The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives
Abstract
1. Introduction
2. Insecticides: Importance and Increasing Demand
3. Prominent Insecticides and Their Adverse Effects
4. Major Known Targets for Insecticidal Activity
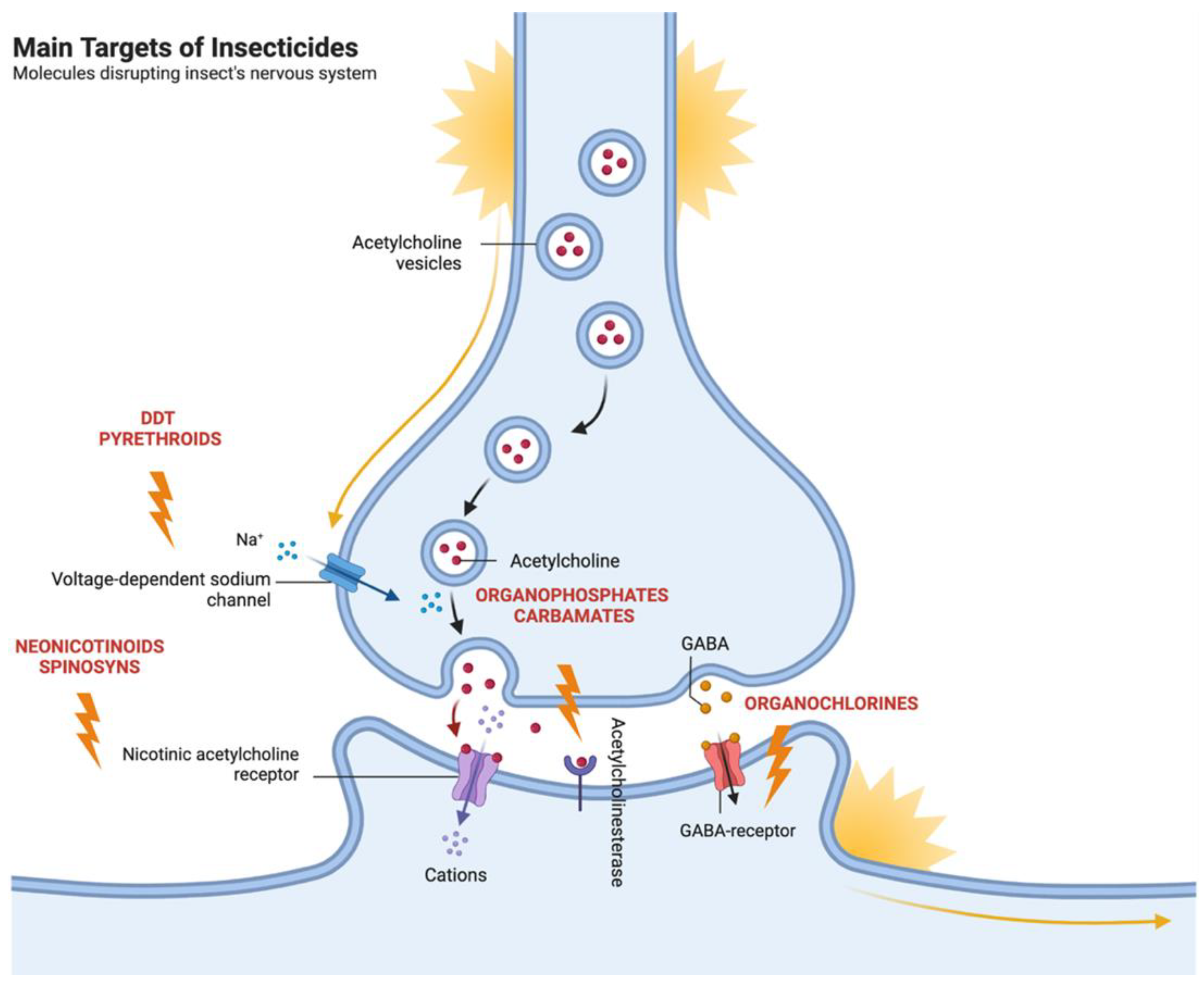
4.1. Molecules Disrupting Insect’s Nervous Systems
4.2. Metabolic Targets
4.3. Growth Regulators and Others
5. The Problem of Resistance
6. Alternatives for Conventional Insecticides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corso, I.C.; Gazzoni, D.L. Sodium Chloride: An Insecticide Enhancer for Controlling Pentatomids on Soybeans. Pesqui. Agropecu. Bras. 1998, 33, 1563–1571. [Google Scholar]
- Wang, C.; Yang, Y.; Wu, N.; Gao, M.; Tan, Y. Combined Toxicity of Pyrethroid Insecticides and Heavy Metals: A Review. Environ. Chem. Lett. 2019, 17, 1693–1706. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A Short History of Insecticides. J. Plant. Prot. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef]
- McLaughlin, G.A. History of Pyrethrum. In Pyrethrum: The Natural Insecticide; Casida, J.E., Ed.; Academic Press: New York, NY, USA, 1973; pp. 3–15. [Google Scholar]
- Unsworth, J. History of Pesticide Use. Available online: http://agrochemicals.iupac.org/index.php?option=%20com_sobi2&sobi2Task=sobi2Details&sobi2Id=31&ItemId=19/ (accessed on 4 October 2022).
