Abscopal Effect, Extracellular Vesicles and Their Immunotherapeutic Potential in Cancer Treatment
Abstract
1. Introduction
2. The Abscopal Effect in Cancer
3. Extracellular Vesicles: Main Characteristics and Their Role in Cancer Progression
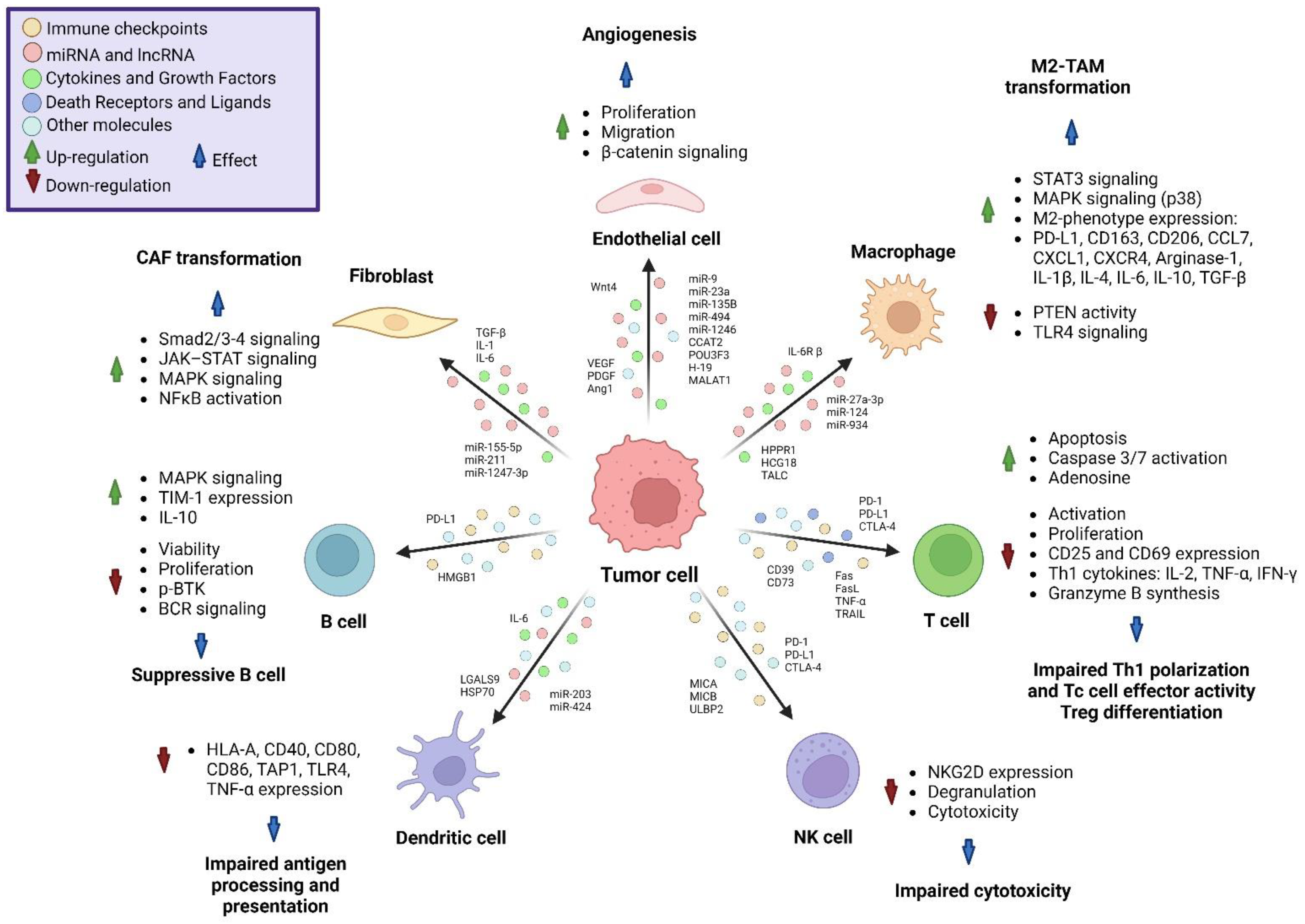
4. Interaction of Tumor-Derived Extracellular Vesicles with Non-Cancer Cells
5. Interaction of Tumor-Derived Extracellular Vesicles with Immune System
5.1. Monocytes
5.2. Dendritic Cells
5.3. T Cells
5.4. NK Cells
5.5. B Cells
6. Extracellular Vesicles Mediating the Abscopal Effect
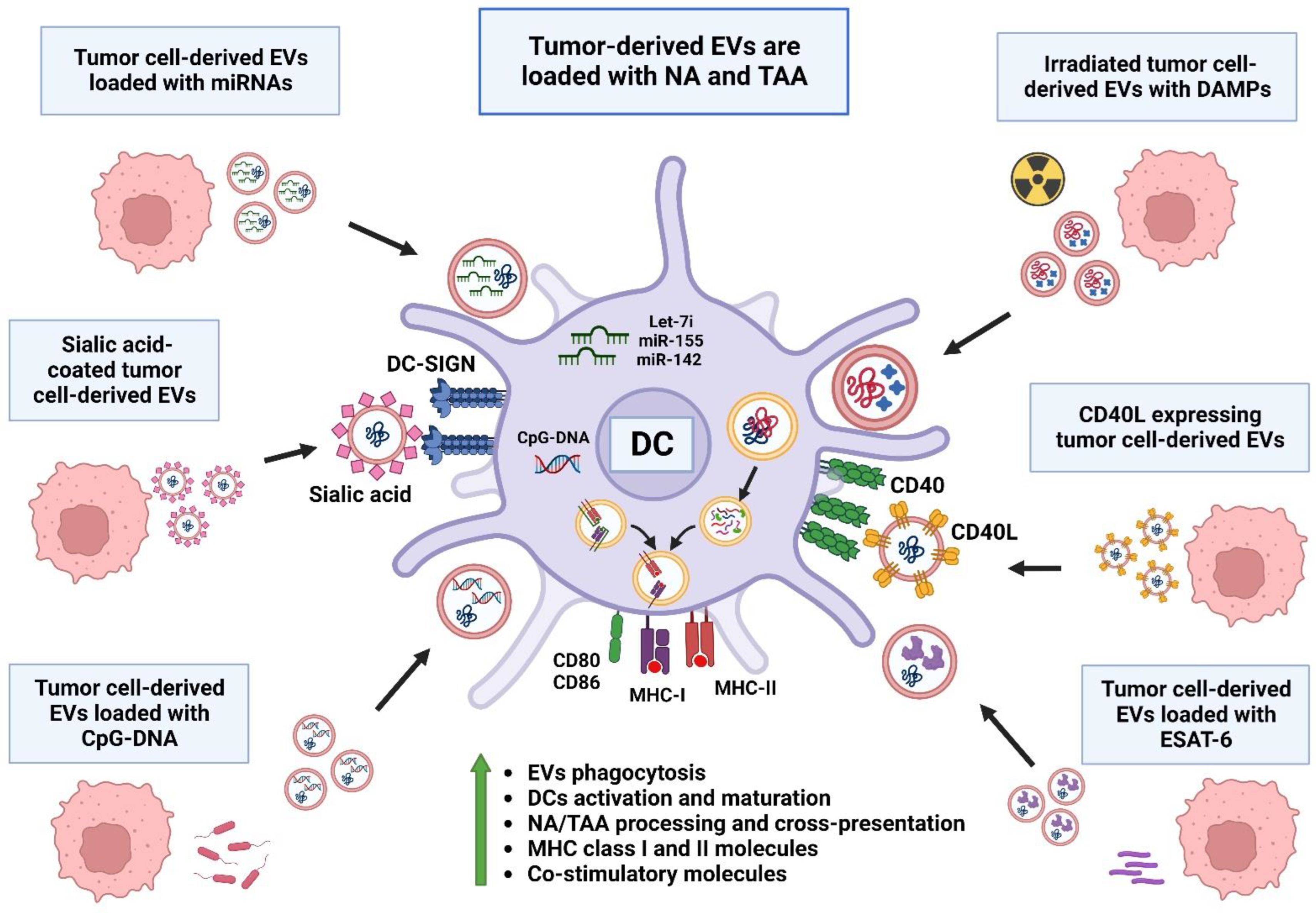
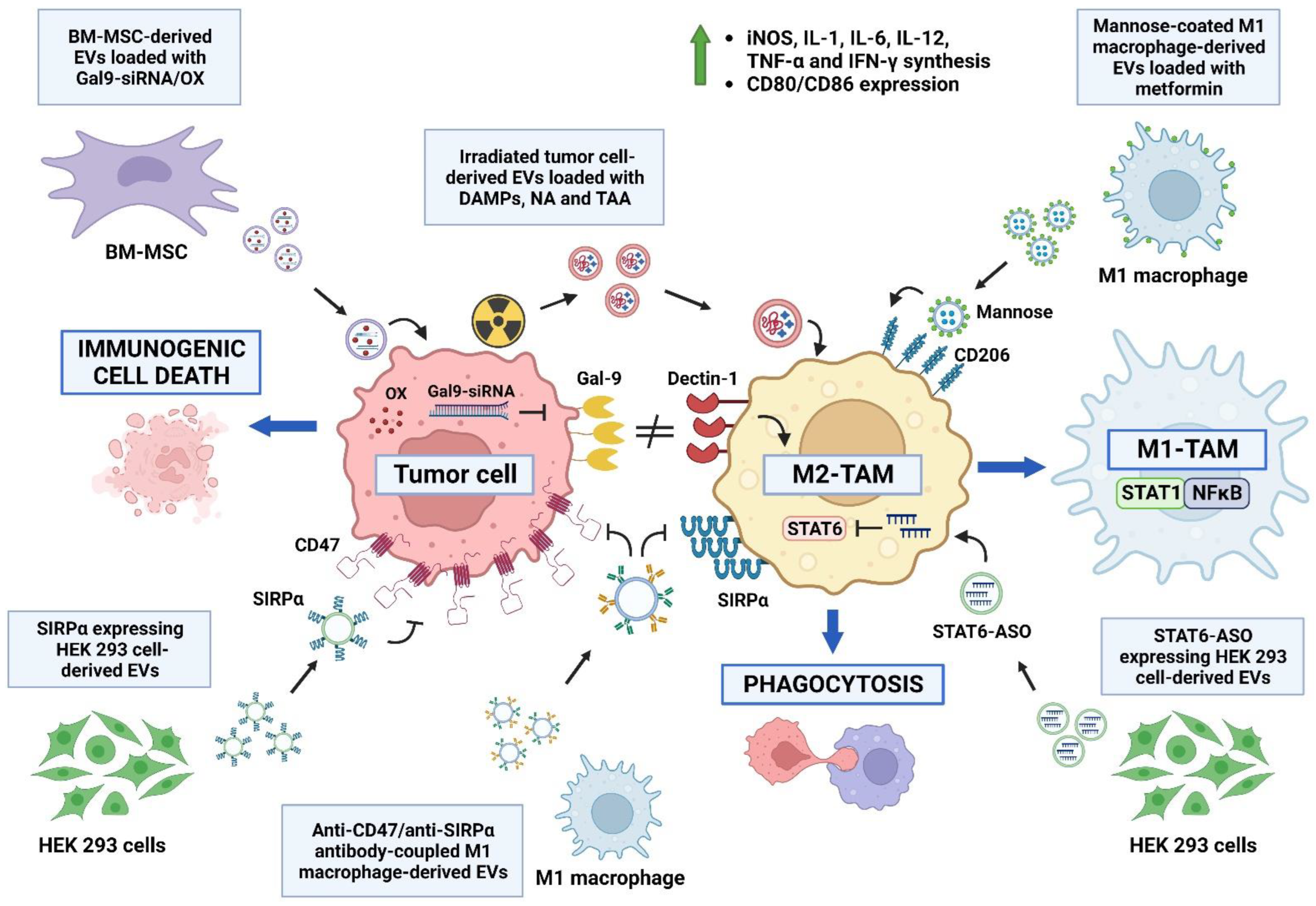
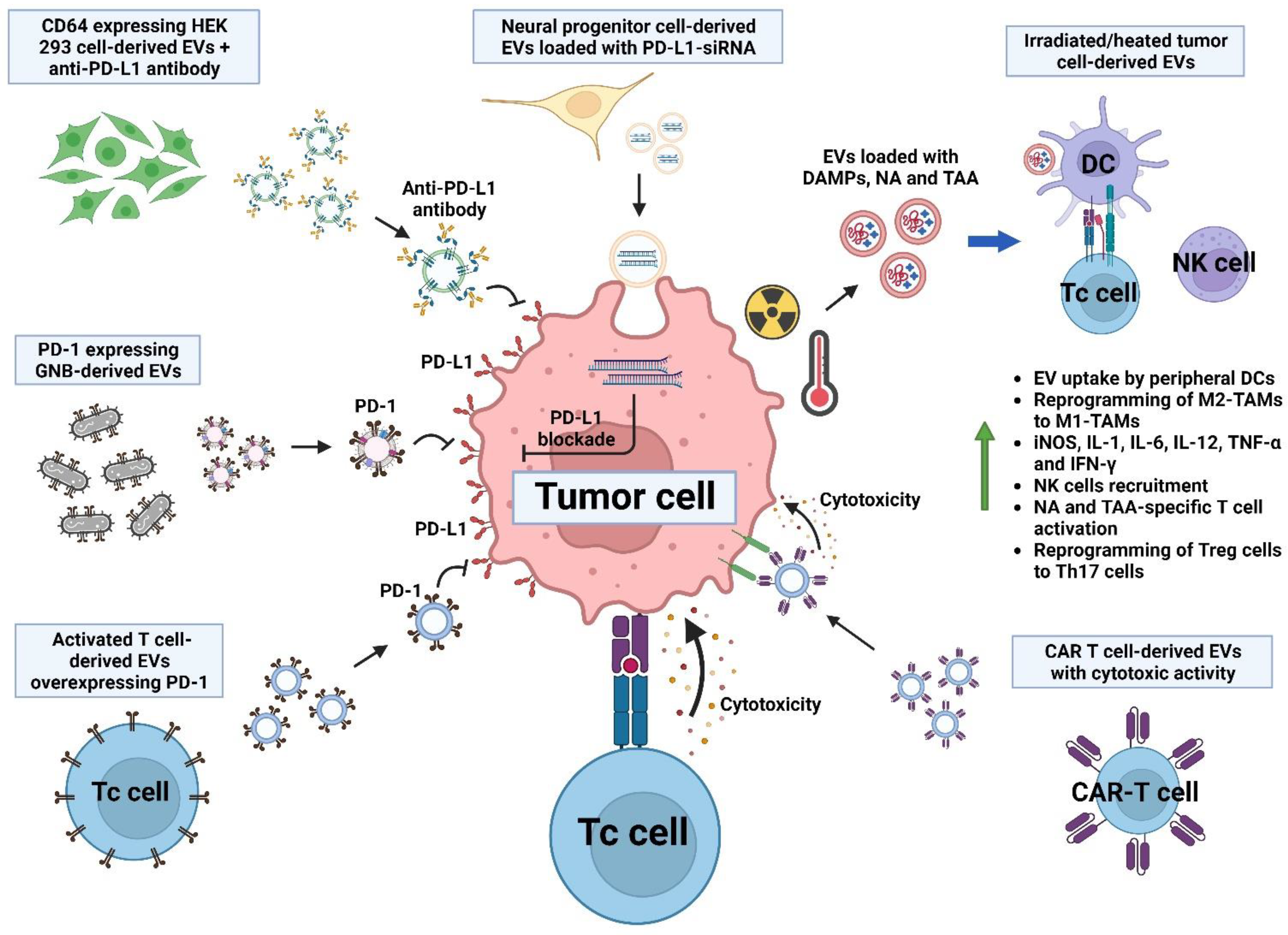
7. Modifying Extracellular Vesicles for Therapeutic Purposes
7.1. Anti-Tumoral Effects of Modified Extracellular Vesicles
7.2. DC Activation via Extracellular Vesicles
7.3. TAM Reprograming via Extracellular Vesicles
7.4. Stimulation of T Cell Response via Extracellular Vesicles and Their Interplay with Immune Checkpoint Inhibition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krieghoff-Henning, E.; Folkerts, J.; Penzkofer, A.; Weg-Remers, S. Cancer—An overview. Med. Monatsschr. Pharm. 2017, 40, 48–54. [Google Scholar]
- Sasieni, P.D.; Shelton, J.; Ormiston-Smith, N.; Thomson, C.S.; Silcocks, P.B. What is the lifetime risk of developing cancer? The effect of adjusting for multiple primaries. Br. J. Cancer 2011, 105, 460–465. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Gelband, H. (Eds.) Cancer Control Opportunities in Low- and Middle-Income Countries; The National Academies Collection: Reports Funded by National Institutes of Health; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Chen, H.Z.; Zhu, J.; Yang, Y.L.; Zhang, Y.H.; Huang, P.X.; Chen, Y.S.; Zhu, C.Y.; Yang, L.P.; Shen, K.; et al. Cancer survival in patients from a hospital-based cancer registry, China. J. Cancer 2018, 9, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef]
- Reale, A.; Khong, T.; Spencer, A. Extracellular Vesicles and Their Roles in the Tumor Immune Microenvironment. J. Clin. Med. 2022, 11, 6892. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Gao, Y.; Lin, C.; An, Q.; Feng, Y.; Liu, Y.; Liu, D.; Luo, H.; Wang, D. The Biology and Function of Extracellular Vesicles in Cancer Development. Front. Cell Dev. Biol. 2021, 9, 777441. [Google Scholar] [CrossRef]
- Tesei, A.; Arienti, C.; Bossi, G.; Santi, S.; De Santis, I.; Bevilacqua, A.; Zanoni, M.; Pignatta, S.; Cortesi, M.; Zamagni, A.; et al. TP53 drives abscopal effect by secretion of senescence-associated molecular signals in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 89. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Chen, X.; Liu, J.; Weng, Y.; Zhuang, Q.; Lin, F.; Huang, Z.; Wu, S.; Ding, J.; et al. Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics 2020, 10, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Elmali, A.; Yazici, G. Abscopal Effect, From Myth to Reality: From Radiation Oncologists’ Perspective. Cureus 2019, 11, e3860. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Formenti, S.C. The abscopal effect 67 years later: From a side story to center stage. Br. J. Radiol. 2020, 93, 20200042. [Google Scholar] [CrossRef]
- Eckert, F.; Jelas, I.; Oehme, M.; Huber, S.M.; Sonntag, K.; Welker, C.; Gillies, S.D.; Strittmatter, W.; Zips, D.; Handgretinger, R.; et al. Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects. Oncoimmunology 2017, 6, e1323161. [Google Scholar] [CrossRef]
- Britschgi, C.; Riesterer, O.; Burger, I.A.; Guckenberger, M.; Curioni-Fontecedro, A. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non-small cell lung cancer. Radiat. Oncol. 2018, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.I.; Cortez, M.A.; Erasmus, J.J.; de Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef]
