A Lifetime of a Dispenser-Release Rates of Olive Fruit Fly-Associated Yeast Volatile Compounds and Their Influence on Olive Fruit Fly (Bactrocera oleae Rossi) Attraction
Abstract
1. Introduction
2. Results and Discussion
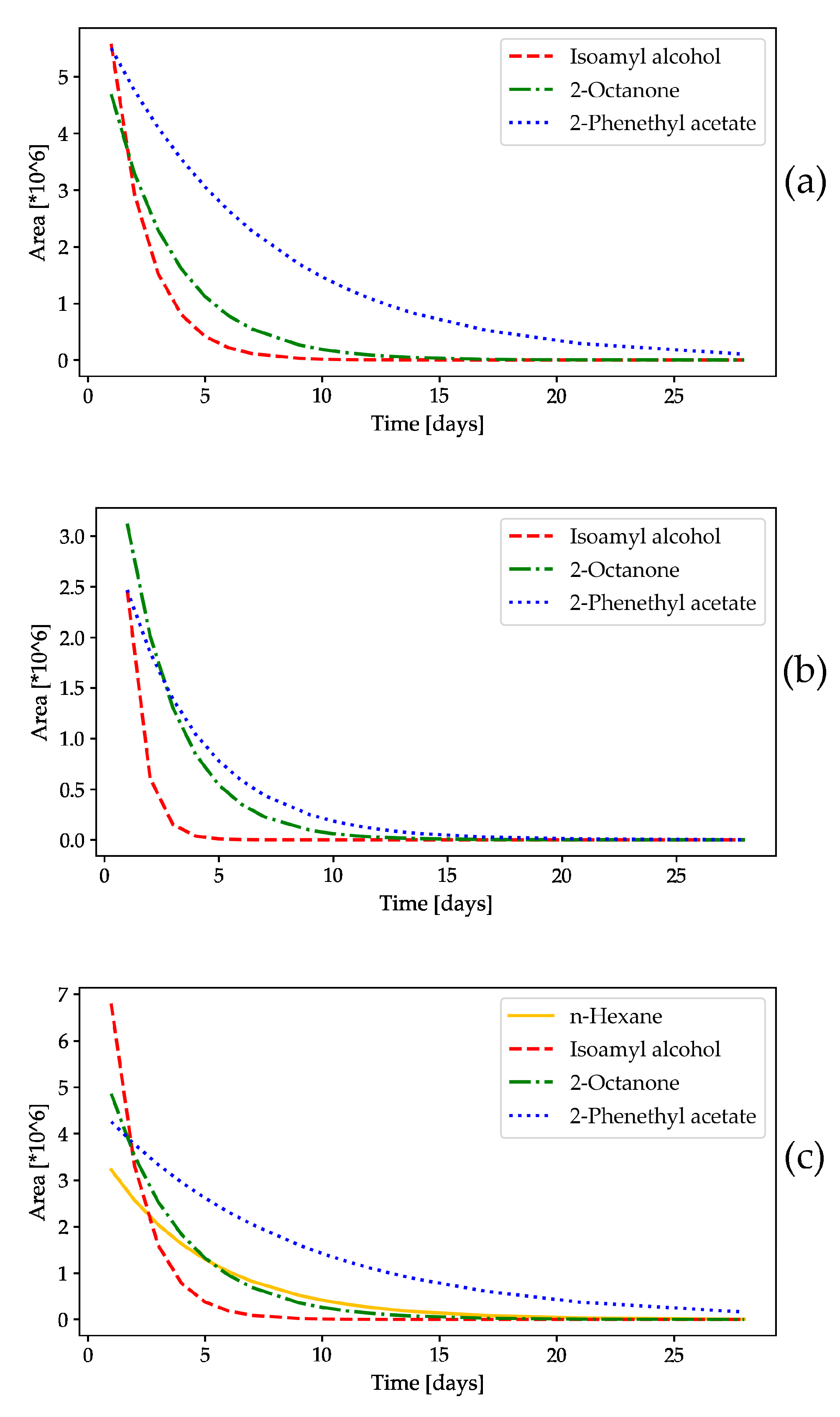
2.1. Release Rates of the Tested Volatile Compounds
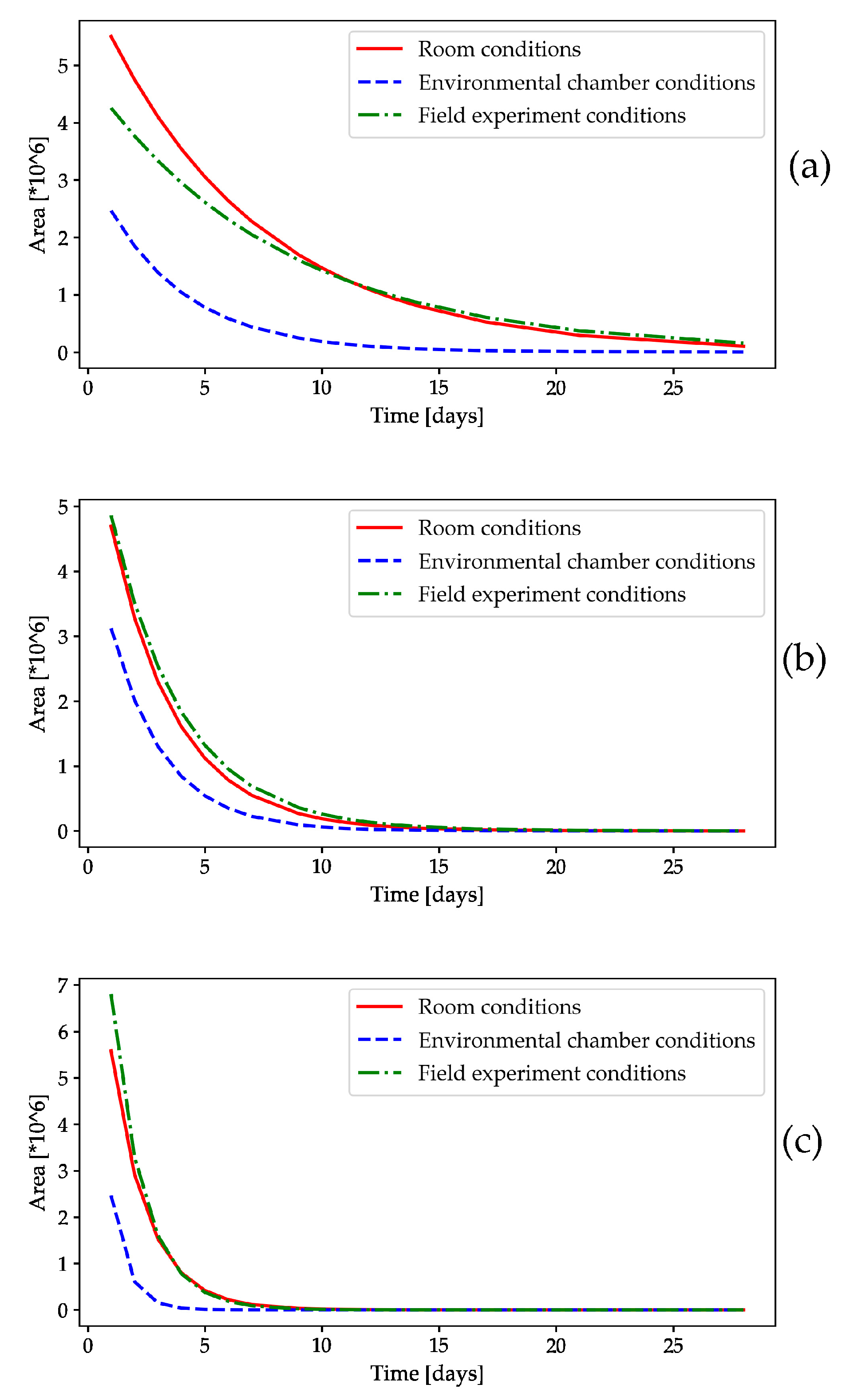
2.1.1. Monitoring the Mass Change in the Volatile Compounds in the Slow-Release Dispensers
2.1.2. HS-GC/FID Analysis of Volatile Compounds
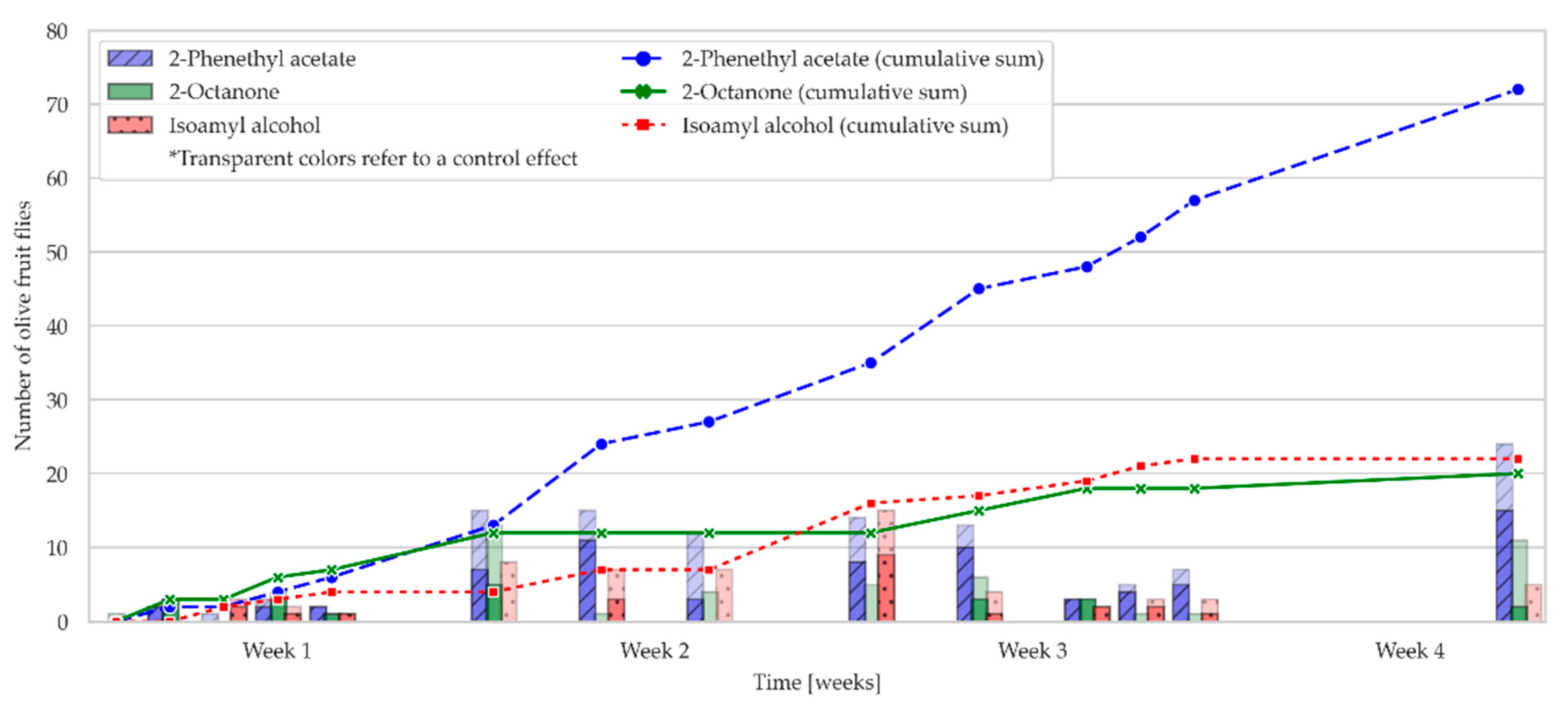
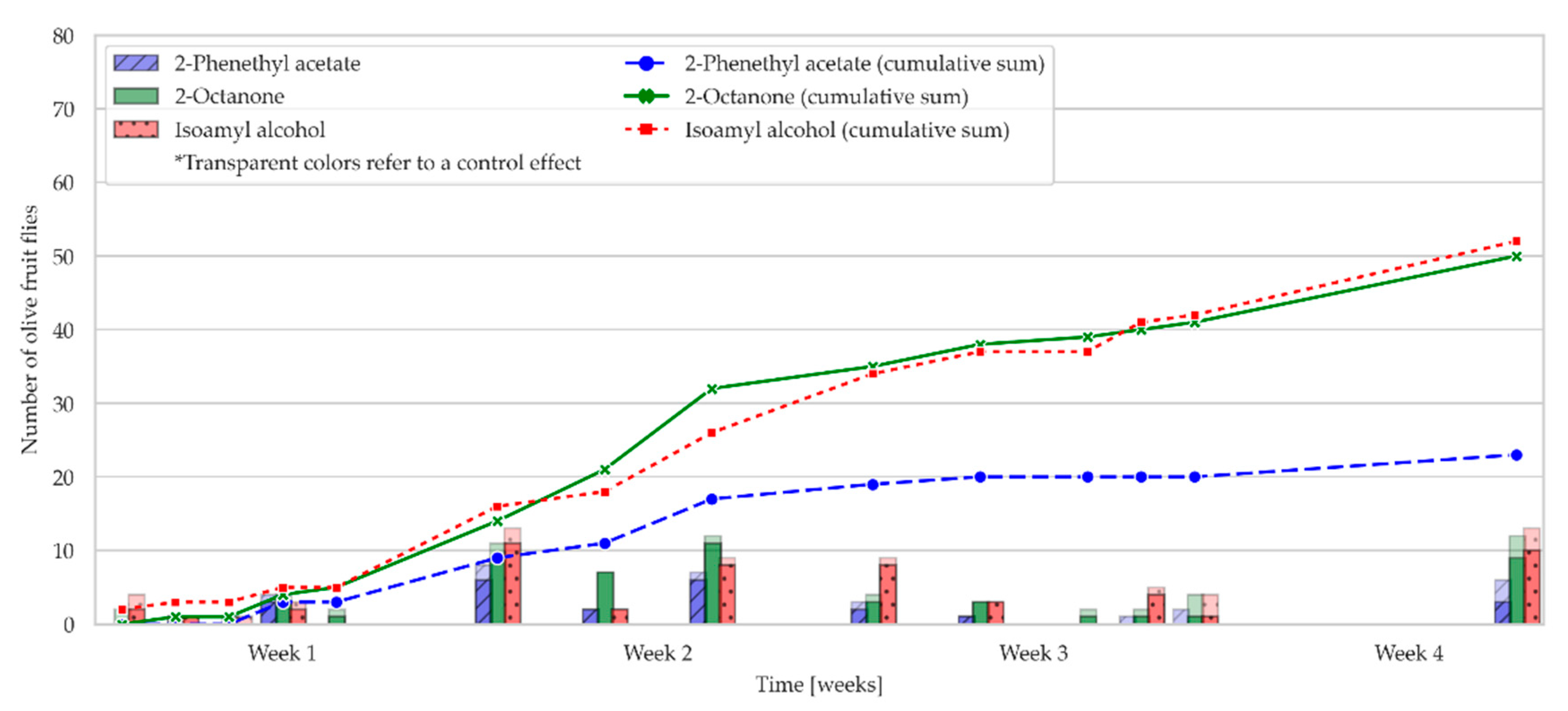
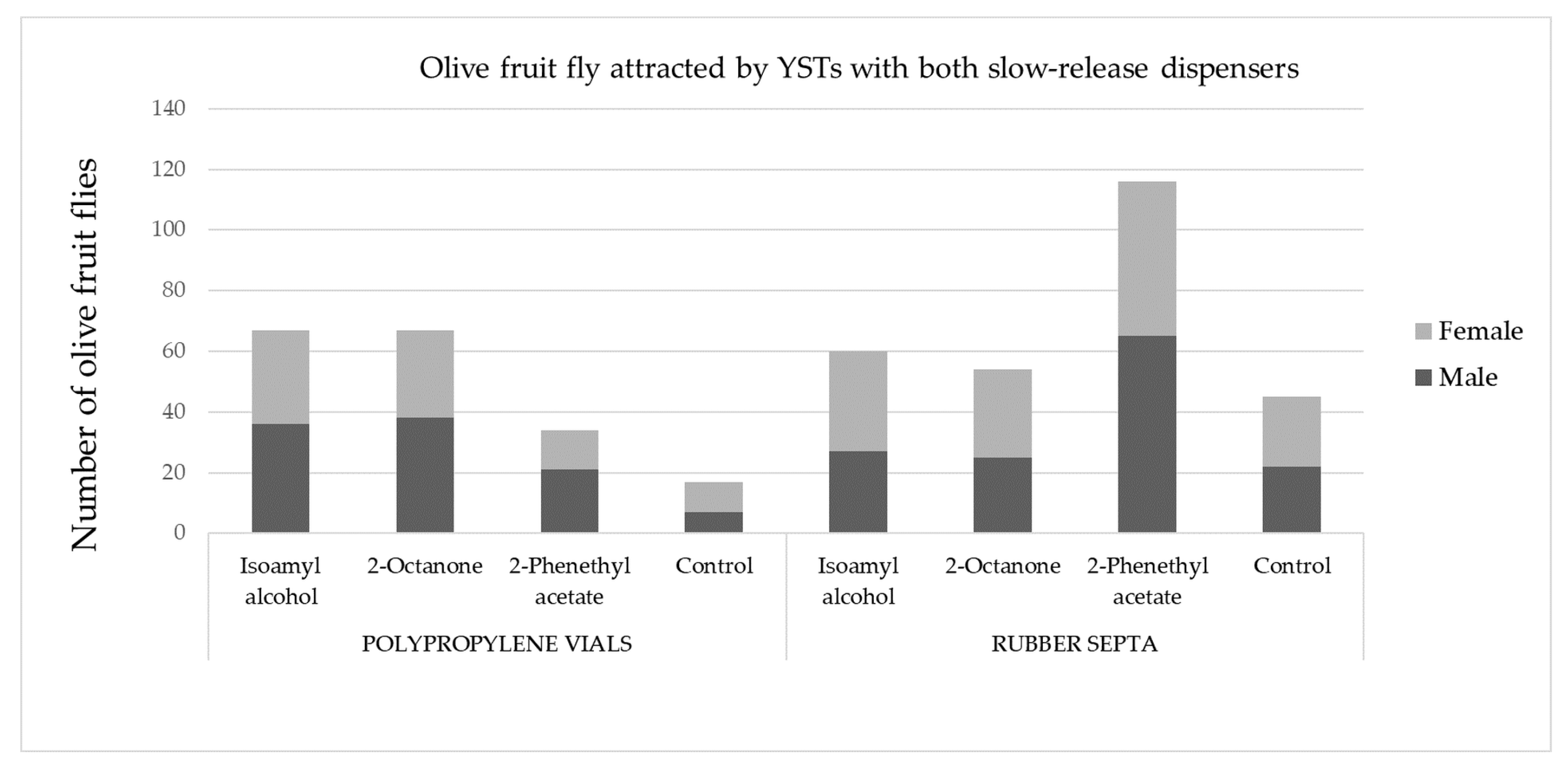
2.2. Field Bioassay: Olive Fruit Fly Attraction to OFF-Associated Yeast Volatile Compounds in an Olive Grove
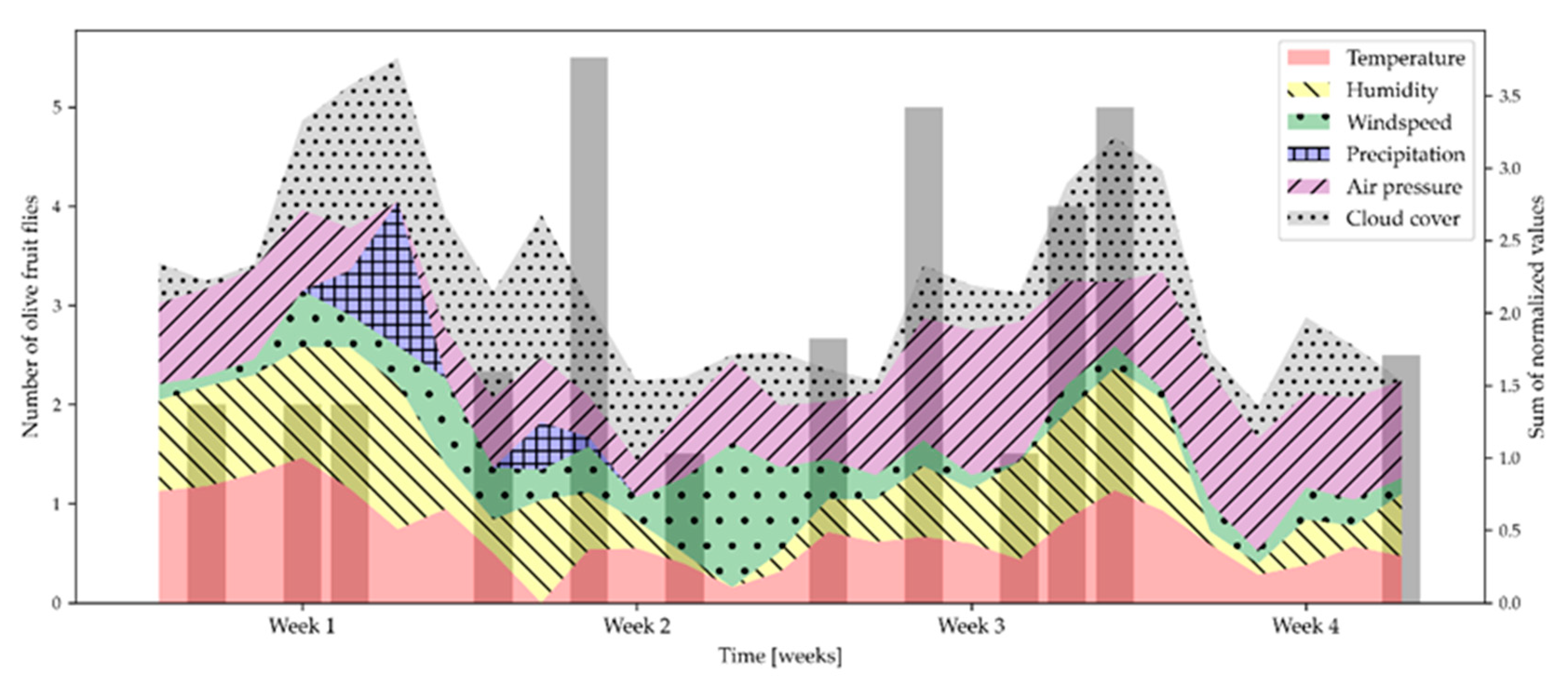
2.3. The Influence of Climatic Parameters on the Attraction of the Olive Fruit Fly
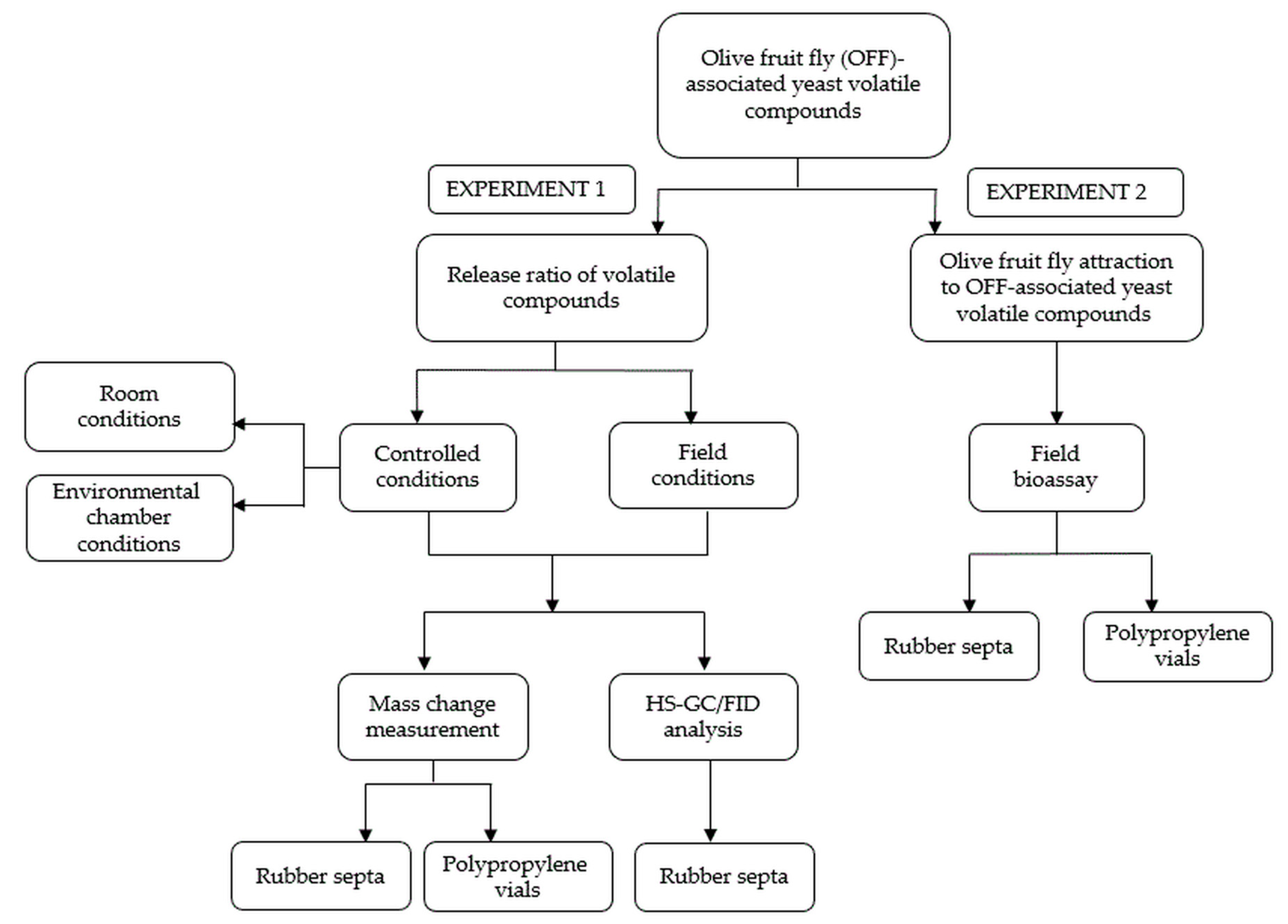
3. Materials and Methods
3.1. Synthetic Volatile Compounds
3.2. Slow-Release Dispensers
3.3. Experiment Design
Monitoring of the Release Rate of the Tested Volatile Compounds: Experiment 1
3.4. Field Bioassay: Experiment 2
Olive Fruit Flies Trapping
3.5. HS-GC/FID Analysis
3.6. Data Analysis
3.6.1. Pearson Correlation Coefficient
3.6.2. Mann–Whitney U Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boller, E.F. Biotehnical Methods for the Management of Fruit Fly Populations. In Fruit Flies of Economic Importance; Cavalloro, R., Ed.; A.A.Balkema: Rotterdam, The Netherlands, 1983; pp. 342–352. [Google Scholar]
- Bakthavatsalam, N. Semiochemicals. In Ecofriendly Pest Management for Food Security; Omkar, I., Ed.; Academic Press: Lucknow, India, 2016; pp. 563–611. [Google Scholar]
- Ezzat, S.M.; Jeevanandam, J.; Egbuna, C.; Merghany, R.M.; Akram, M.; Daniyal, M.; Nisar, J.; Sharif, A. Semiochemicals: A Green Approach to Pest and Disease Control; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128193044. [Google Scholar]
