Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment
Abstract
1. Introduction
2. Results
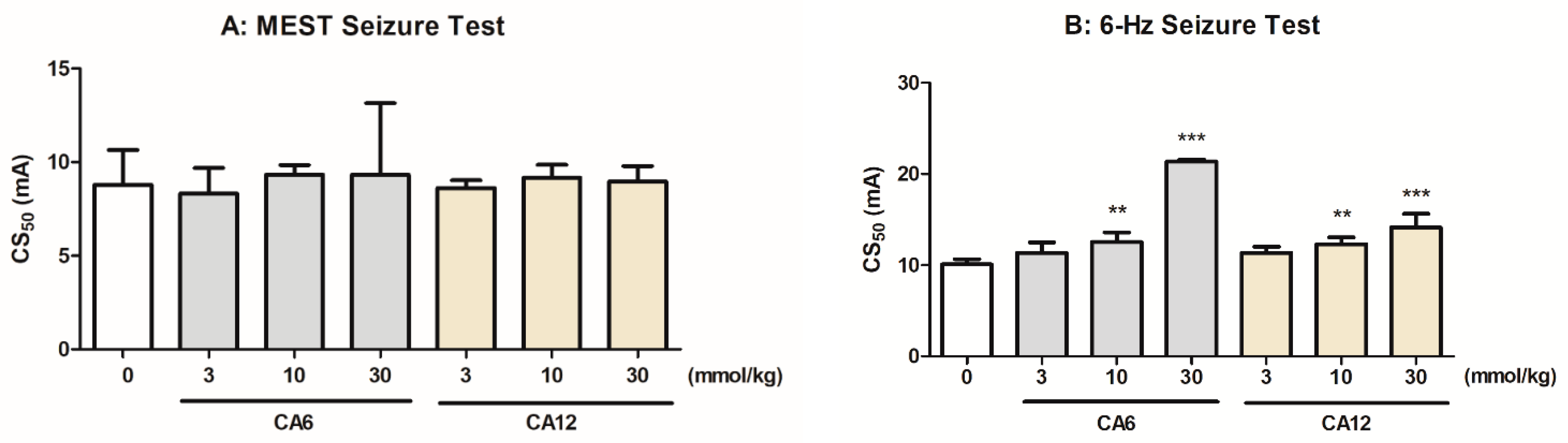
2.1. Effects of CA6 and CA12 in the MEST and 6 Hz-Induced Seizure Threshold Tests
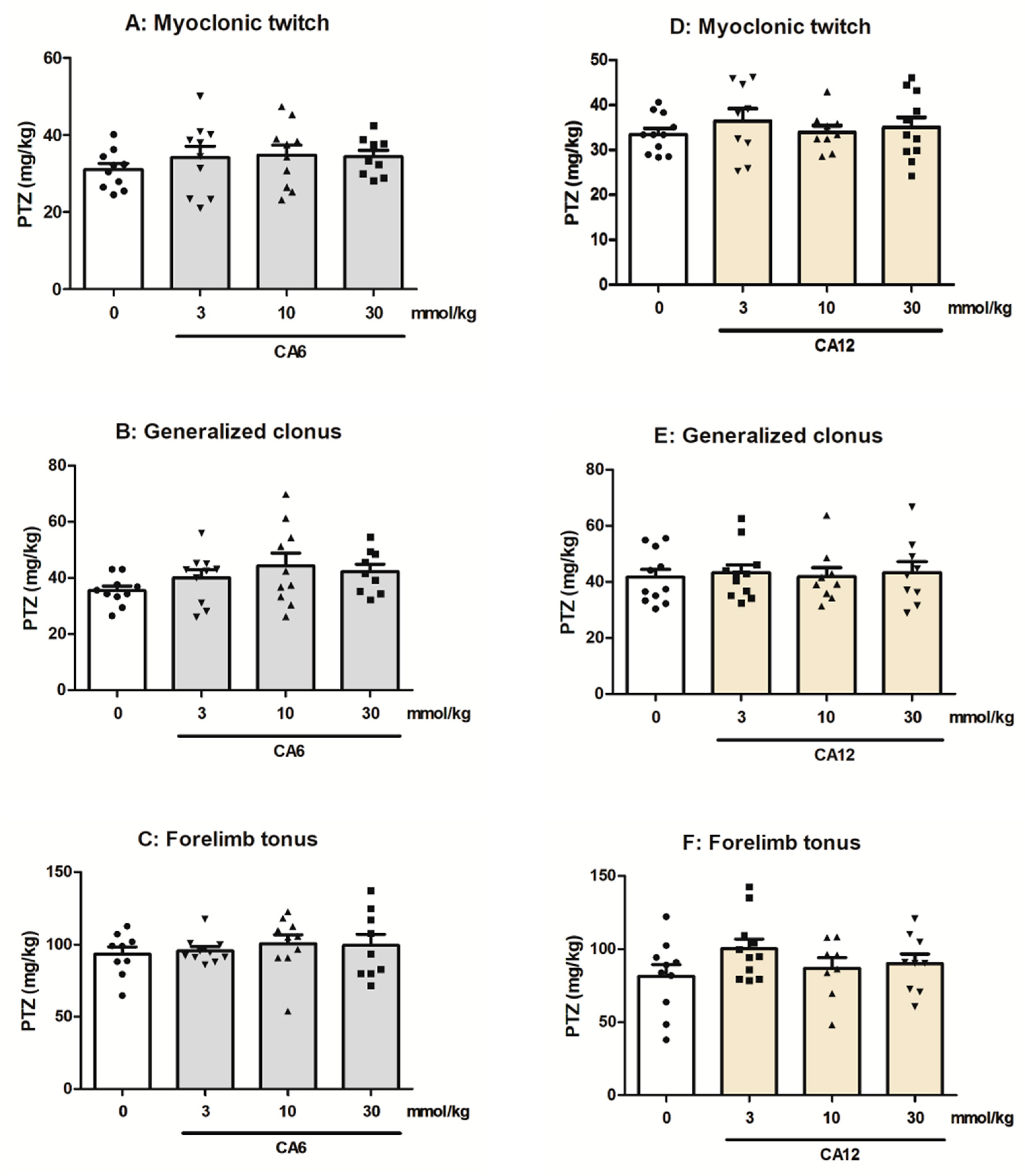
2.2. Effects of CA6 and CA12 on the Seizure Thresholds in the i.v. PTZ-Induced Seizure Test
2.3. Effects of CA6 and CA12 on Motor Coordination and Muscular Strength in Mice
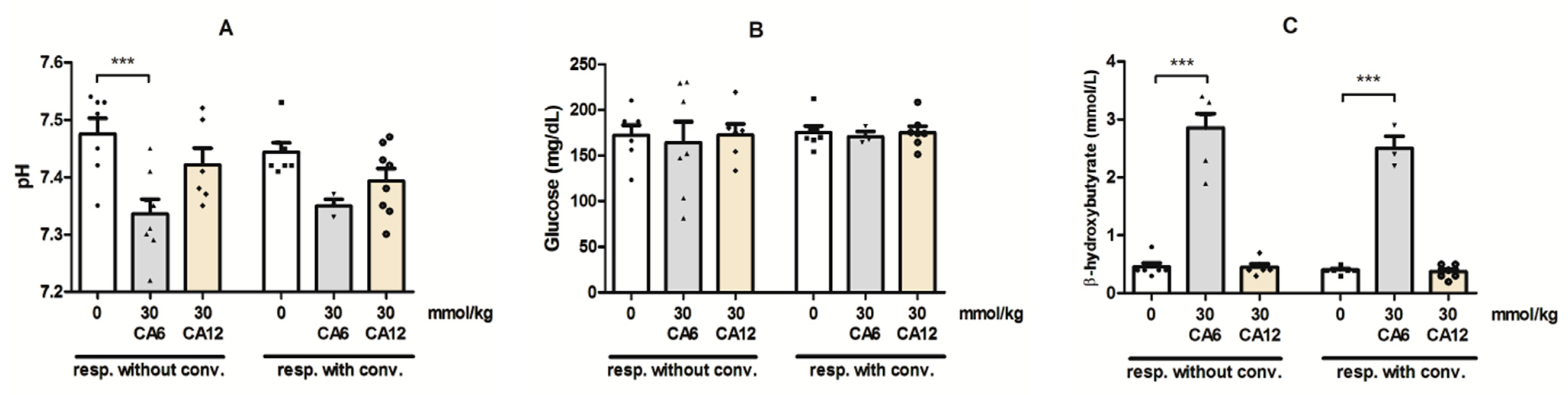
2.4. Effects of CA6 and CA12 on the Blood pH and Concentrations of Glucose and β-Hydroxybutyrate
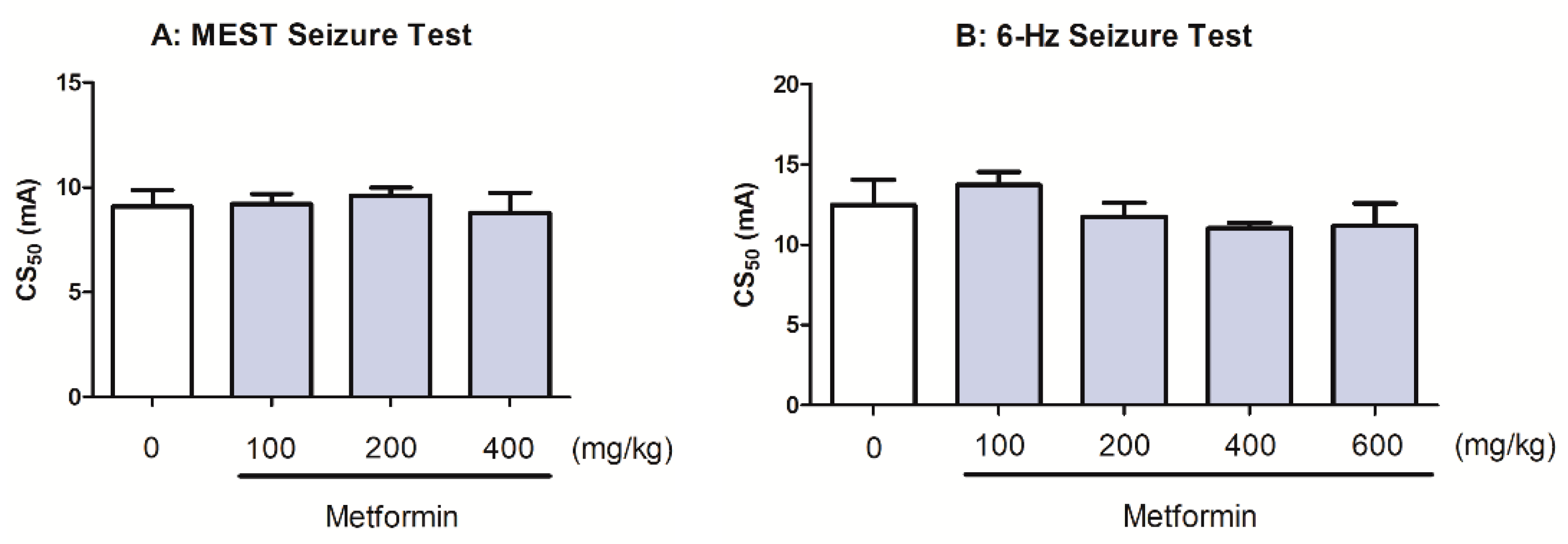