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, Pyrethrins, Pyrethroids and Insect Sodium Channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Casagrande, R.A. The Colorado Potato Beetle: 125 Years of Mismanagement. Bull. Entomol. Soc. Am. 1987, 33, 142–150. [Google Scholar] [CrossRef]
- Majori, G. Short History of Malaria and Its Eradication in Italy. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012016. [Google Scholar] [CrossRef]
- Schwardt, H.H. Borax as an Insecticide for Protecting Seed. J. Econ. Entomol. 1930, 23, 401–404. [Google Scholar] [CrossRef]
- Tanaka, K. γ-BHC: Its History and Mystery—Why Is Only γ-BHC Insecticidal? Pestic. Biochem. Physiol. 2015, 120, 91–100. [Google Scholar] [CrossRef]
- Metcalf, R.L. A Century of DDT. J. Agric. Food Chem 1973, 21, 511–519. [Google Scholar] [CrossRef]
- Nobel Prize Outreach The Nobel Prize in Physiology or Medicine 1948. Available online: https://www.nobelprize.org/prizes/medicine/1948/muller/biographical/ (accessed on 5 October 2022).
- Thuy, T.T. Effects of DDT on Environment and Human Health. J. Educ. Hum. Soc. Sci. 2015, 2, 108–114. [Google Scholar]
- Carson, R. Silent Spring; Fawcett Publications: Greenwich, CT, USA, 1964. [Google Scholar]
- Ullah, S.; Faiz, P.; Aamir, M.; Sabir, M.A.; Mahmood, Q. Occurrence and Spatio-Vertical Distribution of DDT in Soils of Abandoned DDT Factory Area, Amangarh, Pakistan. SN Appl. Sci. 2019, 1, 817. [Google Scholar] [CrossRef]
- Matsuo, N. Discovery and Development of Pyrethroid Insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Castillo, G.; Barrios-Arpi, L.; Ramos-Gonzalez, M.; Vidal, P.; Gonzales-Irribarren, A.; Ramos-Cevallos, N.; Rodríguez, J.L. Neurotoxicity Associated with Oxidative Stress and Inflammasome Gene Expression Induced by Allethrin in SH-SY5Y Cells. Toxicol. Ind. Health 2022, 38, 777–788. [Google Scholar] [CrossRef]
- Elliott, M. The Pyrethroids: Early Discovery, Recent Advances and the Future. Pestic. Sci. 1989, 27, 337–351. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Lores, M.; Llompart, M.; García-Jares, C.; Cela, R. The Photochemical Behaviour of Five Household Pyrethroid Insecticides and a Synergist as Studied by Photo-Solid-Phase Microextraction. Anal. Bioanal. Chem. 2007, 388, 1235–1247. [Google Scholar] [CrossRef]
- Kollmeyer, W.D.; Flattum, R.F.; Foster, J.P.; Powell, J.E.; Schroeder, M.E.; Soloway, S.B. Discovery of the Nitromethylene Heterocycle Insecticides. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 71–89. [Google Scholar]
- Casida, J.E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef]
- Ihara, M.; Matsuda, K. Neonicotinoids: Molecular Mechanisms of Action, Insights into Resistance and Impact on Pollinators. Curr. Opin. Insect. Sci. 2018, 30, 86–92. [Google Scholar] [CrossRef]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to Neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- Mora-Gutiérrez, A.; Rubio, C.; Romero-López, Á.A.; Rubio-Osornio, M. Neurotoxic Effects of Insecticides Chlorpyrifos, Carbaryl, Imidacloprid, in Different Animal Species. In Neurotoxicity-New Advances; Sabuncuoglu, S., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Knight, J.L.; Weaver, D.F. A Computational Quantitative Structure-Activity Relationship Study of Carbamate Anticonvulsants Using Quantum Pharmacological Methods. Seizure 1998, 7, 347–354. [Google Scholar] [CrossRef]
- Mustapha, M.U.; Halimoon, N.B.; Lutfi, W.; Johari, W.; Yunus, M.; Shukor, A.; Umar Mustapha, M.; Halimoon, N.; Johar, W.; Yunus, M. An Overview on Biodegradation of Carbamate Pesticides by Soil Bacteria. Pertanika J. Sci. Technol. 2019, 27, 547–563. [Google Scholar]
- Rano, S.; Singh, M. Strategy for the Inspection of Pesticide Residues in Food and Agriculture. In Sustainable Agriculture Reviews 47; Inamuddin, A.M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 309–333. [Google Scholar]
