Abstract
Unhealthy dietary habits have been identified as a risk factor for the development and progression of cancer. Therefore, adopting a healthy eating pattern is currently recommended to prevent the onset of different types of cancers, including breast carcinoma. In particular, the Mediterranean diet, based on high consumption of omega-3 polyunsaturated fatty acids (N-3 PUFAs), such as those found in cold-water fish and other seafood, nuts, and seeds, is recommended to reduce the incidence of several chronic-degenerative diseases. Indeed, the consumption of N-3 PUFAs, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), reduced the risk of different types of cancer, including breast cancer. Moreover, they can counteract breast cancer progression and reduce the side effects of chemotherapy in breast cancer survival. Studies have demonstrated that DHA, exhibiting greater antitumor activity than EPA in breast cancer, can be attributed to its direct impact on breast cancer cells and also due to its conversion into various metabolites. N-docosahexaenoyl ethanolamine, DHEA, is the most studied DHA derivative for its therapeutic potential in breast cancer. In this review, we emphasize the significance of dietary habits and the consumption of N-3 polyunsaturated fatty acids, particularly DHA, and we describe the current knowledge on the antitumoral action of DHA and its derivative DHEA in the treatment of breast cancer.
1. Introduction
According to the data reported by the World Health Organization, around 30–50% of cancer cases can be prevented by decreasing exposure to risk factors associated with cancer and adopting healthy lifestyles [1]. Dietary habits have been recognized as a risk factor for several types of cancers, including breast carcinoma, which represents the most frequently diagnosed type of cancer and the primary cause of cancer-related deaths among women globally [2,3]. In the last decades, despite the fact that molecular characterizations of breast cancer can predict tumor behavior and prognosis and identify potential targets for personalized drug development and systemic therapy [4,5,6,7], metastatic disease represents the most important cause of death related to breast cancer [8]. Thus, the main goal of public health is to adopt strategies to prevent breast cancer risk. There are a variety of preventive approaches for breast cancer risk factors, among which dietary modification is currently an accepted target for breast cancer prevention [9,10]. In particular, it has been proved that a high intake of saturated and animal monounsaturated fats, which are found in red meat, cheese, and butter, has been associated with an increased risk of breast cancer, due, at least in part, to their ability to increase the levels of estrogen in the blood and supporting inflammation-driven cancer [11]. In contrast, the consumption of polyunsaturated fatty acids (PUFAs), fruits, vegetables, whole grains, and lean protein sources is linked to a reduced breast cancer risk [12,13]. PUFAs, essential fatty acids that the body cannot produce on its own and must be obtained through the diet, are divided into two classes based on the double bond of the methyl terminal, called omega-6 (N-6) and omega-3 (N-3) PUFAs. N-6 and N-3 PUFAs mediate opposite metabolic functions in the human body. In particular, N-3 PUFAs have anti-inflammatory and cardioprotective effects, whereas N-6 PUFAs promote inflammation and blood clotting. Although inflammation is a natural part of the immune response, chronic inflammation can contribute to the development of metabolic and chronic diseases such as type 2 diabetes, obesity, cardiovascular and neurodegenerative diseases, and cancer. Thus, a balanced intake of N-3 and N-6 PUFAs is recommended to maintain a healthy status [14]. In addition, several studies showed the healthy benefits of diets enriched in N-3 PUFAs compared to diets based on the consumption of N-6 PUFAs, supporting their importance in counteracting chronic-degenerative diseases. The Mediterranean diet, which suggests high consumption of fruits and vegetables, whole-grain cereals, legumes, nuts, seeds, and olive oil and moderate amounts of dairy, poultry, and red wine along with fish and seafood, is the foremost dietary pattern that promotes the consumption of N-3 PUFAs. Indeed, fish and other seafood, which are staples of the Mediterranean diet, are rich sources of two types of omega-3 PUFAs: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), whereas nuts and seeds, another key component of the Mediterranean diet, are a good source of alpha-linolenic acid (ALA), which can be converted into EPA and DHA in the body. EPA and DHA exert several positive effects in humans, including reducing inflammation, improving heart health, and preventing different types of cancer, such as breast carcinoma [15,16]. Although both N-3 PUFAs exert antitumoral effects in breast cancer, it has been proved that DHA has a higher antitumoral activity than EPA [17]. The reason why DHA is a more potent antitumoral compound than EPA is not well understood. It is hypothesized that its molecular structure could lead to differences in the way that this fatty acid interacts with cell membranes and signaling pathways, which could determine its stronger antitumor effects than EPA. Indeed, DHA can alter the properties of the membranes, influencing their fluidity and permeability as well as the lipid draft composition [18]. Moreover, DHA can modulate signaling proteins included in lipid microdomains, impacting the downstream signaling pathways [19]. Finally, DHA can activate different nuclear receptors than EPA influencing the expression of several genes involved in tumorigenesis [20]. Thus, based on its unique physicochemical characteristics and biochemical and physiological properties, DHA results in a very interesting molecule to be further investigated in clinical models as a potential compound for the treatment of different diseases, including breast cancer [21]. The positive effects of DHA are related to its own activity in breast cancer cells, as well as to the conversion of other metabolites. Recently, N-3 PUFA amides emerged as new fascinating molecules for the prevention and treatment of breast cancer [22]. These compounds, derived from the conjugation of DHA with amino acids or neurotransmitters, showed higher biological activity compared to the parental compounds, becoming promising tools for breast cancer treatment. Among the derivatives of DHA, N-docosahexaenoyl ethanolamine (DHEA), also called synaptamide, is the most studied molecule showing a wild spectrum of activity in various breast cancer experimental models [22,23]. The aim of this review is to highlight the importance of healthy dietary habits, specifically the consumption of N-3 PUFAs, and to dissect the molecular mechanism underlying the antitumor activity of DHA and its derivative DHEA in reducing the risk of breast carcinoma and potentially serving as a treatment option for breast cancer patients.
