In/Ga-Doped Si as Anodes for Si–Air Batteries with Restrained Self-Corrosion and Surface Passivation: A First-Principles Study
Abstract
1. Introduction
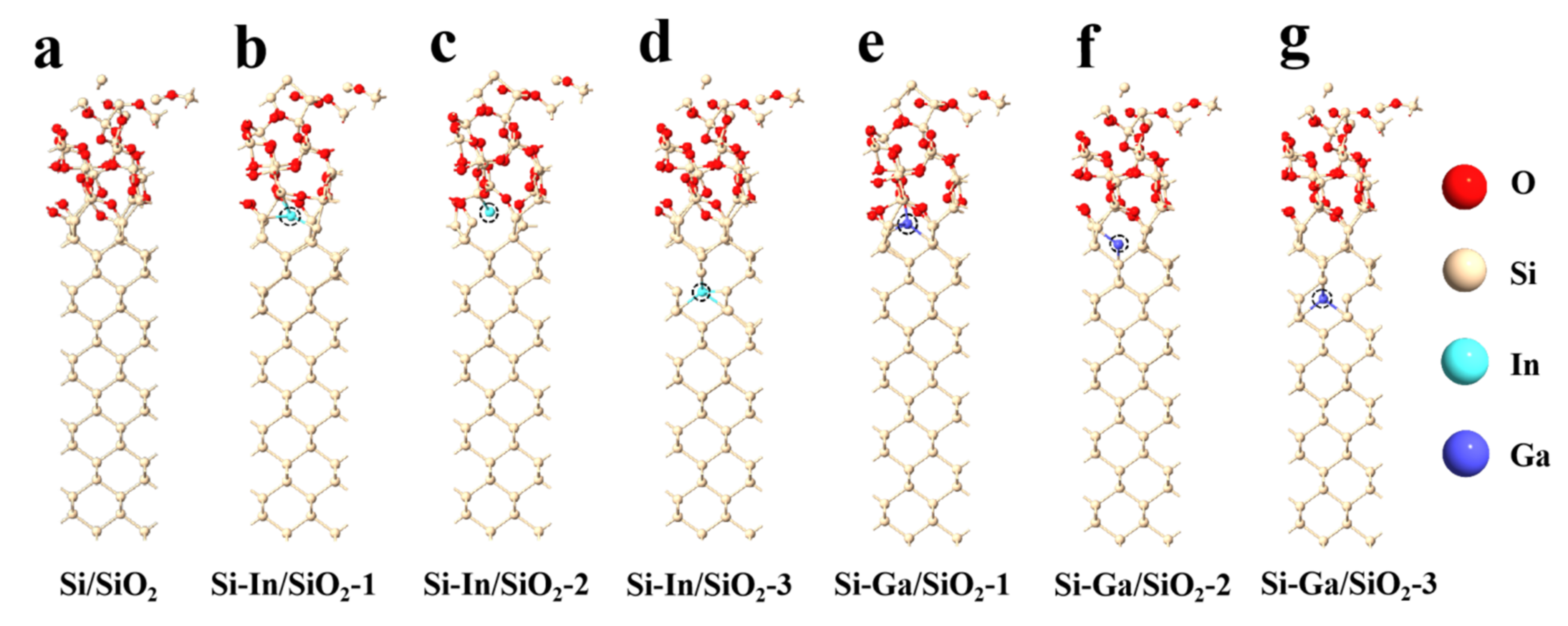
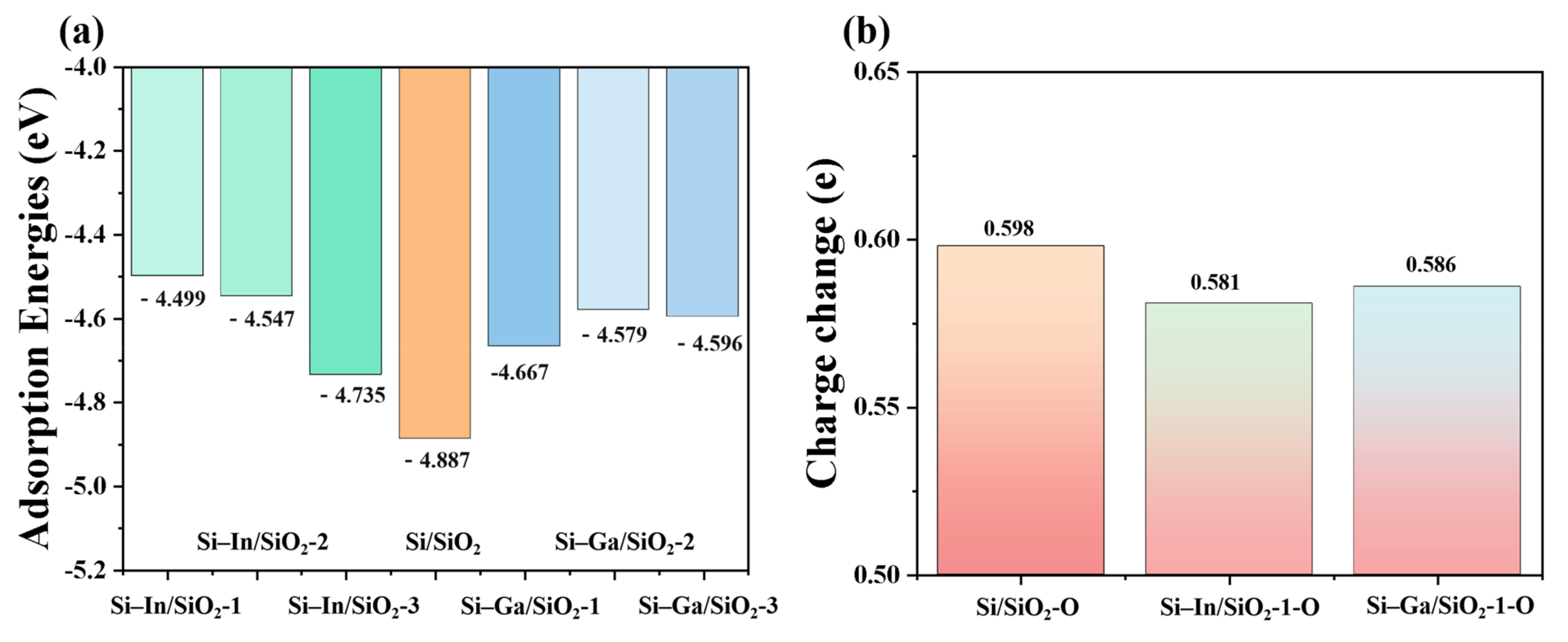
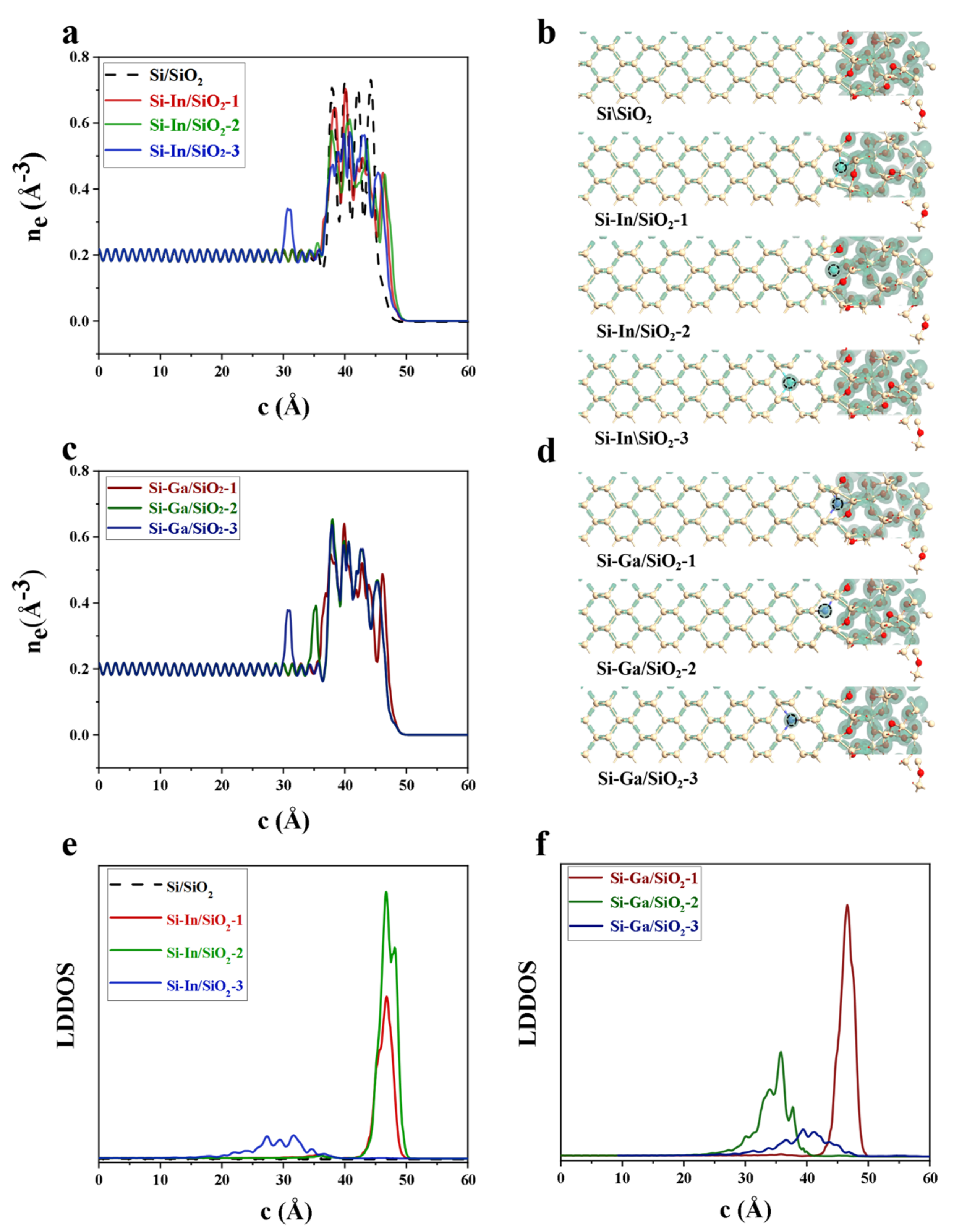
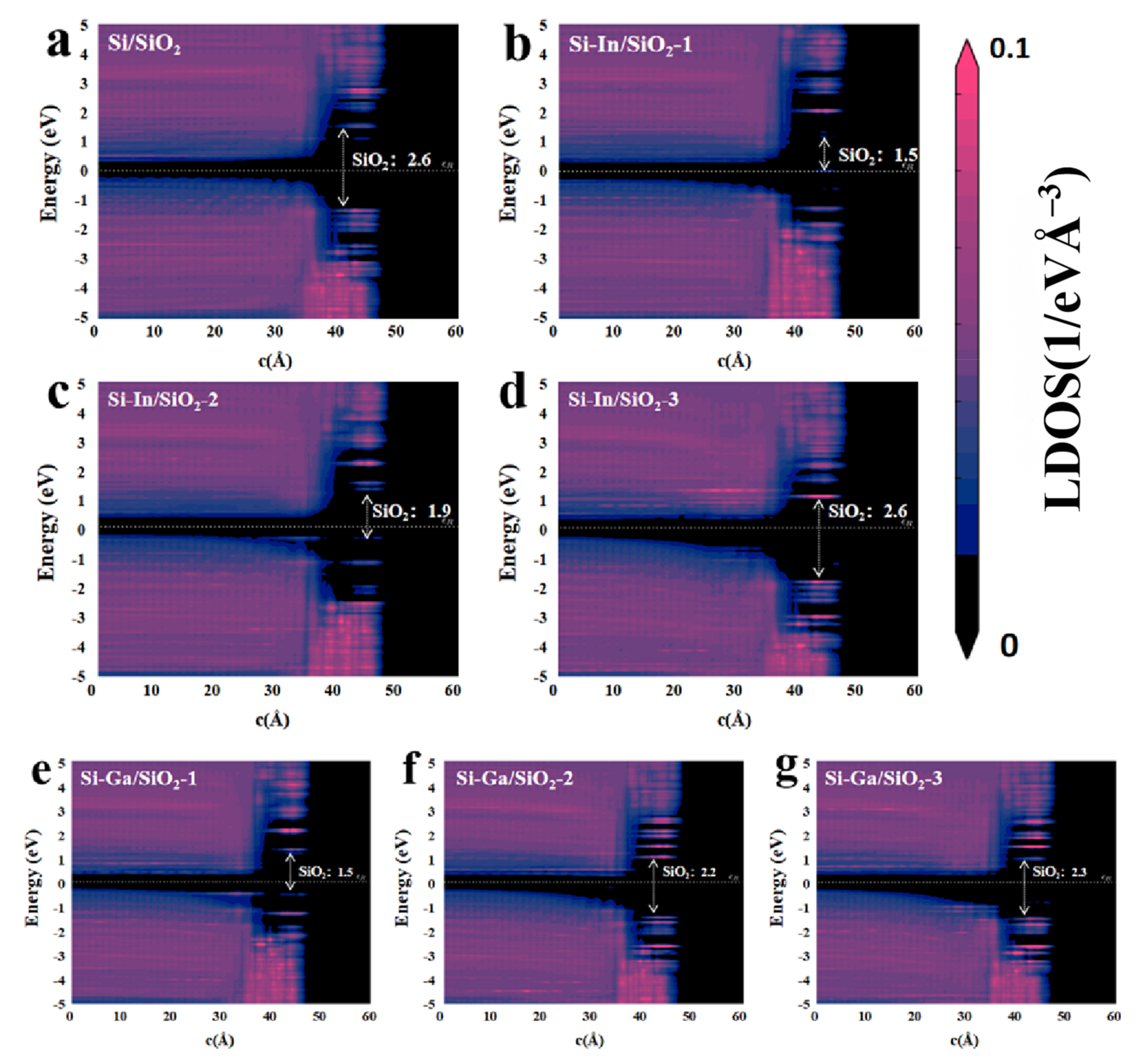
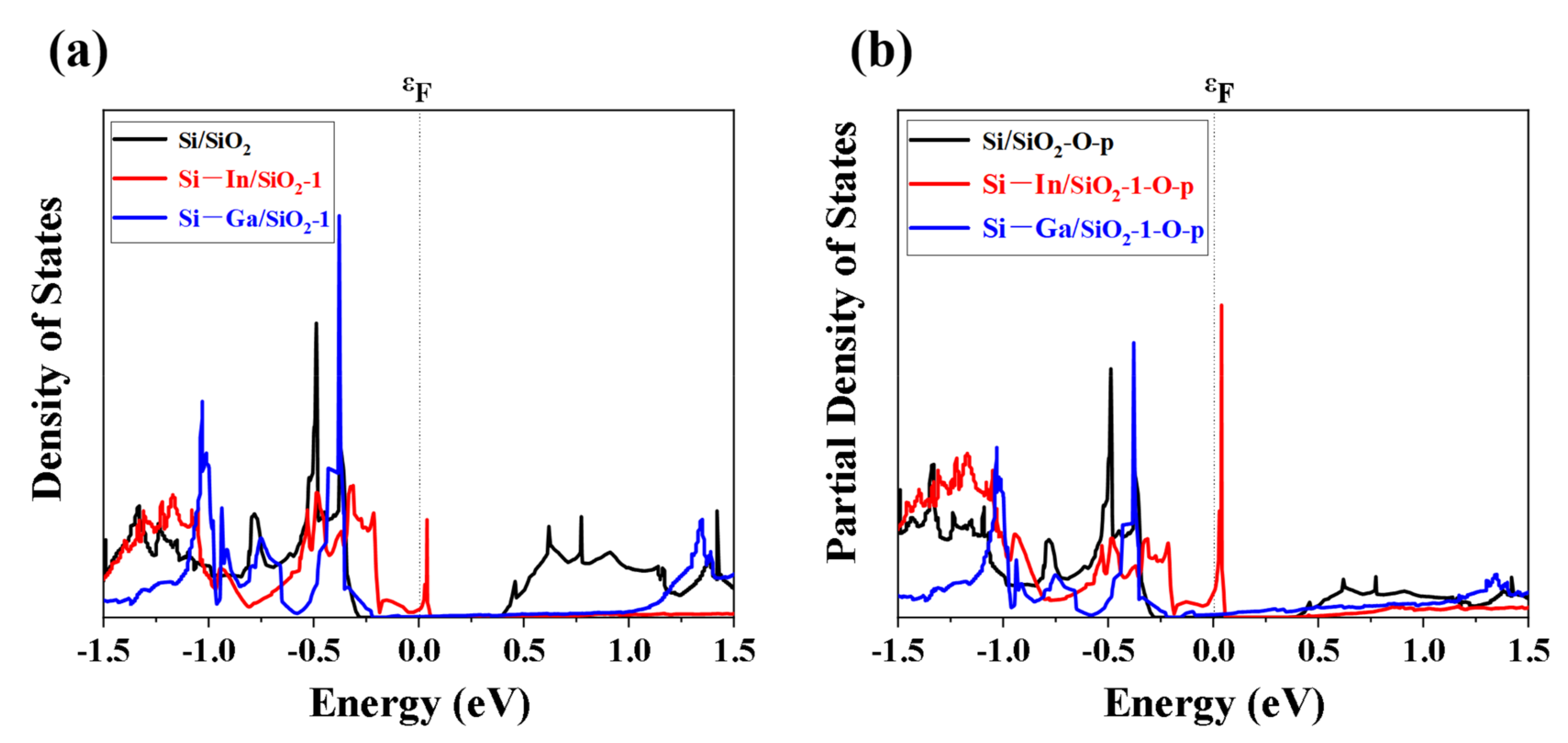
2. Results and Discussion
3. Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, H.; Wen, X.; Zhang, J.; Tang, H.; Deng, Y.; Liao, S.; Tian, X. Recent advances in MOFs/MOF derived nanomaterials toward high-efficiency aqueous zinc ion batteries. Coordin. Chem. Rev. 2022, 468, 214642. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.L.; Zhao, Y.Z.; Ye, Y.H.; Li, Y.Y.; Liu, J.P. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2023, 35, 2110423. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.Y.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Xie, Q.; Manthiram, A. A Cobalt-and Manganese-Free High-Nickel Layered Oxide Cathode for Long-Life, Safer Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102421. [Google Scholar] [CrossRef]