- Zhang, X.; Niedermann, G. Abscopal Effects With Hypofractionated Schedules Extending Into the Effector Phase of the Tumor-Specific T-Cell Response. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 63–73. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Farhood, B.; Eleojo Musa, A.; Taeb, S.; Rezaeyan, A.; Najafi, M. Abscopal effect in radioimmunotherapy. Int. Immunopharmacol. 2020, 85, 106663. [Google Scholar] [CrossRef]
- Krombach, J.; Hennel, R.; Brix, N.; Orth, M.; Schoetz, U.; Ernst, A.; Schuster, J.; Zuchtriegel, G.; Reichel, C.A.; Bierschenk, S.; et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 2019, 8, e1523097. [Google Scholar] [CrossRef]
- Yang, W.; Xiu, Z.; He, Y.; Huang, W.; Li, Y.; Sun, T. Bip inhibition in glioma stem cells promotes radiation-induced immunogenic cell death. Cell Death Dis. 2020, 11, 786. [Google Scholar] [CrossRef]
- Venereau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Ran, J.; Wang, J.; Dai, Z.; Miao, Y.; Gan, J.; Zhao, C.; Guan, Q. Irradiation-Induced Changes in the Immunogenicity of Lung Cancer Cell Lines: Based on Comparison of X-rays and Carbon Ions. Front. Public Health 2021, 9, 666282. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Farhood, B.; Eleojo Musa, A.; Taeb, S.; Najafi, M. Damage-associated molecular patterns in tumor radiotherapy. Int. Immunopharmacol. 2020, 86, 106761. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Hennel, R.; Krombach, J.; Kapfhammer, H.; Brix, N.; Zuchtriegel, G.; Uhl, B.; Reichel, C.A.; Frey, B.; Gaipl, U.S.; et al. Priming of Anti-tumor Immune Mechanisms by Radiotherapy Is Augmented by Inhibition of Heat Shock Protein 90. Front. Oncol. 2020, 10, 1668. [Google Scholar] [CrossRef]
- Podolska, M.J.; Shan, X.; Janko, C.; Boukherroub, R.; Gaipl, U.S.; Szunerits, S.; Frey, B.; Munoz, L.E. Graphene-Induced Hyperthermia (GIHT) Combined With Radiotherapy Fosters Immunogenic Cell Death. Front. Oncol 2021, 11, 664615. [Google Scholar] [CrossRef]
- Abramowicz, A.; Wojakowska, A.; Marczak, L.; Lysek-Gladysinska, M.; Smolarz, M.; Story, M.D.; Polanska, J.; Widlak, P.; Pietrowska, M. Ionizing radiation affects the composition of the proteome of extracellular vesicles released by head-and-neck cancer cells in vitro. J. Radiat. Res. 2019, 60, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harris, S.L.; Levine, A.J. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, S.; Chen, Q.; Zhang, H.; Fu, J.; Zhou, Y.; Bai, Y.; Pan, Y.; Shao, C. Macrophage contributes to radiation-induced anti-tumor abscopal effect on transplanted breast cancer by HMGB1/TNF-alpha signaling factors. Int. J. Biol. Sci. 2021, 17, 926–941. [Google Scholar] [CrossRef]
- Luo, R.; Onyshchenko, K.; Wang, L.; Gaedicke, S.; Grosu, A.L.; Firat, E.; Niedermann, G. Necroptosis-dependent immunogenicity of cisplatin: Implications for enhancing the radiation-induced abscopal effect. Clin. Cancer Res. 2023, 29, 667–683. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, N.; Wang, Y.; Li, M.; Zhang, W.; Fan, L.; Liu, L.; Tang, Z.; Chen, X. Cisplatin nanoparticles boost abscopal effect of radiation plus anti-PD1 therapy. Biomater. Sci. 2021, 9, 3019–3027. [Google Scholar] [CrossRef]
- Mahdikia, H.; Saadati, F.; Freund, E.; Gaipl, U.S.; Majidzadeh, A.K.; Shokri, B.; Bekeschus, S. Gas plasma irradiation of breast cancers promotes immunogenicity, tumor reduction, and an abscopal effect in vivo. Oncoimmunology 2020, 10, 1859731. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, Y.L.; Lin, H.W.; Chang, C.F.; Huang, B.S.; Sun, W.Z.; Cheng, W.F. Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and DAMPs. Cancer Lett. 2021, 523, 149–161. [Google Scholar] [CrossRef]
