hillman Adducts with Azo Esters: A Simple Protocol to Access 3-Spiropyrazole-2-oxindoles. Adv. Synth. Catal. 2018, 360, 3176–3180. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.-N.; Ma, C.; Mei, G.-J.; Shi, F. Diastereo- and Enantioselective Construction of Dihydrobenzo[e]indole Scaffolds via Catalytic Asymmetric [3 + 2] Cycloannulations. J. Org. Chem. 2018, 83, 9190–9200. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, L.-G.; Zhang, J.; Wang, L. DMAP-Catalyzed [2+4] Cycloadditions of Allenoates with N-Acyldiazenes: Direct Method to 1,3,4-Oxadiazine Derivatives. Org. Lett. 2015, 17, 3272–3275. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Cheng, Y.; Chang, X.; Li, X.; Li, P. Organocatalytic enantioselective [2 + 4]-annulation of γ-substituted allenoates with N-acyldiazenes for the synthesis of optically active 1,3,4-oxadiazines. Org. Biomol. Chem. 2021, 19, 1727–1731. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J. Aerobic Oxidation of Alkyl 2-Phenylhydrazinecarboxylates Catalyzed by CuCl and DMAP. J. Org. Chem. 2018, 83, 1673–1679. [Google Scholar] [CrossRef]
- Jo, G.; Kim, M.H.; Kim, J. A practical route to azo compounds by metal-free aerobic oxidation of arylhydrazides using an NOx system. Org. Chem. Front. 2020, 7, 834–839. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.H.; Kim, J. Cu-Catalyzed Aerobic Oxidative Azo-Ene Cyclization. Adv. Synth. Catal. 2021, 363, 4728–4733. [Google Scholar] [CrossRef]
- Jung, D.; Kim, M.H.; Kim, J. Cu-Catalyzed Aerobic Oxidation of Di-tert-butyl Hydrazodicarboxylate to Di-tert-butyl Azodicarboxylate and Its Application on Dehydrogenation of 1,2,3,4-Tetrahydroquinolines under Mild Conditions. Org. Lett. 2016, 18, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Jang, S.H.; Yim, T.; Kim, J. Oxidation Potential Tunable Organic Molecules and Their Catalytic Application to Aerobic Dehydrogenation of Tetrahydroquinolines. Org. Lett. 2018, 20, 6436–6439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ren, L.; Hou, J.; Yu, W.; Chang, J. Annulation Reactions of In-Situ-Generated N-(Het)aroyldiazenes with Isothiocyanates Leading to 2-Imino-1,3,4-oxadiazolines. Org. Lett. 2019, 21, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Q.; Zhao, Q.; Yu, W.; Chang, J. Synthesis of 2-Imino-1,3,4-thiadiazoles from Hydrazides and Isothiocyanates via Sequential Oxidation and P(NMe2)3-Mediated Annulation Reactions. Org. Lett. 2020, 22, 4378–4382. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Q.; Yi, X.; Zhao, Z.; Yu, W.; Chang, J. Synthesis of 2-Imino-1,3,4-Selenadiazoles via Tributylphosphine-Mediated Annulation of N-Aroyldiazenes with Isoselenocyanates. Adv. Synth. Catal. 2021, 363, 4894–4898. [Google Scholar] [CrossRef]
- Kim, S.B.; Baek, S.E.; Lim, J.H.; Kim, J. One-pot synthesis of 2-imino-1,3,4-thiadiazolines from acylhydrazides and isothiocyanates. Bull. Korean Chem. Soc. 2022, 43, 1014–1018. [Google Scholar] [CrossRef]