- Liu, F.; Xu, R.; Wu, Y.; Boyle, D.; Yang, A.; Xu, J.; Zhu, Y.; Ye, Y.; Yu, Z.; Zhang, Z.; et al. Dynamic spatial progression of isolated lithium during battery operations. Nature 2021, 600, 659–663. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, C.; Wu, P.; Yuan, H.; Feng, W.; Liu, Z.; Lu, Y.; Sun, S.; Fu, Z.; Hu, J.; et al. Anode-Free Solid-State Lithium Batteries: A Review. Adv. Energy Mater. 2022, 12, 2201044. [Google Scholar] [CrossRef]
- Weinrich, H.; Durmus, Y.E.; Tempel, H.; Kung, H.; Eichel, R.A. Silicon and Iron as Resource-Efficient Anode Materials for Ambient-Temperature Metal-Air Batteries: A Review. Materials 2019, 12, 2134. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Xia, D.; Zou, R. Metal-organic frameworks and their derivatives for metal-air batteries. Energy Storage Mater. 2019, 23, 757–771. [Google Scholar] [CrossRef]
- Cohn, G.; Starosvetsky, D.; Hagiwara, R.; Macdonald, D.D.; Ein-Eli, Y. Silicon-air batteries. Electrochem. Commun. 2009, 11, 1916–1918. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Aslanbas, Ö.; Kayser, S.; Tempel, H.; Hausen, F.; Haart, L.G.J.; Granwehr, J.; Ein-Elid, Y.; Eichel, R.A.; Kungl, H. Long run discharge, performance and efficiency of primary Silicon–air cells with alkaline electrolyte. Electrochim. Acta 2017, 225, 215–224. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Guerrero, S.S.; Aslanbas, Ö.; Tempel, H.; Hausen, F.; Haart, L.G.J.; Ein-Eli, Y.; Eichel, R.; Kungl, H. Investigation of the corrosion behavior of highly As-doped crystalline Si in alkaline Si-air batteries. Electrochim. Acta 2018, 265, 292–302. [Google Scholar] [CrossRef]
- Cohn, G.; Macdonald, D.D.; Ein-Eli, Y. Remarkable Impact of Water on the Discharge Performance of a Silicon–Air Battery. ChemSusChem 2011, 4, 1124–1129. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, H.; Liu, Y.; Bai, J.; Liao, L.; Huang, Y.; Duan, X. High-Capacity Silicon–Air Battery in Alkaline Solution. ChemSusChem 2012, 5, 177–180. [Google Scholar] [CrossRef]
- Xiao, P.; Li, H.; Fu, J.; Zeng, C.; Zhao, Y.; Zhai, T.; Li, H. An anticorrosive zinc metal anode with ultra-long cycle life over one year. Energy Environ. Sci. 2022, 15, 1638–1646. [Google Scholar] [CrossRef]
- Legrain, F.; Manzhos, S. Aluminum doping improves the energetics of lithium, sodium, and magnesium storage in silicon: A first-principles study. J. Power Sources 2015, 274, 65–70. [Google Scholar] [CrossRef]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2017, 30, 1702944. [Google Scholar] [CrossRef]