- Macke, J.; Bozhikin, I.; Sarate, J.A.R. Feeding a Growing Population without Deforestation: Agroforestry System Partnerships and Mechanisms. Agrofor. Syst. 2021, 95, 687–706. [Google Scholar] [CrossRef]
- Doggart, N.; Morgan-Brown, T.; Lyimo, E.; Mbilinyi, B.; Meshack, C.K.; Sallu, S.M.; Spracklen, D.V. Agriculture Is the Main Driver of Deforestation in Tanzania. Environ. Res. Lett. 2020, 15, 034028. [Google Scholar] [CrossRef]
- Sparks, T.C. Insecticide Discovery: An Evaluation and Analysis. Pestic. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef]
- Rivero, A.; Vézilier, J.; Weill, M.; Read, A.F.; Gandon, S. Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem? PLoS Pathog. 2010, 6, 5–6. [Google Scholar] [CrossRef]
- Göldel, B.; Lemic, D.; Bažok, R. Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa Decemlineata Say) and Their Environmental Benefits. Agriculture 2020, 10, 611. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as Reproductive Toxicant and Endocrine Disruptor: Investigations in Laboratory Animals. Arh. Hig. Rada. Toksikol. 2018, 69, 103–108. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.T.H. Adverse Effects of Exposure to Low Doses of Chlorpyrifos in Lactating Rats. Toxicol. Ind. Health 2011, 27, 213–224. [Google Scholar] [CrossRef]
- Uchendu, C.; Ambali, S.F.; Ayo, A.J. The Organophosphate, Chlorpyrifos, Oxidative Stress and the Role of Some Antioxidants: A Review. Afr. J. Agric. Res. 2012, 7, 2720–2728. [Google Scholar]
- Branch, R.A.; Jacqz, E. Is Carbaryl as Safe as Its Reputation? Does It Have a Potential for Causing Chronic Neurotoxicity in Humans? Am. J. Med. 1986, 80, 659–664. [Google Scholar] [CrossRef]
- Fattahi, E.; Gholam Ali Jorsaraei, S.; Gardaneh, M. The Effect of Carbaryl on the Pituitary-Gonad Axis in Male Rats. Iran. J. Reprod. Med. 2012, 10, 419–424. [Google Scholar]
- Farag, A.T.; Eweidah, M.H.; El-Okazy, A.M. Reproductive Toxicology of Acephate in Male Mice. Reprod. Toxicol. 2000, 14, 457–462. [Google Scholar] [CrossRef]
- Van Scoy, A.; Pennell, A.; Zhang, X. Environmental Fate and Toxicology of Dimethoate. Rev. Environ. Contam. Toxicol. 2016, 237, 53–70. [Google Scholar]
- Elhamalawy, O.H.; Al-Anany, F.S.; el Makawy, A.I. Thiamethoxam-Induced Hematological, Biochemical, and Genetic Alterations and the Ameliorated Effect of Moringa Oleifera in Male Mice. Toxicol. Rep. 2022, 9, 94–101. [Google Scholar] [CrossRef]
- Khan, A.; Khatoon, A. Effect of Sub Lethal Doses of Thiamethoxam (A Pesticide) on Hemato-Biochemical Values in Cockerels. Pak. Vet. J. 2017, 37, 135–138. [Google Scholar]
- Badr, A.M. Organophosphate Toxicity: Updates of Malathion Potential Toxic Effects in Mammals and Potential Treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef]
- Ji, Q.; Lee, J.; Lin, Y.H.; Jing, G.; Tsai, L.J.; Chen, A.; Hetrick, L.; Jocoy, D.; Liu, J. Atrazine and Malathion Shorten the Maturation Process of Xenopus Laevis Oocytes and Have an Adverse Effect on Early Embryo Development. Toxicol. In Vitro 2016, 32, 63–69. [Google Scholar] [CrossRef]
- Çaliskan, M.; Erkmen, B.; Yerli, S.V. The Effects of Zeta Cypermethrin on the Gills of Common Guppy Lebistes Reticulatus. Environ. Toxicol. Pharmacol. 2003, 14, 117–120. [Google Scholar] [CrossRef]