- He, C.; Huang, X.; Zhang, Y.; Lin, X.; Li, S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, S. Changes in growth kinetics of jejunal epithelium in mice maintained on an elemental diet. Cell Tissue Kinet. 1979, 12, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Mobius, W.; Goebbels, S.; Nave, K.A.; et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.P.; Chen, H.Z. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. 2021, 516, 48–56. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic Application of Exosomes in Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef]
- Pineda, B.; Sanchez Garcia, F.J.; Olascoaga, N.K.; Perez de la Cruz, V.; Salazar, A.; Moreno-Jimenez, S.; Hernandez Pedro, N.; Marquez-Navarro, A.; Ortiz Plata, A.; Sotelo, J. Malignant Glioma Therapy by Vaccination with Irradiated C6 Cell-Derived Microvesicles Promotes an Antitumoral Immune Response. Mol. Ther. 2019, 27, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Hsing, C.Y.; Trejo Vazquez, J.; Garnis, C. Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis. Cells 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Zanetti-Domingues, L.C.; Bonner, S.E.; Iyer, R.S.; Martin-Fernandez, M.L.; Huber, V. Cooperation and Interplay between EGFR Signalling and Extracellular Vesicle Biogenesis in Cancer. Cells 2020, 9, 2639. [Google Scholar] [CrossRef] [PubMed]
- Maeshige, N.; Langston, P.K.; Yuan, Z.M.; Kondo, H.; Fujino, H. High-intensity ultrasound irradiation promotes the release of extracellular vesicles from C2C12 myotubes. Ultrasonics 2021, 110, 106243. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Nicolas-Boluda, A.; Mulens-Arias, V.; Richard, S.; Rahmi, G.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Extracellular vesicles for personalized medicine: The input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 2019, 138, 247–258. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell Mol. Life Sci. 2017, 74, 697–713. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, L.; Li, L.; Zhu, G. Extracellular vesicle-orchestrated crosstalk between cancer-associated fibroblasts and tumors. Transl. Oncol. 2021, 14, 101231. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target Ther. 2021, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Taniguchi, K.; Lee, S.W.; Ito, Y.; Kuranaga, Y.; Hashiguchi, Y.; Inomata, Y.; Imai, Y.; Tanaka, R.; Tashiro, K.; et al. Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients. Int. J. Mol. Sci. 2019, 20, 953. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Chang, W.A.; Lin, Y.S.; Pan, Y.C.; Tsai, P.H.; Wu, C.Y.; Kuo, P.L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Lin, C.W.; Chang, Y.L.; Chang, Y.C.; Lin, J.C.; Chen, C.C.; Pan, S.H.; Wu, C.T.; Chen, H.Y.; Yang, S.C.; Hong, T.M.; et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013, 4, 1877. [Google Scholar] [CrossRef]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef]
- Yamada, N.; Tsujimura, N.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Nakagawa, Y.; Naoe, T.; Akao, Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim. Biophys. Acta 2014, 1839, 1256–1272. [Google Scholar] [CrossRef]
- Jung, K.O.; Youn, H.; Lee, C.H.; Kang, K.W.; Chung, J.K. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 2017, 8, 9899–9910. [Google Scholar] [CrossRef]
- Conigliaro, A.; Costa, V.; Lo Dico, A.; Saieva, L.; Buccheri, S.; Dieli, F.; Manno, M.; Raccosta, S.; Mancone, C.; Tripodi, M.; et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 2015, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Gezer, U.; Ozgur, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.L.; Hu, G.W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017, 38, 785–798. [Google Scholar] [CrossRef]
- Lang, H.L.; Hu, G.W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.M.; Wu, L.; Xu, G.H. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972. [Google Scholar] [PubMed]
- Yamada, N.O. Extracellular vesicles: Emerging mediators of intercellular communication and tumor angiogenesis. Ann. Transl. Med. 2017, 5, 59. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced beta-Catenin Signaling in Endothelial Cells. Oncol. Res. 2017, 25, 651–661. [Google Scholar] [CrossRef]