2. Docosahexaenoic Acid: Dietary Sources and Biosynthesis
Docosahexaenoic acid, DHA, is a long-chain, highly unsaturated omega-3 fatty acid composed of 22 carbons in its acyl chain. It is characterized by the presence of six double bonds in its chain and represents the longest chain and most unsaturated fatty acid in the human body [24]. The main dietary sources of DHA are seafood, including fatty fish (tuna, mackerel, codfish, salmon, sardine, and anchovy), shellfish, micro- and macroalgae, and mother’s milk. Low amounts of DHA have been also found in meat and eggs. DHA, as well as EPA, can also be biosynthesized from its precursor, α-linolenic acid (ALA), which represents an essential fatty acid. The conversion of ALA in DHA depends on several factors, including the type of tissue and sex [25,26]. The highest conversion of ALA into DHA occurs in the liver, where the enzymes involved in this reaction, desaturase and elongase, are highly expressed. Indeed, these enzymes catalyze elongation and desaturation reactions to produce EPA from ALA into the endoplasmic reticulum. EPA can be transferred to the peroxisome and be β-oxidized to form DHA. Thus, DHA can be sent back to the endoplasmic reticulum where it can undergo esterification, lipoprotein packaging, and secretion to the blood. The N-3 PUFA biosynthesis pathway, called the “Sprecher pathway”, is also used by N-6 fatty acids, which exert important opposing physiological effects compared to N-3 fatty acids in the human body. In particular, the precursor of N-6 fatty acid, linoleic acid (LA), competes with N-3 ALA for access to the delta(Δ)-6 desaturase, which represents the first enzyme involved in the metabolism of PUFAs, in order to produce long-chain fatty acids [27]. The metabolic biosynthesis pathway of both PUFAs is shown in Figure 1. Thus, a balanced intake of N-3 and N-6 PUFAs is required to ensure the optimal synthesis of N-3 PUFAs. The ratio of N-6/N-3 varies from 1/1 to 4/1 depending on health status. In general, a low N-6/N-3 ratio is recommended to lower the risk of chronic diseases, including inflammation and cancer [28,29,30]. The recommended intake of EPA and DHA for the health of the general population is 200 to 500 mg/day EPA + DHA in the form of fish or fish oil, krill oil, or algae oil supplements. Higher amounts of EPA and DHA are suggested for the treatment of diseases including hypertriglyceridemia or inflammatory disorders such as rheumatoid arthritis [31,32]. Fish oil capsules supplemented with DHA or both DHA and EPA are safe and may cause only minor side effects, such as fishy taste, eructation, dyspepsia, diarrhea, gas, nausea, and arthralgia [33,34,35]. Recently, it has been reported that long-chain fatty acids may be beneficial for weight loss, improving appetite, body weight, post-surgical morbidity, and quality of life in cancer patients. Similarly, in a population undergoing chemotherapy and/or radiotherapy, long-chain N-3 fatty acids have been found to have positive effects, particularly in preserving body composition compared to control groups and protecting against chemotherapy-induced peripheral neuropathy [36].
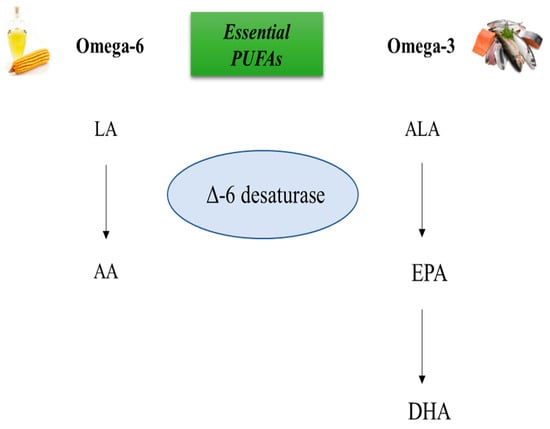
Figure 1.
Biosynthesis of N-3 and N-6 PUFAs. The biosynthesis of N-3 and N-6 polyunsaturated fatty acids (PUFAs) involves competition between the N-6 PUFA linoleic acid (LA), and the N-3 PUFA alpha-linolenic acid (ALA), for binding to delta (∆)-6 desaturase. Depending on the amount of dietary intake of PUFAs, one of these metabolic pathways is activated. If the intake of N-6 PUFAs is higher than N-3 PUFAs, the metabolic pathway leads to the synthesis of arachidonic acid (AA). On the other hand, if the intake of N-3 PUFAs is higher than N-6 PUFAs, the metabolic pathway leads to the synthesis of eicosapentaenoic acid (EPA) and docosapentaenoic acid (DHA).
Biological Properties of DHA
After ingestion or biosynthesis, DHA can be stored in the membranes as esters with glycerophospholipids, conferring fluidity and selective permeability to the membranes in several tissues including the brain, heart, and retina. It has been found that 60% of the total fatty acids in the rod outer segment of the retina are composed of DHA [26], which regulates the fluidity of photoreceptor membranes, retinal integrity, and visual function [37]. Moreover, it has been demonstrated that DHA is essential for the healthy development of children’s brains, increasing the size and complexity of this tissue, and is important for the maintenance of normal brain function in adults, influencing mental, behavioral, and motor skills characterized by cognitive elements [38]. Interestingly, the brain can only convert 1% of the ALA in DHA. Thus, an adequate intake of DHA is required especially in pregnancy for the development of the growing fetal brain [39]. Apart from its role in brain development, DHA modulates inflammatory processes [40]. Indeed, it has been demonstrated that DHA reduced the production of arachnoid acid-eicosanoids and produced d-series resolvins and protectin, which exert anti-inflammatory and inflammation-resolving properties [41]. The mechanism by which DHA exerts anti-inflammatory effects has been widely investigated. It has been found that DHA reduced the production of several pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor α, and IL-1β; decreased the expression of adhesion molecules; lowered the interactions between leucocytes and endothelial cells; and reduced the chemotactic response of leucocytes. Moreover, DHA can modulate the activity of key signaling pathways involved in inflammation. For example, it inhibits the nuclear factor kappa B (NF-κB) activity and enhances the expression of peroxisome proliferator-activated receptor (PPAR) γ, lowering the inflammatory cascade [41]. In the last years, it has been demonstrated that the effects of DHA are also mediated by its conversion into the acid amides, which represent an interesting class of DHA metabolites showing different biological activities to be further investigated. In particular, DHA can be conjugated with neurotransmitter or amino acids giving rise to a new class of N-acyl derivatives.
