Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry
Abstract
:1. Introduction
2. Results and Discussion
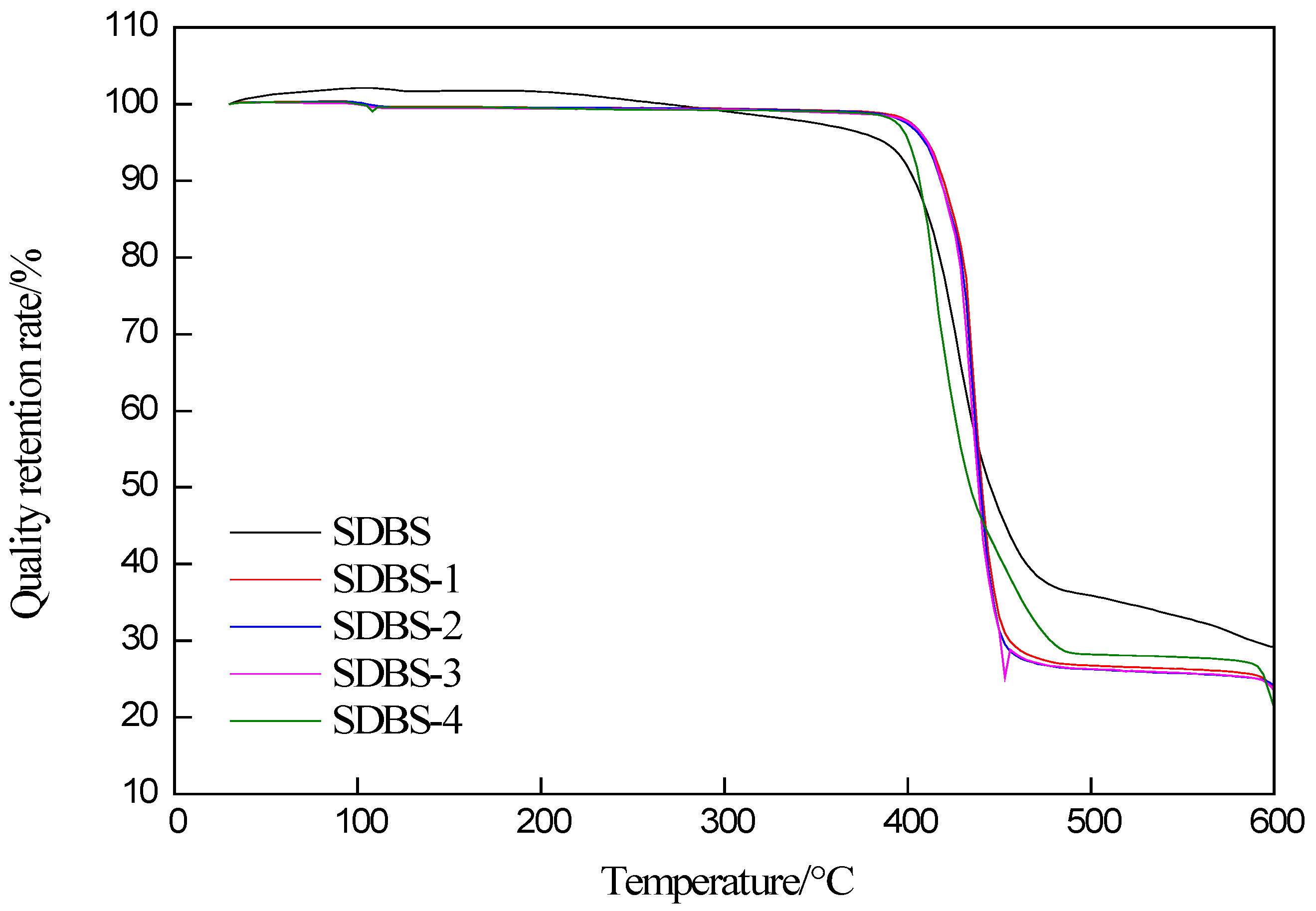