- El-Ghany, N.M.A. Semiochemicals for Controlling Insect Pests. J. Plant Prot. Res. 2019, 59, 1–11. [Google Scholar] [CrossRef]
- El-Shafie, H.A.F.; Faleiro, J.R. Semiochemicals and Their Potential Use in Pest Management. In Biological Control of Pest and Vector Insects; Vonnie, D.C.S., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–22. [Google Scholar]
- Heuskin, S.; Verheggen, F.J.; Haubruge, E.; Wathelet, J.P.; Lognay, G. The Use of Semiochemical Slow-Release Devices in Integrated Pest Management Strategies. Biotechnol. Agron. Soc. Environ. 2011, 15, 459–470. [Google Scholar]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A Review of Bactrocera Oleae (Rossi) Impact in Olive Products: From the Tree to the Table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Kogan, M. Plant Volatiles as Insect Attractants. CRC Crit. Rev. Plant Sci. 1987, 5, 251–301. [Google Scholar] [CrossRef]
- Quilici, S.; Atiama-Nurbel, T.; Brevault, T. Plant Odors as Fruit Fly Attractants. In Trapping and the Detection, Control and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 119–144. [Google Scholar]
- Scarpati, M.L.; Scalzo, R.L.; Vita, G. Olea Europaea Volatiles Attractive and Repellent to the Olive Fruit Fly (Dacus Oleae, Gmelin). J. Chem. Ecol. 1993, 19, 881–891. [Google Scholar] [CrossRef]
- Kokkari, A.I.; Pliakou, O.D.; Floros, G.D.; Kouloussis, N.A.; Koveos, D.S. Effect of Fruit Volatiles and Light Intensity on the Reproduction of Bactrocera (Dacus) Oleae. J. Appl. Entomol. 2017, 141, 841–847. [Google Scholar] [CrossRef]
- Kokkari, A.I.; Milonas, P.G.; Anastasaki, E.; Floros, G.D.; Kouloussis, N.A.; Koveos, D.S. Determination of Volatile Substances in Olives and Their Effect on Reproduction of the Olive Fruit Fly. J. Appl. Entomol. 2021, 145, 841–855. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Identification of Leaf Volatiles from Olive (Olea Europaea) and Their Possible Role in the Ovipositional Preferences of Olive Fly, Bactrocera Oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 2016, 121, 11–19. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Algeri, G.M.; Palmeri, V. Olive Fruit Volatiles Route Intraspecific Interactions and Chemotaxis in Bactrocera Oleae (Rossi) (Diptera: Tephritidae) Females. Sci. Rep. 2020, 10, 2020. [Google Scholar] [CrossRef]
- Vitanović, E.; Aldrich, J.R.; Boundy-Mills, K.; Čagalj, M.; Ebeler, S.E.; Burrack, H.; Zalom, F.G. Olive Fruit Fly, Bactrocera Oleae (Diptera: Tephritidae), Attraction to Volatile Compounds Produced by Host and Insect-Associated Yeast Strains. J. Econ. Entomol. 2020, 113, 752–759. [Google Scholar] [CrossRef]
- Vitanović, E.; Lopez, J.M.; Aldrich, J.R.; Špika, M.J.; Boundy-Mills, K.; Zalom, F.G. Yeasts Associated with the Olive Fruit Fly Bactrocera Oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants. Agronomy 2020, 10, 1501. [Google Scholar] [CrossRef]
- Bego, A.; Burul, F.; Popović, M.; Špika, M.J.; Bratinčević, M.V.; Pošćić, F.; Vitanović, E. Bactrocera Oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants. Agronomy 2022, 12, 72. [Google Scholar] [CrossRef]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical Signaling and Insect Attraction Is a Conserved Trait in Yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Mweresa, C.K.; Mukabana, W.R.; van Loon, J.J.A.; Dicke, M.; Takken, W. Use of Semiochemicals for Surveillance and Control of Hematophagous Insects. Chemoecology 2020, 30, 277–286. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 June 2022).
- Kuenen, L.P.S.; Siegel, J.P. Measure Your Septa Release Ratios: Pheromone Release Ratio Variability Affected by Rubber Septa and Solvent. J. Chem. Ecol. 2015, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.I.; McDonough, L.M. Insect Sex Pheromones: Evaporation Rates of Alcohols and Acetates from Natural Rubber Septa. J. Chem. Ecol. 1981, 7, 627–633. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Sansom, C.E.; Larsen, L.; Worner, S.P.; Rostás, M.; Chapman, R.B.; Butler, R.C.; de Kogel, W.J.; Davidson, M.M.; Perry, N.B.; et al. Volatile Compounds as Insect Lures: Factors Affecting Release from Passive Dispenser Systems. New Zeal. J. Crop Hortic. Sci. 2019, 47, 208–223. [Google Scholar] [CrossRef]
- Douglas, M.; Bates, D.G.W. Nonlinear Regression Analysis and Its Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1988. [Google Scholar]
- Davis, T.S.; Landolt, P.J. A Survey of Insect Assemblages Responding to Volatiles from a Ubiquitous Fungus in an Agricultural Landscape. J. Chem. Ecol. 2013, 39, 860–868. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid Fruit Fly Semiochemicals: Current Knowledge and Future Perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef]
- Yokoyama, V.Y.; Miller, G.T.; Stewart-Leslie, J.; Rice, R.E.; Phillips, P.A. Olive Fruit Fly (Diptera: Tephritidae) Populations in Relation to Region, Trap Type, Season, and Availability of Fruit. J. Econ. Entomol. 2006, 99, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Zineb, B.; Kada, R.; Fatiha, R. First Study of Ovipositional Preference and Developmental Performance of Bactrocera Oleae (Diptera: Tephritidae) in an Arid Zone (Laghouat: Algeria). J. Entomol. Res. 2022, 46, 816–823. [Google Scholar] [CrossRef]
- Broufas, G.D.; Pappas, M.L.; Koveos, D.S. Effect of Relative Humidity on Longevity, Ovarian Maturation, and Egg Production in the Olive Fruit Fly (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2009, 102, 70–75. [Google Scholar] [CrossRef]
- Fletcher, B.S.; Pappas, S.; Kapatos, E. Changes in the Ovaries of Olive Flies (Dacus Oleae (Gmelin)) during the Summer, and Their Relationship to Temperature, Humidity and Fruit Availability. Ecol. Entomol. 1978, 3, 99–107. [Google Scholar] [CrossRef]