3. The Conjugate of DHA with Ethanolamine: Dietary Sources and Biosynthesis
There are limited dietary sources of N-docosahexaenoyl ethanolamine DHEA as it is a relatively new molecule that has primarily been studied in the context of research and as a supplement. Recently, it has been revealed in the human milk of postpartum women [42]. DHEA derivatives can be synthesized from the parental compound DHA. Indeed, DHA can be derivatized with amines, alcohols, or amino acids (from the group of neurotransmitters) leading to fatty acid amides or esters showing a range of different biological activity and receptor affinity than DHA [43]. For example, fatty acid amides are divided into two subclasses: N-acyl amines and N-acyl ethanolamines. The N-acyl amines group includes more than 80 compounds composed of fatty acids and amino acids or neurotransmitters, such as the conjugate of DHA with dopamine, serotonin, glutamic acid and glutamine, GABA, phenylalanine, and histidine. N-acyl ethanolamines include the conjugates of DHA or EPA with ethanolamine, DHEA, or EPEA, respectively. The conjugate DHEA is by far the most studied fatty acid amide. As shown in Figure 2, N-docosahexaenoyl amine (DHEA) can be synthesized from ethanolamines and DHA through an enzymatic process that involves a direct condensation reaction between these two compounds at a molar ratio of 1:1. The reaction is carried out in N-hexane at a temperature of 40 °C for a duration of 15 h, and it uses the catalyst Novozym 435, which contains immobilized Candida antarctica Lipase B (Figure 2).
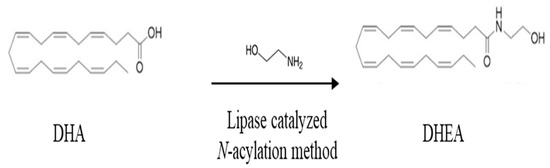
Figure 2.
Synthesis of N-3 docosahexaenoic acid amide. Chemical structures of docosahexaenoic acid (DHA) and N-docosahexaenoyl ethanolamine (DHEA).
Based on the data reported in the literature, the formation of N-acyl ethanolamines depends on the intake of N-3 PUFAs. Animal and human studies showed that a diet enriched in N-3 PUFAs increases the production of the conjugates of DHA and EPA with ethanolamine. Indeed, it has been demonstrated that dietary consumption of fish oil increases the production of DHEA in the rodent brain, jejunum, liver, and adipose tissue [44,45,46,47]. Similarly, in healthy volunteers, it has been found that a daily intake of 480 mg EPA plus 360 mg DHA as fish oil food supplements doubled plasma DHEA levels in 3 weeks [43]. Moreover, DHEA has been recently detected in human milk from postpartum women and a positive correlation between DHA and DHEA concentrations has been revealed [48]. The synthesis of DHEA mainly occurs where there is local availability of DHEA precursors. For example, high concentrations of DHEA have been found in the brain and in the retina, since these tissues are characterized by the increased availability of DHA in the phospholipids. Moreover, DHEA biosynthesis can also occur from hydrolysis of the lipid N-acylphosphatidyl ethanolamine into N-acylethanolamine by the phospholipase D, whereas it can be hydrolyzed by the fatty acid amide hydrolase, FAAH, localized on the endoplasmatic reticulum [49]. Interestingly, it has been demonstrated that different cancer cell lines, including prostate and breast cancer cells, can produce DHEA upon treatment with DHA [23].
Biological Activity of DHA with Ethanolamine
To date, little is known about the biological activity of DHEA. Although only a few studies have been carried out, DHEA seems to be a very interesting molecule showing anti-inflammatory properties. In particular, it has been demonstrated that DHEA reduced the release of nitric oxide (NO) and the secretion of the chemokine CCL2, also called Monocyte chemoattractant protein-1 (MCP-1), in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages [50] along with a decreased expression of cyclooxygenase (COX-2) and COX-2-derived eicosanoids [51]. Moreover, it has been shown that DHEA reduced the production of CCL2, IL6, and NO in mouse peritoneal macrophages. Finally, the anti-inflammatory effects of DHEA have been also revealed in differentiated 3T3-L1 adipocytes, where DHEA was able to reduce the production of CCL2 and IL6 induced by the treatment with LPS [52]. In addition to the anti-inflammatory properties, both DHA and its precursor compound, DHEA, play a crucial role in brain development and the preservation of cognitive function. It has been shown that DHEA stimulated neurite growth and synaptogenesis and enhanced glutamatergic synaptic activity in hippocampal neurons [43,53,54]. Interestingly, the metabolites of DHEA showed biological activity. Indeed, they are substrates for COXs, lipoxygenases (LOXs), and cytochrome P450 enzymes, modulating the inflammatory response. Moreover, DHEA is converted into oxygenated molecules, such as 17-hydroxy-DHEA, 10,17-dihydroxy-DHEA, and 15-hydroxy-16(17)-epoxy-DHEA, which showed anti-inflammatory properties [55].