- Deng, C.; Xie, X.; Han, J.; Lu, B.; Liang, S.Q.; Zhou, J. Stabilization of Zn Metal Anode through Surface Reconstruction of a Cerium-Based Conversion Film. Adv. Funct. Mater. 2021, 31, 2103227. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, X.; Qin, Z.; Chang, Y.; Mao, D.; Wan, L.; Huang, Y. Reversible passivation in primary aluminum-air batteries via composite anodes. Energy Storage Mater. 2022, 49, 537–545. [Google Scholar] [CrossRef]
- You, X.; Zhang, X.; Yu, C.; Chen, Y.; Li, H.; Hou, Y.; Tian, L.; Yang, N.; Xie, G. Corrosion protection of magnesium alloys anode by cerium-based anodization coating in magnesium-air battery. J. Rare Earth. 2023, 41, 471–476. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 Coatings Enabled Uniform Zn Stripping/ Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Roitzheim, C.; Tempel, H.; Hausen, F.; Ein-Eli, Y.; Kungl, H.; Eichel, R.-A. Analysis on discharge behavior and performance of As- and B-doped silicon anodes in non-aqueous Si-air batteries under pulsed discharge operation. J. Appl. Electrochem. 2019, 50, 93–109. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Jakobi, S.; Beuse, T.; Aslanbas, Ö.; Tempel, H.; Hausen, F.; de Haart, L.G.J.; Ein-Eli, Y.; Eichel, R.