Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay
Abstract
1. Introduction
2. Results
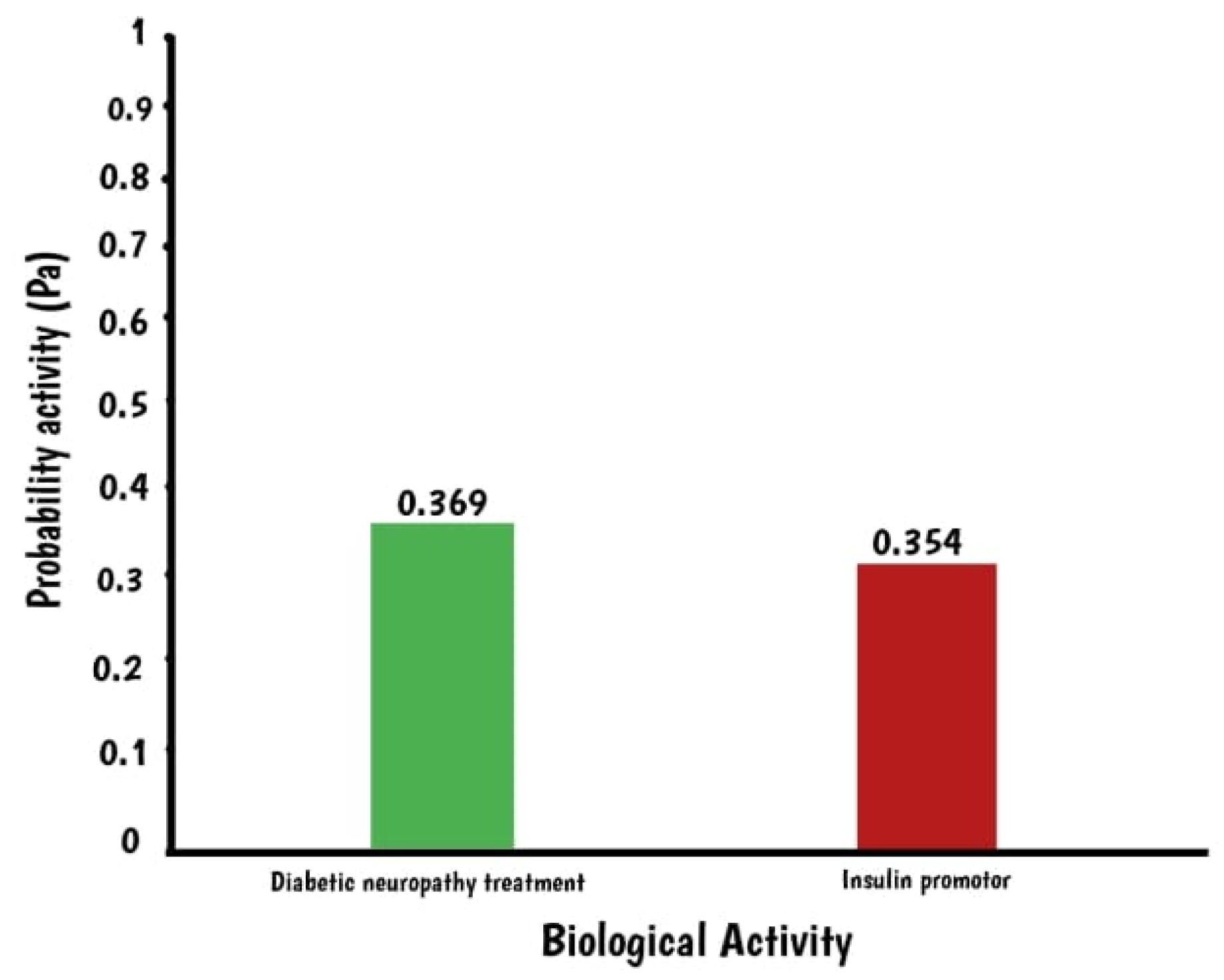
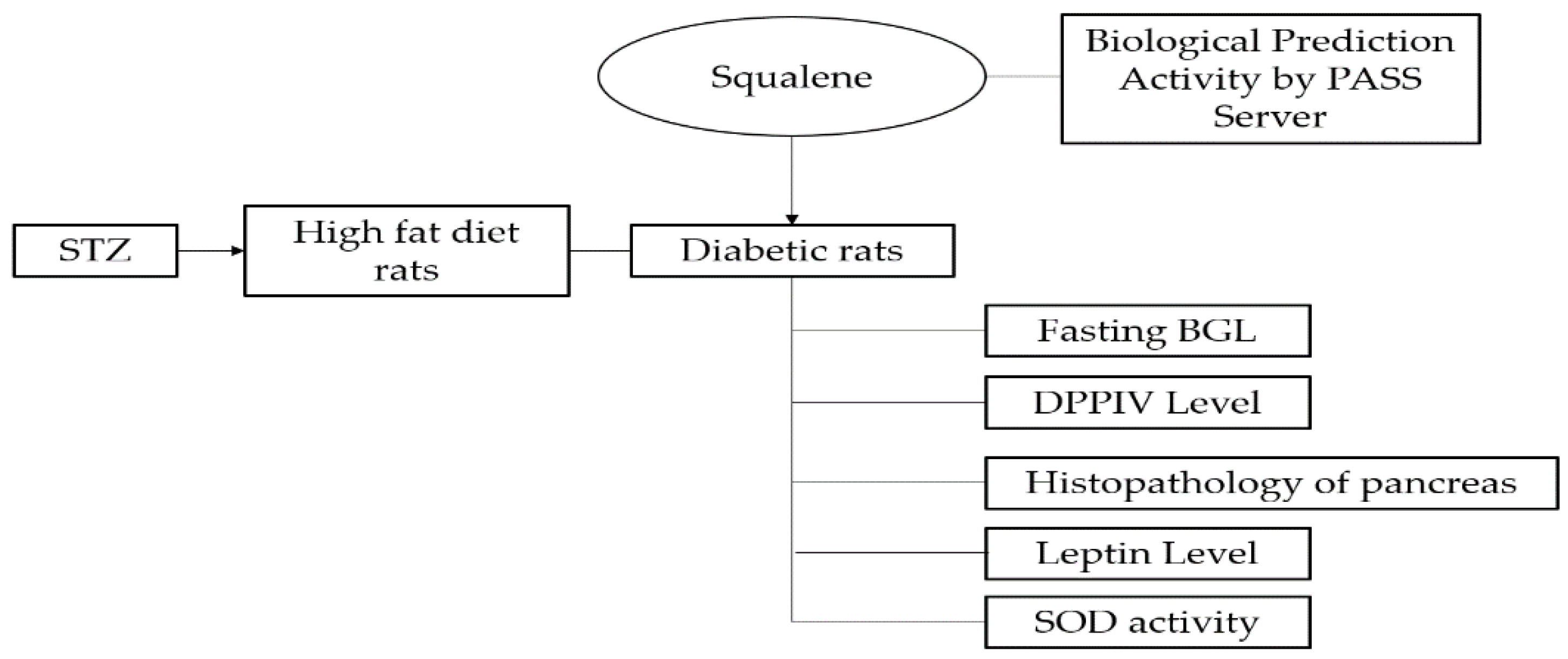
2.1. In Silico Assay: Squalene Biological Prediction Activity by PASS Server
2.2. In Vivo Assay
2.2.1. Effect of Squalene on Fasting Blood Glucose Level (FBGL)
2.2.2. Effect of Squalene on DPPIV Level
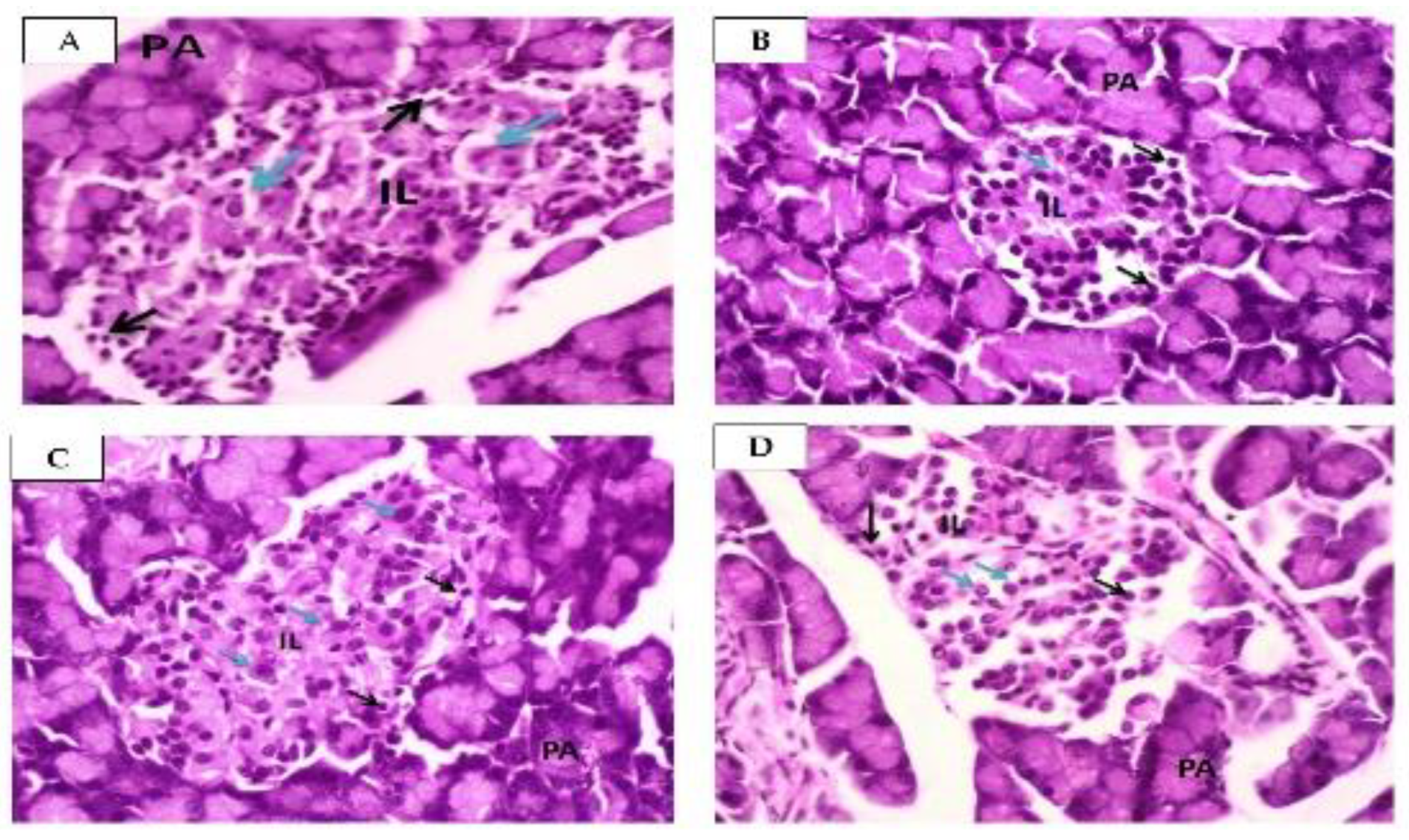
2.2.3. Effect of Squalene on Pancreas
2.2.4. Effect of Squalene on Leptin Levels
2.2.5. Effect of Squalene on SOD Activity
3. Discussion
4. Materials and Methods
4.1. In silico Antidiabetic Analysis
4.2. Materials and Reagent
4.3. Animals
4.4. Diabetic Induction to High Fat Diet Rats
4.5. Experimental Design
4.6. DPPV Level Measurement
4.7. Histopathology of Pancreas
4.8. Leptin Level Measurement
4.9. SOD Activity Measurement
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DC | Diabetic Control |
| DM | Diabetic Mellitus |
| DPPIV | Dipeptidyl peptidase IV |