- Mittal, S.; Gupta, P.; Chaluvally-Raghavan, P.; Pradeep, S. Emerging Role of Extracellular Vesicles in Immune Regulation and Cancer Progression. Cancers 2020, 12, 3563. [Google Scholar] [CrossRef]
- Rincon-Riveros, A.; Lopez, L.; Villegas, E.V.; Antonia Rodriguez, J. Regulation of Antitumor Immune Responses by Exosomes Derived from Tumor and Immune Cells. Cancers 2021, 13, 847. [Google Scholar] [CrossRef]
- Musatova, O.E.; Rubtsov, Y.P. Effects of glioblastoma-derived extracellular vesicles on the functions of immune cells. Front. Cell Dev. Biol. 2023, 11, 1060000. [Google Scholar] [CrossRef] [PubMed]
- Luong, N.; Lenz, J.A.; Modiano, J.F.; Olson, J.K. Extracellular Vesicles Secreted by Tumor Cells Promote the Generation of Suppressive Monocytes. Immunohorizons 2021, 5, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rak, J. Extracellular vesicles—Biomarkers and effectors of the cellular interactome in cancer. Front. Pharmacol. 2013, 4, 21. [Google Scholar] [CrossRef]
- Rabe, D.C.; Walker, N.D.; Rustandy, F.D.; Wallace, J.; Lee, J.; Stott, S.L.; Rosner, M.R. Tumor Extracellular Vesicles Regulate Macrophage-Driven Metastasis through CCL5. Cancers 2021, 13, 3459. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Wu, Y.; Liu, C.; Zeng, F.; Wang, J.L.; Wu, D.Z.; Wu, J.P.; Yue, Z.Q.; Gan, J.H.; Lu, H.; et al. Exosome-mediated transfer of lncRNA HCG18 promotes M2 macrophage polarization in gastric cancer. Mol. Immunol. 2021, 140, 196–205. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liu, H.S.; Wang, F.W.; Xiong, L.; Zhou, C.; Hu, T.; He, X.W.; Wu, X.J.; Xie, D.; Wu, X.R.; et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019, 10, 829. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Wu, P.; Zha, C.; Han, B.; Li, L.; Sun, N.; Qi, T.; Qin, J.; Zhang, Y.; et al. Glioblastoma Cell-Derived lncRNA-Containing Exosomes Induce Microglia to Produce Complement C5, Promoting Chemotherapy Resistance. Cancer Immunol. Res. 2021, 9, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Lima, L.G.; Chai, E.P.Z.; Muller, A.; Lobb, R.J.; Krumeich, S.; Wen, S.W.; Wiegmans, A.P.; Moller, A. Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling. Front. Immunol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Domenis, R.; Cesselli, D.; Toffoletto, B.; Bourkoula, E.; Caponnetto, F.; Manini, I.; Beltrami, A.P.; Ius, T.; Skrap, M.; Di Loreto, C.; et al. Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells. PLoS ONE 2017, 12, e0169932. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M.; et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 896. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Zhou, L.; Chen, W.; Ding, G.; Cao, L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014, 292, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, C.; Wangmo, D.; Subramanian, S. Tumor-Secreted Extracellular Vesicles Regulate T-Cell Costimulation and Can Be Manipulated To Induce Tumor-Specific T-Cell Responses. Gastroenterology 2021, 161, 560–574 e511. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, S.; Sotoodehnejadnematalahi, F.; Fathollahi, A.; Bandehpour, M.; Haji Molla Hoseini, M.; Yeganeh, F. EL4-derived Exosomes Carry Functional TNF-related Apoptosis-inducing Ligand that are Able to Induce Apoptosis and Necrosis in the Target Cells. Int. J. Mol. Cell Med. 2020, 9, 207–215. [Google Scholar] [CrossRef]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.; Rao, A.; Braganhol, E.; Whiteside, T.L. Molecular profiles and immunomodulatory activities of glioblastoma-derived exosomes. Neurooncol. Adv. 2020, 2, vdaa056. [Google Scholar] [CrossRef]
- Bono, M.R.; Fernandez, D.; Flores-Santibanez, F.; Rosemblatt, M.; Sauma, D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015, 589, 3454–3460. [Google Scholar] [CrossRef]