-A.; Kungl, H. Influence of Dopant Type and Orientation of Silicon Anodes on Performance, Efficiency and Corrosion of Silicon—Air batteries with EMIm(HF)2.3F Electrolyte. J. Electrochem. Soc. 2017, 164, 2310–2320. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S.; Huang, J. Germanium-modified silicon as anodes in Si-Ge air batteries with enhanced properties. J. Phys. Chem. Solids. 2021, 157, 110226. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, S.; Hu, S. Si modified by Zn and Fe as anodes in Si-air batteries with ameliorative properties. J. Alloys Compd. 2021, 883, 160902. [Google Scholar] [CrossRef]
- Jamil, S.; Yue, L.; Li, C.; Fasehullah, M.; Din, M.A.U.; Yang, W.T.; Bao, S.J.; Xu, M.W. Significance of gallium doping for high Ni, low Co/Mn layered oxide cathode material. Chem. Eng. J. 2022, 441, 135821. [Google Scholar] [CrossRef]
- Lang, Y.; Hao, J.; Du, X.; Mao, H.; Li, J. Wettability of Gallium-Based Liquid Metals on Different Media Surfaces. Chin. J. Rare Met. 2021, 45, 306–311. [Google Scholar]
- Yin, J.; Wang, Y.; Zhu, Y.; Jin, J.; Chen, C.; Yuan, Y.; Bayhan, Z.; Salah, N.; Alhebshi, N.A.; Zhang, W.; et al. Regulating the redox reversibility of zinc anode toward stable aqueous zinc batteries. Nano Energy 2022, 99, 107331. [Google Scholar] [CrossRef]
- Elsayed, A.E.R.; Shilkamy, H.A.E.S.; Elrouby, M. The impact of indium metal as a minor bimetal on the anodic dissolution and passivation performance of zinc for alkaline batteries: Part I—Potentiodynamic, potentiostatic, XRD, SEM, and EDAX studies. J. Solid State Electr. 2021, 25, 2161–2174. [Google Scholar] [CrossRef]
- Wang, W.; Zuo, P.; Yin, G.; Du, C.; Huo, H.; Ma, Y.; Gao, Y. A dendrite-free Ga-In-Sn-Zn solid-liquid composite anode for rechargeable zinc batteries. Energy Storage Mater. 2023, 58, 195–203. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, S.; Hou, Y.; Li, S.; Wang, C.; Xiong, W.; Zhang, Q.; Miao, X.; Liu, J.; Cao, Y.; et al. Enhanced Density of States Facilitates High Thermoelectric Performance in Solution-Grown Ge- and In-Codoped SnSe Nanoplates. ACS Nano 2023, 17, 801–810. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Yi, C.; Liu, H.; Chen, S.; Sun, X.; Ma, C.; Li, D.; He, C.; Luo, Z.; et al. Efficient control of emission and carrier polarity in WS2 monolayer by indium doping. Sci. China Mater. 2021, 64, 1449–1456. [Google Scholar] [CrossRef]
