The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma
Abstract
1. Introduction
2. MMP-9 Inhibition in Different Diseases
2.1. Inhibitory Mechanisms and Representative Agents of MMP-9 Inhibition
2.2. MMP-9 Inhibition in Different Diseases
2.2.1. Natural MMP-9 Inhibitors or MMP-9 Inhibitory Molecules Applied to Different Diseases
| Reference | Molecules | Research Methods | Research Diseases | Research Subjects | Effective Dose |
|---|---|---|---|---|---|
| [18] | Morus alba Stem Extract (MSE) | ELISA RT-PCR | Periodontitis | THP-1 cells | 20 µg/mL, 40 µg/mL |
| [24] | Epigallocatechin-3-gallate (EGCG) | Gelatin zymography | Nasopharyngeal carcinoma | TW01 cells NA cells | 10 μM, 20 μM, 30 μM, 50 μM |
| [25] | Epigallocatechin-3-gallate (EGCG) | Gelatin zymography | Myeloid leukemia | HL-60 cells | 0.3 µM, 1 µM, 3 µM, 10 µM, 30 µM |
| [26] | Epigallocatechin-3-gallate (EGCG) | Gelatin zymography | Transient global cerebral ischemia | A C57BL/6 mouse model of transient global cerebral ischemia | 50 mg/kg |
| [27] | Mangiferin | Gelatin zymography RT-PCR Western blotting | Astroglioma | U87MG cells U373MG cells CRT-MG cells | 30–300 μM |
| [28] | Mangiferin | Gelatin zymography RT-PCR Western blotting | Prostate carcinoma | LNCaP cells | 400 μM |
| [29] | Mangiferin | Gelatin zymography RT-PCR | Glioma | U87 cells | 50 μM, 100 μM |
| [30] | Mangiferin | ELISA Western blotting | Breast carcinoma | MDA-MB-231 cells BT-549 cells | 12.5 μM, 25 μM, 50 μM |
| [30] | Mangiferin | Western blotting | Breast carcinoma | An SCID mouse MDA-MB-231 xenograft model | 100 mg/kg |
| [31] | β-sitosterol (SITO) | Western blotting | Colon carcinoma | CT26/luc cells | 16 µM |
| [31] | Liposomal β-sitosterol (LS) | Western blotting | Colon carcinoma | CT26/luc cells | 16 µM |
| [32] | Resveratrol (RES) | Gelatin zymography | Vascular leakage | THP-1 cells | 30 μM |
| [32] | Resveratrol-Linoleate (RES-LA) | Gelatin zymography | Vascular leakage | THP-1 cells | 10 μM, 20 μM, 30 μM, 40 μM |
| [33] | Salvianolic acid A (SAA) | Western blotting | Ischemia reperfusion | An SD rat model of cerebral ischemia reperfusion | 5 mg/kg, 10 mg/kg, 20 mg/kg |
| [34] | Theaflavin | RT-PCR | Periodontitis | A Wistar rat model of ligatured periodontitis | 10 mg/mL, 100 mg/mL |
| [35] | Curcumin | RT-PCR Western blotting | Atherosclerosis (AS) | THP-1 cells | 6.25 µM, 25 µM, 50 µM |
| [36] | β-elemene | Immunohistochemistry RT-PCR Western blotting | Melanoma | A C57BL/6J mouse model with subretinal injection of B16F10 cells | 2 μL every 2 days |
| [37] | Angelica gigas (AG) | RT-PCR | Periodontitis | An SD rat model of ligature-induced periodontitis | 1 mg/mL, 100 mg/mL |
| [37] | Angelica gigas (AG) | ELISA | Periodontitis | HDF cells | 1 µg/mL, 10 µg/mL, 100 µg/mL |
2.2.2. Non-Natural MMP-9 Inhibitors or MMP-9 Inhibitory Molecules Applied to Different Diseases
2.2.3. MMP-9 Inhibitors or MMP-9 Inhibitory Molecules Involved in Clinical Trials
3. MMP-9 Expression in Thyroid Carcinoma
3.1. Introduction of Thyroid Carcinoma Diagnosis
3.2. MMP-9 Expression Levels in Thyroid Carcinoma
| Reference | Type of Thyroid Carcinoma | Type of Samples | Research Objects | Research Methods | MMP-9 Expression Data | MMP-9 Expression Levels |
|---|---|---|---|---|---|---|
| [91] | PTC | Tissue | 86 patients with PTC | Immunohistochemistry Gelatin zymography Western blotting | IHC positive staining ratio: PTC tumor tissues: 71/86 (82.56%) PTC non-tumor tissues: 53/86 (61.63%) | MMP-9 had a statistically significant higher level in tumor tissues than non-tumor tissues in PTC (p < 0.05). |
| [92] | PTC | Tissue | 25 patients with non-metastatic small PTC 19 patients with metastatic small PTC | Immunohistochemistry | IHC scores: Non-metastatic PTC tumor tissues: 1.44 Non-metastatic PTC non-tumor tissues: 0.80 Metastatic PTC tumor tissues: 2.00 Metastatic PTC non-tumor tissues: 0.82 | MMP-9 had a statistically significant higher level in tumor tissues than non-tumor tissues in both non-metastatic PTC (p < 0.01) and metastatic PTC (p < 0.001). |
| [93] | PTC | Tissue | 83 patients with PTC | Immunohistochemistry RT-PCR | IHC positive staining ratio: PTC tumor tissues: 48/83 (57.83%) PTC non-tumor tissues: 2/83 (2.41%) | MMP-9 had a statistically significant higher level in tumor tissues than non-tumor tissues in PTC (p < 0.001). |
| [94] | PTC | Tissue | 50 patients with PTC | Immunohistochemistry RT-PCR | / | MMP-9 had a statistically significant higher level in tumor tissues than non-tumor tissues in PTC (p = 0.01). |
| [95] | PTC | Tissue | 60 patients with PTC 30 patients with MNG | ELISA RT-PCR | mRNA level: PTC tumor tissues: 8.05 ± 16.48 PTC non-tumor tissues: 3.06 ± 3.63 Protein level: PTC tumor tissues: 429.60 ± 288.54 PTC non-tumor tissues: 223.15 ± 137.68 | MMP-9 had a statistically significant higher level in tumor tissues than non-tumor tissues in PTC (p < 0.05). |
| [82] | PTC | Tissue | 112 patients with PTC 42 patients with BTN | Immunohistochemistry | IHC scores: PTC tumor tissues: median 4.0, IQR 2.0–8.0 BTN tumor tissues: median 1.0, IQR 0.0–1.0 | MMP-9 had a statistically significant higher level in PTC tissues than in BTN tissues (p < 0.001). |
| [84] | PTC | Serum | 182 patients with PTC 86 patients with BTN 62 HCs | ELISA | Protein level: PTC serums: median 79.45, IQR 64.06–113.15 BTN serums: median 47.35, IQR 38.05–68.14 HC serums: median 47.71, IQR 36.70–59.52 | MMP-9 had a statistically significant higher level in PTC serums than in BTN serums (p < 0.001) and HC serums (p < 0.001). |
| [85] | PTC MTC FTC ATC | Tissue | 47 patients with TC 22 patients with FA | ELISA | / | MMP-9 had a statistically significant higher level in TC tissues than in FA tissues (p = 0.001). |
| [95] | PTC | Tissue | 60 patients with PTC 30 patients with MNG | ELISA RT-PCR | mRNA level: PTC tumor tissues: 6.77 ± 7.16 MNG tumor tissues: 2.49 ± 3.70 Protein level: PTC tumor tissues: 429.60 ± 288.54 MNG tumor tissues: 218.14 ± 113.74 | MMP-9 had a statistically significant higher level in PTC tissues than in MNG tissues (p < 0.05). |
| [96] | FTC | Tissue | 6 patients with WIFC 15 patients with MIFC 19 patients with FA 10 patients with AG | Immunohistochemistry | IHC positive staining ratio: MIFC tumor tissues: 13/15 (86.67%) FA tumor tissues: 11/19 (57.89%) AG tumor tissues: 3/10 (30.00%) | MMP-9 had a statistically significant higher level in MIFC tissues than in FA tissues (p < 0.05) and AG tissues (p < 0.005). |
| [97] | PTC | Tissue | 66 patients with PTC 40 patients with BTN | Immunohistochemistry | IHC positive staining ratio: PTC tumor tissues: 61/66 (92.42%) BTN tumor tissues: 8/40 (20.00%) | MMP-9 had a statistically significant higher level in PTC tissues than in BTN tissues (p < 0.001). |
| [98] | DTC | Serum | 57 patients with DTC 49 patients with BTN 20 HCs | ELISA RT-PCR | Protein level: Mean ± SD DTC serums: 134.70 ± 32.52 BTN serums: 47.60 ± 20.10 HC serums: 40.52 ± 10.20 | MMP-9 had a statistically significant higher level in DTC serums than in BTN serums (p < 0.05) and HC serums (p < 0.05). |