- Bueno, A.M.; Jones, O. Alternative Methods for Controlling the Olive Fly, Bactrocera Oleae, Involving Semiochemicals. In Proceedings of the IOBC wprs Bulletin Pheromones and Other Semio-Chemicals in Integrated Production, Samos, Greece, 25–29 September 2002; Witzgall, P., Mazomenos, B., Konstantopoulou, M., Eds.; Volume 25, pp. 147–156. [Google Scholar]
- Mazomenos, B.E.; Pantazi-Mazomenou, A.; Stefanou, D. Attract and Kill of the Olive Fruit Fly Bactrocera Oleae in Greece as a Part of an Integrated Control System. IOBC WPRS Bull. 2002, 25, 137–146. [Google Scholar]
- Bradley, S.J.; Suckling, D.M.; McNaughton, K.G.; Wearing, C.H.; Karg, G. A Temperature-Dependent Model for Predicting Release Rates of Pheromone from a Polyethylene Tubing Dispenser. J. Chem. Ecol. 1995, 21, 745–760. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Hicks, M.N. Gas Chromatography Column as an Ambient-Temperature Volatile Trap. Entomol. Exp. Appl. 2015, 154, 35–44. [Google Scholar] [CrossRef]
- DHMZ—Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/index_en.php (accessed on 6 June 2022).
- Python Language Reference, Version 3. 8. 12. Python Software Foundation. Available online: http://www.python.org (accessed on 22 July 2022).
| Compound | Chemical Structure | Molecular Formula | Density (g/cm3) | Molecular Weight (g/mol) | Classification |
|---|---|---|---|---|---|
| Isoamyl alcohol | 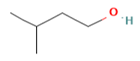 | C5H12O | 0.810 | 88.15 | Primary ALCOHOL |
| 2-Octanone |  | C8H16O | 0.820 | 128.21 | Methyl KETONE |
| 2-Phenethyl acetate | 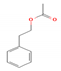 | C10H12O2 | 1.032 | 164.20 | Acetate ESTER |
| n-Hexane | 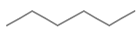 | C6H14 | 0.655 | 86.18 | Straight-chain ALKANE |
| Tested Volatile | Room Conditions | Environmental Chamber Conditions | Field Conditions |
|---|---|---|---|
| Isoamyl alcohol | r = 0.2605 | r = 0.4418 | r = 0.2739 |