2.5. Effects of Metformin on the Seizure Threshold in the MEST and 6 Hz-Iduced Seizure Tests
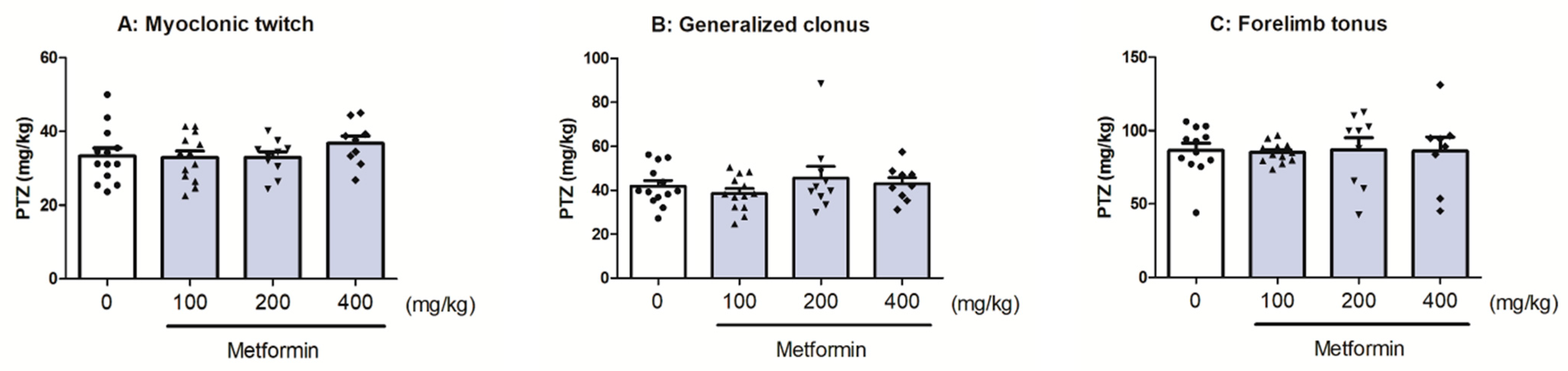
2.6. The Effect of Metformin in the i.v. PTZ-Induced Seizure Threshold Test
2.7. Effects of Metformin on Motor Coordination and Muscular Strength in Mice
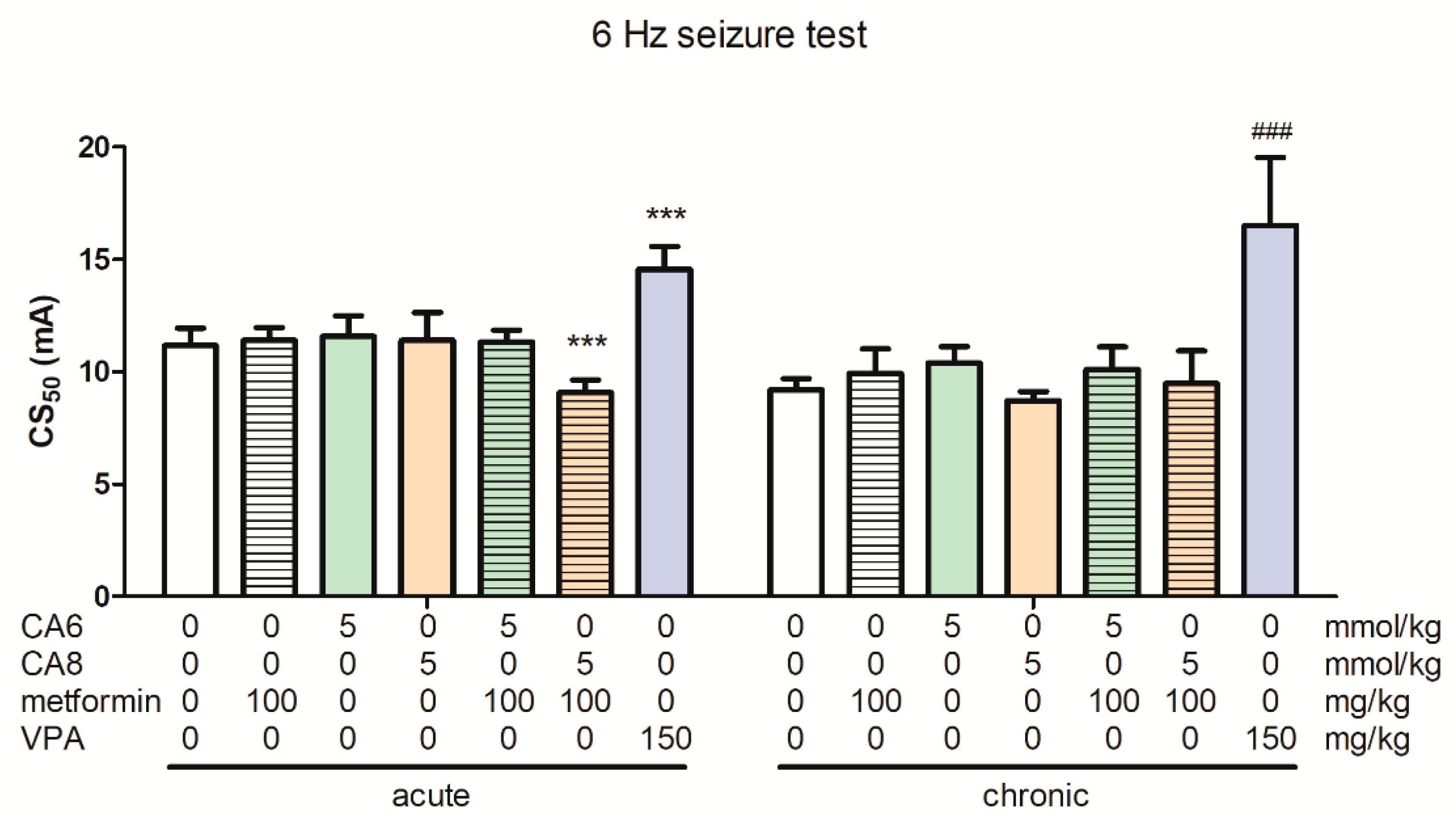
2.8. Effects of Acute and Chronic Treatment with Metformin, CA6, CA8 and Their Combination in the 6 Hz-Induced Seizure Test
2.9. Effects of Acute and Chronic Co-Treatment with Metformin and CA6/CA8 on Motor Coordination and Muscular Strength in Mice
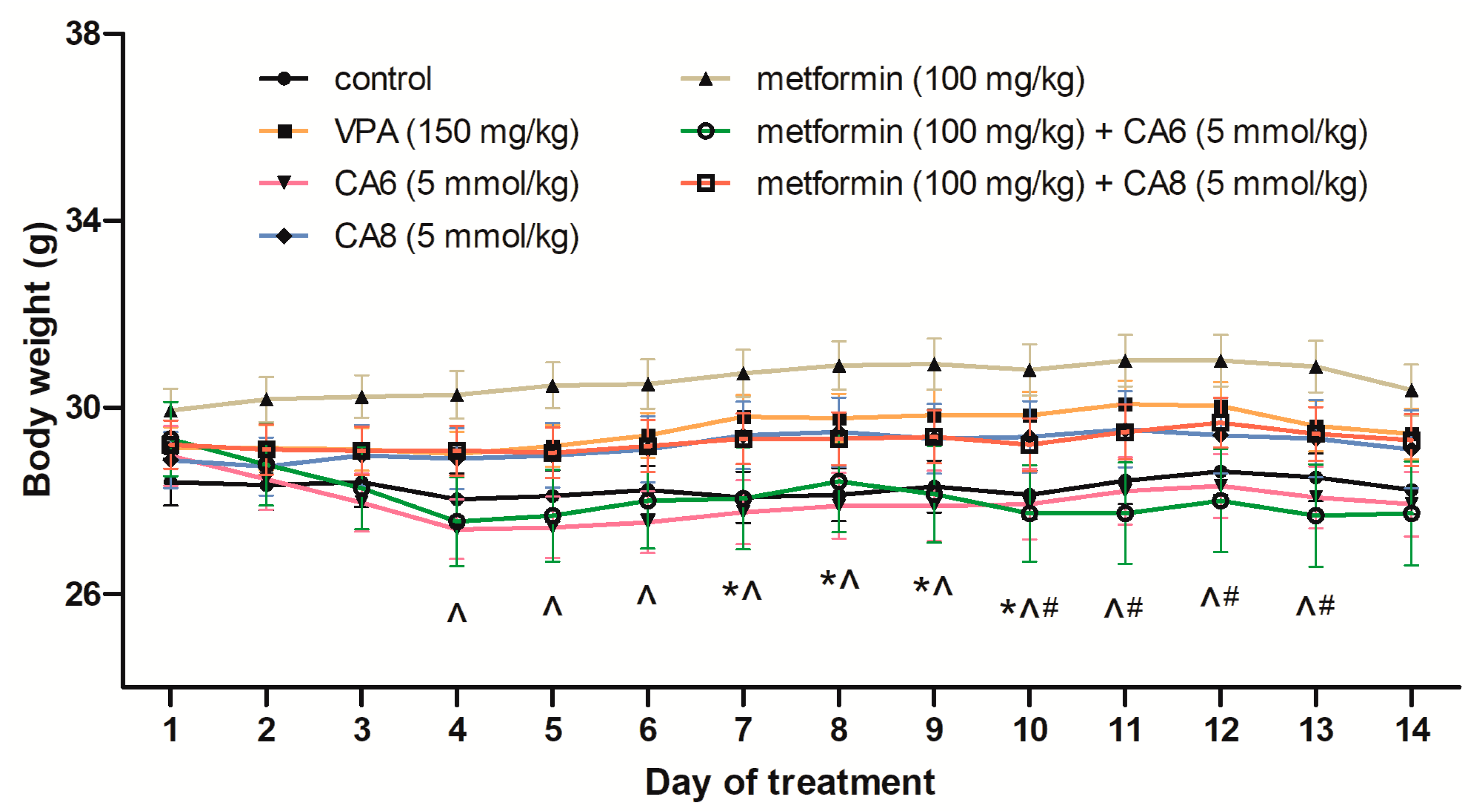