4. Antitumoral Effects of Docosahexaenoic Acid in Breast Cancer
The effects of DHA have been widely investigated in preclinical and clinical studies of breast cancer. In vitro studies have shown that DHA-induced apoptosis in different breast cancer cells either alone or in combination with chemotherapeutic drugs through several mechanisms that are not fully understood [56,57]. To date, it has been demonstrated that the incorporation of DHA into the cell membrane is required for the activation of the apoptosis cascade in breast cancer cells. In triple-negative MDA-MB-231 breast cancer cells, DHA led to modifications in the biophysical characteristics of lipid rafts. This, in turn, resulted in a reduction of breast cancer cell proliferation by decreasing the cholesterol content and potentially altering the distribution of crucial proteins in the lipid rafts [58]. In addition, in the HB4aC5.2 cell normal breast cell line overexpressing HER2, it has been observed that DHA treatment disrupted lipid raft, and inhibited HER2 signaling, enhancing apoptosis [59]. The mechanism by which the change in DHA content of membrane-induced breast cancer cell apoptosis is still under investigation. Several routes are currently taking place, including the increase in intracellular oxidative stress, lipid peroxidation, and the modulation of eicosanoid metabolites [60]. Interestingly, it is known that DHA modulates the activation of nuclear receptors, leading to a change in the expression of the genes involved in tumorigenesis. Among the transcription factors regulated by DHA, the most important are NF-κB (70), activator protein 1 (AP-1) (71,72), c-myc (10,73), p53 (74,75), and PPARs. Another mechanism by which DHA reduced breast cancer cell proliferation is related to its ability to modulate the expression of cell-cycle molecules, thus inducing arrest in the G1 phase and G2M phase of the cell cycle [61,62,63,64,65]. DHA has been shown to downregulate the expression of cyclin D1 and cyclin-dependent kinase (CDK)4/6, which are required for the G1/S transition of the cell cycle. Moreover, in MCF-7 breast cancer cells, it has been found that DHA reduced cell proliferation through proteasome-dependent degradation of the ERα, as well as a decrease in the expression of the cyclin D1 and inhibiting the mitogen-activated protein kinase MAPK signaling. Additionally, DHA has been shown to upregulate the expression of CDK inhibitors, including p21 and p27, which promote cell-cycle arrest in the G1 and G2/M phases. Recently, it has been demonstrated that DHA can trigger pyroptosis, a programmed process mediated by proteases so-called inflammatory caspases, increasing the activation of caspase-1 and gasdermin D, as well as increasing the secretion of IL-1β, which results in the membrane ore formation and cell death [66]. The effects of DHA in breast cancer treatment have been investigated not only in preclinical studies but also in animal models. However, most of the in vivo studies have investigated the action of a mixture of N-3 PUFAs, particularly DHA and EPA, in counteracting breast cancer development. Moreover, only a few studies have evaluated the action of DHA in the treatment of established tumors, whereas the role of DHA in preventing breast cancer development has been widely investigated. For example, it has been reported that increasing the ratio of N-3 PUFA to N-6 PUFA reduced mammary cancer incidence.
Interestingly, it has been observed that DHA is a chemosensitizer agent in different types of cancer, including breast cancer. In MDA-MB-231 breast cancer cells, it has been demonstrated that DHA increased the sensibility of several chemotherapeutic drugs, including paclitaxel, doxorubicin, and docetaxel [67,68,69]. Moreover, it has been demonstrated that DHA administration reduced the tumor vascular density in rats treated with epirubicin [70]. The mechanism by which DHA enhanced the effects of chemotherapeutic molecules is not fully understood. However, it has been demonstrated that the chemosensitizer effects of DHA are related, at least in part, to its ability to increase the peroxidative processes and modulate the oncoprotein expression [71]. Moreover, DHA showed higher chemosensitizer properties when it was administered in combination with EPA. For example, supplementation with DHA alone did not enhance the efficacy of 5-fluorouracil, cyclophosphamide, and gemcitabine in mouse models of colon cancer, supporting the concept that a mixture of N-3 PUFAs is more effective than single supplementation of DHA or EPA.
Clinical studies have, to date, been variable results on the benefits of PUFAs in breast cancer. For example, a clinical trial investigating the effects of N-3 PUFA supplementation with raloxifene in postmenopausal women did not show changes in secondary endpoint blood risk biomarkers, whereas a favorable modulation of different tissue risk markers has been observed for breast cancer in premenopausal and postmenopausal women [35,72]. In contrast, positive results have been obtained in breast cancer patient survival. Indeed, a reduction in breast cancer recurrence and an improvement in overall mortality has been found in women with early-stage breast cancer followed for a median of 7 years [73]. Moreover, DHA was shown to ameliorate the outcome of metastatic breast cancer patients in a phase II trial [74]. To date, N-3 PUFAs are considered immunonutrients to be prescribed to cancer patients along with glutamine, arginine, and ribonucleotides to maintain immunocompetence during the chemotherapeutic treatment. Moreover, N-3 PUFAs represent pharmaconutrients used to reduce treatment-related complications, including cancer-related pain, anorexia-cachexia syndrome, and depression disorder [75]. These results underscore the significance of taking into account the interaction between diet and chemotherapeutic drugs to optimize the efficacy of pharmacological treatments, reduce the risk of recurrence, and minimize treatment-related toxicity and mortality. However, our comprehension of this interaction remains limited, and more extensive research is required to explore this area in greater depth. As the current studies have been conducted on relatively small patient populations, there is a pressing need for larger and more comprehensive studies. Consequently, various organizations have issued dietary recommendations, including guidelines for omega-3 dietary intake [76].
In the last years, different preclinical and clinical studies showed that DHA conjugate with chemotherapeutic drugs has a strong antitumoral effect used as a single agent, reducing the side effects of multiple drug therapy. In particular, it has been found that DHA conjugated with gemcitabine displayed a stronger activity than gemcitabine alone, reducing breast cancer tumor growth [77]. In addition, DHA conjugated with paclitaxel reduced the side effects induced by paclitaxel treatment in patients with resistant solid tumors, including breast cancer patients [78]. In vitro experiments have also shown that DHA conjugated with propofol reduced breast cancer cell migration and increased apoptosis [79]. Recently, it has been demonstrated that the codelivery of doxorubicin, DHA, and α-tocopherol succinate by nanostructured lipid carriers displayed synergistically antitumor effects in both breast cancer cells and mice models, decreasing doxorubicin-induced toxicity in animals [80]. Thus, these codelivery nanosystems improving therapeutic efficiency may be considered promising tools to be used for breast cancer treatment.