| p = 0.2814 | p = 0.0582 | p = 0.2565 | |
| 2-Octanone | r = 0.3446 | r = 0.5139 | r = 0.3149 |
| p = 0.1485 | p = 0.0244 | p = 0.1891 | |
| 2-Phenethyl acetate | r = 0.557 | r = 0.647 | r = 0.5774 |
| p = 0.0133 | p = 0.0026 | p = 0.0096 | |
| n-Hexane | r = 0.6923 | r = 0.7141 | r = 0.7179 |
| p = 0.0010 | p = 0.0059 | p = 0.0005 |
| Climatic Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Environmental Conditions | Volatile Compound | Temperature (°C) | Relative Humidity (%) | Air Pressure (hPa) | Precipitation (mm) | Cloud Cover (okta) | Wind (Bf) |
| Room conditions | Isoamyl alcohol | r = −0.3727 | r = 0.2845 | r = 0.346 | Not analyzed | ||
| p = 0.1560 | p = 0.2855 | p = 0.1893 | |||||
| 2-Octanone | r = −0.399 | r = 0.3299 | r = 0.3295 | Not analyzed | |||
| p = 0.1258 | p = 0.2121 | p = 0.2127 | |||||
| 2-Phenethyl acetate | r = −0.8269 | r = 0.3378 | r = 0.2646 | Not analyzed | |||
| p = 0.0001 | p = 0.2007 | p = 0.3225 | |||||
| Environmental chamber conditions | Isoamyl alcohol | r = −0.2906 | r = 0.1582 | r = 0.2887 | Not analyzed | ||
| p = 0.2759 | p = 0.5584 | p = 0.2872 | |||||
| 2-Octanone | r = −0.3201 | r = 0.3678 | r = 0.3738 | Not analyzed | |||
| p = 0.2269 | p = 0.1610 | p = 0.1538 | |||||
| 2-Phenethyl acetate | r = −0.6483 | r = 0.384 | r = 0.3606 | Not analyzed | |||
| p = 0.0066 | p = 0.1420 | p = 0.1700 | |||||
| Field conditions | Isoamyl alcohol | r = −0.4246 | r = 0.2665 | r = 0.2773 | r = −0.1217 | r = −0.2321 | r = −0.2569 |
| p = 0.1152 | p = 0.3184 | p = 0.3170 | p = 0.6553 | p = 0.3873 | p = 0.3386 | ||
| 2-Octanone | r = −0.4253 | r = 0.3713 | r = 0.2638 | r = −0.0604 | r = −0.2403 | r = −0.2679 | |
| p = 0.1108 | p = 0.1568 | p = 0.3235 | p = 0.8253 | p = 0.3706 | p = 0.3175 | ||
| 2-Phenethyl acetate | r = −0.6632 | r = 0.2679 | r = 0.2315 | r = −0.0414 | r = −0.2384 | r = −0.0927 | |
| p = 0.0051 | p = 0.3158 | p = 0.3883 | p = 0.8802 | p = 0.3747 | p = 0.7347 | ||
