Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer
Abstract
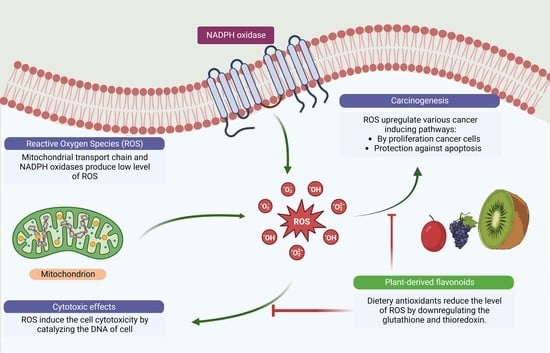
1. Introduction
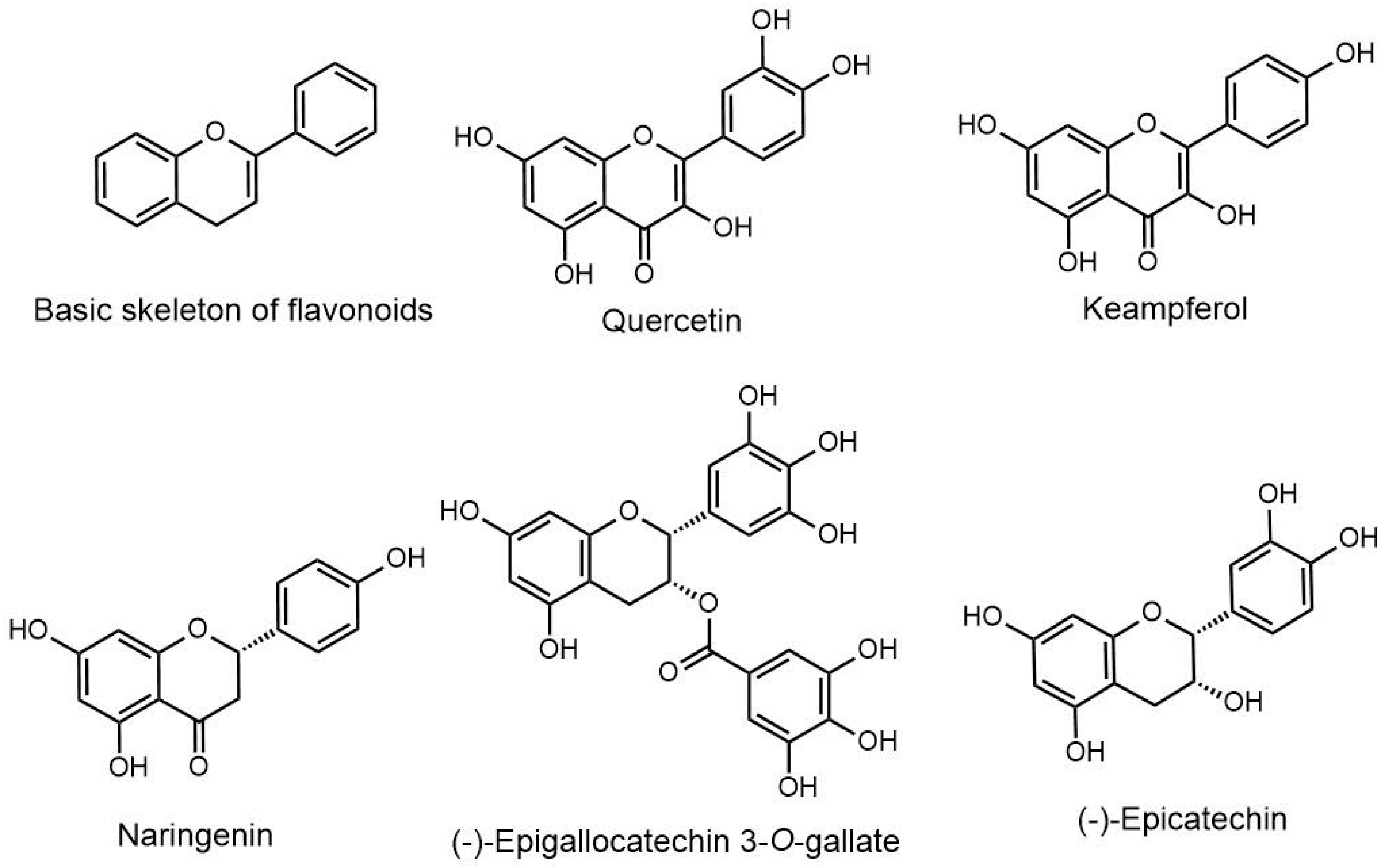
2. Molecular Mechanism of Flavonoids in Different Type of Cancers
2.1. Epicatechin
2.2. Epicatechin Gallate
2.3. Kaempferol
2.4. Naringenin
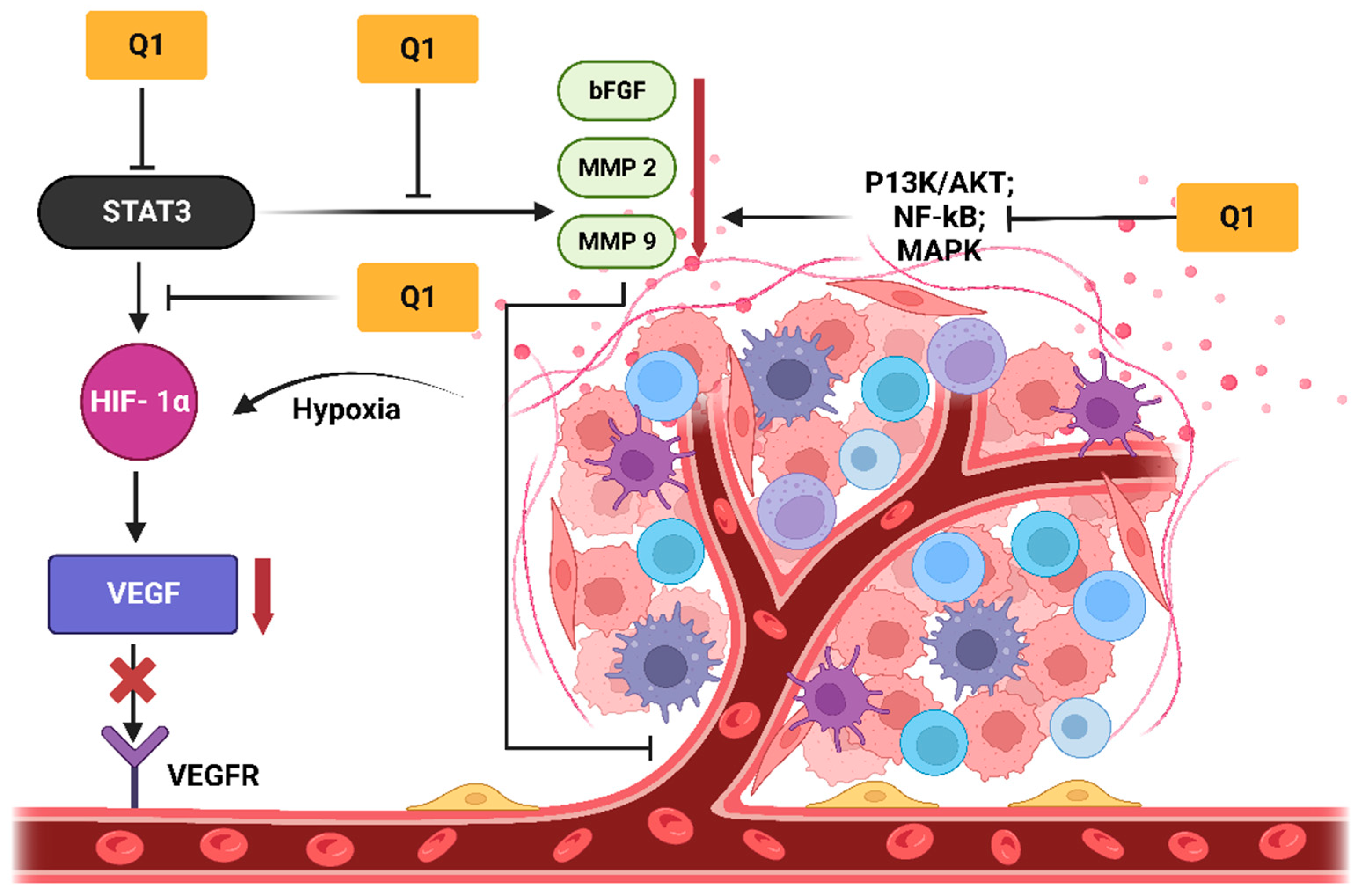
2.5. Quercetin
3. Pharmaceutical Application of Flavonoids against Various Cancers Using Nanotechnological Approaches
3.1. Epigallocatechin-3-Gallate (EGCG)
3.2. Quercetin
3.3. Naringenin
3.4. Kaempferol
3.5. Epicatechin
4. Combinations of Flavonoids with Synthetic Anticancer Agents
4.1. Prostate Cancer
4.2. Oral Cancer
4.3. Brain Cancer
4.4. Colorectal Cancer
4.5. Breast Cancer
| S. No | Flavonoid | Flavonoid | Cancer Type | Findings | Model | Cell Type | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Quercetin, Naringenin | Kaempferol | Liver; prostate | Exhibited synergistic chemotherapeutic potential against two different cells. | In vitro | LNCaP; Hepa 1c1c-7 | [85] |
| 2 | Quercetin | Kaempferol | Gut; breast | Exhibited synergistic effect against HuTu-80 and Caco-2. | In vitro | HuTu-80; Caco-2 | [84] |
| 3 | Ellagic acid | Quercetin | Leukemia | Exhibited apoptosis and reduction of cell growth in human leukemia cells (MOLT-4). | In vitro | MOLT-4 | [86] |
| 4 | Resveratrol | Quercetin | Colon | Enhanced chemotherapeutic potential was observed. | In vitro | HT-29 | [90] |
| 5 | Resveratrol | Quercetin | Glioma | Induced senescence-like growth arrest in C6 rat glioma cells. | In vitro | C6 | [121] |
| 6 | Quercetin | Catechin | Breast | Inhibited mammary tumor growth and metastasis in nude mice. | In vivo | MDA-MB-231 | [122] |
| 7 | Kaempferol | Resveratrol | Prostate | Inhibited TNF-α and cytokine IL-10. | In vitro | RAW-264.7 | [123] |
| 8 | Naringenin | Quercetin | Breast | Showed anticancer potential against MCF-7 breast cancer cells. | In vitro | MCF-7 | [124] |
| 9 | Quercetin | ECGC | Prostate | Enhanced antiproliferative activity in androgen-independent PC-3 cells and in androgen-dependent LNCaP prostate cancer cells. | In vitro | PC-3; LNCaP | [125] |
| 10 | Quercetin | Catechin | Breast | Inhibited the primary tumor growth of breast cancer xenografts in a nude mouse model. | In vivo; in vitro | MDA-MB-231 | [83] |
| 11 | Quercetin | Naringenin | Liver | Exhibited significant potential in reduction of carcinogenesis. | In vivo | - | [126] |
5. Regulatory Prospects for Polyphenolic Compounds
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally Occurring Anti-Cancer Compounds: Shining from Chinese Herbal Medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Al-awsi, G.R.L.; Mubarak, S.M.H.; Heidarifard, M.; Ghasemian, A. Anticancer Effects of Herbal Medicine Compounds and Novel Formulations: A Literature Review. J. Gastrointest. Cancer 2020, 51, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saleem, S.; Chaudhuri, A.; Ali, J.; Baboota, S. Docetaxel: An Update on Its Molecular Mechanisms, Therapeutic Trajectory and Nanotechnology in the Treatment of Breast, Lung and Prostate Cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 101959. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Muralidhar Reddy, P.; Velmurugan, B.K. A Review on the Effects of Current Chemotherapy Drugs and Natural Agents in Treating Non-Small Cell Lung Cancer. Biomed. 2017, 7, 12–23. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Tony Kong, A.-N. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Bahrami, A.; Fereidouni, M.; Pirro, M.; Bianconi, V.; Sahebkar, A. Modulation of Regulatory T Cells by Natural Products in Cancer. Cancer Lett. 2019, 459, 72–85. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Kesharwani, P.; Khanna, K.; Karwasra, R.; Sharma, N.; Rawat, S.; Sharma, D.; Ahmad, F.J.; Jain, G.K.; et al. Intranasal Delivery of Naloxone-Loaded Solid Lipid Nanoparticles as a Promising Simple and Non-Invasive Approach for the Management of Opioid Overdose. Int. J. Pharm. 2021, 599, 120428. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells Is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dong, J. (−)-Epicatechin Acts as a Potent Agonist of the Membrane Androgen Receptor, ZIP9 (SLC39A9), to Promote Apoptosis of Breast and Prostate Cancer Cells. J. Steroid Biochem. Mol. Biol. 2021, 211, 105906. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Suganuma, M.; Fujiki, H. New Role of (−)-Epicatechin in Enhancing the Induction of Growth Inhibition and Apoptosis in Human Lung Cancer Cells by Curcumin. Cancer Prev. Res. 2010, 3, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin Stimulates Mitochondrial Activity and Selectively Sensitizes Cancer Cells to Radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Min, K.; Kwon, T.K. Anticancer Effects and Molecular Mechanisms of Epigallocatechin-3-Gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bachrach, U. The Specific Anti-Cancer Activity of Green Tea (−)-Epigallocatechin-3-Gallate (EGCG). Amino Acids 2002, 22, 131–143. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A Review on Anti-Cancer Effect of Green Tea Catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Kong, A.N.T.; Owuor, E.; Yu, R.; Hebbar, V.; Chen, C.; Hu, R.; Mandlekar, S. Induction of Xenobiotic Enzymes by the Map Kinase Pathway and the Antioxidant or Electrophile Response Element (ARE/EpRE). Drug Metab. Rev. 2001, 33, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-Induced Growth Inhibition and Apoptosis in A549 Lung Cancer Cells Is Mediated by Activation of MEK-MAPK. J. Cell. Physiol. 2003, 197, 110–121. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Wang, Y.; Zheng, X.; Chu, Q. Kaempferol-3-O-Rutinoside, a Flavone Derived from Tetrastigma Hemsleyanum, Suppresses Lung Adenocarcinoma via the Calcium Signaling Pathway. Food Funct. 2021, 12, 8351–8365. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, L.; Gao, M.; Wang, S.; Meng, L.; Guo, L. Molecular Docking and in Vitro Experiments Verified That Kaempferol Induced Apoptosis and Inhibited Human HepG2 Cell Proliferation by Targeting BAX, CDK1, and JUN. Mol. Cell. Biochem. 2022, 478, 767–780. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol Inhibits the Growth and Metastasis of Cholangiocarcinoma in Vitro and in Vivo. Acta Biochim. Biophys. Sin. 2015, 48, 238–245. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, K.; Chen, H.; Liu, K.; Langfald, A.; Yang, G.; Zhang, Y.; Yu, D.H.; Kim, M.O.; Lee, M.H.; et al. Kaempferol Targets RSK2 and MSK1 to Suppress UV Radiation-Induced Skin Cancer. Cancer Prev. Res. 2014, 7, 958–967. [Google Scholar] [CrossRef]
- Amjad, E.; Sokouti, B.; Asnaashari, S. A Systematic Review of Anti-Cancer Roles and Mechanisms of Kaempferol as a Natural Compound. Cancer Cell Int. 2022, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin Exerts Anticancer Effects by Inducing Tumor Cell Death and Inhibiting Angiogenesis in Malignant Melanoma. Int. J. Med. Sci. 2020, 17, 3049. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharmacol. 2021, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin Inhibits Migration, Invasion, Induces Apoptosis in Human Lung Cancer Cells and Arrests Tumour Progression in Vitro. J. Cell. Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and Naringenin as Anticancer Agents and Adjuvants in Cancer Combination Therapy: Efficacy and Molecular Mechanisms of Action, a Comprehensive Narrative Review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive Review on Naringenin and Naringin Polyphenols as a Potent Anticancer Agent. Environ. Sci. Pollut. Res. 2022, 29, 31025–31041. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented Anticancer Activity of Naringenin-Loaded TPGS Polymeric Nanosuspension for Drug Resistive MCF-7 Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761. [Google Scholar] [CrossRef]