- Calderón-Segura, M.E.; Gómez-Arroyo, S.; Cortés-Eslava, J.; Martínez-Valenzuela, C.; Mojica-Vázquez, L.H.; Sosa-López, M.; Flores-Ramírez, D.; Romero-Velázquez, Z.E. In Vitro Cytotoxicity and Genotoxicity of Furia®180 SC (Zeta-Cypermethrin) and Bulldock 125®SC (β-Cyfluthrin) Pyrethroid Insecticides in Human Peripheral Blood Lymphocytes. Toxicol. Mech. Methods. 2018, 28, 268–278. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Wang, C. Toxicity of the Pyrethroid Bifenthrin Insecticide. Environ. Chem. Lett. 2018, 16, 1377–1391. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.; Wu, Q.; Wang, Q. The Combined Adverse Effects of Cis-Bifenthrin and Graphene Oxide on Lipid Homeostasis in Xenopus Laevis. J. Hazard. Mater. 2021, 407, 124876. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Du, J.; Zhao, M. Environmentally Relevant Levels of Λ-Cyhalothrin, Fenvalerate, and Permethrin Cause Developmental Toxicity and Disrupt Endocrine System in Zebrafish (Danio Rerio) Embryo. Chemosphere 2017, 185, 1173–1180. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Fetoui, H.; Zribi, N.; Fakhfakh, F.; Keskes, L. Quercetin Attenuates Lambda Cyhalothrin-Induced Reproductive Toxicity in Male Rats. Environ. Toxicol. 2013, 28, 673–680. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine Pesticides, Their Toxic Effects on Living Organisms and Their Fate in the Environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Kaushik, P.; Kaushik, G. An Assessment of Structure and Toxicity Correlation in Organochlorine Pesticides. J. Hazard. Mater. 2007, 143, 102–111. [Google Scholar] [CrossRef]
- Wolfe, Z.M.; Scharf, M.E. Differential Microbial Responses to Antibiotic Treatments by Insecticide-Resistant and Susceptible Cockroach Strains (Blattella Germanica L.). Sci. Rep. 2021, 11, 24196. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Yang, Y.; Li, H.; Wang, X.; Yang, B.; Zhang, J.; Li, C.; Millar, N.S.; Liu, Z. Synergistic and Compensatory Effects of Two Point Mutations Conferring Target-Site Resistance to Fipronil in the Insect GABA Receptor RDL. Sci. Rep. 2016, 6, 32335. [Google Scholar] [CrossRef]
- Gaio, A.D.O.; Gusmão, D.S.; Santos, A.V.; Berbert-Molina, M.A.; Pimenta, P.F.P.; Lemos, F.J.A. Contribution of Midgut Bacteria to Blood Digestion and Egg Production in Aedes Aegypti (Diptera: Culicidae) (L.). Parasit. Vectors. 2011, 4, 105. [Google Scholar] [CrossRef]
- Kirst, H.A. The Spinosyn Family of Insecticides: Realizing the Potential of Natural Products Research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef]
- Salgado, V.L.; Sparks, T.C. The Spinosyns: Chemistry, Biochemistry, Mode of Action, and Resistance. In Comprehensive Molecular Insect Science; Gilbert, L.I., Latrou, K., Gill, S.S., Eds.; Elsevier: Oxford, UK, 2005; pp. 137–173. [Google Scholar]
- Ujihara, K. The History of Extensive Structural Modifications of Pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Clark, J.M.; Symington, S.B. Advances in the Mode of Action of Pyrethroids. Top. Curr. Chem. 2012, 314, 49–72. [Google Scholar]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid Insecticides Mode of Action on Insect Nicotinic Acetylcholine Receptors Using Binding Studies. Pestic. Biochem. Physiol. 2018, 151, 59–66. [Google Scholar] [CrossRef]
- Hollingworth, R.M.; Leister, J.; Ghali, G. Mode of Action of Formamidine Pesticides: An Evaluation of Monoamine Oxidase as the Target. Chem. Biol. Interact. 1979, 24, 35–49. [Google Scholar] [CrossRef]
- Rathnayake, L.K.; Northrup, S.H. Structure and Mode of Action of Organophosphate Pesticides: A Computational Study. Comput. Theor. Chem. 2016, 1088, 9–23. [Google Scholar] [CrossRef]
- Kuhr, R.J.; Dorough, H.W. Carbamate Insecticides: Chemistry, Biochemistry, and Toxicology; CRC Press Inc.: Cleveland, OH, USA, 1976. [Google Scholar]
- Auger, P.; Guichou, S.; Kreiter, S. Variations in Acaricidal Effect of Wettable Sulfur on Tetranychus Urticae (Acari: Tetranychidae): Effect of Temperature, Humidity and Life Stage. Pest. Manag. Sci. 2003, 59, 559–565. [Google Scholar] [CrossRef]
- Ware, G.W.; Whitacre, D.M. The Pesticide Book: An Introduction to Insecticides; Meister Publications: Willoughby, OH, USA, 2004. [Google Scholar]
- Pieper, G.R.; Casida, J.E. House Fly Adenosine Triphosphatases and Their Inhibition by Insecticidal Organotin Compounds. J. Econ. Entomol. 1965, 58, 392–400. [Google Scholar] [CrossRef]