| [99] | PTC | Serum | 41 patients with PTC 56 patients with BTN | ELISA | Protein level: PTC serums: 299.98 ± 70.48 BTN serums: 126.62 ± 19.26 | MMP-9 had a statistically significant higher level in PTC serums than in BTN serums (p < 0.01). |
| [100] | PTC | Plasma | 30 patients with PTC 30 patients with BTN 23 HCs | ELISA | Protein level: Mean ± SD PTC plasma: 72.3 ± 23.3 BTN plasma: 23.0 ± 2.2 HC plasma: 22.1 ± 3.0 | MMP-9 had a statistically significant higher level in PTC plasma than in BTN plasma (p < 0.05) and HC plasma (p < 0.05). |
| [101] | PTC FTC ATC | Cell | IHH-4 cells FTC-133 cells 8505C cells HT-ori3 cells | Western blot RT-PCR | Protein level (the relative gray value): HT-ori3 cells: 1.01 ± 0.43 IHH-4 cells: 6.59 ± 1.24 FTC-133 cells: 5.10 ± 0.91 8505C cells: 5.42 ± 0.86 mRNA level: HT-ori3 cells: 1.01 ± 0.09 IHH-4 cells: 4.56 ± 0.61 FTC-133 cells: 3.41 ± 0.42 8505C cells: 2.79 ± 0.26 | MMP-9 had a statistically significant higher level in IHH-4 cells, FTC-133 cells, and 8505C cells than HT-ori3 cells (p < 0.05). |
| [98] | DTC | Serum | 57 patients with DTC 49 patients with BTN 20 HCs | ELISA RT-PCR | Protein level: DTC preoperative serums: 134.70 ± 32.52 DTC postoperative serums (1 month after surgery): 51.46 ± 18.34 | MMP-9 had a statistically significant higher level in DTC serums before operation than after operation (p < 0.05). |
| [99] | PTC | Serum | 41 patients with PTC 56 patients with BTN | ELISA | Protein level: PTC preoperative serums: 299.98 ± 70.48 PTC postoperative serums (3 months after surgery): 201.65 ± 65.31 PTC postoperative serums (6 months after surgery): 184.64 ± 64.82 PTC postoperative serums (12 months after surgery): 169.07 ± 64.16 | MMP-9 had a statistically significant higher level in PTC serums before operation than after operation (p < 0.05). |
| [102] | PTC | Tissue | 27 patients with metastatic PTC 31 patients with non-metastatic PTC | Immunohistochemistry | IHC positive and strongly positive staining ratio: Metastatic PTC tumor tissues: 19/27 (70.37%) Non-metastatic PTC tumor tissues: 11/31 (35.48%) | MMP-9 had a statistically significant higher level in PTC tumor tissues with metastasis than without metastasis (p < 0.05). |
| [103] | PTC | Tissue | 71 patients with cervical lymph node metastatic PTC 85 patients with non-metastatic PTC | Immunohistochemistry | IHC intense positive staining ratio: Metastatic PTC tumor tissues: 17/21 (80.95%)Non-metastatic PTC tumor tissues: 4/21 (19.05%) | MMP-9 had a statistically significant higher level in PTC tumor tissues with metastasis than without metastasis (p = 0.000). |
| [104] | PTC | Tissue | 17 patients with CPTC 25 patients with FPTC | Immunohistochemistry | IHC positive staining ratio: FPTC tumor tissues: 20/25 (80.00%) CPTC tumor tissues: 17/17 (100.00%) | MMP-9 had a statistically significant higher level in CPTC tissues than in FPTC tissues (p < 0.004). |
| Reference | Type of Thyroid Carcinoma | Type of Samples | Lymph Node Metastasis (LNM) | Extrathyroidal Invasion (EI) | Degree of Tumor Infiltration (DTI) | TNM Stage | Tumor Size | Distant Metastasis | Age | Lymphovascular Invasion |
|---|---|---|---|---|---|---|---|---|---|---|
| [81] | PTC | Tissue | p = 0.028 | p = 0.001 | p = 0.005 | p = 0.031 | ||||
| [82] | PTC | Tissue | Central LNM p = 0.002 Lateral LNM p < 0.001 | p = 0.004 | ||||||
| [83] | PTC | Tissue | p = 0.004 | p < 0.001 | p < 0.001 | p = 0.034 | ||||
| [91] | PTC | Tissue | p = 0.014 | |||||||
| [93] | PTC | Tissue | p < 0.001 (protein) p = 0.038 (mRNA) | p = 0.008 | ||||||
| [94] | PTC | Tissue | p = 0.019 | p = 0.019 | ||||||
| [95] | PTC | Tissue | p = 0.011 (mRNA) p = 0.001 (protein) | p = 0.015 (mRNA) p = 0.001 (protein) | p = 0.003 (mRNA) p = 0.036 (protein) | |||||
| [97] | PTC | Tissue | p = 0.006 | p < 0.001 | p = 0.003 | |||||
| [105] | FTC | Tissue | p = 0.001 | p = 0.016 | ||||||
| [84] | PTC | Serum | Lateral LNM p < 0.001 | p = 0.022 | p < 0.001 | p = 0.029 | p = 0.003 | |||
| [98] | DTC | Serum | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ||||
| [100] | PTC | Plasma | p < 0.001 | p = 0.037 | p = 0.003 | p = 0.002 | p = 0.034 |
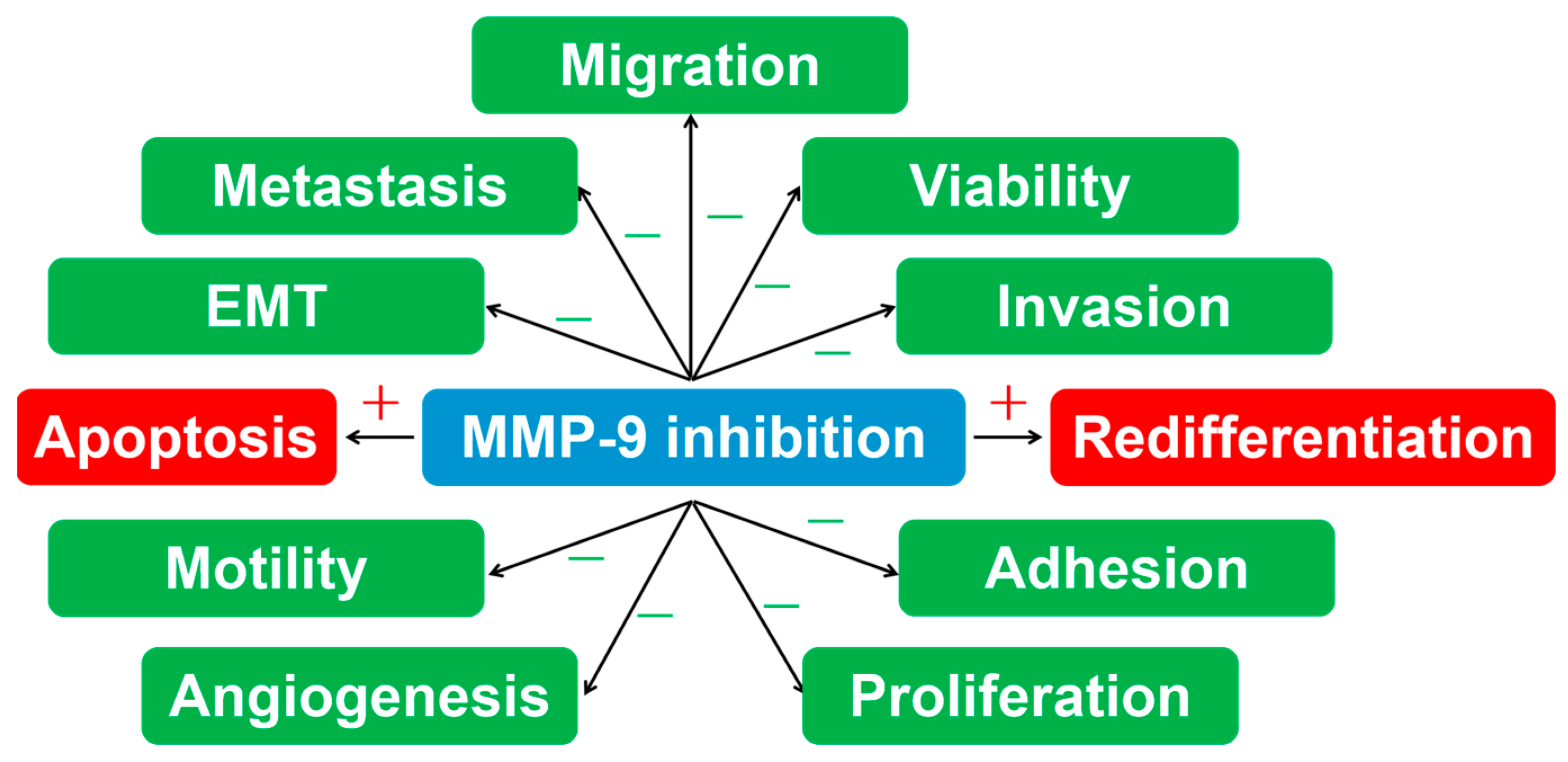
4. MMP-9 Inhibition in Thyroid Carcinoma
4.1. Introduction of Thyroid Carcinoma Therapy
4.2. MMP-9 Inhibition in Thyroid Carcinoma
4.2.1. Natural MMP-9 Inhibitors or MMP-9 Inhibitory Molecules Applied to Thyroid Carcinoma
4.2.2. Non-Natural MMP-9 Inhibitors or MMP-9 Inhibitory Molecules Applied to Thyroid Carcinoma
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Q.; Yuan, T.X.; Song, Q.L.; Zhang, Y.; Wei, Q.; Zhou, L.; Luo, J.; Zuo, G.; Tang, M.; et al. Matrix metalloproteinase 9 (MMP-9) in osteosarcoma: Review and meta-analysis. Clin. Chim. Acta 2014, 433, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Atkinson, J.J.; Senior, R.M. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell Mol. Biol. 2003, 28, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Muroski, M.E.; Roycik, M.D.; Newcomer, R.G.; Van den Steen, P.E.; Opdenakker, G.; Monroe, H.R.; Sahab, Z.J.; Sang, Q.X. Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr. Pharm. Biotechnol. 2008, 9, 34–46. [Google Scholar]
- Deleon-Pennell, K.Y.; Altara, R.; Yabluchanskiy, A.; Modesti, A.; Lindsey, M.L. The circular relationship between matrix metalloproteinase-9 and inflammation following myocardial infarction. IUBMB Life 2015, 67, 611–618. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Wang, L.; Wei, C.; Deng, L.; Wang, Z.; Song, M.; Xiong, Y.; Liu, M. The Accuracy of Serum Matrix Metalloproteinase-9 for Predicting Hemorrhagic Transformation After Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 1653–1665. [Google Scholar] [CrossRef]
- Ramos-Fernandez, M.; Bellolio, M.F.; Stead, L.G. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: A systematic review. J. Stroke Cerebrovasc. Dis. 2011, 20, 47–54. [Google Scholar] [CrossRef]
- Bronisz, E.; Kurkowska-Jastrzebska, I. Matrix Metalloproteinase 9 in Epilepsy: The Role of Neuroinflammation in Seizure Development. Mediat. Inflamm. 2016, 2016, 7369020. [Google Scholar] [CrossRef]
- Li, H.; Sheng, Z.; Khan, S.; Zhang, R.; Liu, Y.; Zhang, Y.; Yong, V.W.; Xue, M. Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression. Front. Neurol. 2022, 13, 861843. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Kanavi, M.R.; Rasaee, M.J. Inhibition of matrix metalloproteinase-9 for the treatment of dry eye syndrome; a review study. Exp. Eye Res. 2021, 205, 108523. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Sherer, Y.; Shoenfeld, Y. Matrix Metalloproteinase-9 and Autoimmune Diseases. J. Clin. Immunol. 2006, 26, 299–307. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Bianconi, V.; Avila-Rodriguez, M.F.; Barreto, G.E.; Jamialahmadi, T.; Pirro, M.; Sahebkar, A. Curcumin: A phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol. Res. 2020, 156, 104765. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.L.; Cheng, Y.W.; Yu, M.; Ho, J.D.; Kuo, Y.C.; Chiou, G.C.Y.; Chang, H.M.; Lee, T.H.; Hsiao, G. The fungus-derived retinoprotectant theissenolactone C improves glaucoma-like injury mediated by MMP-9 inhibition. Phytomedicine 2019, 56, 207–214. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Leveraging Natural Variance towards Enhanced Understanding of Phytochemical Sunscreens. Trends Plant Sci. 2017, 22, 308–315. [Google Scholar] [CrossRef]