- Chirumbolo, S. Quercetin in Cancer Prevention and Therapy. Integr. Cancer Ther. 2013, 12, 97–102. [Google Scholar] [CrossRef]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical Nanostructured Lipid Carrier Gel of Quercetin and Resveratrol: Formulation, Optimization, in Vitro and Ex Vivo Study for the Treatment of Skin Cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid. -Based Complement. Altern. Med. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Fan, J.J.; Hsu, W.H.; Lee, K.H.; Chen, K.C.; Lin, C.W.; Lee, Y.L.A.; Ko, T.P.; Lee, L.T.; Lee, M.T.; Chang, M.S.; et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100a7 Signaling. Antioxidants 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Rodríguez-Gómez, M.J.; Justino, G.C.; Charro, N.; Florencio, M.H.; Mira, L. Influence of the Metabolic Profile on the in Vivo Antioxidant Activity of Quercetin under a Low Dosage Oral Regimen in Rats. Br. J. Pharmacol. 2008, 153, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Agarwal, C.; Agarwal, R. Differential Responses of Skin Cancer-Chemopreventive Agents Silibinin, Quercetin, and Epigallocatechin 3-Gallate on Mitogenic Signaling and Cell Cycle Regulators in Human Epidermoid Carcinoma A431 Cells. Nutr. Cancer 2001, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with Green Tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef]
- Imran, M.; Gowd, V.; Saha, P.; Rashid, S.; Ahmad Chaudhary, A.; Mohamed, M.Y.A.; Alawam, A.S.; Khan, R. Biologically Inspired Stealth—Camouflaged Strategies in Nanotechnology for the Improved Therapies in Various Diseases. Int. J. Pharm. 2023, 631, 122407. [Google Scholar] [CrossRef]
- Subedi, L.; Pandey, P.; Khadka, B.; Shim, J.H.; Cho, S.S.; Kweon, S.; Byun, Y.; Kim, K.T.; Park, J.W. Enhancement of the Anticancer Effect of Atorvastatin-Loaded Nanoemulsions by Improving Oral Absorption via Multivalent Intestinal Transporter-Targeting Lipids. Drug Deliv. 2022, 29, 3397–3413. [Google Scholar] [CrossRef]
- Subedi, L.; Song, S.Y.; Jha, S.K.; Lee, S.H.; Pangeni, R.; Koo, K.T.; Kim, B.J.; Cho, S.S.; Park, J.W. Preparation of Topical Itraconazole with Enhanced Skin/Nail Permeability and In Vivo Antifungal Efficacy against Superficial Mycosis. Pharmaceutics 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of Drug Delivery by Encapsulating Nanoparticles in Natural Cell Membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef]
- Peng, J.; Liang, X.; Calderon, L. Progress in Research on Gold Nanoparticles in Cancer Management. Medicine 2019, 98, e15311. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Imran, M.; Nadeem, M.; Jain, D.; Haider, K.; Moshahid Alam Rizvi, M.; Sheikh, A.; Kesharwani, P.; Kumar jain, G.; Jalees Ahmad, F. Formulation and Development of Novel Lipid-Based Combinatorial Advanced Nanoformulation for Effective Treatment of Non-Melanoma Skin Cancer. Int. J. Pharm. 2023, 632, 122580. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and Future Perspectives in Epithelial Drug Delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin Gallate-Loaded Polysaccharide Nanoparticles for Prostate Cancer Chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin Gallate (EGCG)-Nanoethosomes as a Transdermal Delivery System for Docetaxel to Treat Implanted Human Melanoma Cell Tumors in Mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (−)-Epigallocatechin-3-Gallate Vesicular Systems for Prevention and Treatment of Skin Cancer: A Comprehensive Experimental Study with Preclinical Investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Adhikary, G.; Eckert, R.L. The Bmi-1 Polycomb Protein Antagonizes the (−)-Epigallocatechin-3-Gallate-Dependent Suppression of Skin Cancer Cell Survival. Carcinogenesis 2010, 31, 496–503. [Google Scholar] [CrossRef]