- Turiello, R.; Capone, M.; Morretta, E.; Monti, M.C.; Madonna, G.; Azzaro, R.; Del Gaudio, P.; Simeone, E.; Sorrentino, A.; Ascierto, P.A.; et al. Exosomal CD73 from serum of patients with melanoma suppresses lymphocyte functions and is associated with therapy resistance to anti-PD-1 agents. J. Immunother. Cancer 2022, 10, e004043. [Google Scholar] [CrossRef] [PubMed]
- Siemaszko, J.; Marzec-Przyszlak, A.; Bogunia-Kubik, K. NKG2D Natural Killer Cell Receptor-A Short Description and Potential Clinical Applications. Cells 2021, 10, 1420. [Google Scholar] [CrossRef]
- Lundholm, M.; Schroder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikstrom, P. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS ONE 2014, 9, e108925. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, Q.; Cheng, Y.; Chen, X.; Wang, G.; Shi, M.; Zhang, T.; Cao, Y.; Pan, H.; Zhang, L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 2018, 6, 145. [Google Scholar] [CrossRef]
- Schroeder, J.C.; Puntigam, L.; Hofmann, L.; Jeske, S.S.; Beccard, I.J.; Doescher, J.; Laban, S.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.N.; et al. Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers 2020, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Orlowski, G.; Song, W. Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells. J. Immunol. 2009, 182, 329–339. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Kuroda, S.; Kanaya, N.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Yagi, C.; Sugimoto, R.; Hamada, Y.; Kikuchi, S.; et al. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol. Ther. 2021, 29, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wang, H.; Zhang, Y.; Min, W. The Role of Tumor-Derived Exosomes in the Abscopal Effect and Immunotherapy. Life 2021, 11, 381. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Prise, K.M.; Little, M.P. Non-targeted effects of ionising radiation–Implications for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef]
- Jokar, S.; Marques, I.A.; Khazaei, S.; Martins-Marques, T.; Girao, H.; Laranjo, M.; Botelho, M.F. The Footprint of Exosomes in the Radiation-Induced Bystander Effects. Bioengineering 2022, 9, 243. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Moore, S.R.; Goodwin, E.H. Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutat. Res. 2004, 568, 21–32. [Google Scholar] [CrossRef]
- Azzam, E.I.; Little, J.B. The radiation-induced bystander effect: Evidence and significance. Hum. Exp. Toxicol. 2004, 23, 61–65. [Google Scholar] [CrossRef]
- Morgan, W.F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003, 159, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.S. Extracellular Vesicles: Biology and Potentials in Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 9586. [Google Scholar] [CrossRef]
- Gilligan, K.E.; Dwyer, R.M. Engineering Exosomes for Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1122. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Mukherjee, S.; Ali, S.; Bhardwaj, U.; Choudhary, R.K.; Balakrishnan, S.; Naseem, A.; Mir, S.A.; Banawas, S.; Alaidarous, M.; et al. Pioneer Role of Extracellular Vesicles as Modulators of Cancer Initiation in Progression, Drug Therapy, and Vaccine Prospects. Cells 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Baghaei, K.; Amani, D.; Ebtekar, M. Tumor-derived exosomes encapsulating miR-34a promote apoptosis and inhibit migration and tumor progression of colorectal cancer cells under in vitro condition. Daru 2021, 29, 267–278. [Google Scholar] [CrossRef]