2.10. Effects of Chronic Treatment with Metformin, CA6, CA8 and Their Combinations on Body Weight
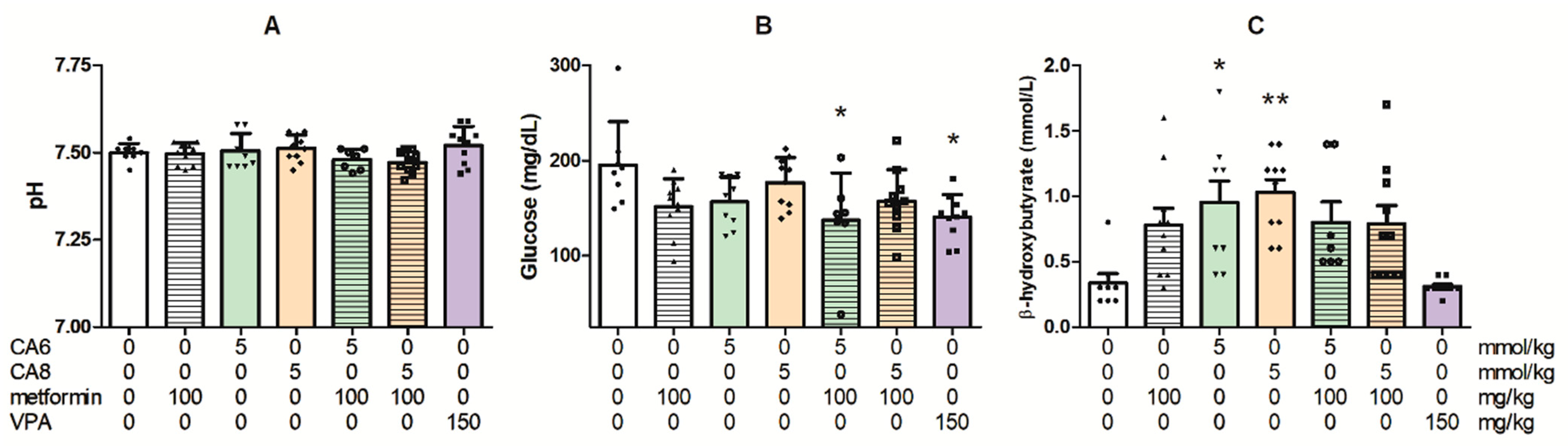
2.11. Effects of Chronic Treatment with Metformin, CA6, CA8 and Their Combinations on the Blood pH and Concentrations of Glucose and β-Hydroxybutyrate
3. Discussion
4. Materials and Methods
4.1. Animals and Experiment Design
- Procedures with CA6 and CA12: 15 animals/group in the i.v. PTZ test, 15 animals/group in the MEST test, 15 animals/group in the 6 Hz-induced psychomotor seizure test, and 10 animals/group in the grip-strength test and the chimney test. Before the 6 Hz seizure test, three mice died after treatment with 30 mmol/kg CA6 and were excluded from the analysis, as well as one mouse administered with 30 mmol/kg of CA12;
- Procedures with metformin: 15 animals/group in the i.v. PTZ test, 20 animals/group in the MEST test, 20 animals/group in the 6 Hz-induced psychomotor seizure test, and 10 animals/group in the grip-strength test and the chimney test;
- Procedures with a combination of metformin and selected MCFAs (CA6 and CA8) after acute and chronic treatment:
- (1)
- Acute effects—20 animals/group in the 6 Hz-induced psychomotor seizure test, and 10 animals/group in the grip-strength test and the chimney test. In all groups, vehicles were injected repeatedly every 24 h for 13 consecutive days. The acute effect of substances on the seizure threshold was assessed on the following (14th) day;
- (2)
- Chronic effects—20 animals/group in the 6 Hz-induced psychomotor seizure test, 10 animals/group in the grip-strength test and the chimney test, and 10 animals/group were used to measure the glucose level, acidosis and ketosis. In all groups, substances and vehicles were injected repeatedly every 24 h for 14 consecutive days. The chronic effect of substances on the seizure threshold was assessed on the last day of treatment (after 14 injections).