- Lim, J.H.; Baek, S.E.; Lad, B.; Kim, J. Synthesis of 2-Imino-1,3,4-oxadiazolines from Acylhydrazides and Isothiocyanates via Aerobic Oxidation and DMAP-Mediated Annulation Sequence. ACS Omega 2022, 7, 28148–28159. [Google Scholar] [CrossRef]
- Hirose, D.; Taniguchi, T.; Ishibashi, H. Recyclable Mitsunobu Reagents: Catalytic Mitsunobu Reactions with an Iron Catalyst and Atmospheric Oxygen. Angew. Chem. Int. Ed. 2013, 52, 4613–4617. [Google Scholar] [CrossRef]
- Fang, T.; Tan, O.; Ding, Z.; Liu, B.; Bin Xu, B. Pd-Catalyzed Oxidative Annulation of Hydrazides with Isocyanides: Synthesis of 2-Amino-1,3,4-oxadiazoles. Org. Lett. 2014, 16, 2342–2345. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Phosphane-Catalyzed [3 + 2] Annulation of Allenoates with Aldehydes: A Simple and Efficient Synthesis of 2-Alkylidenetetrahydrofurans. Chem. Eur. J. 2009, 15, 8698–8702. [Google Scholar] [CrossRef] [PubMed]

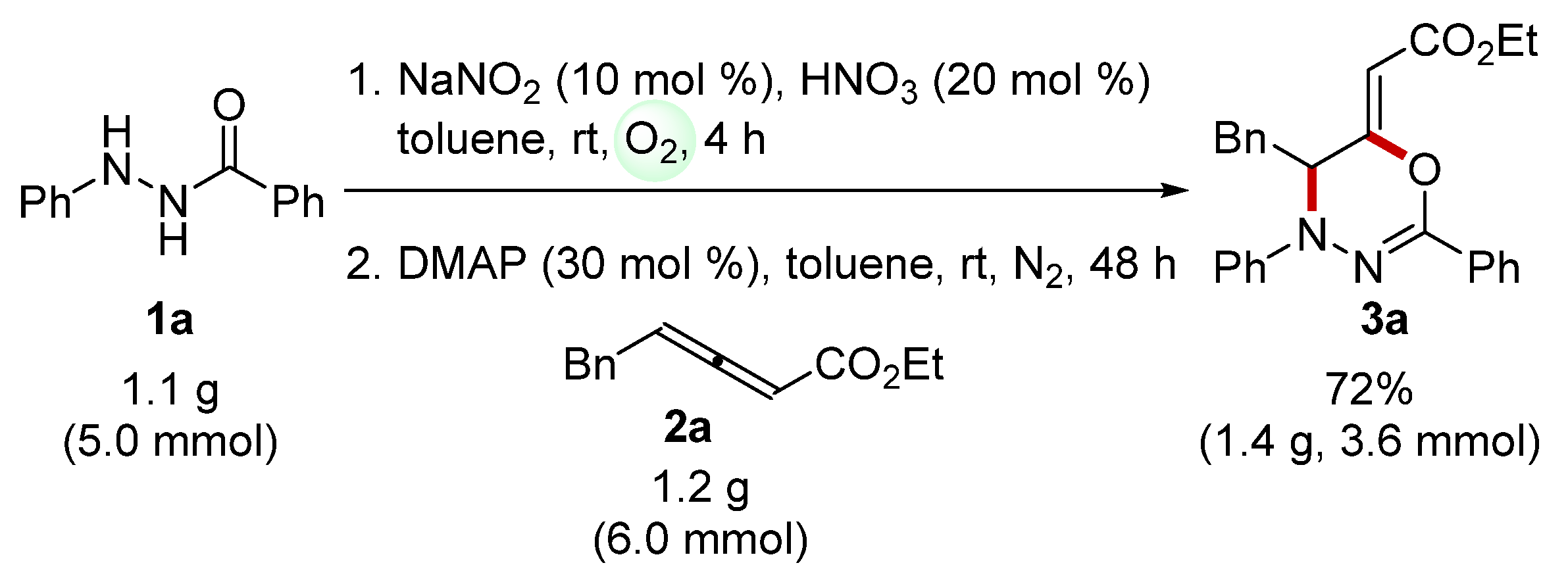
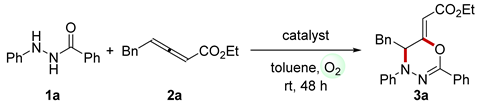 | ||
|---|---|---|
| Entry | Catalyst (mol %) | Yield (%) b |
| 1 | CuCl (10)/DMAP (60) | 14 |
| 2 | Fe(Pc) (10)/DMAP (40) | 15 |
| 3 | Mn(Pc) (10)/DMAP (40) | 19 |
| 4 | NaNO2 (10)/HNO3 (20)/DMAP (40) | 30 |
 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol %) | Base | Solvent | Yield (%) b |
| 1 | CuCl (10)/DMAP (20) | DMAP | toluene | 24 |
| 2 | Fe(Pc) (10) | DMAP | toluene | 30 |
| 3 | Mn(Pc) (10) | DMAP | toluene | 13 |
| 4 | NaNO2 (10)/HNO3 (20) | DMAP | toluene | 75 |
| 5 | NaNO2 (10)/HNO3 (20) | pyridine | toluene | 13 |
| 6 | NaNO2 (10)/HNO3 (20) | DBU | toluene | 0 |
| 7 | NaNO2 (10)/HNO3 (20) | DMAP | CH3CN | 20 |
| 8 | NaNO2 (10)/HNO3 (20) | DMAP | CH2Cl2 | 46 |
| 9 | NaNO2 (10)/HNO3 (20) | DMAP | 1,4-dioxane | 45 |
| 10 | NaNO2 (10)/HNO3 (20) | DMAP | EtOH | 25 |
| 11 c | NaNO2 (10)/HNO3 (20) | DMAP | toluene | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.B.; Maiti, S.; Park, E.S.; Kim, G.Y.; Choun, Y.; Ahn, S.K.; Kim, J.K.; Kim, J. One-Pot Synthesis of 1,3,4-Oxadiazines from Acylhydrazides and Allenoates. Molecules 2023, 28, 3815. https://doi.org/10.3390/molecules28093815
Kim SB, Maiti S, Park ES, Kim GY, Choun Y, Ahn SK, Kim JK, Kim J. One-Pot Synthesis of 1,3,4-Oxadiazines from Acylhydrazides and Allenoates. Molecules. 2023; 28(9):3815. https://doi.org/10.3390/molecules28093815
Chicago/Turabian StyleKim, Su Been, Santanu Maiti, Eun Sun Park, Ga Young Kim, Yunji Choun, Soon Kil Ahn, Jae Kwang Kim, and Jinho Kim. 2023. "One-Pot Synthesis of 1,3,4-Oxadiazines from Acylhydrazides and Allenoates" Molecules 28, no. 9: 3815. https://doi.org/10.3390/molecules28093815
APA StyleKim, S. B., Maiti, S., Park, E. S., Kim, G. Y., Choun, Y., Ahn, S. K., Kim, J. K., & Kim, J. (2023). One-Pot Synthesis of 1,3,4-Oxadiazines from Acylhydrazides and Allenoates. Molecules, 28(9), 3815. https://doi.org/10.3390/molecules28093815