- Hosseini, M.; Baghaei, K.; Hajivalili, M.; Zali, M.R.; Ebtekar, M.; Amani, D. The anti-tumor effects of CT-26 derived exosomes enriched by MicroRNA-34a on murine model of colorectal cancer. Life Sci. 2022, 290, 120234. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Baghaei, K.; Hashemi, S.M.; Zali, M.R.; Ghanbarian, H.; Amani, D. Tumor-Derived Exosomes Enriched by miRNA-124 Promote Anti-tumor Immune Response in CT-26 Tumor-Bearing Mice. Front. Med. 2021, 8, 619939. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-kappaB Signaling. Reprod. Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W.; Jing, D.; Yang, L.; Guo, H.; Wang, L.; Zhang, W.; Pu, F.; Shao, Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Control. Release 2022, 343, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Lin, Z.; Xu, J.; Lu, Y.; Meng, Q.; Wang, C.; Yang, Y.; Xin, X.; Li, X.; Pu, H.; et al. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting beta-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018, 9, 253. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Xiong, Q.; Bai, Y.; Shi, R.; Wang, J.; Xu, W.; Zhang, M.; Song, T. Preferentially released miR-122 from cyclodextrin-based star copolymer nanoparticle enhances hepatoma chemotherapy by apoptosis induction and cytotoxics efflux inhibition. Bioact. Mater. 2021, 6, 3744–3755. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Callegari, E.; D’Abundo, L.; Guerriero, P.; Simioni, C.; Elamin, B.K.; Russo, M.; Cani, A.; Bassi, C.; Zagatti, B.; Giacomelli, L.; et al. miR-199a-3p Modulates MTOR and PAK4 Pathways and Inhibits Tumor Growth in a Hepatocellular Carcinoma Transgenic Mouse Model. Mol. Ther. Nucleic Acids 2018, 11, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhang, H.; Liu, R.; Deng, T.; Ning, T.; Bai, M.; Yang, Y.; Zhu, K.; Wang, J.; Duan, J.; et al. iRGD-modified exosomes effectively deliver CPT1A siRNA to colon cancer cells, reversing oxaliplatin resistance by regulating fatty acid oxidation. Mol. Oncol. 2021, 15, 3430–3446. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Yi, K.; Qi, H.; Li, S.; Li, X.; Wang, Q.; Wang, Y.; Liu, C.; Qiu, M.; Yuan, X.; et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics 2020, 10, 7889–7905. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Wurdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef]
- Horrevorts, S.K.; Stolk, D.A.; van de Ven, R.; Hulst, M.; van Het Hof, B.; Duinkerken, S.; Heineke, M.H.; Ma, W.; Dusoswa, S.A.; Nieuwland, R.; et al. Glycan-Modified Apoptotic Melanoma-Derived Extracellular Vesicles as Antigen Source for Anti-Tumor Vaccination. Cancers 2019, 11, 1266. [Google Scholar] [CrossRef]
- Liu, W.; Takahashi, Y.; Morishita, M.; Nishikawa, M.; Takakura, Y. Development of CD40L-modified tumor small extracellular vesicles for effective induction of antitumor immune response. Nanomedicine 2020, 15, 1641–1652. [Google Scholar] [CrossRef]
- Taghikhani, A.; Hassan, Z.M.; Ebrahimi, M.; Moazzeni, S.M. microRNA modified tumor-derived exosomes as novel tools for maturation of dendritic cells. J. Cell Physiol. 2019, 234, 9417–9427. [Google Scholar] [CrossRef]
- Khani, A.T.; Sharifzad, F.; Mardpour, S.; Hassan, Z.M.; Ebrahimi, M. Tumor extracellular vesicles loaded with exogenous Let-7i and miR-142 can modulate both immune response and tumor microenvironment to initiate a powerful anti-tumor response. Cancer Lett. 2021, 501, 200–209. [Google Scholar] [CrossRef]
- Escamilla-Tilch, M.; Filio-Rodríguez, G.; García-Rocha, R.; Mancilla-Herrera, I.; Mitchison, N.A.; Ruiz-Pacheco, J.A.; Sánchez-García, F.J.; Sandoval-Borrego, D.; Vázquez-Sánchez, E.A. The interplay between pathogen-associated and danger-associated molecular patterns: An inflammatory code in cancer? Immunol. Cell Biol. 2013, 91, 601–610. [Google Scholar] [CrossRef]