4.2. Drugs
4.3. I.v. PTZ Seizure Threshold Test
4.4. Maximal Electroconvulsions
4.5. Psychomotor Seizure Threshold Test
4.6. Behavioral Effects in Grip-Strength Tests
4.7. Behavioral Effects in Chimney Test
4.8. Determination of Blood pH and Concentrations of Glucose and β-Hydroxybutyrate
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Billakota, S.; Devinsky, O.; Kim, K.W. Why we urgently need improved epilepsy therapies for adult patients. Neuropharmacology 2020, 170, 107855. [Google Scholar] [CrossRef] [PubMed]
- Moshe, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef]
- Gasior, M.; French, A.; Joy, M.T.; Tang, R.S.; Hartman, A.L.; Rogawski, M.A. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia 2007, 48, 793–800. [Google Scholar] [CrossRef]
- Likhodii, S.S.; Serbanescu, I.; Cortez, M.A.; Murphy, P.; Snead, O.C., 3rd; Burnham, W.M. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 2003, 54, 219–226. [Google Scholar] [CrossRef]
- Rho, J.M.; Anderson, G.D.; Donevan, S.D.; White, H.S. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia 2002, 43, 358–361. [Google Scholar] [CrossRef]
- Zarnowska, I.; Luszczki, J.J.; Zarnowski, T.; Buszewicz, G.; Madro, R.; Czuczwar, S.J.; Gasior, M. Pharmacodynamic and pharmacokinetic interactions between common antiepileptic drugs and acetone, the chief anticonvulsant ketone body elevated in the ketogenic diet in mice. Epilepsia 2009, 50, 1132–1140. [Google Scholar] [CrossRef]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic control of vesicular glutamate transport and release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef]
- Masino, S.A.; Li, T.; Theofilas, P.; Sandau, U.S.; Ruskin, D.N.; Fredholm, B.B.; Geiger, J.D.; Aronica, E.; Boison, D. A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J. Clin. Investig. 2011, 121, 2679–2683. [Google Scholar] [CrossRef]
- Boison, D. Adenosine kinase, epilepsy and stroke: Mechanisms and therapies. Trends Pharmacol. Sci. 2006, 27, 652–658. [Google Scholar] [CrossRef]
- Dragunow, M. Purinergic mechanisms in epilepsy. Prog. Neurobiol. 1988, 31, 85–108. [Google Scholar] [CrossRef]
- Huber, A.; Padrun, V.; Deglon, N.; Aebischer, P.; Mohler, H.; Boison, D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc. Natl. Acad. Sci. USA 2001, 98, 7611–7616. [Google Scholar] [CrossRef]
- Kumaria, A.; Tolias, C.M.; Burnstock, G. ATP signalling in epilepsy. Purinergic Signal. 2008, 4, 339–346. [Google Scholar] [CrossRef]
- Li, T.; Steinbeck, J.A.; Lusardi, T.; Koch, P.; Lan, J.Q.; Wilz, A.; Segschneider, M.; Simon, R.P.; Brustle, O.; Boison, D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain 2007, 130 Pt 5, 1276–1288. [Google Scholar] [CrossRef]
- Wlaz, P.; Socala, K.; Nieoczym, D.; Zarnowski, T.; Zarnowska, I.; Czuczwar, S.J.; Gasior, M. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 110–116. [Google Scholar] [CrossRef]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Nakamura, J.; Miwa, T.; Sasaki, H.; Shibasaki, J.; Kaneto, H. Effect of straight chain fatty acids on seizures induced by picrotoxin and pentylenetetrazole in mice. J. Pharmacobiodyn 1990, 13, 76–81. [Google Scholar] [CrossRef]
- Wlaz, P.; Socala, K.; Nieoczym, D.; Luszczki, J.J.; Zarnowska, I.; Zarnowski, T.; Czuczwar, S.J.; Gasior, M. Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 2012, 62, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Terbach, N.; Plant, N.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013, 69, 105–114. [Google Scholar] [CrossRef]
- Takahashi, S. Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological conditions of the neurovascular unit. Neuropathology 2020, 40, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.R.; Xu, X.C.; Xu, F.; Zhang, W.L.; Zhang, W.L.; Liu, L.M.; Wang, W.P. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem. Biophys. Res. Commun. 2014, 448, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, B.; Zheng, F.; Li, Y.; Zhang, Y.; Hu, Y.; Wang, X. Chronic metformin treatment facilitates seizure termination. Biochem. Biophys. Res. Commun. 2017, 484, 450–455. [Google Scholar] [CrossRef]
- Mehrabi, S.; Sanadgol, N.; Barati, M.; Shahbazi, A.; Vahabzadeh, G.; Barzroudi, M.; Seifi, M.; Gholipourmalekabadi, M.; Golab, F. Evaluation of metformin effects in the chronic phase of spontaneous seizures in pilocarpine model of temporal lobe epilepsy. Metab. Brain Dis. 2018, 33, 107–114. [Google Scholar] [CrossRef]
- Hussein, A.M.; Eldosoky, M.; El-Shafey, M.; El-Mesery, M.; Ali, A.N.; Abbas, K.M.; Abulseoud, O.A. Effects of metformin on apoptosis and alpha-synuclein in a rat model of pentylenetetrazole-induced epilepsy. Can. J. Physiol. Pharmacol. 2019, 97, 37–46. [Google Scholar] [CrossRef]
- Yimer, E.M.; Surur, A.; Wondafrash, D.Z.; Gebre, A.K. The Effect of Metformin in Experimentally Induced Animal Models of Epileptic Seizure. Behav. Neurol. 2019, 2019, 6234758. [Google Scholar] [CrossRef]