- Xing, L.; Lyu, J.Y.; Yang, Y.; Cui, P.F.; Gu, L.Q.; Qiao, J.B.; He, Y.J.; Zhang, T.Q.; Sun, M.; Lu, J.J.; et al. PH-Responsive de-PEGylated Nanoparticles Based on Triphenylphosphine-Quercetin Self-Assemblies for Mitochondria-Targeted Cancer Therapy. Chem. Commun. 2017, 53, 8790–8793. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Ghosh, D.; Sarkar, S.; Ghosh, A.; Swarnakar, S.; Das, N. Nanocapsulated Quercetin Downregulates Rat Hepatic MMP-13 and Controls Diethylnitrosamine-Induced Carcinoma. Nanomedicine 2014, 9, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of Nanotechnology in Improving Bioavailability and Bioactivity of Diet-Derived Phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.L.; Silva, L.F.C.; Mazzarino, L.; Siqueira, J.M.; Fernandes, D.; Pacheco, L.K.; Maioral, M.F.; Santos-Silva, M.C.; Baisch, A.L.M.; Assreuy, J.; et al. Oral Delivery of a High Quercetin Payload Nanosized Emulsion: In Vitro and In Vivo Activity Against B16-F10 Melanoma. J. Nanosci. Nanotechnol. 2016, 16, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Li, Y.H.; Wu, G.; Ren, J.Z.; Lu, H.B.; Li, Z.M.; Han, X.W. Quercetin Nanoparticles Display Antitumor Activity via Proliferation Inhibition and Apoptosis Induction in Liver Cancer Cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef]
- Tan, B.J.; Liu, Y.; Chang, K.L.; Lim, B.K.W.; Chiu, G.N.C. Perorally Active Nanomicellar Formulation of Quercetin in the Treatment of Lung Cancer. Int. J. Nanomed. 2012, 7, 651. [Google Scholar] [CrossRef]
- Askar, M.A.; El-Nashar, H.A.S.; Al-Azzawi, M.A.; Rahman, S.S.A.; Elshawi, O.E. Synergistic Effect of Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of Breast Cancer. Breast Cancer Basic Clin. Res. 2022, 16, 1–17. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-Inflammatory and Anticancer Activities of Naringenin-Loaded Liquid Crystalline Nanoparticles in Vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T.R. Antioxidant Studies of Chitosan Nanoparticles Containing Naringenin and Their Cytotoxicity Effects in Lung Cancer Cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef]
- Desai, P.; Thumma, N.J.; Wagh, P.R.; Zhan, S.; Ann, D.; Wang, J.; Prabhu, S. Cancer Chemoprevention Using Nanotechnology-Based Approaches. Front. Pharmacol. 2020, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The Mechanism of Kaempferol Induced Apoptosis and Inhibited Proliferation in Human Cervical Cancer SiHa Cell: From Macro to Nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.H.; Li, B.; Li, Z.; Jiang, B.H.; Chen, Y.C. Kaempferol Nanoparticles Achieve Strong and Selective Inhibition of Ovarian Cancer Cell Viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-Loaded Mucoadhesive Nanoemulsion for Intranasal Administration Reduces Glioma Growth in Vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol Conjugated Gold Nanoclusters Enabled Efficient for Anticancer Therapeutics to A549 Lung Cancer Cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Chao, Y.; Huang, C.T.; Fu, L.T.; Huang, Y.B.; Tsai, Y.H.; Wu, P.C. The Effect of Submicron Emulsion Systems on Transdermal Delivery of Kaempferol. Chem. Pharm. Bull. 2012, 60, 1171–1175. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J.; Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of Natural Compounds for Potential Skin Cancer Treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- Perez-Ruiz, A.G.; Ganem, A.; Olivares-Corichi, I.M.; García-Sánchez, J.R. Lecithin-Chitosan-TPGS Nanoparticles as Nanocarriers of (−)-Epicatechin Enhanced Its Anticancer Activity in Breast Cancer Cells. RSC Adv. 2018, 8, 34773–34782. [Google Scholar] [CrossRef]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan Mediated Targeted Drug Delivery System: A Review. J. Pharm. Pharm. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Saravanan, T.S.; Monteclaro, C.C.; Presser, N.; Ye, X.; Selvan, S.R.; Brosman, S. Epicatechins Purified from Green Tea (Camellia Sinensis) Differentially Suppress Growth of Gender-Dependent Human Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2006, 3, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Valance Washington, A.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Leigh Ackland, M.; Van De Waarsenburg, S.; Jones, R. Synergistic Antiproliferation Action of the Flavonols Quercetin and Kaempferol in Cultured Human Cancer Cell Lines. In Vivo 2005, 19, 69–76. [Google Scholar]