- Lummen, P. Complex I Inhibitors as Insecticides and Acaricides. Biochim. Biophys. Acta 1998, 1364, 287–296. [Google Scholar] [CrossRef]
- Das, S.K. Mode of Action of Pesticides and the Novel Trends—A critical Review. Int. Res. J. Agric. Soil Sci. 2013, 3, 393–401. [Google Scholar]
- Ahmed, N.E.; Asif, M. Various Chemical and Biological Activities of Pyridazinone Derivatives. Eur. J. Exp. Biol. 2017, 5, 1–19. [Google Scholar]
- Chou, A.P.; Li, S.; Fitzmaurice, A.G.; Bronstein, J.M. Mechanisms of Rotenone-Induced Proteasome Inhibition. Neurotoxicology 2010, 31, 367–372. [Google Scholar] [CrossRef]
- Jennifer Mordue, A.; Nisbet, A.J. Azadirachtin from the Neem Tree Azadirachta Indica: Its Action Against Insects. An. Soc. Entomol. Bras. 2000, 29, 615–632. [Google Scholar]
- Price, N.R. The Mode of Action of Fumigants. J. Stored Prod. Res. 1985, 21, 157–164. [Google Scholar] [CrossRef]
- Sarwar, M. Inorganic Insecticides Used in Landscape Settings and Insect Pests. Chem. Res. J. 2016, 1, 50–57. [Google Scholar]
- Sullivan, J.J.; Goh, K.S. Environmental Fate and Properties of Pyriproxyfen. J. Pestic. Sci. 2008, 33, 339–350. [Google Scholar] [CrossRef]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational Insecticides in Pest Management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Khater, H.F. Ecosmart Biorational Insecticides: Alternative Insect Control Strategies. In Insecticides—Advances in Integrated Pest Management; Perveen, F., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 17–60. [Google Scholar]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef]
- Joo, S.H.; Keum, Y.S. Oxidative Metabolism of Quinazoline Insecticide Fenazaquin by Aspergillus Niger. Appl. Biol. Chem. 2018, 61, 681–687. [Google Scholar] [CrossRef]
- Guo, X.C.; Zhang, Y.H.; Gao, W.B.; Pan, L.; Zhu, H.J.; Cao, F. Absolute Configurations and Chitinase Inhibitions of Quinazoline-Containing Diketopiperazines from the Marine-Derived Fungus Penicillium polonicum. Mar. Drugs 2020, 18, 479. [Google Scholar] [CrossRef]
- Tozzi, A. A. A Brief History of the Development of Piperonyl Butoxide as an Insecticide Synergist. In Piperonyl Butoxide—The Insecticide Synergist; Jones, D.G., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 1–5. [Google Scholar]
- Tak, J.H.; Isman, M.B. Enhanced Cuticular Penetration as the Mechanism for Synergy of Insecticidal Constituents of Rosemary Essential Oil in Trichoplusia Ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- B-Bernard, C.; Philogfene, B.J.R. Insecticide Synergists: Role, Importance, and Perspectives. J. Toxicol. Environ. Health 1993, 38, 199–233. [Google Scholar] [CrossRef]
- Park, N.J.; Oh, S.C.; Choi, Y.H.; Choi, K.R.; Cho, K.Y. Inheritance and Cross Resistance of Phenthoate-Selected Diamondback Moth, Plutella xylostella (Lepidoptera: Yponomeutidae). J. Asia Pac. Entomol. 2004, 7, 233–237. [Google Scholar] [CrossRef]
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Al-Mutairi, K.A.; Salman, M. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In Global Decline of Insects; El-Shafie, H.A.F., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Marasigan, K.; Toews, M.; Kemerait, R.; Abney, M.R.; Culbreath, A.; Srinivasan, R. Evaluation of Alternatives to an Organophosphate Insecticide with Selected Cultural Practices: Effects on Thrips, Frankliniella Fusca, and Incidence of Spotted Wilt in Peanut Farmscapes. J. Econ. Entomol. 2018, 111, 1030–1041. [Google Scholar] [CrossRef]
- Ben-Issa, R.; Gomez, L.; Gautier, H. Companion Plants for Aphid Pest Management. Insects 2017, 8, 112. [Google Scholar] [CrossRef]
- Endersby, N.M.; Morgan, W.C. Alternatives to Synthetic Chemical Insecticides for Use in Crucifer Crops. Biol. Agric. Hortic. 1991, 8, 33–52. [Google Scholar] [CrossRef]