- Brueggeman, L.; Sturgeon, M.L.; Martin, R.M.; Grossbach, A.J.; Nagahama, Y.; Zhang, A.; Howard, M.A., 3rd; Kawasaki, H.; Wu, S.; Cornell, R.A.; et al. Drug repositioning in epilepsy reveals novel antiseizure candidates. Ann. Clin. Transl. Neurol. 2018, 6, 295–309. [Google Scholar] [CrossRef]
- Amin, S.; Mallick, A.A.; Edwards, H.; Cortina-Borja, M.; Laugharne, M.; Likeman, M.; O’Callaghan, F.J.K. The metformin in tuberous sclerosis (MiTS) study: A randomised double-blind placebo-controlled trial. EClinicalMedicine 2021, 32, 100715. [Google Scholar] [CrossRef]
- Bibi, F.; Ullah, I.; Kim, M.O.; Naseer, M.I. Metformin attenuate PTZ-induced apoptotic neurodegeneration in human cortical neuronal cells. Pak. J. Med. Sci. 2017, 33, 581–585. [Google Scholar] [CrossRef]
- Wilcock, C.; Bailey, C.J. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 1994, 24, 49–57. [Google Scholar] [CrossRef]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopien, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Socala, K.; Nieoczym, D.; Pierog, M.; Wlaz, P. Role of the adenosine system and glucose restriction in the acute anticonvulsant effect of caprylic acid in the 6 Hz psychomotor seizure test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 44–51. [Google Scholar] [CrossRef]
- Keane, P.E.; Simiand, J.; Mendes, E.; Santucci, V.; Morre, M. The effects of analogues of valproic acid on seizures induced by pentylenetetrazol and GABA content in brain of mice. Neuropharmacology 1983, 22, 875–879. [Google Scholar] [CrossRef]
- Chapman, A.G.; Meldrum, B.S.; Mendes, E. Acute anticonvulsant activity of structural analogues of valproic acid and changes in brain GABA and aspartate content. Life Sci. 1983, 32, 2023–2031. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Chow, C.; Cunnane, S.; McIntyre Burnham, W. The effect of the ‘classic’ ketogenic diet on animal seizure models. Brain Res. 2003, 959, 206–213. [Google Scholar] [CrossRef]
- Hartman, A.L.; Zheng, X.; Bergbower, E.; Kennedy, M.; Hardwick, J.M. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia 2010, 51, 1395–1402. [Google Scholar] [CrossRef]
- Zarnowska, I.; Luszczki, J.J.; Zarnowski, T.; Wlaz, P.; Czuczwar, S.J.; Gasior, M. Proconvulsant effects of the ketogenic diet in electroshock-induced seizures in mice. Metab. Brain Dis. 2017, 32, 351–358. [Google Scholar] [CrossRef]
- Smyth, H.F.J.; Carpenter, C.P. The place of the range finding test in the industrial toxicology laboratory. Journal. Ind. Hyg. Toxicol. 1944, 26, 269–273. [Google Scholar]
- Ziemann, A.E.; Schnizler, M.K.; Albert, G.W.; Severson, M.A.; Howard, M.A., 3rd; Welsh, M.J.; Wemmie, J.A. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008, 11, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Matthews, D.R. Recent advances in the monitoring and management of diabetic ketoacidosis. QJM 2004, 97, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.G.; Schall, J.I.; Richard, E.L.; Gallagher, P.R.; Stallings, V.A. Predictive power of first morning glucose and the ketogenic diet. Neuropediatrics 2007, 38, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Lyle, M.; Rogawski, M.A.; Gasior, M. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia 2008, 49, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Bough, K.J.; Chen, R.S.; Eagles, D.A. Path analysis shows that increasing ketogenic ratio, but not beta-hydroxybutyrate, elevates seizure threshold in the Rat. Dev. Neurosci. 1999, 21, 400–406. [Google Scholar] [CrossRef]
- Bough, K.J.; Yao, S.G.; Eagles, D.A. Higher ketogenic diet ratios confer protection from seizures without neurotoxicity. Epilepsy Res. 2000, 38, 15–25. [Google Scholar] [CrossRef]
- Todorova, M.T.; Tandon, P.; Madore, R.A.; Stafstrom, C.E.; Seyfried, T.N. The ketogenic diet inhibits epileptogenesis in EL mice: A genetic model for idiopathic epilepsy. Epilepsia 2000, 41, 933–940. [Google Scholar] [CrossRef]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Zhou, G.C.; Myers, R.; Li, Y.; Chen, Y.L.; Shen, X.L.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Avery, L.B.; Bumpus, N.N. Valproic acid is a novel activator of AMP-activated protein kinase and decreases liver mass, hepatic fat accumulation, and serum glucose in obese mice. Mol. Pharmacol. 2014, 85, 1–10. [Google Scholar] [CrossRef]