- Campbell, J.K.; King, J.L.; Harmston, M.; Lila, M.A.; Erdman, J.W. Synergistic Effects of Flavonoids on Cell Proliferation in Hepa-1c1c7 and LNCaP Cancer Cell Lines. J. Food Sci. 2006, 71, 358–363. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic Acid and Quercetin Interact Synergistically with Resveratrol in the Induction of Apoptosis and Cause Transient Cell Cycle Arrest in Human Leukemia Cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low Concentrations of Quercetin and Ellagic Acid Synergistically Influence Proliferation, Cytotoxicity and Apoptosis in MOLT-4 Human Leukemia Cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef]
- Rodgers, E.H.; Grant, M.H. The Effect of the Flavonoids, Quercetin, Myricetin and Epicatechin on the Growth and Enzyme Activities of MCF7 Human Breast Cancer Cells. Chem. Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- ElAttar, T.M.A.; Virji, A.S. Modulating Effect of Resveratrol and Quercetin on Oral Cancer Cell Growth and Proliferation. Anticancer Drugs 1999, 10, 187–193. [Google Scholar] [CrossRef]
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and Quercetin in Combination Have Anticancer Activity in Colon Cancer Cells and Repress Oncogenic MicroRNA-27a. Nutr. Cancer 2013, 65, 494–504. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Lopez, S.; Abia, R.; Rodriguez-Arcos, R.; Jimenez, A.; Guillen, R.; Muriana, F.J.G. Combination of Quercetin and Kaempferol Enhances in Vitro Cytotoxicity on Human Colon Cancer (HCT-116) Cells. Rec. Nat. Prod. 2014, 8, 262–271. [Google Scholar]
- Chuang-Xin, L.; Wen-Yu, W.; Yao, C.; Xiao-Yan, L.; Yun, Z. Quercetin Enhances the Effects of 5-Fluorouracil-Mediated Growth Inhibition and Apoptosis of Esophageal Cancer Cells by Inhibiting NF-ΚB. Oncol. Lett. 2012, 4, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.K.; Ağca, C.A.; Aşkın, H. Quercetin and Luteolin Improve the Anticancer Effects of 5-Fluorouracil in Human Colorectal Adenocarcinoma In Vitro Model: A Mechanistic Insight. Nutr. Cancer 2022, 74, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin Reverses Docetaxel Resistance in Prostate Cancer via Androgen Receptor and PI3K/AKT Signaling Pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Shimomura, I.; Kogure, A.; Usuba, W.; Takahashi, R.U.; Ochiya, T.; Yamamoto, Y. Quercetin Inhibits Lef1 and Resensitizes Docetaxel-Resistant Breast Cancer Cells. Molecules 2020, 25, 2576. [Google Scholar] [CrossRef]
- Xu, C.; Ding, Y.; Ni, J.; Yin, L.; Zhou, J.; Yao, J. Tumor-Targeted Docetaxel-Loaded Hyaluronic Acid-Quercetin Polymeric Micelles with p-Gp Inhibitory Property for Hepatic Cancer Therapy. RSC Adv. 2016, 6, 27542–27556. [Google Scholar] [CrossRef]
- Wong, M.Y.; Chiu, G.N.C. Simultaneous Liposomal Delivery of Quercetin and Vincristine for Enhanced Estrogen-Receptor-Negative Breast Cancer Treatment. Anticancer Drugs 2010, 21, 401–410. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination Therapy for the Treatment of Pancreatic Cancer through Hyaluronic Acid-Decorated Nanoparticles Loaded with Quercetin and Gemcitabine: A Preliminary in Vitro Study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- Mohammadi, E.; Alemi, F.; Maleki, M.; Malakoti, F.; Farsad-Akhtar, N.; Yousefi, B. Quercetin and Methotrexate in Combination Have Anticancer Activity in Osteosarcoma Cells and Repress Oncogenic MicroRNA-223. Drug Res. 2022, 72, 226–233. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic Compound Kaempferol Overcomes 5-Fluorouracil Resistance in Human Resistant LS174 Colon Cancer Cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol Can Reverse the 5-Fu Resistance of Colorectal Cancer Cells by Inhibiting PKM2-Mediated Glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.; Rodríguez, C.; Olmedo, I.; Carrasco-Rojas, J.; Rojas, D.; Molina-Berríos, A.; Díaz-Dosque, M.; Jara, J.A. Kaempferol Induces Cell Death and Sensitizes Human Head and Neck Squamous Cell Carcinoma Cell Lines to Cisplatin. Adv. Exp. Med. Biol. 2021, 1326, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D.; Vadgama, J.V. Sensitization to Docetaxel in Prostate Cancer Cells by Green Tea and Quercetin. J. Nutr. Biochem. 