- Yiemwattana, I.; Kaomongkolgit, R.; Wirojchanasak, S.; Chaisomboon, N. Morus alba Stem Extract Suppresses Matrix Metalloproteinases (MMP)-1, MMP-9, and Tissue Inhibitors of Metalloproteinase (TIMP)-1 Expression via Inhibition of IkappaBalpha Degradation Induced by Porphyromonas gingivalis LPS Signal in THP-1 Cells. Eur. J. Dent. 2019, 13, 229–234. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P. Tanshinone IIA: A phytochemical as a promising drug candidate for neurodegenerative diseases. Pharmacol. Res. 2021, 169, 105661. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, C.; Lozano-Sanchez, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef]
- Fang, C.Y.; Wu, C.C.; Hsu, H.Y.; Chuang, H.Y.; Huang, S.Y.; Tsai, C.H.; Chang, Y.; Tsao, G.S.; Chen, C.L.; Chen, J.Y. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int. J. Mol. Sci. 2015, 16, 2530–2558. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Currie, J.C.; Moghrabi, A.; Beliveau, R. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leuk. Res. 2007, 31, 1277–1284. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, Y.H.; Kim, J.M.; Lee, H.; Park, W.K.; Lim, M.B.; Chu, Y.K.; Lo, E.H.; Lee, S.R. Green tea polyphenol (-)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 2009, 87, 567–575. [Google Scholar] [CrossRef]
- Jung, J.S.; Jung, K.; Kim, D.H.; Kim, H.S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dilshara, M.G.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Mangiferin inhibits tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-kappaB activity. BMB Rep. 2015, 48, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, L.; Zhong, Z.; Xiao, C.; Zhang, J. Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol. Rep. 2015, 33, 2815–2820. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and beta-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lee, C.F.; Chou, W.T.; Hwang, J.J.; Tyan, Y.S.; Chuang, H.Y. Liposomal beta-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics 2022, 14, 1214. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Pavlickova, T.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol-Linoleate protects from exacerbated endothelial permeability via a drastic inhibition of the MMP-9 activity. Biosci. Rep. 2018, 38, BSR20171712. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.-K.; Zhang, X.; Zhou, Q.-M.; He, G.-R.; Xu, X.-N.; Rong, Y.; Zhou, W.-X.; Du, G.-H. Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin. J. Nat. Med. 2018, 16, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Kuraji, R.; Taya, Y.; Ito, H.; Numabe, Y. Effects of theaflavins on tissue inflammation and bone resorption on experimental periodontitis in rats. J. Periodontal Res. 2018, 53, 1009–1019. [Google Scholar] [CrossRef]
- Cao, J.; Ye, B.; Lin, L.; Tian, L.; Yang, H.; Wang, C.; Huang, W.; Huang, Z. Curcumin Alleviates oxLDL Induced MMP-9 and EMMPRIN Expression through the Inhibition of NF-kappaB and MAPK Pathways in Macrophages. Front. Pharmacol. 2017, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, L.; Liu, L.M.; Geng, J.; Chen, L. Inhibition of tumor growth by beta-elemene through downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9 in a murine intraocular melanoma model. Melanoma Res. 2015, 25, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, H.; Yang, W.M. Angelica gigas ameliorates the destruction of gingival tissues via inhibition of MMP-9 activity. RSC Adv. 2018, 8, 13089–13093. [Google Scholar] [CrossRef]
- Cui, J.; Chen, S.; Zhang, C.; Meng, F.; Wu, W.; Hu, R.; Hadass, O.; Lehmidi, T.; Blair, G.J.; Lee, M.; et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol. Neurodegener. 2012, 7, 21. [Google Scholar] [CrossRef]
- Jia, F.; Yin, Y.H.; Gao, G.Y.; Wang, Y.; Cen, L.; Jiang, J.Y. MMP-9 inhibitor SB-3CT attenuates behavioral impairments and hippocampal loss after traumatic brain injury in rat. J. Neurotrauma 2014, 31, 1225–1234. [Google Scholar] [CrossRef]
- Du, H.T.; Du, L.L.; Tang, X.L.; Ge, H.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Ahmed, Z.; Alhajlah, S.; Thompson, A.M.; Fairclough, R.J. Clinic-ready inhibitor of MMP-9/-12 restores sensory and functional decline in rodent models of spinal cord injury. Clin. Transl. Med. 2022, 12, e884. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Titlestad, I.; Lindqvist, A.; Wielders, P.; Wray, H.; Wang, M.; Samuelsson, V.; Mo, J.; Holt, A. Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: A randomised controlled trial. Pulm. Pharmacol. Ther. 2012, 25, 169–177. [Google Scholar] [CrossRef]
- Churg, A.; Wang, R.; Wang, X.; Onnervik, P.O.; Thim, K.; Wright, J.L. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax 2007, 62, 706–713. [Google Scholar] [CrossRef]
- Nanjan, P.; Nambiar, J.; Nair, B.G.; Banerji, A. Synthesis and discovery of (I-3, II-3)-biacacetin as a novel non-zinc binding inhibitor of MMP-2 and MMP-9. Bioorg. Med. Chem. 2015, 23, 3781–3787. [Google Scholar] [CrossRef]
- Krarup, P.M.; Eld, M.; Heinemeier, K.; Jorgensen, L.N.; Hansen, M.B.; Agren, M.S. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int. J. Color. Dis. 2013, 28, 1151–1159. [Google Scholar] [CrossRef]
- Kalani, A.; Pushpakumar, S.B.; Vacek, J.C.; Tyagi, S.C.; Tyagi, N. Inhibition of MMP-9 attenuates hypertensive cerebrovascular dysfunction in Dahl salt-sensitive rats. Mol. Cell. Biochem. 2016, 413, 25–35. [Google Scholar] [CrossRef]
- Lin, C.; Wu, W.; Lu, H.; Li, W.; Bao, Z.; Wang, Y.; Zhao, L.; Guo, T.; Cai, N.; Li, Z.; et al. MMP-9 Inhibitor GM6001 Prevents the Development of ssTBI-Induced Parkinson’s Disease via the Autophagy Pathway. Cell. Mol. Neurobiol. 2021, 41, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Perez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Nguyen, T.T.; Song, W.; Anderson, B.; Wolter, W.R.; Schroeder, V.A.; Hesek, D.; Lee, M.; Mobashery, S.; Chang, M. Selective MMP-9 Inhibitor (R)-ND-336 Alone or in Combination with Linezolid Accelerates Wound Healing in Infected Diabetic Mice. ACS Pharmacol. Transl. Sci. 2021, 4, 107–117. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, H.; Liu, Y.; Wang, L.; Zhang, N.; Zhang, G.; Liu, R.; Han, M. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells. J. Cancer 2019, 10, 6481–6490. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Maione, F.; Capano, S.; Meda, C.; Picconi, O.; Brundu, S.; Pisacane, A.; Sapino, A.; Palladino, C.; Barillari, G.; et al. HIV Protease Inhibitors Block HPV16-Induced Murine Cervical Carcinoma and Promote Vessel Normalization in Association with MMP-9 Inhibition and TIMP-3 Induction. Mol. Cancer Ther. 2020, 19, 2476–2489. [Google Scholar] [CrossRef]