| FBGL | Fasting blood glucose level |
| H&E | Hematoxylin and Eosin |
| SOD | Superoxide Dismutase |
| IL | Islets of Langerhans |
| T2DM | Type 2 DM |
References
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Manimaran, A.; Rajneesh, C.P. Activities of antioxidant enzyme and lipid peroxidation in ovarian cancer patients. Acad. J. Cancer Res. 2009, 2, 68–72. [Google Scholar]
- Mallick, M.; Bose, A.; Mukhi, S. Comparative evaluation of the antioxidant activity of some commonly used spices. Int. J. Pharm. Tech. Res. 2016, 9, 1–8. [Google Scholar]
- Yusoff, N.; Ahmad, M.; al Hindi, B.; Widyawati, T.; Yam, M.; Mahmud, R.; Asmawi, M. Aqueous extract of Nypa fruticans Wurmb. vinegar alleviates postprandial hyperglycemia in normoglycemic rats. Nutrients 2015, 7, 7012–7026. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Garvey, W.T.; et al. AACE comprehensive diabetes management algorithm 2013. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2013, 19, 327–336. [Google Scholar]
- De Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Widyawati, T.; Yusoff, N.A.; Bello, I.; Asmawi, M.Z.; Ahmad, M. Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model. Molecules 2022, 27, 6814. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; DCCT/Edic Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Widyawati, T.; Purnawan, W.W.; Atangwho, I.J.; Yusoff, N.A.; Ahmad, M.; Asmawi, M.Z. Anti-diabetic activity of Syzygium polyanthum (Wight) leaf extract, the most commonly used herb among diabetic patients in Medan, North Sumatera, Indonesia. Int. J. Pharm. Sci. Res. 2015, 6, 1698. [Google Scholar]
- Farijati. Pengaruh Dari Sediaan Yang Mengandung Squalene Dan Lecithin Yang Beredar di Pasaran Terhadap Kadar Glukosa Darah Kelinci Normal Dengan Menggunakan uji Tolerensi Glukosa Dan Tanpa Toleransi Glukosa Oral. 1995. Available online: http://repository.ubaya.ac.id/10137/ (accessed on 3 January 2020).