2015, 26, 408–415. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-ΚB/MiR-155-5p/MDR1 Pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Pons-Fuster López, E.; Gómez García, F.; López Jornet, P. Combination of 5-Florouracil and Polyphenol EGCG Exerts Suppressive Effects on Oral Cancer Cells Exposed to Radiation. Arch. Oral Biol. 2019, 101, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wirkus, J.; Yang, Z.; Machuca, J.; Esparza, Y.; Mackenzie, G.G. EGCG Sensitizes Chemotherapeutic-Induced Cytotoxicity by Targeting the ERK Pathway in Multiple Cancer Cell Lines. Arch. Biochem. Biophys. 2020, 692, 107546. [Google Scholar] [CrossRef]
- Tang, S.N.; Fu, J.; Shankar, S.; Srivastava, R.K. EGCG Enhances the Therapeutic Potential of Gemcitabine and CP690550 by Inhibiting STAT3 Signaling Pathway in Human Pancreatic Cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Magyar, C.E.; Elshimali, Y.; Heber, D.; Vadgama, J.V. Green Tea and Quercetin Sensitize PC-3 Xenograft Prostate Tumors to Docetaxel Chemotherapy. J. Exp. Clin. Cancer Res. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin Sensitizes Human Prostate Cancer Cells to Paclitaxel Therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, O.P.; González-Torres, A.; Álvarez-Salas, L.M.; Hernández-Sánchez, H.; García-Pérez, B.E.; Thompson-Bonilla, M.d.R.; Jaramillo-Flores, M.E. Effect of Naringenin and Its Combination with Cisplatin in Cell Death, Proliferation and Invasion of Cervical Cancer Spheroids. RSC Adv. 2020, 11, 129–141. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, M.; Kim, H.J.; Jang, S.B.; Bae, S.J.; Lee, I.K.; Ryu, D.; Ha, K.T. Targeting Lactate Dehydrogenase A with Catechin Resensitizes SNU620/5FU Gastric Cancer Cells to 5-Fluorouracil. Int. J. Mol. Sci. 2021, 22, 5406. [Google Scholar] [CrossRef] [PubMed]
- Núñez-iglesias, M.J.; Novio, S.; García, C.; Pérez-muñuzuri, M.E.; Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P.; Freire-garabal, M. Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro. Int. J. Mol. Sci. 2021, 22, 7178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Zhang, Y.; Zhang, Z.J.; Qiu, Q.W.; Cao, J.G.; He, Z.M. Effects of VBMDMP on the Reversal of Cisplatin Resistance in Human Lung Cancer A549/DDP Cells. Oncol. Rep. 2015, 33, 372–382. [Google Scholar] [CrossRef]
- Alshehri, O.Y.; Al-Abbasi, F.A.; El-Bassossy, H.M.; Abdallah, H.M.; Al-Abd, A.M. Abstract 263: Epicatechin Protects from Doxorubicin Induced Cardiotoxicity without Affecting Its Cytotoxic Profile in Breast Cancer Cells. Cancer Res. 2016, 76, 263. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and Anticancer Activity of Flavonoids and Its Possibility for Clinical Use by Combining with Conventional Chemotherapeutic Agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar] [PubMed]
- Hsieh, T.C.; Wu, J.M. Targeting CWR22Rv1 Prostate Cancer Cell Proliferation and Gene Expression by Combinations of the Phytochemicals EGCG, Genistein and Quercetin. Anticancer. Res. 2009, 29, 4025–4032. [Google Scholar] [PubMed]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and Metformin-Mediated Chemoprevention of Oral Cancer Is Associated with Inhibition of Cancer Stem Cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Nieh, S.; Jao, S.W.; Liu, C.L.; Wu, C.H.; Chang, Y.C.; Yang, C.Y.; Lin, Y.S. Quercetin Suppresses Drug-Resistant Spheres via the P38 MAPK-Hsp27 Apoptotic Pathway in Oral Cancer Cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic Enhancement of Anticancer Effects on Numerous Human Cancer Cell Lines Treated with the Combination of EGCG, Other Green Tea Catechins, and Anticancer Compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P.; et al. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C.; Dillenburg-Pilla, P.; Horn, F.; Salbego, C.; Lenz, G. Resveratrol and Quercetin Cooperate to Induce Senescence-like Growth Arrest in C6 Rat Glioma Cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape Polyphenols Inhibit Akt/Mammalian Target of Rapamycin Signaling and Potentiate the Effects of Gefitinib in Breast Cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of Apigenin, Kaempferol and Resveratrol on the Gene Expression and Protein Secretion of Tumor Necrosis Factor Alpha (TNF-α) and Interleukin-10 (IL-10) in RAW-264.7 Macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Rhman, M.A.; Devnarain, N.; Khan, R.; Owira, P.M.O. Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 3437. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin Increased the Antiproliferative Activity of Green Tea Polyphenol (−)-Epigallocatechin Gallate in Prostate Cancer Cells. Nutr. Cancer 2012, 64, 580–587. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, A.A.; Fahim, H.I.; Zaky, M.Y. Quercetin and Naringenin Abate Diethylnitrosamine/Acetylaminofluorene-Induced Hepatocarcinogenesis in Wistar Rats: The Roles of Oxidative Stress, Inflammation and Cell Apoptosis. Drug Chem. Toxicol. 2022, 45, 262–273. [Google Scholar] [CrossRef]


| S. No | Flavonoid | Synthetic Anticancer Drug | Cancer Type | Result | Model | IC50 Value | Cell Type | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Quercetin | 5-Flurouracil | Esophageal | Inhibited the growth of EC9706 cells and induced higher apoptosis compared to 5-Flurouracil alone. | In vitro | QCN = 100 µM; 5-FU= 0.2 mM | EC9706; Eca109 | [92] |
| 2 | Quercetin | 5-Flurouracil | Colorectal | Combination of quercetin and 5-Flurouracil reduced the growth of HT29 cells significantly compared to quercetin alone. | In vitro | QCN = 176.6 µg/mL; 5-FU = 107 µg/mL | HT-29 | [93] |
| 3 | Quercetin | Docetaxel | Prostate | Quercetin in combination with docetaxel reversed drug resistance via P13K/AkT signaling pathways. | In vitro | QCN = 20 µM; DTX = 5 nM | LNCaP/R; PC-3/R | [94] |
| 4 | Quercetin | Docetaxel | Breast | These two drugs in combination provided synergistic effects and resensitized the cancer cells to cancer treatment. | In vitro | QCN = 64.8 µM; DTX = 5 nM | MCF-7 | [95] |
| 5 | Quercetin | Docetaxel | Hepatic | Demonstrated superior anticancer efficacy with accumulation in tumor cells. | In vitro; In vivo | DTX-QCN = 0.00639 µg/mL | HepG2 | [96] |
| 6 | Quercetin | Vincristine | Breast | Provided synergistic anticancer effects by delivery of both compounds to the cancer cells. | In vitro | - | MCF-7 | [97] |
| 7 | Quercetin | Gemcitabine | Pancreatic | Demonstrated enhanced cellular uptake and improved cytotoxicity towards cancer cells. Interestingly, in combination, these drugs showed better therapeutic effects. | In vitro | GMC = 0.97 µM; QCN = 97µM | Mia-PaCa-2; PANC-1 | [98] |
| 8 | Quercetin | Methotrexate | Osteosarcoma | Quercetin increased methotrexate cytotoxicity in cancer cells. | In vitro | QCN = 142.3 µM; MTX = 13.7 ng/mL | Saos-2 | [99] |
| 9 | Kaempferol | 5-Flurouracil | Colon | Showed synergistic inhibitory effects with respect to cell cytotoxicity. In addition, both drugs induced apoptosis and initiated cell cycle arrest. The blockade of ROS production by kaempferol and the modulation of various proteins validated the success of chemotherapy. | In vitro | KMP = 44 µM; 5FU = 26 µM | LS174 | [100] |
| 10 | Kaempferol | 5-Flurouracil | Colorectal | Kaempferol reversed 5-Fluorouracil resistance by downregulating PKM2-mediated glycolysis. | In vitro | KMP = 70µM; 5FU = 37 µM | LS174 | [101] |
| 11 | Kaempferol | Cisplatin | Head and Neck Squamous | The combination was shown to inhibit the consumption of oxygen and metabolism, and reduced the ATP content in cancer cells. | In vitro | KMP = 120 µM; 40 µM | Cal-27; Hep-2 | [102] |
| 12 | EGCG | Docetaxel | Prostate | EGCG in combination with docetaxel reduced the resistance of docetaxel towards cancer cells and increased the chemotherapeutic effects. | In vitro | EGCG = 40 µM; DTX = 5 nM | LAPC-4-AI; PA-3 | [103] |