- Ito, T.; Sugiura, K.; Hasegawa, A.; Ouchi, W.; Yoshimoto, T.; Mizoguchi, I.; Inaba, T.; Hamada, K.; Eriguchi, M.; Koyama, Y. Microbial Antigen-Presenting Extracellular Vesicles Derived from Genetically Modified Tumor Cells Promote Antitumor Activity of Dendritic Cells. Pharmaceutics 2021, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Ito, T.; Hasegawa, A.; Eriguchi, M.; Inaba, T.; Ushigusa, T.; Sugiura, K. Exosomes derived from tumor cells genetically modified to express Mycobacterium tuberculosis antigen: A novel vaccine for cancer therapy. Biotechnol. Lett. 2016, 38, 1857–1866. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Han, D.; Zhong, J.; Yang, C.; Chen, X. Dose-dependent immunomodulatory effects of metformin on human neonatal monocyte-derived macrophages. Cell Immunol. 2022, 377, 104557. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, X.; Yong, T.; Bie, N.; Zhan, G.; Li, X.; Liang, Q.; Li, J.; Yu, J.; Huang, G.; et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.S.B.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Kamerkar, S.; Leng, C.; Burenkova, O.; Jang, S.C.; McCoy, C.; Zhang, K.; Dooley, K.; Kasera, S.; Zi, T.; Siso, S.; et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci. Adv. 2022, 8, eabj7002. [Google Scholar] [CrossRef]
- Feng, C.; Xiong, Z.; Wang, C.; Xiao, W.; Xiao, H.; Xie, K.; Chen, K.; Liang, H.; Zhang, X.; Yang, H. Folic acid-modified Exosome-PH20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact. Mater. 2021, 6, 963–974. [Google Scholar] [CrossRef]
- Wan, C.; Sun, Y.; Tian, Y.; Lu, L.; Dai, X.; Meng, J.; Huang, J.; He, Q.; Wu, B.; Zhang, Z.; et al. Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming. Sci. Adv. 2020, 6, eaay9789. [Google Scholar] [CrossRef]
- Lu, J.; Wei, N.; Zhu, S.; Chen, X.; Gong, H.; Mi, R.; Huang, Y.; Chen, Z.; Li, G. Exosomes Derived From Dendritic Cells Infected With Toxoplasma gondii Show Antitumoral Activity in a Mouse Model of Colorectal Cancer. Front. Oncol. 2022, 12, 899737. [Google Scholar] [CrossRef]
- Liang, Y.B.; Tang, H.; Chen, Z.B.; Zeng, L.J.; Wu, J.G.; Yang, W.; Li, Z.Y.; Ma, Z.F. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Mol. Med. Rep. 2017, 16, 6405–6411. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPalpha, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 2018–2022. [Google Scholar] [CrossRef]
- Li, L.; Miao, Q.; Meng, F.; Li, B.; Xue, T.; Fang, T.; Zhang, Z.; Zhang, J.; Ye, X.; Kang, Y.; et al. Genetic engineering cellular vesicles expressing CD64 as checkpoint antibody carrier for cancer immunotherapy. Theranostics 2021, 11, 6033–6043. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Johnson, L.R.; Lee, D.Y.; Eacret, J.S.; Ye, D.; June, C.H.; Minn, A.J. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 2021, 184, 4981–4995 e4914. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Jie, J. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, Y.; Yang, R.; Liu, C.; Hsu, J.M.; Jiang, Z.; Sun, L.; Wei, Y.; Li, C.W.; Yu, D.; et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene 2021, 40, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liang, R.; Erel-Akbaba, G.; Saad, L.; Obeid, P.J.; Gao, J.; Chiocca, E.A.; Weissleder, R.; Tannous, B.A. Immune Checkpoint Inhibition in GBM Primed with Radiation by Engineered Extracellular Vesicles. ACS Nano 2022, 16, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, Y.K.; Kim, G.B.; Nam, G.H.; Kim, S.A.; Park, Y.; Yang, Y.; Kim, I.S. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103+ dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J. Extracell. Vesicles 2019, 8, 1670893. [Google Scholar] [CrossRef]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Shao, B.; Xu, F.; Huang, X.; Guo, X.; Zhou, S. Self-Blockade of PD-L1 with Bacteria-Derived Outer-Membrane Vesicle for Enhanced Cancer Immunotherapy. Adv. Mater. 2022, 34, e2106307. [Google Scholar] [CrossRef]
| Cancer Cell | Radiation Dose | DAMPs | Effect on Non-Cancer Cells | References |
|---|---|---|---|---|
| HCC1937 human breast cancer cells | Single ablative dose of 20 Gy | HSP70 HMGB1 S100A8/A9 | Endothelial cell activation and surface expression of adhesion molecules ICAM-1, VCAM-1 and E-selectin and release of IL-6, CXCL1, CXCL2 and CCL7. Recruitment of neutrophils and monocytes, and differentiation and maturation of antigen-presenting cells. | [22] |