- Widyawati, T.; Yusoff, N.; Asmawi, M.; Ahmad, M. Antihyperglycemic effect of methanol extract of Syzygium polyanthum (Wight.) leaf in streptozotocin-induced diabetic rats. Nutrients 2015, 7, 7764–7780. [Google Scholar] [CrossRef]
- Bandaru, P.; Shankar, A. Association between plasma leptin levels and diabetes mellitus. Metab. Syndr. Relat. Disord. 2011, 9, 19–23. [Google Scholar] [CrossRef]
- Fischer, S.; Hanefeld, M.; Haffner, S.M.; Fusch, C.; Schwanebeck, U.; Köhler, C.; Julius, U. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 2002, 39, 105–110. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Danusantoso, H. Peran radikal bebas terhadap beberapa penyakit paru. J. Kedokt. Trisakti 2003, 22, 31–36. [Google Scholar]
- Al-mahmood, S.M.A.; Razak, T.A.; Abdullah, S.T.C.; Fatnoon, N.N.; Ahmad, N.; Mohamed, A.H.; Al-ani, I.M. A comprehensive study of chronic diabetes complications in streptozotocin-induced diabetic rat. Makara. J. Health Res. 2016, 20, 48–56. [Google Scholar] [CrossRef]
- Reed, M.J.; Meszaros, K.; Entes, L.J.; Claypool, M.D.; Pinkett, J.G.; Gadbois, T.M.; Reaven, G.M. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000, 49, 1390–1394. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Spanova, M.; Daum, G. Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid. Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Relas, H.; Gylling, H.; Miettinen, T.A. Fate of intravenously administered squalene and plant sterols in human subjects. J. Lipid. Res. 2001, 42, 988–994. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Von Tungeln, L.S.; Mitkus, R.J.; Anderson, S.A.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Pharmacokinetics and biodistribution of squalene-containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul. Toxicol. Pharmacol. 2016, 1, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Baldwin, S.L.; Duthie, M.S.; Reed, S.G.; Vedvick, T.S. Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. AAPS PharmSciTech 2012, 13, 498–506. [Google Scholar] [CrossRef]
- Fox, C.B. Squalene emulsions for parenteral vaccine and drug delivery. Molecules 2009, 14, 3286–3312. [Google Scholar] [CrossRef]
- Martirosyan, D.; Ashoori, M.R.; Mikaeili, A.S.; Pezeshki, S.; Serani, A.; Lee, M.; Mirmiranpour, H. Inflammatory factors and immunoglobulins alterations in subjects with type 2 diabetes mellitus treated with squalene. Funct. Food Sci. 2022, 2, 181–197. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene stimulates a key innate immune cell to foster wound healing and tissue repair. Evid.-Based Complement. Altern. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Farhadian, N.; Amiri, A.R.; Hataminia, F.; Soflaei, S.S.; Karimi, M. Evaluating the efficacy of extracted squalene from seed oil in the form of microemulsion for the treatment of COVID-19: A clinical study. J. Med. Virol. 2022, 94, 119–130. [Google Scholar] [CrossRef]