| 13 | EGCG | 5-Flurouracil | Colorectal | EGCG was revealed to improve the sensitivity of colorectal cells for 5-Flurouracil by inhibiting and downregulating the GRP78/NF-kB/miR-155-p/MDR1 pathway. | In vitro | 5FU = 5 µM; EGCG= 50 µM | HCT-116; DLD1 | [104] |
| 14 | EGCG | 5-Flurouracil | Oral Squamous cell | It was revealed that this combination significantly reduced both cell viability and cell migration compared to 5-Flurouracil alone. | In vitro | - | PE/CA-PJ15 | [105] |
| 15 | EGCG | Doxorubicin | Pancreatic; Colon | This combination significantly induced apoptosis and blocked cell metastasis and progression by downregulating the ERK pathway. | In vitro | EGCG = 62 µM; DOX = 5 µM | Panc-1; MIA PaCa-2; BxPc-3; HCT15 | [106] |
| 16 | EGCG | Gemcitabine | Pancreatic | EGCG with gemcitabine was revealed to downregulate the growth, invasion, and migration of cancer cells, causing apoptosis by hampering the STAT3 signaling pathway. | In vitro | EGCG = 60µM; GCM = 20 µM | AsPC-1; PANC-1 | [107] |
| 17 | EGCG | Docetaxel | Prostate | The combination of these two reduced the tumor growth by 62 fold. | In vivo | - | CRPC | [108] |
| 18 | Naringenin | Paclitaxel | Prostate | It was revealed that naringenin sensitized the cancer cells for paclitaxel therapy by inducing apoptosis and cell cycle arrest in the G1 phase. | In vitro | NGN = 150 µM; PTX = 5 nM | DU145; PC3 | [109] |
| 19 | Naringenin | Cisplatin | Cervical | It was revealed that naringenin impaired cell growth by initiating apoptosis, proliferation, and cytotoxicity. | In vitro | NGN = 500 µM; CSP = 16 µM | HeLa | [110] |
| 20 | Naringenin | Cisplatin | Lung | In combination with naringenin, the chemotherapeutic effects of cisplatin were significantly increased, with naringenin increasing the expression of caspase-3, and recuing the expression of MMP-2, and MMP-9. | In vitro | CSP = 28 µL/mL; NGN = 200 µM | A549 | [34] |
| 21 | Epicatechin | 5-Flurouracil | Gastric | In combination, epicatechin showed higher inhibitory effects on the production of lactate and exhibited higher cytotoxicity and ROS-mediated apoptosis in SNU620/FU cells. | In vitro | - | SNU620 | [111] |
| 22 | Epicatechin | Docetaxel | Prostate/Breast | Higher chemotherapeutic effects were observed through the upregulation of CDKN1A, BAX, and caspase 9. | In vitro | - | PC3; DU-145; MCF-7 | [112] |
| 23 | Epicatechin | Cisplatin | Lung | Epicatechin showed concentration-dependent cytotoxicity with cisplatin and promoted cell death by a exerting synergistic effect. | In vitro | - | A549/DDP | [113] |
| 24 | Epicatechin | Doxorubicin | Breast | In combination with doxorubicin, epicatechin reduced the chances of cardiotoxicity without altering the chemotherapeutic effects of doxorubicin in MDA-MB231 cells. | In vitro; In vivo | - | MCF-7; T47D; MDA-MB-231 | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Insaf, A.; Hasan, N.; Sugandhi, V.V.; Shrestha, D.; Paudel, K.R.; Jha, S.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P.; et al. Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer. Molecules 2023, 28, 3475. https://doi.org/10.3390/molecules28083475
Imran M, Insaf A, Hasan N, Sugandhi VV, Shrestha D, Paudel KR, Jha SK, Hansbro PM, Dua K, Devkota HP, et al. Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer. Molecules. 2023; 28(8):3475. https://doi.org/10.3390/molecules28083475
Chicago/Turabian StyleImran, Mohammad, Areeba Insaf, Nazeer Hasan, Vrushabh V. Sugandhi, Deumaya Shrestha, Keshav Raj Paudel, Saurav Kumar Jha, Philip M. Hansbro, Kamal Dua, Hari Prasad Devkota, and et al. 2023. "Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer" Molecules 28, no. 8: 3475. https://doi.org/10.3390/molecules28083475
APA StyleImran, M., Insaf, A., Hasan, N., Sugandhi, V. V., Shrestha, D., Paudel, K. R., Jha, S. K., Hansbro, P. M., Dua, K., Devkota, H. P., & Mohammed, Y. (2023). Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer. Molecules, 28(8), 3475. https://doi.org/10.3390/molecules28083475