5. Antitumoral Effects of DHEA in Breast Cancer
Since breast cancer cells have the ability to convert DHA into DHEA, exerting a higher antitumoral activity, several studies have investigated the effects of this compound in breast cancer. According to the data presented in the literature, DHEA has higher antiproliferative effects than DHA in breast cancer cells while exhibiting no adverse impact on the viability of non-tumorigenic mammary epithelial cells [81]. These observations shed light on DHEA as a promising candidate for the treatment of breast cancer. However, the research is still far from a conclusion and additional investigations are required to suggest this molecule as a potential drug for breast cancer. In estrogen receptor α-positive breast cancer cells, Rovito et al., have demonstrated that DHEA inhibited cancer cell proliferation in a concentration-dependent manner, reaching an IC50 value of 0.8 µM after 96 h in MCF-7 cells [82]. The antiproliferative effects of DHEA in MCF-7 breast cancer cells are mediated by the activation of the PPARγ, a nuclear receptor involved in several diseases, such as inflammation, metabolic disorders, cardiovascular disease, and different types of cancer [83,84]. In particular, it has been documented that activation of PPARγ by agonists induced breast cancer cell death and inhibits breast cancer progression [85]. Rovito et al., have shown that DHEA, acting as a ligand, upregulated PPARγ mRNA and protein levels as well as the expression of the PPARγ target gene phosphatase and tensin homolog on chromosome ten (PTEN). Interestingly, the enhanced PTEN protein levels were associated with the decrease in phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of the rapamycin (mTOR) signaling pathway. The PI3K/Akt/mTOR signaling pathway is one of the most common dysregulated signaling pathways in breast cancer, leading to increased cell proliferation and survival. This dysregulation can occur through multiple mechanisms, including activating mutations in PI3K or loss of negative regulators, such as the tumor suppressor PTEN. DHEA not only decreases the activation of the PI3K/Akt/mTOR signaling pathway but also increases the expression of Beclin-1, which triggers autophagy of breast cancer cells enhancing the formation of the autophagosome. Specifically, Beclin-1 binds to the Bcl-2 homology-3 (BH3) domain, resulting in the inhibition of Beclin-1 autophagic function. It has been demonstrated that DHEA promotes autophagosome formation by increasing the phosphorylation of Bcl-2 at serine 70, thereby reducing its physical interaction with Beclin-1 and resulting in elevated levels of the unbound Beclin-1 protein [82]. DHEA can exert antiproliferative effects in breast cancer cells not only through the activation of PPARγ but also by binding to the cannabinoid receptors (CBs) [86]. Brown et al., have demonstrated that the antiproliferative effects of DHEA in ERα positive MCF-7 and triple-negative MDA-MB-231 breast cancer cells are, at least in part, mediated by its binding to the CB1, since the inhibition of the CB1 by specific antagonists decreased the antiproliferative and antitumoral activities of DHEA in breast cancer cells. The effects of DHEA in reducing cell proliferation involve the MAPK signaling pathway. It has been widely demonstrated that the activation of the MAPK signaling pathway promotes cancer cell proliferation by inducing cell-cycle progression, increasing cell survival, and inhibiting apoptosis. Moreover, it can also promote cancer cell migration and invasion by regulating cytoskeletal dynamics and modulating the expression of genes involved in epithelial-mesenchymal transition. The authors found that DHEA treatment decreases the expression of p38 MAPK and phosphorylated p38 MAPK (pp38) as well as the phosphorylated ERK, thus impacting breast cancer proliferation [81]. Recently, the effects of DHEA in triple-negative breast cancer cells have been investigated, demonstrating its potential antitumoral action [87]. In particular, it has been reported that DHEA reduced the viability of both MDA-MB-231 and MDA-MB-436 breast cancer cells in a concentration-dependent manner, showing IC50 values of 27.29 μM and 19.76 μM, respectively. Moreover, nontoxic concentrations of DHEA shifted MDA-MB-231 and MDA-MB-436 cells from an energetic to a quiescent state. Interestingly, DHEA affected cell motility and invasiveness of triple-negative breast cancer cells, which represent two distinct characteristics of this aggressive breast cancer subtype. These results are partially in agreement with the data published by Brown et al., who showed that DHEA reduces cell invasion without affecting cell migration [81]. To better understand the mechanism of action of DHEA in triple-negative breast cancer cells, Augimeri et al., analyzed the breast cancer cell secretome upon DHEA treatment [87]. Indeed, the role of secreted factors in mediating the phenotype of cancer cells as well as their interaction with the cells of the tumor microenvironment has been widely demonstrated. DHEA treatment has modified the secretion of 28 molecules interconnected in a protein–protein network. Among these proteins, we observed that the C-C motif chemokine ligand 5 (CCL5) represented a key regulator factor and mediated the effect of DHEA in reducing the motility of triple-negative breast cancer cells. Moreover, the authors have demonstrated that DHEA, by decreasing CCL5 secretion, affected the inflammatory tumor microenvironment, reducing macrophage recruitment. In addition, DHEA reduced macrophage viability as well as the expression of tumor-associated macrophage (TAM) markers in a paracrine fashion. Interestingly, DHEA can also affect the macrophage phenotype through a direct effect on these cells. Indeed, Gionfriddo et al., have demonstrated that DHEA attenuated the secretion of cytokines associated with the M1 and M2 macrophage phenotype in a PPARγ-dependent manner in breast TAMs [88].
The specific mechanisms involved in DHEA antineoplastic effects in breast cancer cells are summarized in Figure 3.
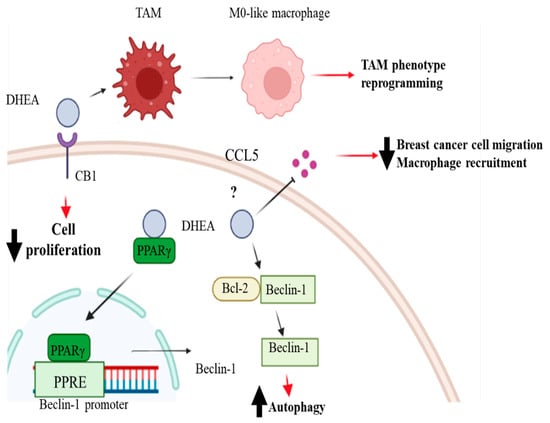
Figure 3.