- Rajasinghe, L.D.; Pindiprolu, R.H.; Gupta, S.V. Delta-tocotrienol inhibits non-small-cell lung cancer cell invasion via the inhibition of NF-kappaB, uPA activator, and MMP-9. OncoTargets Ther. 2018, 11, 4301–4314. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.L.; Sung, K.R.; Kwon, J.; Shin, J.A. Statins Suppress TGF-beta2-Mediated MMP-2 and MMP-9 Expression and Activation Through RhoA/ROCK Inhibition in Astrocytes of the Human Optic Nerve Head. Invest. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Cui, Y.; Gao, J.; Wang, K.; Cui, J. Lipoxin A4 Methyl Ester Reduces Early Brain Injury by Inhibition of the Nuclear Factor Kappa B (NF-kappaB)-Dependent Matrix Metallopeptidase 9 (MMP-9) Pathway in a Rat Model of Intracerebral Hemorrhage. Med. Sci. Monit. 2019, 25, 1838–1847. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCalpha and PKCbeta1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Dang, Q.; Wu, D.; Li, Y.; Fang, L.; Liu, C.; Wang, X.; Liu, X.; Min, W. Walnut-derived peptides ameliorate d-galactose-induced memory impairments in a mouse model via inhibition of MMP-9-mediated blood-brain barrier disruption. Food Res. Int. 2022, 162, 112029. [Google Scholar] [CrossRef] [PubMed]
- Lubis, B.; Lelo, A.; Amelia, P.; Prima, A. The Effect of Thiamine, Ascorbic Acid, and the Combination of Them on the Levels of Matrix Metalloproteinase-9 (MMP-9) and Tissue Inhibitor of Matrix Metalloproteinase-1 (TIMP-1) in Sepsis Patients. Infect. Drug Resist. 2022, 15, 5741–5751. [Google Scholar] [CrossRef]
- Yin, P.; Chen, J.; Wu, Y.; Gao, F.; Wen, J.; Zhang, W.; Su, Y.; Zhang, X. Chemoprevention of 4NQO-Induced Mouse Tongue Carcinogenesis by AKT Inhibitor through the MMP-9/RhoC Signaling Pathway and Autophagy. Anal. Cell. Pathol. 2022, 2022, 3770715. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Mairaj Siddiquei, M.; Imtiaz Nawaz, M.; Abu El-Asrar, A.M. The ERK1/2 Inhibitor U0126 Attenuates Diabetes-Induced Upregulation of MMP-9 and Biomarkers of Inflammation in the Retina. J. Diabetes Res. 2013, 2013, 658548. [Google Scholar] [CrossRef]
- Ermolli, M.; Schumacher, M.; Lods, N.; Hammoud, M.; Marti, H.P. Differential expression of MMP-2/MMP-9 and potential benefit of an MMP inhibitor in experimental acute kidney allograft rejection. Transpl. Immunol. 2003, 11, 137–145. [Google Scholar] [CrossRef]
- Roach, D.M.; Fitridge, R.A.; Laws, P.E.; Millard, S.H.; Varelias, A.; Cowled, P.A. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 260–269. [Google Scholar] [CrossRef]
- Tai, S.H.; Chen, H.Y.; Lee, E.J.; Chen, T.Y.; Lin, H.W.; Hung, Y.C.; Huang, S.Y.; Chen, Y.H.; Lee, W.T.; Wu, T.S. Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J. Pineal Res. 2010, 49, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Selvaraj, P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin. Immunol. 2009, 133, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Ino, J.; Ishii, H.; Nitta, K.; Yoshida, M. MMP-9 inhibition by ACE inhibitor reduces oxidized LDL-mediated foam-cell formation. J. Atheroscler. Thromb. 2010, 17, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kuang, X.; Xie, Z.; Liang, L.; Zhang, Z.; Zhang, Y.; Ma, F.; Gao, Q.; Chang, R.; Lee, H.H.; et al. Small-molecule MMP2/MMP9 inhibitor SB-3CT modulates tumor immune surveillance by regulating PD-L1. Genome Med. 2020, 12, 83. [Google Scholar] [CrossRef]
- Lee, M.; Chen, Z.; Tomlinson, B.N.; Gooyit, M.; Hesek, D.; Juarez, M.R.; Nizam, R.; Boggess, B.; Lastochkin, E.; Schroeder, V.A.; et al. Water-Soluble MMP-9 Inhibitor Reduces Lesion Volume after Severe Traumatic Brain Injury. ACS Chem. Neurosci. 2015, 6, 1658–1664. [Google Scholar] [CrossRef]
- Magnussen, H.; Watz, H.; Kirsten, A.; Wang, M.; Wray, H.; Samuelsson, V.; Mo, J.; Kay, R. Safety and tolerability of an oral MMP-9 and -12 inhibitor, AZD1236, in patients with moderate-to-severe COPD: A randomised controlled 6-week trial. Pulm. Pharmacol. Ther. 2011, 24, 563–570. [Google Scholar] [CrossRef]
- Kwan, M.Y.; Choo, A.; Hanania, T.; Ghavami, A.; Beltran, J.; Shea, J.; Barboza, A.; Hu, A.; Fowler, M.; Neelagiri, V.R.; et al. Biomarker Analysis of Orally Dosed, Dual Active, Matrix Metalloproteinase (MMP)-2 and MMP-9 Inhibitor, AQU-118, in the Spinal Nerve Ligation (SNL) Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 811. [Google Scholar] [CrossRef]
- Shirian, J.; Arkadash, V.; Cohen, I.; Sapir, T.; Radisky, E.S.; Papo, N.; Shifman, J.M. Converting a broad matrix metalloproteinase family inhibitor into a specific inhibitor of MMP-9 and MMP-14. FEBS Lett. 2018, 592, 1122–1134. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Alexander, R.; Haarlander, T.M.; Burke, S.L.; Singer, M.; Huo, C.; Zhang, Y.M.; Maguire, D.; Spurlino, J.; Deckman, I.; et al. Discovery of a highly selective chemical inhibitor of matrix metalloproteinase-9 (MMP-9) that allosterically inhibits zymogen activation. J. Biol. Chem. 2017, 292, 17963–17974. [Google Scholar] [CrossRef] [PubMed]
- Mogharrabi, M.; Rahimi, H.R.; Hasanzadeh, S.; Dastani, M.; Kazemi-Oskuee, R.; Akhlaghi, S.; Soukhtanloo, M. The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial. ARYA Atheroscler. 2020, 16, 136–145. [Google Scholar]
- Sandborn, W.J.; Bhandari, B.R.; Fogel, R.; Onken, J.; Yen, E.; Zhao, X.; Jiang, Z.; Ge, D.; Xin, Y.; Ye, Z.; et al. Randomised clinical trial: A phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis. Aliment. Pharmacol. Ther. 2016, 44, 157–169. [Google Scholar] [CrossRef]
- Rudek, M.A.; Figg, W.D.; Dyer, V.; Dahut, W.; Turner, M.L.; Steinberg, S.M.; Liewehr, D.J.; Kohler, D.R.; Pluda, J.M.; Reed, E. Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J. Clin. Oncol. 2001, 19, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Cunningham, D.; Metges, J.P.; Van Cutsem, E.; Wainberg, Z.; Elboudwarej, E.; Lin, K.W.; Turner, S.; Zavodovskaya, M.; Inzunza, D.; et al. Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival. J. Immunother. Cancer 2021, 9, e003580. [Google Scholar] [CrossRef]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Todsen, T.; Bennedbaek, F.N.; Kiss, K.; Hegedus, L. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Head Neck 2021, 43, 1009–1013. [Google Scholar] [CrossRef]
- Son, J.I.; Rhee, S.Y.; Woo, J.T.; Park, W.S.; Byun, J.K.; Kim, Y.J.; Byun, J.M.; Chin, S.O.; Chon, S.; Oh, S.; et al. Insufficient experience in thyroid fine-needle aspiration leads to misdiagnosis of thyroid cancer. Endocrinol. Metab. 2014, 29, 293–299. [Google Scholar] [CrossRef]
- Roncevic, J.; Djoric, I.; Selemetjev, S.; Jankovic, J.; Dencic, T.I.; Bozic, V.; Cvejic, D. MMP-9-1562 C/T single nucleotide polymorphism associates with increased MMP-9 level and activity during papillary thyroid carcinoma progression. Pathology 2019, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, C.; Xu, J.; Zhou, D.; Yan, H.; Li, W.; Chen, G.; Zhang, N.; Xu, D.; Hu, H. Immunohistochemical analysis of matrix metalloproteinase-9 predicts papillary thyroid carcinoma prognosis. Oncol. Lett. 2019, 17, 2308–2316. [Google Scholar] [CrossRef] [PubMed]