- Saputri, K.E.; Fakhmi, N.; Kusumaningtyas, E.; Priyatama, D.; Santoso, B. Docking molekular potensi anti diabetes melitus tipe 2 turunan zerumbon sebagai inhibitor aldosa reduktase dengan autodock-vina. Chim. Et Nat. Acta 2016, 4, 16–20. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Chen, Z. Insights into the molecular mechanisms of protein-ligand interactions by molecular docking and molecular dynamics simulation: A case of oligopeptide binding protein. Comput. Math. Methods Med. 2018, 2018, 3502514. [Google Scholar] [CrossRef]
- Pham, E.C.; Truong, T.N.; Dong, N.H.; Vo, D.D.; Hong Do, T.T. Synthesis of a series of novel 2-amino-5-substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as potential anticancer, antifungal and antibacterial agents. Med. Chem. 2021, 17, 558–573. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Popa, O.; Băbeanu, N.E.; Popa, I.; Niță, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. BioMed. Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Kinjo, T.; Saitoh, I.; Watanabe, M. Mutations in the C1 element of the insulin promoter lead to diabetic phenotypes in homozygous mice. Commun. Biol. 2020, 3, 309. [Google Scholar] [CrossRef] [PubMed]
- Mirmiranpour, H.; Ashoori, M.R.; Mikaeili, A.S.; Pezeshki, S.; Serani, A.; Vassar, R.; Martirosyan, D. The effect of squalene on lipid profile and some oxidative biomarkers in patients with type 2 diabetes mellitus. Funct. Food Sci. 2022, 2, 144–156. [Google Scholar] [CrossRef]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Anim. Model. Diabetes Res. 2012, 933, 161–174. [Google Scholar]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Widyawati, T.; Syarifah, S.; Sumantri, I.B. Squalene decreased malondialdehyde level of diabetic rats. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 24–25 August 2021; IOP Publishing: Bristol, UK, 2021; Volume 912, p. 012054. [Google Scholar]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes–a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem.-Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Kolb, H. Mouse models of insulin dependent diabetes: Low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab. Rev. 1987, 3, 751–778. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- KS Polonsky, The past 200 years in diabetes. N. Engl. J. Med. 2012, 367, 1332–1340. [CrossRef] [PubMed]
- Razak, A.; Mariam, A.; Amirin, S.; Mohd Zaini, A. Assessment on functionality and viability of β cells following repetitive dosage administration of ethanolic extracts of Andrographis paniculata on streptozotocininduced diabetic rats. Int. Med. J. 2010, 9, 21–26. [Google Scholar]
- Andrade-Cetto, A.; Becerra-Jiménez, J.; CárdenasVázquez, R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J. Ethnopharmacol. 2008, 116, 27–32. [Google Scholar] [CrossRef]