Mechanism of action and biological effects of DHEA in breast cancer. Docosahexaenoyl ethanolamide (DHEA) can increase Beclin-1 expression through the peroxisome proliferator-activated receptor (PPAR) γ binding to its response element (PPRE) in the promoter region of Beclin-1 and increase its dissociation from the Beclin-1/Bcl2 complex, inducing autophagy. DHEA can also bind to cannabinoid receptor 1 (CB1), which partially mediates its antiproliferative and antitumoral effects in breast cancer cells. DHEA can also modify the secretion of the C-C motif chemokine ligand 5 (CCL5), reducing breast cancer cell migration and macrophage recruitment. Furthermore, DHEA can affect the inflammatory tumor microenvironment, affecting tumor-associated macrophage (TAM) markers’ expression, and reprogramming the TAM phenotype. Created with BioRender.com.
To date, the effects of DHEA in combination with chemotherapeutic drugs are still unknown. Moreover, despite extensive research into the favorable effects of DHEA in mitigating tumor aggressiveness, there is currently a lack of animal and clinical studies investigating this phenomenon.
6. Conclusions
Adopting a healthy eating pattern, such as the Mediterranean diet, that includes high consumption of N-3 PUFAs, particularly DHA, is recommended for the prevention and treatment of breast cancer. However, more research is necessary to fully understand the mechanism of action of DHA, particularly the N-docosahexaenoyl ethanolamine for breast cancer. While intriguing data suggest the antitumor effects of DHEA in breast cancer cells, its effect in in vivo models needs to be further investigated. Moreover, until now the potential beneficial effect of the combination of chemotherapy with supplementation/treatment with DHEA is not well recognized. Therefore, in our opinion, more attention should be paid to the application of DHEA in the prevention and treatment of breast cancer.
Author Contributions
Conceptualization, writing—original draft preparation, G.A.; conceptualization, writing—review, editing, funding acquisition D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Department of Excellence (Italian Law 232/2016), the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity, and cancer: A global perspective. In Continuous Update Project Expert Report; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, K.; Lu, S.; He, W.; Du, Z. Targeted Therapy and Immunotherapy for Heterogeneous Breast Cancer. Cancers 2022, 14, 5456. [Google Scholar] [CrossRef]
- Barone, I.; Giordano, C.; Malivindi, R.; Lanzino, M.; Rizza, P.; Casaburi, I.; Bonofiglio, D.; Catalano, S.; Ando, S. Estrogens and PTP1B function in a novel pathway to regulate aromatase enzymatic activity in breast cancer cells. Endocrinology 2012, 153, 5157–5166. [Google Scholar] [CrossRef]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Ando, S.; Catalano, S. Phosphodiesterase type 5 and cancers: Progress and challenges. Oncotarget 2017, 8, 99179–99202. [Google Scholar] [CrossRef]
- Van Mechelen, M.; Van Herck, A.; Punie, K.; Nevelsteen, I.; Smeets, A.; Neven, P.; Weltens, C. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res. Treat. 2020, 181, 115–125. [Google Scholar] [CrossRef]
- Pastore, E.; Caini, S.; Bendinelli, B.; Palli, D.; Ermini, I.; de Bonfioli Cavalcabo, N.; Assedi, M.; Ambrogetti, D.; Fontana, M.; Masala, G. Dietary Patterns, Dietary Interventions, and Mammographic Breast Density: A Systematic Literature Review. Nutrients 2022, 14, 5312. [Google Scholar] [CrossRef]
- Regal, P.; Fente, C.A.; Cepeda, A.; Silva, E.G. Food and omics: Unraveling the role of food in breast cancer development. Curr. Opin. Food Sci. 2021, 39, 197–207. [Google Scholar] [CrossRef]
- Bojkova, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer-Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, M.; Ma, D.W. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res. 2010, 12, 211. [Google Scholar] [CrossRef]
- Fodil, M.; Blanckaert, V.; Ulmann, L.; Mimouni, V.; Chenais, B. Contribution of N-3 Long-Chain Polyunsaturated Fatty Acids to the Prevention of Breast Cancer Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 7936. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczynski, A.; Ogluszka, M.; Nawrocka, A.; Polawska, E.; Grzesiak, A.; Slaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchala, M. Effects of Dietary N-3 and N-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, G.; Bonofiglio, D. The Mediterranean Diet as a Source of Natural Compounds: Does It Represent a Protective Choice against Cancer? Pharmaceuticals 2021, 14, 920. [Google Scholar] [CrossRef]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Ando, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate noN-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Differential anti-cancer effects of purified EPA and DHA and possible mechanisms involved. Curr. Med. Chem. 2011, 18, 4065–4075. [Google Scholar] [CrossRef]
- Casanas-Sanchez, V.; Perez, J.A.; Fabelo, N.; Quinto-Alemany, D.; Diaz, M.L. Docosahexaenoic (DHA) modulates phospholipid-hydroperoxide glutathione peroxidase (Gpx4) gene expression to ensure self-protection from oxidative damage in hippocampal cells. Front. Physiol. 2015, 6, 203. [Google Scholar] [CrossRef]
- Giordano, C.; Plastina, P.; Barone, I.; Catalano, S.; Bonofiglio, D. N-3 Polyunsaturated Fatty Acid Amides: New Avenues in the Prevention and Treatment of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2279. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Wahle, K.W.; Cascio, M.G.; Smoum-Jaouni, R.; Mechoulam, R.; Pertwee, R.G.; Heys, S.D. Omega-3 N-acylethanolamines are endogenously synthesised from omega-3 fatty acids in different human prostate and breast cancer cell lines. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 305–310. [Google Scholar] [CrossRef]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef]