- Li, J.; McCullough, L.D. Effects of AMP-activated protein kinase in cerebral ischemia. J. Cereb. Blood Flow. Metab. 2010, 30, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, T.; Szabo, E.Z.; Numat, M.; Orlowski, J. Activation of AMP-activated protein kinase regulates hippocampal neuronal pH by recruiting Na(+)/H(+) exchanger NHE5 to the cell surface. J. Biol. Chem. 2014, 289, 20879–20897. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Iizumi, T.; Mashima, K.; Abe, T.; Suzuki, N. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro 2014, 6, 1759091414550997. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Rani, E.; Waheed, A.; Rajput, S.K. Pharmacoresistant Epilepsy: A Current Update on Non-Conventional Pharmacological and Non-Pharmacological Interventions. J. Epilepsy Res. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, A.; Veliskova, J.; Velisek, L. Differential effects of low glucose concentrations on seizures and epileptiform activity in vivo and in vitro. Eur. J. Neurosci. 2006, 23, 1512–1522. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef]
- Greene, A.E.; Todorova, M.T.; McGowan, R.; Seyfried, T.N. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia 2001, 42, 1371–1378. [Google Scholar] [CrossRef]
- Hartman, A.L.; Gasior, M.; Vining, E.P.; Rogawski, M.A. The neuropharmacology of the ketogenic diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef]
- Bough, K.J.; Eagles, D.A. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia 1999, 40, 138–143. [Google Scholar] [CrossRef]
- Bough, K.J.; Matthews, P.J.; Eagles, D.A. A ketogenic diet has different effects upon seizures induced by maximal electroshock and by pentylenetetrazole infusion. Epilepsy Res. 2000, 38, 105–114. [Google Scholar] [CrossRef]
- Samala, R.; Willis, S.; Borges, K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008, 81, 119–127. [Google Scholar] [CrossRef]
- Socała, K.; Wlaź, P. Acute Seizure Tests Used in Epilepsy Research: Step-by-Step Protocol of the Maximal Electroshock Seizure (MES) Test, the Maximal Electroshock Seizure Threshold (MEST) Test, and the Pentylenetetrazole (PTZ)-Induced Seizure Test in Rodents. In Experimental and Translational Methods to Screen Drugs Effective Against Seizures and Epilepsy; Vohora, D., Ed.; Springer: New York, NY, USA, 2021; pp. 79–102. [Google Scholar]
- Kimball, A.W.; Burnett, W.T., Jr.; Doherty, D.G. Chemical protection against ionizing radiation. I. Sampling methods for screening compounds in radiation protection studies with mice. Radiat. Res. 1957, 7, 1–12. [Google Scholar] [CrossRef]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Boissier, J.R.; Tardy, J.; Diverres, J.C. Une nouvelle methode simple pour explorer l’action ‘tranquilistante’: Le test de la cheminee. Med. Exp. 1960, 3, 81–84. [Google Scholar]
| Treatment (mmol/kg) | Impairment of Motor Performance (%) | Neuromuscular Strength (mN/g) |
|---|---|---|
| control | 0 | 31.1 ± 1.3 |
| CA6 (3) | 0 | 30.3 ± 0.8 |
| CA6 (10) | 0 | 30.5 ± 0.9 |
| CA6 (30) | 30 | 18.4 ± 4.6 ** |
| CA12 (3) | 0 | 30.9 ± 0.7 |
| CA12 (10) | 0 | 29.5 ± 0.5 |
| CA12 (30) | 0 | 27.9 ± 1.5 |
| Treatment (mg/kg) | Impairment of Motor Performance (%) | Neuromuscular Strength (mN/g) |
|---|---|---|
| control | 0 | 36.3 ± 1.6 |
| metformin (100) | 0 | 33.1 ± 1.2 |
| metformin (200) | 0 | 36.6 ± 1.6 |
| metformin (400) | 0 | 34.1 ± 1.0 |
| metformin (600) | 0 | 33.1 ± 0.9 |
| Treatment | Impairment of Motor Performance (%) | Neuromuscular Strength (mN/g) |
|---|---|---|
| Acute treatment | ||
| control | 0 | 36.0 ± 1.8 |
| metformin (100 mg/kg) | 10 | 33.0 ± 1.2 |
| CA6 (5 mmol/kg) | 10 | 31.0 ± 1.1 |
| CA8 (5mmol/kg) | 0 | 32.0 ± 1.9 |
| metformin (100 mg/kg) + CA6 (5 mmol/kg) | 0 | 35.7 ± 1.3 |
| metformin (100 mg/kg) + CA8 (5 mmol/kg) | 10 | 31.8 ± 1.3 |
| VPA (150 mg/kg) | 0 | 33.6 ± 1.4 |
| Chronic treatment | ||
| control | 0 | 38.0 ± 1.4 |
| metformin (100 mg/kg) | 20 | 37.5 ± 1.5 |
| CA6 (5 mmol/kg) | 0 | 36.5 ± 1.0 |
| CA8 (5mmol/kg) | 0 | 36.9 ± 2.3 |
| metformin (100 mg/kg) + CA6 (5 mmol/kg) | 10 | 35.4 ± 1.5 |
| metformin (100 mg/kg) + CA8 (5 mmol/kg) | 0 | 35.2 ± 1.7 |
| VPA (150 mg/kg) | 0 | 36.6 ± 2.2 |
| Treatment Group | Mortality |
|---|---|
| control | 0/30 |
| metformin (100 mg/kg) | 0/30 |
| CA6 (5 mmol/kg) | 2/30 |
| CA8 (5 mmol/kg) | 0/30 |
| metformin (100 mg/kg) + CA6 (5 mmol/kg) | 8/30 |
| metformin (100 mg/kg) + CA8 (5 mmol/kg) | 0/30 |
| VPA (150 mg/kg) | 0/30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieróg, M.; Socała, K.; Nieoczym, D.; Wyska, E.; Samorek-Pieróg, M.; Wlaź, P. Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment. Molecules 2023, 28, 3810. https://doi.org/10.3390/molecules28093810
Pieróg M, Socała K, Nieoczym D, Wyska E, Samorek-Pieróg M, Wlaź P. Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment. Molecules. 2023; 28(9):3810. https://doi.org/10.3390/molecules28093810
Chicago/Turabian StylePieróg, Mateusz, Katarzyna Socała, Dorota Nieoczym, Elżbieta Wyska, Małgorzata Samorek-Pieróg, and Piotr Wlaź. 2023. "Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment" Molecules 28, no. 9: 3810. https://doi.org/10.3390/molecules28093810
APA StylePieróg, M., Socała, K., Nieoczym, D., Wyska, E., Samorek-Pieróg, M., & Wlaź, P. (2023). Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment. Molecules, 28(9), 3810. https://doi.org/10.3390/molecules28093810