- Marečko, I.; Cvejić, D.; Šelemetjev, S.; Paskaš, S.; Tatić, S.; Paunović, I.; Savin, S. Enhanced activation of matrix metalloproteinase-9 correlates with the degree of papillary thyroid carcinoma infiltration. Croat. Med. J. 2014, 55, 128–137. [Google Scholar] [CrossRef]
- Xu, D.; Su, C.; Guo, L.; Yan, H.; Wang, S.; Yuan, C.; Chen, G.; Pang, L.; Zhang, N. Predictive Significance of Serum MMP-9 in Papillary Thyroid Carcinoma. Open Life Sci. 2019, 14, 275–287. [Google Scholar] [CrossRef]
- Buergy, D.; Weber, T.; Maurer, G.D.; Mudduluru, G.; Medved, F.; Leupold, J.H.; Brauckhoff, M.; Post, S.; Dralle, H.; Allgayer, H. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int. J. Cancer 2009, 125, 894–901. [Google Scholar] [CrossRef]
- Boltze, C.; Riecke, A.; Ruf, C.G.; Port, M.; Nizze, H.; Kügler, C.; Abend, M. Sporadic and radiation-associated papillary thyroid cancers can be distinguished using routine immunohistochemistry. Oncol. Rep. 2009, 22, 459–467. [Google Scholar] [PubMed]
- Selemetjev, S.; Ethoric, I.; Paunovic, I.; Tatic, S.; Cvejic, D. Coexpressed High Levels of VEGF-C and Active MMP-9 Are Associated With Lymphatic Spreading and Local Invasiveness of Papillary Thyroid Carcinoma. Am. J. Clin. Pathol. 2016, 146, 594–602. [Google Scholar] [CrossRef]
- Wajner, S.M.; Capp, C.; Brasil, B.A.; Meurer, L.; Maia, A.L. Reduced tissue inhibitor of metalloproteinase-2 expression is associated with advanced medullary thyroid carcinoma. Oncol. Lett. 2014, 7, 731–737. [Google Scholar] [CrossRef]
- Ivkovic, I.; Limani, Z.; Jakovcevic, A.; Huic, D.; Prgomet, D. Role of Matrix Metalloproteinases and Their Inhibitors in Locally Invasive Papillary Thyroid Cancer. Biomedicines 2022, 10, 3178. [Google Scholar] [CrossRef]
- Bumber, B.; Marjanovic Kavanagh, M.; Jakovcevic, A.; Sincic, N.; Prstacic, R.; Prgomet, D. Role of matrix metalloproteinases and their inhibitors in the development of cervical metastases in papillary thyroid cancer. Clin. Otolaryngol. 2020, 45, 55–62. [Google Scholar] [CrossRef]
- Maeta, H.; Ohgi, S.; Terada, T. Protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in papillary thyroid carcinomas. Virchows Arch. 2001, 438, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bayadsi, H.; Barghout, G.; Gustafsson, M.; Sund, M.; Hennings, J. The expression of stromal biomarkers in small papillary thyroid carcinomas. World J. Surg. Oncol. 2022, 20, 340. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, R.; Yang, J.Y.; Tang, C.; Yang, L.; Xu, M.; Jiang, Q.F.; Liu, Z.M. Expression of TGF-beta1, SNAI1 and MMP-9 is associated with lymph node metastasis in papillary thyroid carcinoma. J. Mol. Histol. 2014, 45, 391–399. [Google Scholar] [CrossRef]
- Roncevic, J.; Jankovic Miljus, J.; Isic Dencic, T.; Bozic, V.; Zivaljevic, V.; Selemetjev, S.; Doric, I. Predictive Significance of Two MMP-9 Promoter Polymorphisms and Acetylated c-Jun Transcription Factor for Papillary Thyroid Carcinoma Advancement. Diagnostics 2022, 12, 1953. [Google Scholar] [CrossRef]
- Zarkesh, M.; Zadeh-Vakili, A.; Akbarzadeh, M.; Fanaei, S.A.; Hedayati, M.; Azizi, F. The role of matrix metalloproteinase-9 as a prognostic biomarker in papillary thyroid cancer. BMC Cancer 2018, 18, 1199. [Google Scholar] [CrossRef]
- Cho Mar, K.; Eimoto, T.; Tateyama, H.; Arai, Y.; Fujiyoshi, Y.; Hamaguchi, M. Expression of matrix metalloproteinases in benign and malignant follicular thyroid lesions. Histopathology 2006, 48, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, Q.; Li, Q.; Lin, S.; Li, J. Immunohistochemical levels of cyclo-oxygenase-2, matrix metalloproteinase-9 and vascular endothelial growth factor in papillary thyroid carcinoma and their clinicopathological correlations. J. Int. Med. Res. 2014, 42, 619–627. [Google Scholar] [CrossRef]
- Zhang, W.J.; Song, B.; Yang, T. MMP-2, MMP-9, TIMP-1, and TIMP-2 in the Peripheral Blood of Patients with Differentiated Thyroid Carcinoma. Cancer Manag. Res. 2019, 11, 10675–10681. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Yuan, T.; Ding, Q. Clinical value of matrix metalloproteinase-2 and -9 in ultrasound-guided radiofrequency ablation treatment for papillary thyroid carcinoma. J. Int. Med. Res. 2020, 48, 300060520917581. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Sheu, W.H. Preoperative plasma concentrations of vascular endothelial growth factor and matrix metalloproteinase 9 are associated with stage progression in papillary thyroid cancer. Clin. Endocrinol. 2003, 58, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.; Wang, F.; Wang, X.; Yang, F.; Zhao, C.; Feng, C.; Li, T. Role of MMP-9 in epithelial-mesenchymal transition of thyroid cancer. World J. Surg. Oncol. 2020, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhong, Y.; Luo, Z.; Huang, Y.; Lin, H.; Luo, M.; Zhan, S.; Xie, K.; Ma, Y.; Li, Q.Q. Assessment of biomarkers for clinical diagnosis of papillary thyroid carcinoma with distant metastasis. Int. J. Biol. Markers 2010, 25, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.K.; Zhang, M.B.; Li, J.; Li, C.T.; Tang, J.; Li, J.L. Values of ultrasound features and MMP-9 of papillary thyroid carcinoma in predicting cervical lymph node metastases. Sci. Rep. 2017, 7, 6670. [Google Scholar] [CrossRef] [PubMed]
- Dragutinovic, V.V.; Tatic, S.B.; Nikolic-Mandic, S.; Savin, S.; Cvejic, D.; Dunderovic, D.; Gajic, M.; Paunovic, I. Matrix metalloproteinase-9 and the Cu/Zn ratio as ancillary diagnostic tools in distinguishing between the classical and follicular variants of papillary thyroid carcinoma. Biol. Trace Elem. Res. 2012, 149, 29–33. [Google Scholar] [CrossRef]
- Zhuang, Z.N.; Xu, Z.J.; Zhou, Q.; Xu, X.Z.; Tian, J.; Liu, Y.F.; Guo, S.; Wang, J.Y.; Xu, K.S. Clinical significance of integrin beta6 as a tumor recurrence factor in follicular thyroid carcinoma. Head Neck 2015, 37, 1439–1447. [Google Scholar] [CrossRef]
- Dobrescu, R.; Picu, C.; Caragheorgheopol, A.; Manda, D.; Ioachim, D.; Goldstein, A.; Badiu, C. Serum Matrix metalloproteinase-9 (MMP-9) can help identify patients with papillary thyroid cancer at high risk of persistent disease: Value and limitations of a potential marker of neoplasia. Cancer Biomark. 2020, 29, 337–346. [Google Scholar] [CrossRef]
- Ouyang, X.; Feng, L.; Yao, L.; Xiao, Y.; Hu, X.; Zhang, G.; Liu, G.; Wang, Z. Testicular orphan receptor 4 (TR4) promotes papillary thyroid cancer invasion via activating circ-FNLA/miR-149-5p/MMP9 signaling. Mol. Ther. Nucleic Acids 2021, 24, 755–767. [Google Scholar] [CrossRef]
- Su, Z.; Bao, W.; Yang, G.; Liu, J.; Zhao, B. SOX12 Promotes Thyroid Cancer Cell Proliferation and Invasion by Regulating the Expression of POU2F1 and POU3F1. Yonsei Med. J. 2022, 63, 591–600. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Liu, Z.; Li, Y.; Xu, Y.; Liu, H. Oncogenic pseudogene DUXAP10 knockdown suppresses proliferation and invasion and induces apoptosis of papillary thyroid carcinoma cells by inhibition of Akt/mTOR pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1473–1483. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Qin, Y.; Wu, C.; He, L.; Zhang, T.; Shao, L.; Zhang, H.; Zhang, P. Knockdown of KDM1A suppresses tumour migration and invasion by epigenetically regulating the TIMP1/MMP9 pathway in papillary thyroid cancer. J. Cell. Mol. Med. 2019, 23, 4933–4944. [Google Scholar] [CrossRef]