- Smith, C.M. Conventional Breeding of Insect-Resistant Crop Plants: Still the Best Way to Feed the World Population. Curr. Opin. Insect. Sci. 2021, 45, 7–13. [Google Scholar] [CrossRef]
- Mustafa, M.; Rehman Bilal, A.; Hamza, M.A.; Hamid, A.; Ijaz, M.; Ghilman, S.; Sherazi, M.; Rashid, H.; Bilal, A.R. Transgenic Crops and Sterile Insect Releases Are Eco-Friendly Approaches for the Management of Major Insect Pests of Crops. Life Sci. J. 2022, 19, 45–51. [Google Scholar]
- Robinson, A.S. Mutations and Their Use in Insect Control. Mutat. Res. 2002, 511, 113–132. [Google Scholar] [CrossRef]
- Kunimi, Y. Current Status and Prospects on Microbial Control in Japan. J. Invertebr. Pathol. 2007, 95, 181–186. [Google Scholar] [CrossRef]
- Guo, J.; Wu, S.; Zhang, F.; Huang, C.; He, K.; Babendreier, D.; Wang, Z. Prospects for Microbial Control of the Fall Armyworm Spodoptera Frugiperda: A Review. BioControl 2020, 65, 647–662. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Gene Amplification and Insecticide Resistance. Pest. Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Crossthwaite, A.J.; Rendine, S.; Stenta, M.; Slater, R. Target-Site Resistance to Neonicotinoids. J. Chem. Biol. 2014, 7, 125–128. [Google Scholar] [CrossRef]
- Wilson, T.G.; Ashok, M. Insecticide Resistance Resulting from an Absence of Target-Site Gene Product. Proc. Natl. Acad. Sci. USA 1998, 95, 14040–14044. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H. The molecular genetics of insecticide resistance. Genetics 2013, 194, 807–815. [Google Scholar] [CrossRef]
- Jensen, S.E. Insecticide Resistance in the Western Flower Thrips, Frankliniella Occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-Based Metabolic Insecticide Resistance in Anopheles and Aedes Mosquito Vectors: Muddying the Waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef]
- Carrasco, D.; Lefèvre, T.; Moiroux, N.; Pennetier, C.; Chandre, F.; Cohuet, A. Behavioural Adaptations of Mosquito Vectors to Insecticide Control. Curr. Opin. Insect. Sci. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Takken, W. Do Insecticide-Treated Bednets Have an Effect on Malaria Vectors? Trop. Med. Int. Health 2002, 7, 1022–1030. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Furlong, M.J. Behavior as a Mechanism of Insecticide Resistance: Evaluation of the Evidence. Curr. Opin. Insect. Sci. 2017, 21, 19–25. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Pereira, E.J.G.; Cordeiro, E.M.G.; Braga, L.S.; Guedes, R.N.C. Insecticide Resistance, Mixture Potentiation and Fitness in Populations of the Maize Weevil (Sitophilus zeamais). Crop Protect. 2011, 30, 1655–1666. [Google Scholar] [CrossRef]
- Foster, S.P.; Tomiczek, M.; Thompson, R.; Denholm, I.; Poppy, G.; Kraaijeveld, A.R.; Powell, W. Behavioural Side-Effects of Insecticide Resistance in Aphids Increase Their Vulnerability to Parasitoid Attack. Anim. Behav. 2007, 74, 621–632. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect Cuticle: A Critical Determinant of Insecticide Resistance. Curr. Opin. Insect. Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Gott, R.C.; Kunkel, G.R.; Zobel, E.S.; Lovett, B.R.; Hawthorne, D.J. Implicating ABC Transporters in Insecticide Resistance: Research Strategies and a Decision Framework. J. Econ. Entomol. 2017, 110, 667–677. [Google Scholar] [CrossRef]
- Shivanandappa, T.; Rajashekar, Y. Mode of Action of Plant-Derived Natural Insecticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: New Delhi, India, 2014; pp. 323–345. [Google Scholar]

| Insecticide | Chemical Formula | Chemical Structure | Adverse Effects |
|---|---|---|---|
| (1) Imidacloprid (Neonicotinoid) | C9H10ClN5O2 | 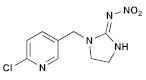 | The residues of this substance can make their way into the food chain and affect both the reproductive capacity of lab rats and that of their offspring. It is a chemical that disrupts endocrine and steroidogenesis [33]. |