- Juárez-Rojop, I.E.; Díaz-Zagoya, J.C.; Ble-Castillo, J.L.; Miranda-Osorio, P.H.; Castell-Rodríguez, A.E.; Tovilla-Zárate, C.A.; Bermúdez-Ocaña, D.Y. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 236. [Google Scholar] [CrossRef]
- Al-masri, I.M.; Mohammad, M.K.; Tahaa, M.O. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzyme Inhibit. Med. Chemist. 2009, 24, 1061–1066. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Pratley, R.E.; Salsali, A. Inhibition of DPP-4: A new therapeutic approach for the treatment of type 2 diabetes. Curr. Med. Res. Opin. 2007, 23, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, N.; Nagakura, T.; Yamazaki, K.; Inoue, T.; Tanaka, I. Improvement of high fat-diet-induced insulin resistance in dipeptidyl peptidase IV-deficient Fischer rats. Life Sci. 2002, 71, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Frerker, N.; Raber, K.; Bode, F.; Skripuletz, T.; Nave, H.; Klemann, C.; Von Hörsten, S. Phenotyping of congenic dipeptidyl peptidase 4 (DP4) deficient Dark Agouti (DA) rats suggests involvement of DP4 in neuro-, endocrine, and immune functions. Clin. Chem. Lab. Med. 2009, 47, 275–287. [Google Scholar] [CrossRef]
- Nikmah, U.A.; Dany, F. Kadar leptin sebagai petanda diabetes pada individu dengan diabetes dan toleransi glukosa terganggu. Indones. Bull. Health Res. 2017, 45, 145–152. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 18, 80–84. [Google Scholar] [CrossRef]
- Shin, A.C.; Balasubramanian, P.; Suryadevara, P.; Zyskowski, J.; Herdt, T.H.; MohanKumar, S.M.; MohanKumar, P.S. Metformin effectively restores the HPA axis function in diet-induced obese rats. Int. J. Obes. 2021, 45, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Westphal, S.; Kraus, D.; Meier, B.; Perwitz, N.; Ott, V.; Fasshauer, M.; Klein, H.H. Metformin inhibits leptin secretion via a mitogen-activated protein kinase signalling pathway in brown adipocytes. J. Endocrinol. 2004, 183, 299–307. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Bi, D.; Wang, X.; Zhang, X.; Dai, H.; Chen, S.; Zhang, W. Influence of squalene feeding on plasma leptin, testosterone & blood pressure in rats. Indian J. Med. Res. 2009, 129, 150–153. [Google Scholar]
- Peltola, P.; Pihlajamäki, J.; Koutnikova, H.; Ruotsalainen, E.; Salmenniemi, U.; Vauhkonen, I.; Kainulainen, S.; Gylling, H.; Miettinen, T.A.; Auwerx, J.; et al. Visceral obesity is associated with high levels of serum squalene. Obesity 2006, 14, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.N.; Xie, J.; Li, R.; Chen, Q.H.; Chen, J.Z.; Wei, Z.H.; Kang, L.N.; Xu, B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc. Disord. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Ni, H.; Wu, M.; Tang, Z.; Halim, M.; Shi, D. ROS-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem. Biophys. Res. Commun. 