- Igarashi, M.; DeMar, J.C., Jr.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary N-3 PUFA deprivation. J. Lipid Res. 2007, 48, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from alpha-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef]
- Murff, H.J.; Shu, X.O.; Li, H.; Yang, G.; Wu, X.; Cai, H.; Wen, W.; Gao, Y.T.; Zheng, W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: A prospective cohort study. Int. J. Cancer 2011, 128, 1434–1441. [Google Scholar] [CrossRef]
- Thiebaut, A.C.; Chajes, V.; Gerber, M.; BoutroN-Ruault, M.C.; Joulin, V.; Lenoir, G.; Berrino, F.; Riboli, E.; Benichou, J.; Clavel-Chapelon, F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer 2009, 124, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Yuan, J.M.; Sun, C.L.; Lee, H.P.; Yu, M.C. Opposing effects of dietary N-3 and N-6 fatty acids on mammary carcinogenesis: The Singapore Chinese Health Study. Br. J. Cancer 2003, 89, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A.; Mason, R.P. Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017, 16, 23. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Phillips, T.A.; Box, J.A.; Kreutzjans, A.L.; Carlson, S.E.; Hidaka, B.H.; Metheny, T.; Zalles, C.M.; Mills, G.B.; et al. Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Premenopausal Women. Cancer Prev. Res. 2015, 8, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Querques, G.; Forte, R.; Souied, E.H. Retina and omega-3. J. Nutr. Metab. 2011, 2011, 748361. [Google Scholar] [CrossRef]
- Richards, M.P.; Pettitt, P.B.; Stiner, M.C.; Trinkaus, E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc. Natl. Acad. Sci. USA 2001, 98, 6528–6532. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Gaitan, A.V.; Wood, J.T.; Liu, Y.; Ji, L.; Nikas, S.P.; Makriyannis, A.; Lammi-Keefe, C.J. Maternal Dietary Fatty Acids and Their Relationship to Derived Endocannabinoids in Human Milk. J. Hum. Lact. 2021, 37, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, J.; Balvers, M.; Witkamp, R. N-Acyl amines of docosahexaenoic acid and other N-3 polyunsatured fatty acids - from fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013, 169, 772–783. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Jilkova, Z.M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic effects of N-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar] [CrossRef]
- Kim, J.; Carlson, M.E.; Kuchel, G.A.; Newman, J.W.; Watkins, B.A. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. Int. J. Obes. 2016, 40, 129–137. [Google Scholar] [CrossRef]
- Smith, S.; Kevala, K.; Cunningham, B.; Rouse, C.; Hunt, C.E.; Kim, H.Y. N-docosahexaenoylethanolamine detected in human breast milk. Prostaglandins Leukot. Essent. Fatty Acids 2018, 137, 1–4. [Google Scholar] [CrossRef]
- Bisogno, T. Endogenous cannabinoids: Structure and metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 1–9. [Google Scholar] [CrossRef]
- Meijerink, J.; Plastina, P.; Vincken, J.P.; Poland, M.; Attya, M.; Balvers, M.; Gruppen, H.; Gabriele, B.; Witkamp, R.F. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: Evidence for a new link between fish oil and inflammation. Br. J. Nutr. 2011, 105, 1798–1807. [Google Scholar] [CrossRef]
- Meijerink, J.; Poland, M.; Balvers, M.G.; Plastina, P.; Lute, C.; Dwarkasing, J.; van Norren, K.; Witkamp, R.F. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. Br. J. Pharmacol. 2015, 172, 24–37. [Google Scholar] [CrossRef]
- Balvers, M.G.; Verhoeckx, K.C.; Plastina, P.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim. Biophys. Acta 2010, 1801, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, D.M.; Akbar, M.; Huang, B.X. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336. [Google Scholar] [CrossRef]
- Kim, H.Y.; Spector, A.A.; Xiong, Z.M. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011, 96, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fredman, G.; Krishnamoorthy, S.; Agrawal, N.; Irimia, D.; Piomelli, D.; Serhan, C.N. Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J. Biol. Chem. 2011, 286, 31532–31541. [Google Scholar] [CrossRef]
- Sun, H.; Hu, Y.; Gu, Z.; Owens, R.T.; Chen, Y.Q.; Edwards, I.J. Omega-3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecaN-1 inhibition of the MEK-Erk pathway. Carcinogenesis 2011, 32, 1518–1524. [Google Scholar] [CrossRef]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Cremona, A.; Montorfano, G.; Jovenitti, I.E.; Orsini, F.; Arosio, P.; Rizzo, A.M. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell Biochem. Biophys. 2012, 64, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Ravacci, G.R.; Brentani, M.M.; Tortelli, T.C.; Torrinhas, R.S.; Santos, J.R.; Logullo, A.F.; Waitzberg, D.L. Docosahexaenoic Acid Modulates a HER2-Associated Lipogenic Phenotype, Induces Apoptosis, and Increases Trastuzumab Action in HER2-Overexpressing Breast Carcinoma Cells. Biomed. Res. Int. 2015, 2015, 838652. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Zhao, J.; Dong, L.; Ge, Y.; Hou, L.; Liu, Y.; Zheng, Z. Docosahexaenoic acid inhibited the Wnt/beta-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J. Nutr. Biochem. 2014, 25, 104–110. [Google Scholar] [CrossRef]
- Lin, G.; Zhu, S.; Wu, Y.; Song, C.; Wang, W.; Zhang, Y.; Chen, Y.L.; He, Z. omega-3 free fatty acids and all-trans retinoic acid synergistically induce growth inhibition of three subtypes of breast cancer cell lines. Sci. Rep. 2017, 7, 2929. [Google Scholar] [CrossRef]