| (2) Chlorpyrifos (Organophosphate) | C9H11Cl3NO3PS | 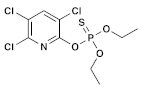 | The laboratory rats that were exposed showed a decrease in body weight and an increase in the relative weights of their liver and kidney. The damage to their liver was significant, and there was a notable increase in total protein and uric acid levels. Additionally, there was an increase in oxidative stress observed in the exposed rats [34,35]. |
| (3) Carbaryl (Carbamate) | C12H11NO2 | 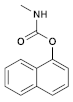 | The toxicity observed is a result of cholinesterase inhibition. When pigs were exposed to this substance for an extended period, it caused a progressive neuromyopathy that resulted in structural damage, which cannot be reversed acutely with atropine. Similarly, in lab rats, there was a significant reduction in their overall weight. Moreover, there was a notable decrease in the number of germ cells, spermatocytes, spermatids, and Leydig cells. Additionally, the testosterone levels significantly declined, while the levels of LH and FSH increased significantly [36,37]. |
| (4) Acephate (Organophosphate) | C4H10NO3PS | 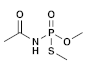 | The highest doses administered to lab rats inhibited the activity of acetylcholinesterase in the brain and skeletal muscles. In the same group, there was a decrease in the number of implantations and live fetuses, along with an increase in the number of early resorptions observed. Furthermore, there was a decrease in sperm motility and count in the exposed rats. Dose-dependent histologic changes, including the degeneration of muscle fibers, were also observed [38]. |
| (5) Dimethoate (Organophosphate) | C5H12NO3PS2 |  | This substance, like other organophosphates, is known to inhibit acetylcholinesterase (AChE) activity, leading to severe nerve damage. In plants, its effects are reflected in reduced photosynthesis and growth, while in birds, the activity of brain enzymes is inhibited, resulting in sublethal effects. Aquatic organisms are expected to be highly affected by direct exposure, leading to changes in their swimming behavior [39]. |
| (6) Thiamethoxam (Neonicotinoid) | C8H10ClN5O3S | 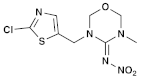 (E isomer) 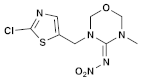 (Z isomer) | In cockerels, exposure to thiamethoxam (TMX) at sub-lethal levels resulted in a dose-dependent reduction in key hematological parameters, including total erythrocyte count, hemoglobin, packed cell volume, and total leukocyte count. The biochemistry of the birds was also impacted, with significant alterations in total proteins, albumin, and globulin. The study indicated that TMX caused substantial changes in the hematological profile and liver and kidney function of the birds. In addition, TMX increased oxidative damage to lipids and DNA in these organs, while reducing the antioxidant activities in liver and kidney cells, leading to oxidative stress [40,41]. |
| (7) Malathion (Organophosphate) | C10H19O6PS2 |  | Malathion (MAL) was found to have adverse effects on frog oocyte maturation, resulting in reduced levels of Emi2, a critical factor for oocyte maturation. In addition, embryos fertilized under the influence of MAL showed a higher rate of abnormal division, leading to embryo death during early embryogenesis. The toxicity mechanisms of MAL include inhibition of acetylcholinesterase, oxidative stress, DNA damage, and apoptotic cell damage. Its toxic effects on the central nervous system are well documented, but it also affects the liver, kidney, testis, ovaries, lung, pancreas, and blood. MAL is considered a genotoxic and carcinogenic chemical compound and evidence shows adverse effects associated with prenatal, and postnatal exposure in both animals and humans. These findings are supported by various studies [42,43]. |