- Kummer, N.T.; Nowicki, T.S.; Azzi, J.P.; Reyes, I.; Iacob, C.; Xie, S.; Swati, I.; Darzynkiewicz, Z.; Gotlinger, K.H.; Suslina, N.; et al. Arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction. J. Cell. Biochem. 2012, 113, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Chen, H.; Li, X.; Lu, P.; Long, M.; Peng, X.; Lin, S.; Tan, L.; Zhu, Y.; Ouyang, N.; et al. Activation of the ROCK1/MMP-9 pathway is associated with the invasion and poor prognosis in papillary thyroid carcinoma. Int. J. Oncol. 2017, 51, 1209–1218. [Google Scholar] [CrossRef]
- Rothhut, B.; Ghoneim, C.; Antonicelli, F.; Soula-Rothhut, M. Epidermal growth factor stimulates matrix metalloproteinase-9 expression and invasion in human follicular thyroid carcinoma cells through Focal adhesion kinase. Biochimie 2007, 89, 613–624. [Google Scholar] [CrossRef]
- Kalhori, V.; Törnquist, K. MMP2 and MMP9 participate in S1P-induced invasion of follicular ML-1 thyroid cancer cells. Mol. Cell. Endocrinol. 2015, 404, 113–122. [Google Scholar] [CrossRef]
- Zhang, X.G.; Lu, X.F.; Jiao, X.M.; Chen, B.; Wu, J.X. PLK1 gene suppresses cell invasion of undifferentiated thyroid carcinoma through the inhibition of CD44v6, MMP-2 and MMP-9. Exp. Ther. Med. 2012, 4, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.Y.; Kemppainen, K.; Lassila, T.; Törnquist, K. Sphingosine 1-phosphate attenuates MMP2 and MMP9 in human anaplastic thyroid cancer C643 cells: Importance of S1P2. PLoS ONE 2018, 13, e0196992. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gao, X.J.; Zhang, Z.D.; Yang, Z.X.; Zhang, G. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1495–1508. [Google Scholar]
- Kim, Y.J.; Hwang, H.J.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Lee, S.J. Enigma Plays Roles in Survival of Thyroid Carcinoma Cells through PI3K/AKT Signaling and Survivin. Anticancer Res. 2018, 38, 3515–3525. [Google Scholar] [CrossRef]
- Ren, L.; Xu, Y.; Liu, C.; Wang, S.; Qin, G. IL-17RB enhances thyroid cancer cell invasion and metastasis via ERK1/2 pathway-mediated MMP-9 expression. Mol. Immunol. 2017, 90, 126–135. [Google Scholar] [CrossRef]
- Schneider, D.F.; Chen, H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J. Clin. 2013, 63, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, F.; Dedhia, P.H.; Ringel, M.D. Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 2021, 149, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, L.; Yu, H.X.; Bao, J.D.; Lu, R.R. Curcumin inhibits the metastasis of K1 papillary thyroid cancer cells via modulating E-cadherin and matrix metalloproteinase-9 expression. Biotechnol. Lett. 2013, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, L.; Cheng, X.; Lin, X.F.; Lu, R.R.; Bao, J.D.; Yu, H.X. Curcumin inhibits hypoxia-induced migration in K1 papillary thyroid cancer cells. Exp. Biol. Med. 2015, 240, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kong, D.; Zhang, Y.; Li, S.; Li, Y.; Dong, L.; Zhang, N.; Ma, J. Curcumin inhibits the viability, migration and invasion of papillary thyroid cancer cells by regulating the miR-301a-3p/STAT3 axis. Exp. Ther. Med. 2021, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Lee, S.J. Evodiamine Suppresses Survival, Proliferation, Migration and Epithelial-Mesenchymal Transition of Thyroid Carcinoma Cells. Anticancer Res. 2018, 38, 6339–6352. [Google Scholar] [CrossRef]
- Ding, Q.; Li, X.; Sun, Y.; Zhang, X. Schizandrin A inhibits proliferation, migration and invasion of thyroid cancer cell line TPC-1 by down regulation of microRNA-429. Cancer Biomark. 2019, 24, 497–508. [Google Scholar] [CrossRef]
- Shi, G.H.; Zhou, L. Emodin suppresses angiogenesis and metastasis in anaplastic thyroid cancer by affecting TRAF6-mediated pathways in vivo and in vitro. Mol. Med. Rep. 2018, 18, 5191–5197. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Q.; Zhao, W.; Gong, Y.; Su, A.; Liu, F.; Liu, Y.; Li, Z.; Zhu, J. Ginsenoside Rg3 Inhibition of Thyroid Cancer Metastasis Is Associated with Alternation of Actin Skeleton. J. Med. Food 2018, 21, 849–857. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Zhang, S.; Liu, J.; Yao, X.; Zhao, Q.; Kou, B.; Han, P.; Wang, X.; Bai, Y.; et al. Taraxasterol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in papillary thyroid cancer cells through regulating the Wnt/beta-catenin signaling. Hum. Exp. Toxicol. 2021, 40 (Suppl. S12), S87–S95. [Google Scholar] [CrossRef]
- De Amicis, F.; Perri, A.; Vizza, D.; Russo, A.; Panno, M.L.; Bonofiglio, D.; Giordano, C.; Mauro, L.; Aquila, S.; Tramontano, D.; et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J. Cell. Physiol. 2013, 228, 2054–2062. [Google Scholar] [CrossRef]
- Rajoria, S.; Suriano, R.; George, A.; Shanmugam, A.; Schantz, S.P.; Geliebter, J.; Tiwari, R.K. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PLoS ONE 2011, 6, e15879. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Wang, S.; Yao, J.; Guo, C.; Dong, J.; Liao, L. Salidroside inhibits migration and invasion of poorly differentiated thyroid cancer cells. Thorac. Cancer 2019, 10, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.F.L.; Hecht, F.; Cazarin, J.; Fortunato, R.S.; Vaisman, M.; Carvalho, D.P.; Ferreira, A.C.F. The flavonoid quercetin reduces cell migration and increases NIS and E-cadherin mRNA in the human thyroid cancer cell line BCPAP. Mol. Cell. Endocrinol. 2021, 529, 111266. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Jung, S.P.; Han, J.; Kim, S.; Kim, J.S.; Nam, S.J.; Lee, J.E.; Kim, J.H. Silibinin inhibits TPA-induced cell migration and MMP-9 expression in thyroid and breast cancer cells. Oncol. Rep. 2013, 29, 1343–1348. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.G.; Qiu, J.L.; Pang, T.; Wang, H.; Zhang, X.X. Sevoflurane inhibits the progression of PTC by downregulating miR-155. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6579–6587. [Google Scholar] [PubMed]
- Kalhori, V.; Magnusson, M.; Asghar, M.Y.; Pulli, I.; Tornquist, K. FTY720 (Fingolimod) attenuates basal and sphingosine-1-phosphate-evoked thyroid cancer cell invasion. Endocr. Relat. Cancer 2016, 23, 457–468. [Google Scholar] [CrossRef]
- Haghpanah, V.; Malehmir, M.; Larijani, B.; Ahmadian, S.; Alimoghaddam, K.; Heshmat, R.; Ghavamzadeh, A.; Adabi, K.; Ghaffari, S.H. The Beneficial Effects of Valproic Acid in Thyroid Cancer Are Mediated through Promoting Redifferentiation and Reducing Stemness Level: An In Vitro Study. J. Thyroid Res. 2014, 2014, 218763. [Google Scholar] [CrossRef]
- Zhang, D.; Wan, L.; Zhang, J.; Liu, C.; Sun, H. Effect of BMAP-28 on human thyroid cancer TT cells is mediated by inducing apoptosis. Oncol. Lett. 2015, 10, 2620–2626. [Google Scholar] [CrossRef]