| n-Hexane | r = −0.4989 | r = 0.3322 | r = 0.2077 | r = 0.0201 | r = −0.2146 | r = −0.1801 | |
| p = 0.0496 | p = 0.2087 | p = 0.4402 | p = 0.9401 | p = 0.4261 | p = 0.5047 | ||
| Compared OFF-Associated Yeast Volatiles | U Statistic | p-Value | |
|---|---|---|---|
| Polypropylene vials | 2-Phenethyl acetate, 2-octanone | 44.0 | 0.05213 |
| 2-Phenethyl acetate, isoamyl alcohol | 50.5 | 0.10561 | |
| 2-Octanone, isoamyl alcohol | 68.5 | 0.43005 | |
| Rubber septa | 2-Phenethyl acetate, 2-octanone | 31.5 | 0.00916 |
| 2-Phenethyl acetate, isoamyl alcohol | 28.0 | 0.00547 | |
| 2-Octanone, isoamyl alcohol | 67.0 | 0.39374 |
| OFF-Associated Yeast Volatile Compound | U Statistic | p-Value |
|---|---|---|
| 2-Phenethyl acetate | 30.0 | 0.00736 |
| Isoamyl alcohol | 57.0 | 0.19644 |
| 2-Octanone | 45.5 | 0.06039 |
| Temperature (°C) | Air Pressure (hPa) | Humidity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Week | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean |
| Room conditions | 1st | 21.1 | 23.6 | 22.76 | 997 | 1012 | 1006.63 | 48.7 | 63 | 56.33 |
| 2nd | 17.2 | 21.2 | 18.95 | 1002 | 1012 | 1006.17 | 39.1 | 52.9 | 45.89 | |
| 3rd | 17.1 | 20.8 | 18.45 | 1003 | 1020 | 1012.44 | 43.1 | 67.6 | 51.31 | |
| 4th | 17.4 | 20 | 18.54 | 1008 | 1019 | 1013.19 | 46.2 | 61.8 | 51.05 | |
| Environmental chamber conditions | 1st–4th | 17 | 23 | 20 | 1013.25 | 1013.25 | 1013.25 | 60 | 70 | 65 |
| Field conditions | 1st | 15.2 | 24.3 | 19.21 | 1007.91 | 1021.65 | 1016.55 | 42 | 95 | 70.96 |
| 2nd | 10.6 | 19.3 | 14.2 | 101.71 | 1020.58 | 1015.47 | 29 | 89 | 47.69 | |
| 3rd | 10.3 | 20.8 | 16.54 | 1012.05 | 1028.31 | 1020.83 | 37 | 89 | 61.12 | |
| 4th | 9.4 | 21.2 | 15.28 | 1017.25 | 1026.85 | 1021.59 | 27 | 90 | 52.25 | |
| Treatment | Trap (Dispenser Type) | Attractants (Volatile Compounds) | Retention System |
|---|---|---|---|