- Khan, N.A.; Nishimura, K.; Aires, V.; Yamashita, T.; Oaxaca-Castillo, D.; Kashiwagi, K.; Igarashi, K. Docosahexaenoic acid inhibits cancer cell growth via p27Kip1, CDK2, ERK1/ERK2, and retinoblastoma phosphorylation. J. Lipid Res. 2006, 47, 2306–2313. [Google Scholar] [CrossRef]
- Rescigno, T.; Capasso, A.; Tecce, M.F. Effect of Docosahexaenoic Acid on Cell Cycle Pathways in Breast Cell Lines With Different Transformation Degree. J. Cell Physiol. 2016, 231, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Barascu, A.; Besson, P.; Le Floch, O.; Bougnoux, P.; Jourdan, M.L. CDK1-cyclin B1 mediates the inhibition of proliferation induced by omega-3 fatty acids in MDA-MB-231 breast cancer cells. Int. J. Biochem. Cell Biol. 2006, 38, 196–208. [Google Scholar] [CrossRef]
- Pizato, N.; Luzete, B.C.; Kiffer, L.; Correa, L.H.; de Oliveira Santos, I.; Assumpcao, J.A.F.; Ito, M.K.; Magalhaes, K.G. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018, 8, 1952. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6N-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef]
- Maheo, K.; Vibet, S.; Steghens, J.P.; Dartigeas, C.; Lehman, M.; Bougnoux, P.; Gore, J. Differential sensitization of cancer cells to doxorubicin by DHA: A role for lipoperoxidation. Free Radic. Biol. Med. 2005, 39, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Goruk, S.; Mazurak, V.; Postovit, L.; Field, C.J. Role of docosahexaenoic acid in enhancement of docetaxel action in patient-derived breast cancer xenografts. Breast Cancer Res. Treat. 2019, 177, 357–367. [Google Scholar] [CrossRef]
- Colas, S.; Maheo, K.; Denis, F.; Goupille, C.; Hoinard, C.; Champeroux, P.; Tranquart, F.; Bougnoux, P. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: A role for tumor vascularization. Clin. Cancer Res. 2006, 12, 5879–5886. [Google Scholar] [CrossRef]
- Vibet, S.; Goupille, C.; Bougnoux, P.; Steghens, J.P.; Gore, J.; Maheo, K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic. Biol. Med. 2008, 44, 1483–1491. [Google Scholar] [CrossRef]
- Signori, C.; DuBrock, C.; Richie, J.P.; Prokopczyk, B.; Demers, L.M.; Hamilton, C.; Hartman, T.J.; Liao, J.; El-Bayoumy, K.; Manni, A. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: Interim feasibility and biomarkers analysis from a clinical trial. Eur. J. Clin. Nutr. 2012, 66, 878–884. [Google Scholar] [CrossRef]
- Patterson, R.E.; Flatt, S.W.; Newman, V.A.; Natarajan, L.; Rock, C.L.; Thomson, C.A.; Caan, B.J.; Parker, B.A.; Pierce, J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr. 2011, 141, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Conigliaro, T.; Boyce, L.M.; Lopez, C.A.; Tonorezos, E.S. Food Intake During Cancer Therapy: A Systematic Review. Am. J. Clin. Oncol. 2020, 43, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, J.; Tian, C.; Cao, J.; Fida, G.; Wang, Z.; Chen, H.; Qian, Z.; Chen, W.R.; Gu, Y. The targeting mechanism of DHA ligand and its conjugate with Gemcitabine for the enhanced tumor therapy. Oncotarget 2014, 5, 3622–3635. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, P.M.; Picus, J.; Wildi, J.D.; Goodner, S.A.; Creekmore, A.N.; Gao, F.; Govindan, R.; Ellis, M.J.; Tan, B.R.; Linette, G.P.; et al. Phase 1 and pharmacokinetic study of weekly docosahexaenoic acid-paclitaxel, Taxoprexin, in resistant solid tumor malignancies. Cancer Chemother. Pharmacol. 2009, 63, 451–458. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Zerouga, M.; Wu, M.; Castillo, A.; Harvey, K.; Zaloga, G.P.; Stillwell, W. Anticancer properties of propofol-docosahexaenoate and propofol-eicosapentaenoate on breast cancer cells. Breast Cancer Res. 2005, 7, R645–R654. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.O.; de Souza, A.M.; Cassali, G.D.; de Barros, A.L.B.; Miranda Ferreira, L.A. Co-delivery of doxorubicin, docosahexaenoic acid, and alpha-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef]
- Brown, I.; Lee, J.; Sneddon, A.A.; Cascio, M.G.; Pertwee, R.G.; Wahle, K.W.J.; Rotondo, D.; Heys, S.D. Anticancer effects of N-3 EPA and DHA and their endocannabinoid derivatives on breast cancer cell growth and invasion. Prostaglandins Leukot. Essent. Fatty Acids 2020, 156, 102024. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; De Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARgamma activation in MCF-7 breast cancer cells. J. Cell Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef]
- Augimeri, G.; Giordano, C.; Gelsomino, L.; Plastina, P.; Barone, I.; Catalano, S.; Ando, S.; Bonofiglio, D. The Role of PPARgamma Ligands in Breast Cancer: From Basic Research to Clinical Studies. Cancers 2020, 12, 2623. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Qi, H.; Gabriele, S.; Catalano, S.; Aquila, S.; Belmonte, M.; Ando, S. Peroxisome proliferator-activated receptor gamma inhibits follicular and anaplastic thyroid carcinoma cells growth by upregulating p21Cip1/WAF1 gene in a Sp1-dependent manner. Endocr. Relat. Cancer 2008, 15, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Rovito, D.; Gionfriddo, G.; Barone, I.; Giordano, C.; Grande, F.; De Amicis, F.; Lanzino, M.; Catalano, S.; Ando, S.; Bonofiglio, D. Ligand-activated PPARgamma downregulates CXCR4 gene expression through a novel identified PPAR response element and inhibits breast cancer progression. Oncotarget 2016, 7, 65109–65124. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, G.; Fiorillo, M.; Morelli, C.; Panza, S.; Giordano, C.; Barone, I.; Catalano, S.; Sisci, D.; Ando, S.; Bonofiglio, D. The Omega-3 Docosahexaenoyl Ethanolamide Reduces CCL5 Secretion in Triple Negative Breast Cancer Cells Affecting Tumor Progression and Macrophage Recruitment. Cancers 2023, 15, 819. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARgamma Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).