- Chiang, K.C.; Kuo, S.F.; Chen, C.H.; Ng, S.; Lin, S.F.; Yeh, C.N.; Chen, L.W.; Takano, M.; Chen, T.C.; Juang, H.H.; et al. MART-10, the vitamin D analog, is a potent drug to inhibit anaplastic thyroid cancer cell metastatic potential. Cancer Lett. 2015, 369, 76–85. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Synergistic cytotoxicity of the dipeptidyl peptidase-IV inhibitor gemigliptin with metformin in thyroid carcinoma cells. Endocrine 2018, 59, 383–394. [Google Scholar] [CrossRef]
- Lee, S.Y. Anti-Metastatic and Anti-Inflammatory Effects of Matrix Metalloproteinase Inhibition by Ginsenosides. Biomedicines 2021, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Qi, B.; Yuan, D.; Dong, S.; Guo, D.; Zhang, C.; Yu, M. Suppression of PMA-induced tumor cell invasion and migration by ginsenoside Rg1 via the inhibition of NF-kappaB-dependent MMP-9 expression. Oncol. Rep. 2014, 32, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; Kim, C.H. Non-thermal atmospheric pressure plasma inhibits thyroid papillary cancer cell invasion via cytoskeletal modulation, altered MMP-2/-9/uPA activity. PLoS ONE 2014, 9, e92198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Vosgha, H.; Ariana, A.; Smith, R.A.; Lam, A.K. miR-205 targets angiogenesis and EMT concurrently in anaplastic thyroid carcinoma. Endocr. Relat. Cancer 2018, 25, 323–337. [Google Scholar] [CrossRef]
- Liu, F.; Lou, K.; Zhao, X.; Zhang, J.; Chen, W.; Qian, Y.; Zhao, Y.; Zhu, Y.; Zhang, Y. miR-214 regulates papillary thyroid carcinoma cell proliferation and metastasis by targeting PSMD10. Int. J. Mol. Med. 2018, 42, 3027–3036. [Google Scholar] [CrossRef]
- You, A.; Fu, L.; Li, Y.; Li, X.; You, B. MicroRNA-203 restrains epithelial-mesenchymal transition, invasion and migration of papillary thyroid cancer by downregulating AKT3. Cell Cycle 2020, 19, 1105–1121. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Tan, L.; Lin, S.; Long, M.; Peng, X. LncRNA-HCG18 regulates the viability, apoptosis, migration, invasion and epithelial-mesenchymal transition of papillary thyroid cancer cells via regulating the miR-106a-5p/PPP2R2A axis. Pathol. Res. Pract. 2021, 221, 153395. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cui, M.; Yu, P.; Li, Q. Correlations of lncRNAs with cervical lymph node metastasis and prognosis of papillary thyroid carcinoma. OncoTargets Ther. 2019, 12, 1269–1278. [Google Scholar] [CrossRef]
- Min, X.; Liu, K.; Zhu, H.; Zhang, J. Long Noncoding RNA LINC003121 Inhibits Proliferation and Invasion of Thyroid Cancer Cells by Suppression of the Phosphatidylinositol-3-Kinase (PI3K)/Akt Signaling Pathway. Med. Sci. Monit. 2018, 24, 4592–4601. [Google Scholar] [CrossRef]
- Cui, W.; Xue, J. Circular RNA DOCK1 downregulates microRNA-124 to induce the growth of human thyroid cancer cell lines. Biofactors 2020, 46, 591–599. [Google Scholar] [CrossRef] [PubMed]
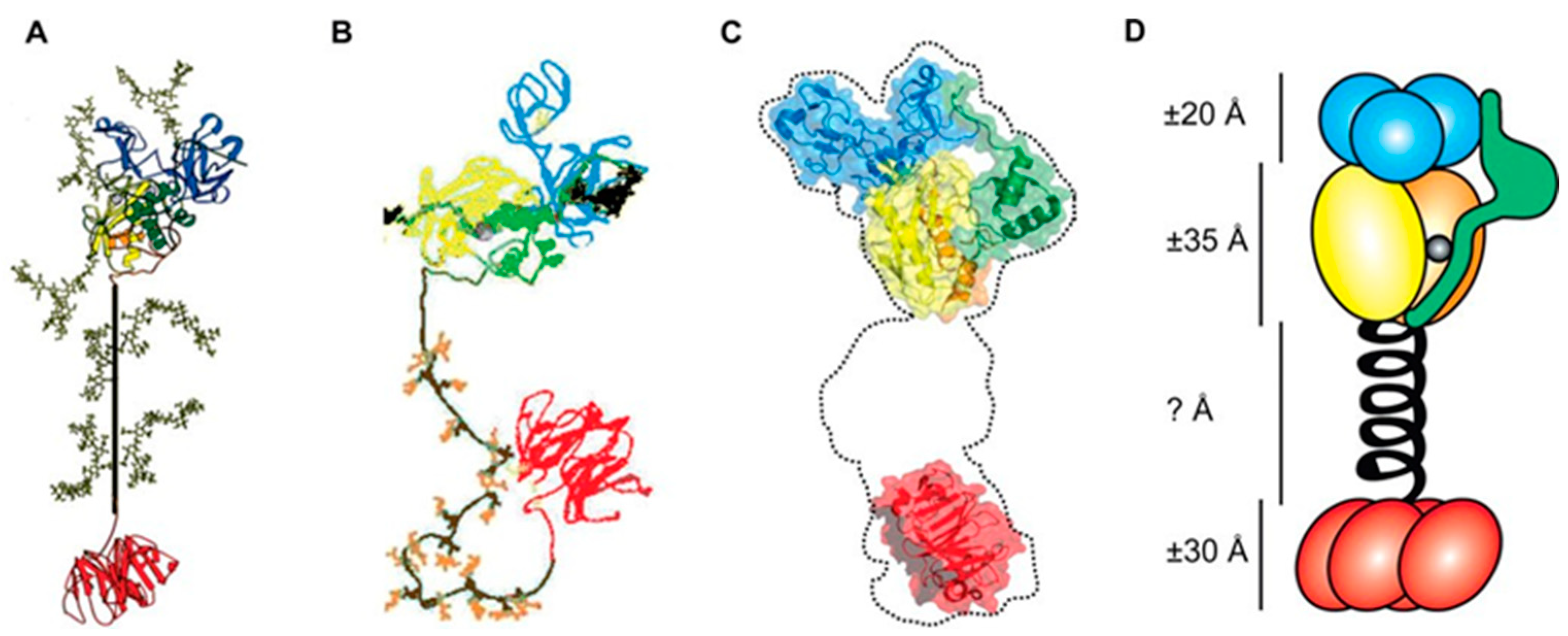
| Reference | Molecules | Research Methods | Research Diseases | Research Subjects | Effective Dose |
|---|---|---|---|---|---|
| [16] | Theissenolactone C (LC53) | Gelatin zymography RT-PCR Western blotting | Glaucoma | MCP-1-stimulated THP-1 cells | 2 µM, 5 µM, 10 µM |
| [16] | Theissenolactone C (LC53) | Gelatin zymography RT-PCR Western blotting | Glaucoma | IL-1β-activated primary astrocytes derived from the rat brain | 2 µM, 5 µM, 10 µM |
| [16] | Theissenolactone C (LC53) | Gelatin zymography RT-PCR Western blotting | Glaucoma | An SD rat model of high intraocular pressure (IOP)-related retinal ischemia reperfusion injury | 10 mg/kg |
| [16] | Memantine | Gelatin zymography RT-PCR Western blotting | Glaucoma | An SD rat model of high intraocular pressure (IOP)-related retinal ischemia reperfusion injury | 20 mg/kg |
| [16] | Memantine | Gelatin zymography RT-PCR Western blotting | Glaucoma | MCP-1-stimulated THP-1 cells | 100 µM |
| [38] | SB-3CT | Gelatin zymography | Embolic focal cerebral ischemia | A C57Bl/6 J mouse model of embolic focal cerebral ischemia | 25 mg/kg |
| [39] | SB-3CT | Immunohistochemistry | Traumatic brain injury (TBI) | An SD rat model of traumatic brain injury induced by a fluid percussion | 50 mg/kg |
| [40] | SB-3CT | RT-PCR | Corneal lymphangiogenesis | A C57BL/6 mouse model of inflammatory corneal neovascularization induced by corneal suture placement | 50 µM, 100 µM, 200 µM |
| [41] | AZD1236 | Gelatin zymography | Spinal cord injury | A mouse and rat model of spinal cord injury | 100 mg/kg, 200 mg/kg, 300 mg/kg (oral administration) 2.5 mg/mL, 5 mg/mL, 10 mg/mL (intrathecal injection) |
| [42] | AZD1236 | / | Chronic obstructive pulmonary disease (COPD) | Patients with stable moderate-to-severe COPD | 75 mg |
| [43] | AZ11557272 | Western blotting | Smoke-induced emphysema | A Hartley strain guinea pig model exposed to the smoke or air | 100 mg/kg |
| [44] | (Ⅰ-3,Ⅱ-3)-biacacetin | Gelatin zymography | Fibrosarcoma | HT1080 cells | 10 μM |
| [45] | AZD3342 | ELISA Gelatin zymography RT-PCR | Colonic anastomoses | An SD rat model constructed of colonic anastomoses | 50 mg/kg |
| [46] | GM6001 | ImmunohistochemistryWestern blotting | Hypertensive cerebropathy | Dahl salt-sensitive (Dahl/SS) and Lewis rat models fed with a high-salt diet | 1.2 mg/kg |
| [47] | GM6001 | Western blotting | Single severe traumatic brain injury (ssTBI) | A C57BL/6 WT mouse model of traumatic brain injury stimulated by a closed head injury | 50 mg/kg |
| [48] | AG-L-66085 | RT-PCR | Retinoblastoma (Rb) | Y79 cells Weri-1 cells | 5 μM |
| [49] | (R)-ND-336 | Gelatin zymography | Diabetic foot ulcers (DFUs) | A db/db mouse model with a single 8-mm diameter full-thickness excisional wound | 2 mg/kg |
| [50] | (R)-ND-336 | Gelatin zymography | Diabetic foot ulcers (DFUs) | A db/db mouse model with an infected wound | 10 μg |
| [51] | β-aminopropionitrile (BAPN) | ELISA Gelatin zymography Western blotting | Gastric carcinoma | A nude mouse model of gastric carcinoma inoculated SGC-7901 cells | 0.1 mM, 0.2 mM, 0.3 mM |
| [52] | Indinavir (IDV) | Gelatin zymography RT-PCR | Cervical carcinoma (CC) | An HPV16/E2 mouse transgenic model of HR-HPV-induced estrogen-promoted CC | 1.4 mg/day |
| [52] | Saquinavir (SQV) | Gelatin zymography RT-PCR | Cervical carcinoma (CC) | An HPV16/E2 mouse transgenic model of HR-HPV-induced estrogen-promoted CC | 1 mg/day |
| [52] | Lopinavir (LPV) | Gelatin zymography RT-PCR | Cervical carcinoma (CC) | An HPV16/E2 mouse transgenic model of HR-HPV-induced estrogen-promoted CC | 0.46 mg/day |
| [53] | Delta-tocotrienol (δT) | Gelatin zymography RT-PCR Western blotting | Non-small-cell lung carcinoma (NSCLC) | A549 cells H1299 cells | 10 μM, 20 μM, 30 μM |
| [54] | Simvastatin | Gelatin zymography | Glaucoma | Primary astrocytes derived from the human optic nerve head | 5 μg/mL |
| [54] | Lovastatin | Gelatin zymography | Glaucoma | Primary astrocytes derived from the human optic nerve head | 5 μg/mL |
| [54] | Atorvastatin | Gelatin zymography | Glaucoma | Primary astrocytes derived from the human optic nerve head | 5 μg/mL |