- Alkauskas, A.; Broqvist, P.; Devynck, F.; Pasquarello, A. Band Offsets at Semiconductor-Oxide Interfaces from Hybrid Density-Functional Calculations. Phys. Rev. Lett. 2008, 101, 106802. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Ailan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Stillinger, F.H.; Weber, T.A. Computer simulation of local order in condensed phases of silicon. Phys. Rev. B 1985, 31, 5262. [Google Scholar] [CrossRef]
- He, Q.; Yu, B.; Li, Z.; Zhao, Y. Density Functional Theory for Battery Materials. Energy Environ. Mater. 2019, 2, 264–279. [Google Scholar] [CrossRef]
- Adekoya, D.; Qian, S.; Gu, X.; Wen, W.; Li, D.; Ma, J.; Zhang, S. DFT-Guided Design and Fabrication of Carbon-Nitride-Based Materials for Energy Storage Devices: A Review. Nano-Micro Lett. 2021, 13, 13. [Google Scholar] [CrossRef]
- Atomistix Toolkit (ATK). Available online: http://www.quantumwise.com (accessed on 23 May 2021).


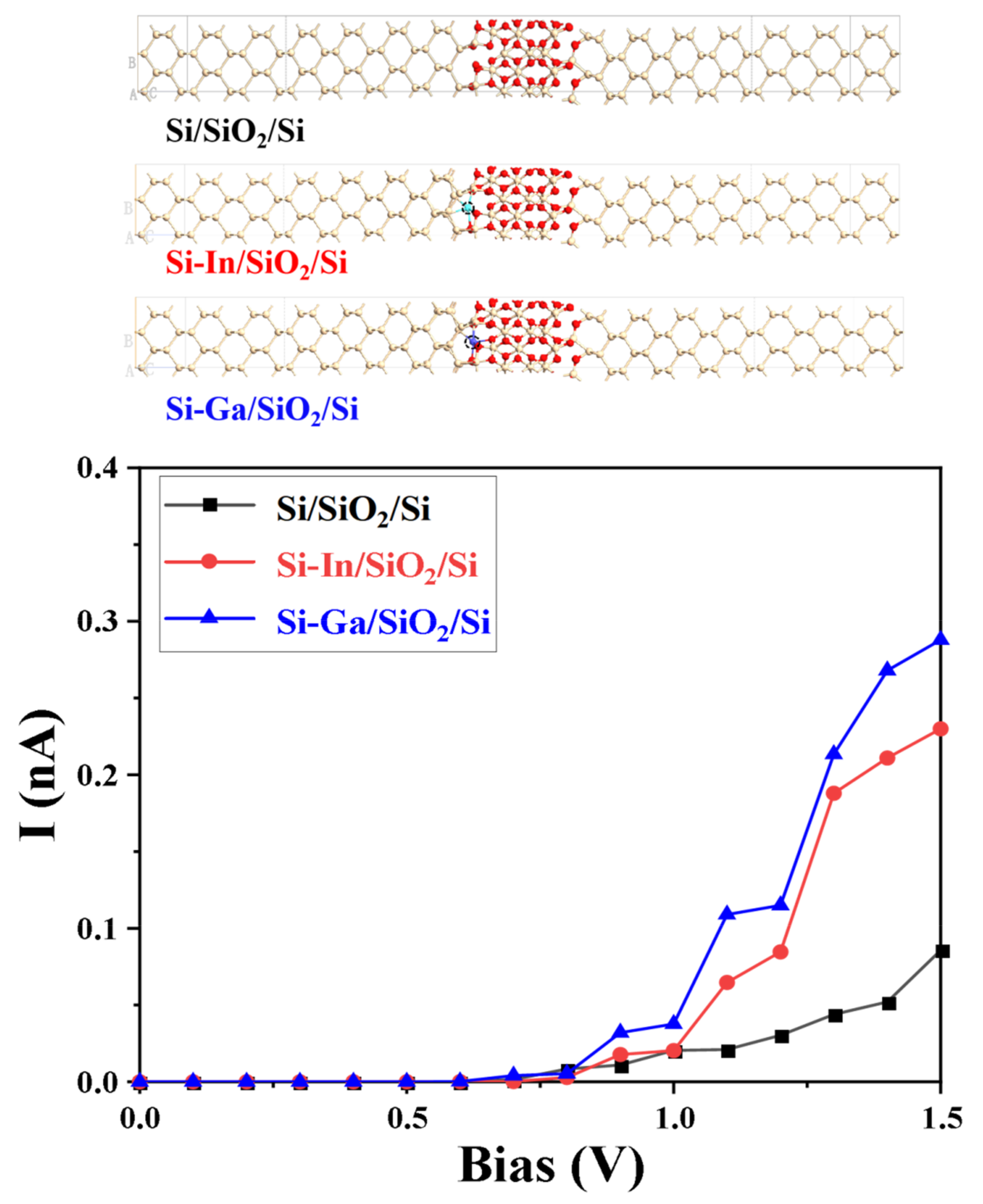
| Modification Strategy | Current | Bias Voltage | References |
|---|---|---|---|
| Si/SiO2/Si | 0.086 nA | 1.5 V | / |
| Si-In/SiO2/Si | 0.23 nA | 1.5 V | |
| Si-Ga/SiO2/Si | 0.29 nA | 1.5 V | |