| A | YST + PPV | Isoamyl Alcohol | Sticky |
| B | YST + PPV | 2-Octanone | Sticky |
| C | YST + PPV | 2-Phenethyl acetate | Sticky |
| D | YST + PPV | n-Hexane | Sticky |
| E | YST + RS | Isoamyl Alcohol | Sticky |
| F | YST + RS | 2-Octanone | Sticky |
| G | YST + RS | 2-Phenethyl acetate | Sticky |
| H | YST + RS | n-Hexane | Sticky |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veršić Bratinčević, M.; Bego, A.; Nižetić Kosović, I.; Jukić Špika, M.; Burul, F.; Popović, M.; Ninčević Runjić, T.; Vitanović, E. A Lifetime of a Dispenser-Release Rates of Olive Fruit Fly-Associated Yeast Volatile Compounds and Their Influence on Olive Fruit Fly (Bactrocera oleae Rossi) Attraction. Molecules 2023, 28, 2431. https://doi.org/10.3390/molecules28062431
Veršić Bratinčević M, Bego A, Nižetić Kosović I, Jukić Špika M, Burul F, Popović M, Ninčević Runjić T, Vitanović E. A Lifetime of a Dispenser-Release Rates of Olive Fruit Fly-Associated Yeast Volatile Compounds and Their Influence on Olive Fruit Fly (Bactrocera oleae Rossi) Attraction. Molecules. 2023; 28(6):2431. https://doi.org/10.3390/molecules28062431
Chicago/Turabian StyleVeršić Bratinčević, Maja, Ana Bego, Ivana Nižetić Kosović, Maja Jukić Špika, Filipa Burul, Marijana Popović, Tonka Ninčević Runjić, and Elda Vitanović. 2023. "A Lifetime of a Dispenser-Release Rates of Olive Fruit Fly-Associated Yeast Volatile Compounds and Their Influence on Olive Fruit Fly (Bactrocera oleae Rossi) Attraction" Molecules 28, no. 6: 2431. https://doi.org/10.3390/molecules28062431
APA StyleVeršić Bratinčević, M., Bego, A., Nižetić Kosović, I., Jukić Špika, M., Burul, F., Popović, M., Ninčević Runjić, T., & Vitanović, E. (2023). A Lifetime of a Dispenser-Release Rates of Olive Fruit Fly-Associated Yeast Volatile Compounds and Their Influence on Olive Fruit Fly (Bactrocera oleae Rossi) Attraction. Molecules, 28(6), 2431. https://doi.org/10.3390/molecules28062431