| Glioma stem cells | 10 Gy X-ray (160 kV) at a dose rate of 0.50 Gy/min |
HSP70/90 ATP HMGB1 | Increased phagocytosis and DC maturation, and proliferation of T cells. | [23,24] |
| A549, NCI-H520 (human) and LLC lung cancer cells | 100 keV, with a dose rate of 1.0 Gy/min Total dose of 6 Gy | HMGB1 | Maturation and activation of DCs, and activation of memory Th cells and effector Tc cells. | [25] |
|
MDA-MB-231 (breast), H522 (lung) and LNCaP (prostate) cancer cells | 100 Gy at a dose rate of 5.56 Gy/min | CRT ATP HMGB1 | Increased sensitivity to antigen-specific Tc cell lysis, and enhanced T-cell recognition of specific HLA-I. | [26,27] |
| HCT116 human colorectal cancer cells | 5 Gy at a dose rate of 1 Gy in 63 s |
HMGB1 ATP UTP | Monocyte migration. | [28] |
| B16F10 mouse melanoma cells | 20 Gy, 120 kV | ATP HSP70 HMGB1 | Upregulation of CD80, CD86, MHC-II and CD40 on DCs and proliferation of Th and Tc cells. | [29] |
| DAMPs | Model | AE Inducer | Immune Effect | Effect over Secondary Tumor | References |
| HMGB1 | 4T1 mouse breast cancer cells in BALB/c mice | 24 Gy (3 × 8 Gy) | M1 polarization of macrophages, recruitment and secretion of TNF-α | Reduced tumor growth, inhibition of proliferation and migration | [32] |
| mtDNA ATP | B16-CD133 melanoma cells in C57BL/6N mice C51 colon carcinoma cells in BALB/c mice | 24 Gy (2 × 12 Gy) + cisplatin + anti-PD-1 16 Gy (2 × 8 Gy) + cisplatin + anti-PD-1 antibody | DC recruitment and activation via secretion of ATP, mtDNA and type I IFN, Tc cell cross-priming and recruitment | Reduced tumor growth and necroptosis | [33] |
| CRT | LLC Lewis lung carcinoma cells in C57BL/6 mice | 10 Gy (5 × 2 Gy) + Cisplatin-loaded nanoparticles + anti-PD-1 | Tc cell activation, proliferation and recruitment | Reduced tumor growth and increased CXCL10 synthesis | [34] |
| CRT HSP-70 HSP-90 | 4T1 mouse breast cancer cells in BALB/cfC3H mice | Helium-driven plasma gas jet with 1 W per 300 s | Increase in tumor-infiltrating DCs and Th cells | Reduced tumor growth, apoptosis and CRT expression | [35] |
| CRT HMGB-1 HSP-70 | TC-1 lung tumor cells in C57BL/6 J mice | 24 Gy (3 × 8 Gy) + oncolytic vaccinia virus | M1 polarization of macrophage, increase in Tc and Th activation and recruitment and decrease in Treg cells | Reduced tumor growth | [36] |
| CRT HMGB1 HSP70 | Pan02 murine pancreatic adenocarcinoma cells in C57BL/6 mice | Electroporation with 1000 V per 100 ms | Increase in effector and memory Tc cells, and Tc cell recruitment | Reduced tumor growth and decreased LOX expression | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, A.; Chavarria, V.; Flores, I.; Ruiz, S.; Pérez de la Cruz, V.; Sánchez-García, F.J.; Pineda, B. Abscopal Effect, Extracellular Vesicles and Their Immunotherapeutic Potential in Cancer Treatment. Molecules 2023, 28, 3816. https://doi.org/10.3390/molecules28093816
Salazar A, Chavarria V, Flores I, Ruiz S, Pérez de la Cruz V, Sánchez-García FJ, Pineda B. Abscopal Effect, Extracellular Vesicles and Their Immunotherapeutic Potential in Cancer Treatment. Molecules. 2023; 28(9):3816. https://doi.org/10.3390/molecules28093816
Chicago/Turabian StyleSalazar, Aleli, Víctor Chavarria, Itamar Flores, Samanta Ruiz, Verónica Pérez de la Cruz, Francisco Javier Sánchez-García, and Benjamin Pineda. 2023. "Abscopal Effect, Extracellular Vesicles and Their Immunotherapeutic Potential in Cancer Treatment" Molecules 28, no. 9: 3816. https://doi.org/10.3390/molecules28093816
APA StyleSalazar, A., Chavarria, V., Flores, I., Ruiz, S., Pérez de la Cruz, V., Sánchez-García, F. J., & Pineda, B. (2023). Abscopal Effect, Extracellular Vesicles and Their Immunotherapeutic Potential in Cancer Treatment. Molecules, 28(9), 3816. https://doi.org/10.3390/molecules28093816