2016, 476, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Al Nahdi, A.M.; John, A.; Raza, H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxidative Med. Cell. Longev. 2017, 2017, 7054272. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Khullar, M.; Al-Shudiefat, A.A.; Ludke, A.; Binepal, G.; Singal, P.K. Oxidative stress: A key contributor to diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 2010, 88, 233–240. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Et Biophys. Acta BBA-Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Widyawati, T.; Syarifah, S.; Ichwan, M.; Anggraini, D.R.; Wahyuni, A.S. Antihyperglycemic and pancreatic protective effect of squalene in streptozotocin-induced diabetic rat. In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), Medan, Indonesia, 4–6 May 2018; pp. 483–486. [Google Scholar]
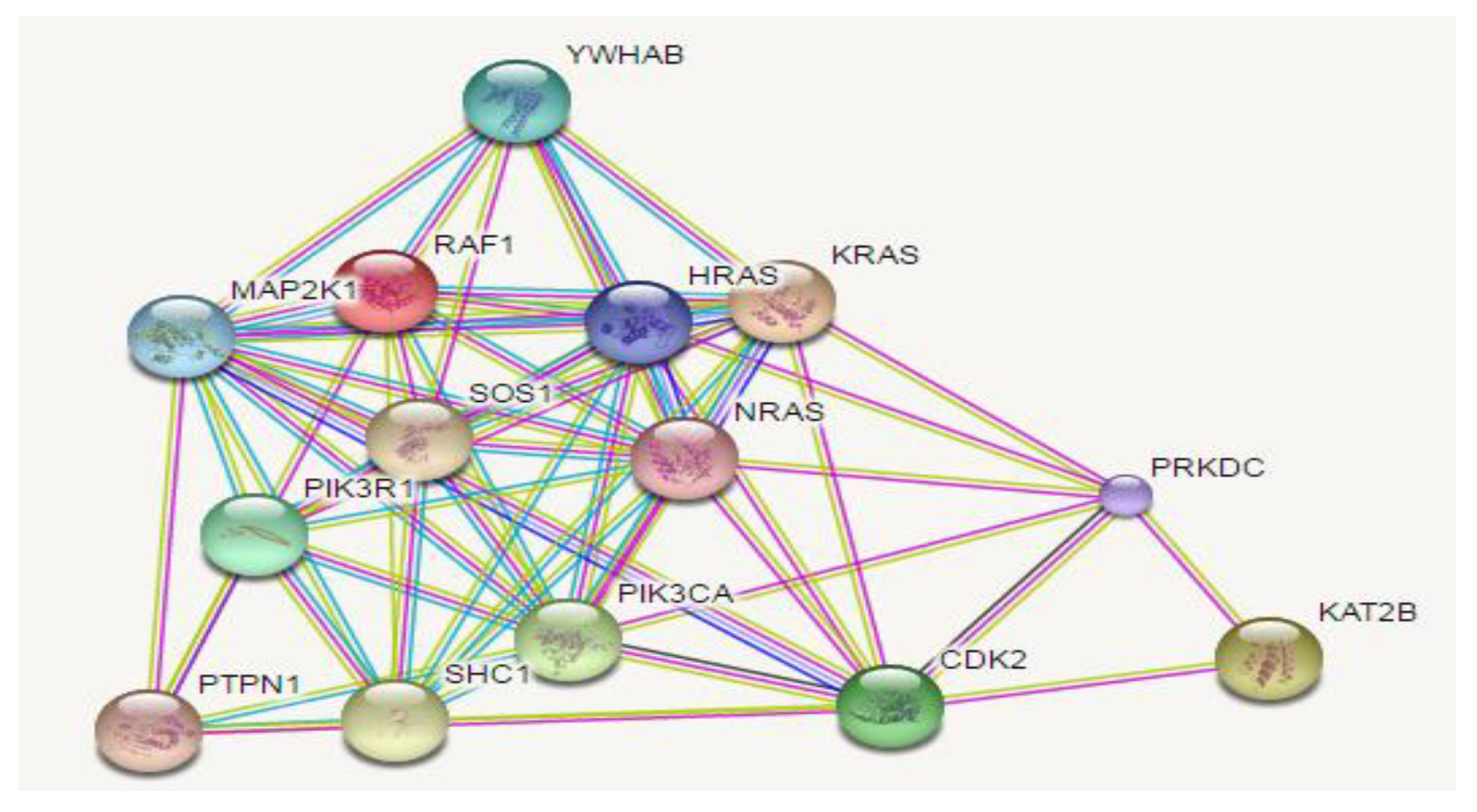
| Active Compound | Potential Target |
|---|---|
| Squalene | FNTA |
| KRAS | |
| RABGGTB | |
| RABGGTA | |
| FNTB | |
| SQUALENELE | |
| TRPV1 | |
| TRPV4 |
| Group | FBGL (mg/dL) Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 14 | |
| Squalene (n = 6) | 318.40 ± 46.03 | 292.60 ± 43.13 | 262.80 ± 4.93 | 220.40 ± 36.15 | 175.00 ± 23.49 | 134.40 ± 16.95 a* |
| Metformin (n = 6) | 338.00 ± 25.13 | 302.40 ± 27.5 | 260.00 ± 26.89 | 215.40 ± 32.27 | 170.40 ± 40.31 | 113.18 ± 33.03 b* |
| Diabetic control (n = 5) | 285.20 ± 74.34 | 294.20 ± 89.90 | 294.40 ± 104.36 | 311.00 ± 118.34 | 319.20 ± 127.70 | 350.30 ± 159.88 |
| p | 0.302 | 0.962 | 0.676 | 0.399 | 0.145 | 0.017 |
| Group | DPPIV (ng/mL) Mean ± SD |
|---|---|
| Squalene (n = 6) | 44.09 ± 5.29 |
| Metformin (n = 6) | 59.09 ± 8.10 |
| Diabetic control (n = 5) | 61.26 ± 15.06 |
| p | 0.105 |
| Group | Leptin (ng/mL) Mean ± SD |
|---|---|
| Squalene (n = 5) | 13.86 ± 0.47 *a |
| Metformin (n = 4) | 9.22 ± 0.84 |
| Diabetic control (n = 5) | 15.39 ± 1.77 *b |
| p | 0.011 |
| Group | SOD (U/mL) Mean ± SD |
|---|---|
| Squalene (n = 6) | 22.42 ± 0.27 |
| Metformin (n = 6) | 22.81 ± 0.08 * |
| Diabetic control (n = 4) | 21.88 ± 0.97 |
| p | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyawati, T.; Syahputra, R.A.; Syarifah, S.; Sumantri, I.B. Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay. Molecules 2023, 28, 3783. https://doi.org/10.3390/molecules28093783
Widyawati T, Syahputra RA, Syarifah S, Sumantri IB. Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay. Molecules. 2023; 28(9):3783. https://doi.org/10.3390/molecules28093783
Chicago/Turabian StyleWidyawati, Tri, Rony Abdi Syahputra, Siti Syarifah, and Imam Bagus Sumantri. 2023. "Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay" Molecules 28, no. 9: 3783. https://doi.org/10.3390/molecules28093783
APA StyleWidyawati, T., Syahputra, R. A., Syarifah, S., & Sumantri, I. B. (2023). Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay. Molecules, 28(9), 3783. https://doi.org/10.3390/molecules28093783