| Si-Zn/SiO2/Si | 0.43 nA | 1.5 V | [24] |
| Si-Fe/SiO2/Si | 0.20 nA | 1.5 V | |
| Si/Ge-2/SiO2/Si | 0.19 nA | 1.5 V | [25] |
| Si/Ge-4/SiO2/Si | 0.19 nA | 1.5 V | |
| Si/Ge-12/SiO2/Si | 0.20 nA | 1.5 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhao, T.; Yu, Y. In/Ga-Doped Si as Anodes for Si–Air Batteries with Restrained Self-Corrosion and Surface Passivation: A First-Principles Study. Molecules 2023, 28, 3784. https://doi.org/10.3390/molecules28093784
Wang D, Zhao T, Yu Y. In/Ga-Doped Si as Anodes for Si–Air Batteries with Restrained Self-Corrosion and Surface Passivation: A First-Principles Study. Molecules. 2023; 28(9):3784. https://doi.org/10.3390/molecules28093784
Chicago/Turabian StyleWang, Dongxu, Tingyu Zhao, and Yingjian Yu. 2023. "In/Ga-Doped Si as Anodes for Si–Air Batteries with Restrained Self-Corrosion and Surface Passivation: A First-Principles Study" Molecules 28, no. 9: 3784. https://doi.org/10.3390/molecules28093784
APA StyleWang, D., Zhao, T., & Yu, Y. (2023). In/Ga-Doped Si as Anodes for Si–Air Batteries with Restrained Self-Corrosion and Surface Passivation: A First-Principles Study. Molecules, 28(9), 3784. https://doi.org/10.3390/molecules28093784