| (8) Zeta-cypermethrin (Pyrethroid) | C22H19Cl2NO3 | 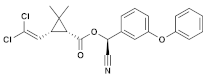 | In common guppies (Leporinus reticulatus), exposure to various doses of zeta-cypermethrin resulted in the lifting of the epithelial layer from gill lamellae and necrosis. Other observed histopathological effects included exudation, hyperplasia, and shortening of secondary lamellae. Additionally, in vitro experiments showed that zeta-cypermethrin caused DNA damage in human peripheral lymphocytes, indicating its genotoxic properties [44,45]. |
| (9) Bifenthrin (Pyrethroid) | C23H22ClF3O2 | 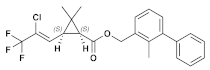 | The hepatic function of tadpoles is negatively affected by cis-bifenthrin. Aquatic species are highly susceptible to the acute lethal toxicity of bifenthrin. Bifenthrin also has sublethal toxic effects on non-target organisms, such as developmental toxicity, neurobehavioral toxicity, oxidative damage, immune toxicity, and endocrine-disrupting effects [46,47]. |
| (10) λ-cyhalothrin (Pyrethroid) | C23H19ClF3NO3 | 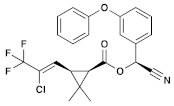 | Previously conducted research has indicated that synthetic pyrethroids, such as λ-cyhalothrin (LCT), have high levels of aquatic toxicity. Exposure of zebrafish to synthetic pyrethroids, including LCT, resulted in a dose-dependent increase in mortality, higher malformation rates, and lower hatching rates. This exposure to LCT led to a significant decrease in thyroid hormone triiodothyronine (T3) levels, indicating potential developmental toxicity by disrupting endocrine signaling at concentrations present in the environment. In other studies, administration of LCT to laboratory rats led to decreased functional sperm parameters, enzymatic and non-enzymatic antioxidant levels, and the presence of irregular seminiferous tubules containing only Sertoli cells [48,49]. |
| Common Name | Class of Insecticide | Targeted System | Mode of Action |
|---|---|---|---|
| Abamectin | Avermectin | Nervous system | Chloride channel activator |
| Azadirachtin | Botanical from neem oil | Growth and development/metabolic processes | Prothoracicotropic hormone (PTTH) inhibitor; phagostimulant disruptor |
| Bacillus thuringiensis | Microbial | Metabolic processes | Insect midgut membrane disruptor |
| Cinnamaldehyde | Botanical | Energy production | Exact mode of action not well understood; possibly interference with glucose uptake or utilization |
| Decalesides I and II | Botanical (natural trisaccharides) | Nervous system | Inhibition of sodium pump |
| Emamectin benzoate | Avermectin | GABA-gated chloride channels | Chloride channel activators |
| Pyrethrins I and II | Botanical (pyrethrum) | Nervous system | Sodium channel modulator |
| Rotenone | Botanical | Mitochondrial electron transport system | Electron transport inhibitor—site 1 |
| Ryanodine | Botanical | Calcium channels (ryanodine receptor) | Activation |
| Spinosad | Spinosyn | Nervous system | Nicotinic acetylcholine receptor agonist (mimic) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. https://doi.org/10.3390/molecules28083641
Araújo MF, Castanheira EMS, Sousa SF. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules. 2023; 28(8):3641. https://doi.org/10.3390/molecules28083641
Chicago/Turabian StyleAraújo, Maria F., Elisabete M. S. Castanheira, and Sérgio F. Sousa. 2023. "The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives" Molecules 28, no. 8: 3641. https://doi.org/10.3390/molecules28083641
APA StyleAraújo, M. F., Castanheira, E. M. S., & Sousa, S. F. (2023). The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules, 28(8), 3641. https://doi.org/10.3390/molecules28083641