| [55] | Lipoxin A4 methyl ester (LXA4 ME) | Western blotting | Intracerebral hemorrhage (ICH) | An SD rat model of intracerebral hemorrhage | 10 ng/day, 100 ng/day |
| [56] | Hydroxytyrosol (HT) | ELISA Gelatin zymography RT-PCR | Atherosclerosis (AS) | PBMCs U937 cells | 1–10 μM IC50 = 10 μM |
| [57] | Walnut-derived peptide TWLPLPR (TW-7) | Immunohistochemistry | Alzheimer’s disease (AD) | β-amyloid 25–35-injured bEnd.3 cells | 100 μM |
| [58] | Thiamine | ELISA | Sepsis | 86 blood samples of septic patients | 200 mg |
| [58] | Ascorbic acid | ELISA | Sepsis | 86 blood samples of septic patients | 50 mg/kg |
| [59] | MK2206 2HCl | ImmunohistochemistryRT-PCR Western blotting | Oral squamous cell carcinoma (OSCC) | CAL27 cells SCC25 cells | 6 μM, 10 μM |
| [60] | U0126 | Gelatin zymography RT-PCR | Diabetic retinopathy (DR) | An SD rate model of diabetes induced by streptozotocin | 0.1 mM |
| [61] | BB-94 (Batimastat) | Gelatin zymography RT-PCR | Acute kidney allograft rejection | A Lewis rat model of orthotopic kidney allotransplantation | 30 mg/kg |
| [62] | Doxycycline | Gelatin zymography | Skeletal muscle ischemia-reperfusion injury | An SD rat model of skeletal muscle ischemia-reperfusion injury | 50 mg/kg, 200 mg/kg |
| [63] | Melatonin | Gelatin zymography Western blotting | Transient focal cerebral ischemia | A C57BL/B6 mouse model of transient focal cerebral ischemia | 5 mg/kg |
| [64] | 1, 25(OH)2D3 | ELISA | Pulmonary tuberculosis (PTB) | Peripheral blood mononuclear cells (PBMCs) from 43 PTB patients | 0.1 μM |
| [65] | Imidaprilat | Gelatin zymography | Atherosclerosis | THP-1 cells | 100 nM, 1000 nM |
| Reference | Type of Thyroid Carcinoma | Signaling Pathways |
|---|---|---|
| [107] | PTC | TR4/circ-FNLA/miR-149-5p/MMP-9 |
| [108] | PTC | SOX12/POU2F1, POU3F1/MMP-9 |
| [109] | PTC | DUXAP10/Akt/mTOR/MMP-9 |
| [110] | PTC | KDM1A/TIMP-1/MMP-9 |
| [111] | PTC | ALOX5/ MMP9 |
| [112] | PTC | ROCK1/MMP-9 |
| [113] | FTC | FRNK/FAK/EGF/MMP-9 |
| [114] | FTC | S1P/S1P1/MMP-9 |
| [115] | ATC | PLK1/MMP-9 |
| [116] | ATC | S1P/S1P2/MMP-9 |
| [117] | PTC, FTC | S100A4/MMP-9 |
| [118] | PTC, ATC | Enigma/PI3K/AKT/MMP-9 |
| [119] | PTC, FTC, ATC | IL-17RB/ERK1/2/MMP-9 |
| Reference | Molecules | Research Methods | Type of Thyroid Carcinoma | Research Models | Doses | Results |
|---|---|---|---|---|---|---|
| [122] | Curcumin | Gelatin zymography Western blotting | PTC | K1 cells | 12.5 μM, 25 μM, 50 μM | Migration inhibition Metastasis inhibition EMT inhibition Viability inhibition |
| [123] | Curcumin | Gelatin zymography | PTC | K1 cells | 25 μM, 50 μM | Migration inhibition |
| [124] | Curcumin | Western blotting | PTC | TPC-1 cells | 23.31 μM | Migration inhibition Invasion inhibition EMT inhibition Viability inhibition |
| [125] | Evodiamine | Western blotting | PTC | TPC-1 cells | 5 μM | Proliferation inhibition Migration inhibition EMT inhibition Viability inhibition |
| [125] | Evodiamine | Western blotting | ATC | SW1736 cells | 5 μM | Proliferation inhibitionMigration inhibitionEMT inhibitionViability inhibition |
| [126] | Schizandrin A (SchA) | RT-PCR Western blotting | PTC | TPC-1 cells | 50 µM | Proliferation inhibition Migration inhibition Invasion inhibition |
| [127] | Emodin | Western blotting | ATC | 8505C cells SW1736 cells | 10 µM, 15 µM, 20 µM, 25 µM | Proliferation inhibition Metastasis inhibition Angiogenesis inhibition |
| [128] | Ginsenoside Rg3 | Western blotting | PTC | TPC-1 cells BCPAP cells | 50 µM, 100 µM | Metastasis inhibition |
| [128] | Ginsenoside Rg3 | Western blotting | ATC | C643 cells Ocut-2c cells | 50 µM, 100 µM | Metastasis inhibition |
| [129] | Taraxasterol (TAR) | Western blotting | PTC | TPC-1 cells BCPAP cells | 2.5 µg/mL, 5 µg/mL, 10 µg/mL | Migration inhibition Invasion inhibition EMT inhibition |
| [130] | Epigallocatechin-3-gallate (EGCG) | Gelatin zymography Western blotting | PTC | FB-2 cells | 10 μM, 40 μM, 60 μM | Proliferation inhibition EMT inhibition Motility inhibition |
| [131] | Diindolylmethane (DIM) | Gelatin zymography Western blotting | PTC | BCPAP cells | 25 µM | Proliferation inhibition Migration inhibition Invasion inhibition Metastasis inhibition Adhesion inhibition |
| [131] | Diindolylmethane (DIM) | Gelatin zymography Western blotting | ATC | 8505C cells | 25 µM | Proliferation inhibition Migration inhibition Invasion inhibition Metastasis inhibition Adhesion inhibition |
| [131] | Diindolylmethane (DIM) | Gelatin zymography Western blotting | FTC | CGTHW-1 cells ML-1 cells | 25 µM | Proliferation inhibition Migration inhibition Invasion inhibition Metastasis inhibition Adhesion inhibition |
| [132] | Salidroside | RT-PCR Western blotting | ATC | WRO cells | 10 µM, 20 µM, 40 µM | Migration inhibition Invasion inhibition |
| [133] | Quercetin | RT-PCR | PTC | BCPAP cells | 100 μM | Migration inhibition Invasion inhibition Adhesion inhibition Apoptosis promotion |
| [134] | Silibinin | Gelatin zymography RT-PCR | PTC | TPC-1 cells | 50 µM | Migration inhibition |
| [135] | Sevoflurane | Western blotting | PTC | TPC-1 cells IHH-4 cells | 2.5% | Migration inhibition Invasion inhibition Viability inhibition Apoptosis promotion |
| [136] | Fingolimod (FTY720) | Gelatin zymography Western blotting | FTC | ML-1 cells | 10 μM | Proliferation inhibition Invasion inhibition |
| [137] | Valproic Acid (VPA) | RT-PCR | ATC | 8305C cells | 0.1 mM, 1 mM, 5 mM | Redifferentiation promotion |
| [137] | Valproic Acid (VPA) | RT-PCR | PTC | BCPAP cells | 0.1 mM, 1 mM | Redifferentiation promotion |
| [138] | BMAP-28 | Colorimetry RT-PCR | MTC | TT cells | 4 µM | Proliferation inhibition |
| [139] | 1α,25(OH)2D3 | Gelatin zymography Western blotting | ATC | 8505C cells | 0.1 µM | Migration inhibition Invasion inhibition |
| [139] | MART-10 | Gelatin zymography Western blotting | ATC | 8505C cells | 0.1 µM | Migration inhibition Invasion inhibition |
| [140] | Gemigliptin and metformin | Western blotting | PTC | TPC-1 cells | 1 mM Gemigliptin and 30 mM metformin | Proliferation inhibition Migration inhibition Viability inhibition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wei, J.; Chen, B.; Wang, Y.; Yang, S.; Wu, K.; Meng, X. The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma. Molecules 2023, 28, 3705. https://doi.org/10.3390/molecules28093705
Li Z, Wei J, Chen B, Wang Y, Yang S, Wu K, Meng X. The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma. Molecules. 2023; 28(9):3705. https://doi.org/10.3390/molecules28093705
Chicago/Turabian StyleLi, Zhenshengnan, Jia Wei, Bowen Chen, Yaoqi Wang, Shuai Yang, Kehui Wu, and Xianying Meng. 2023. "The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma" Molecules 28, no. 9: 3705. https://doi.org/10.3390/molecules28093705
APA StyleLi, Z., Wei, J., Chen, B., Wang, Y., Yang, S., Wu, K., & Meng, X. (2023). The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma. Molecules, 28(9), 3705. https://doi.org/10.3390/molecules28093705