2.1. Thermogravimetric Analysis
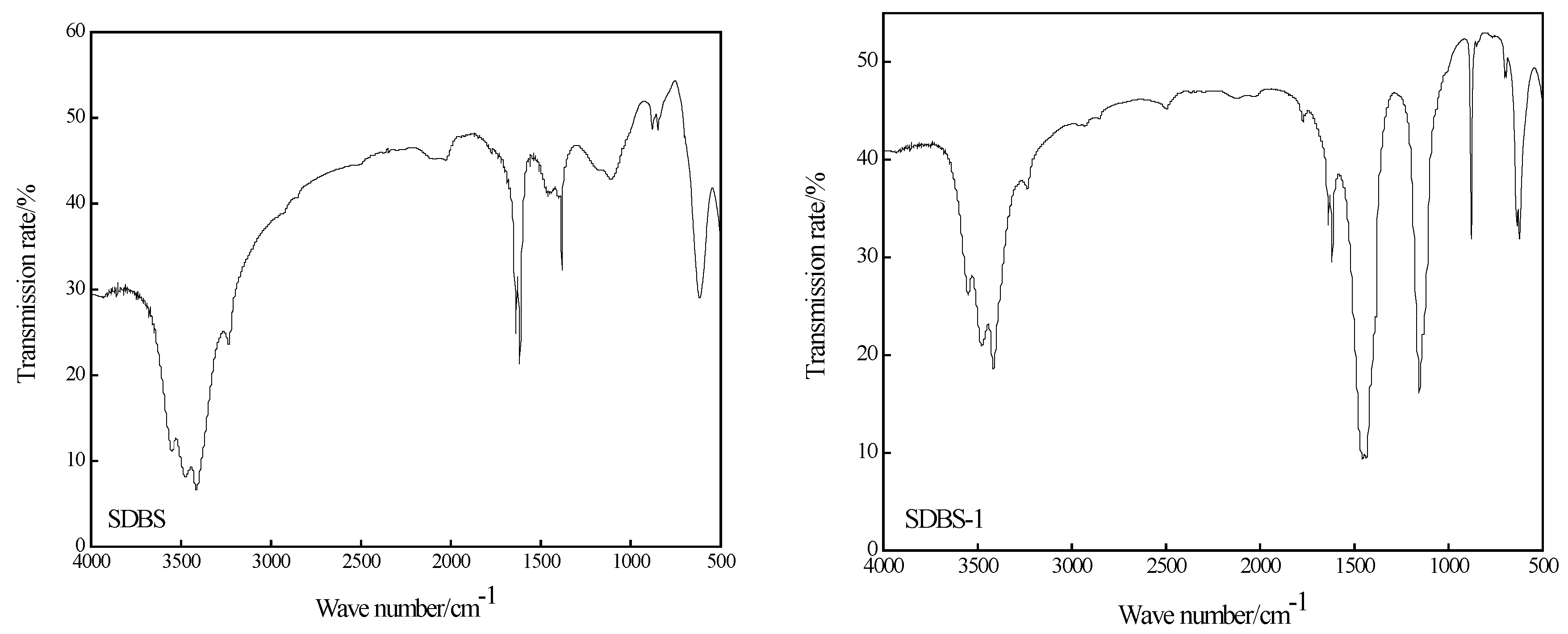
2.2. Infrared Analysis
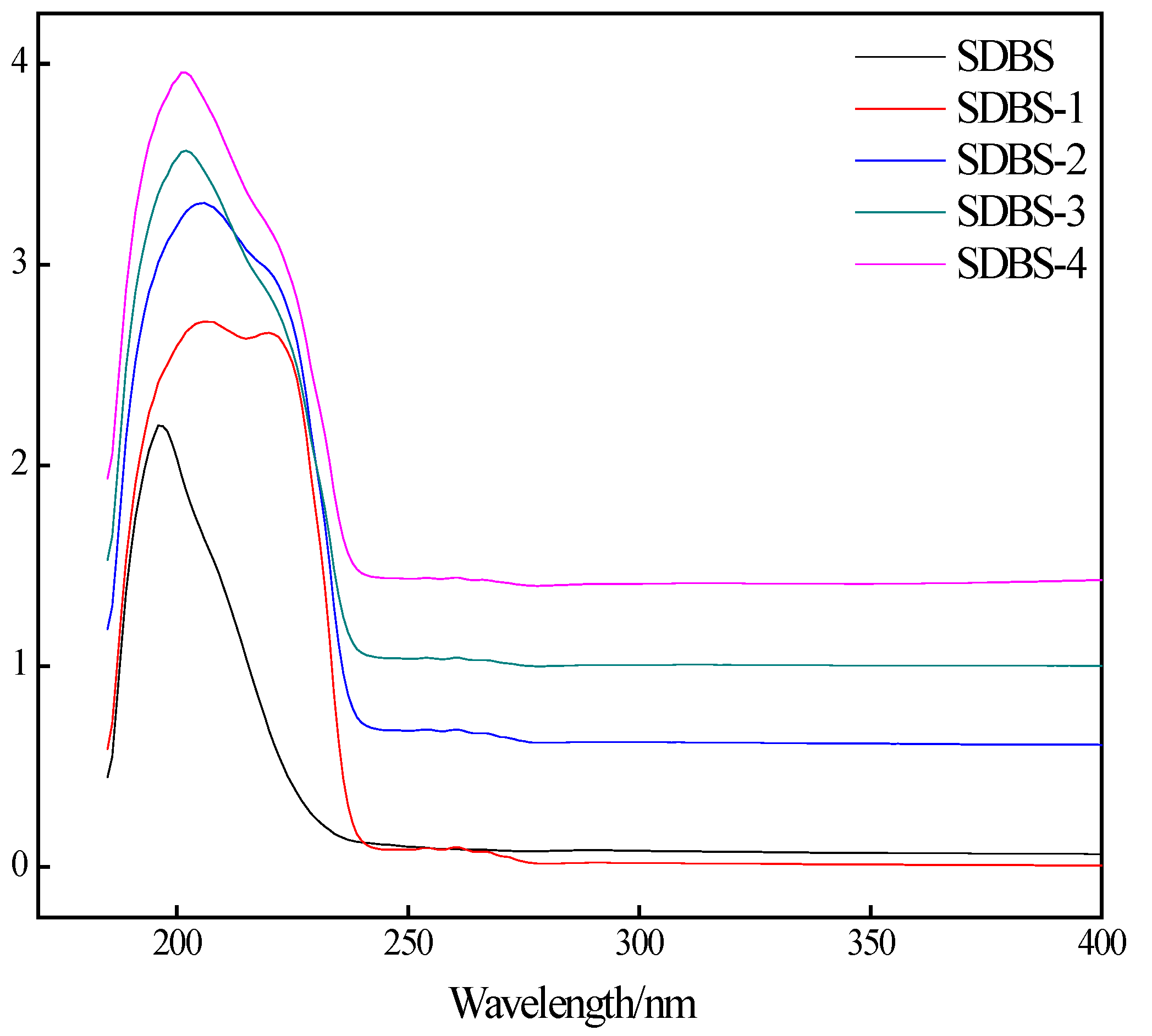
2.3. Ultraviolet Spectroscopy
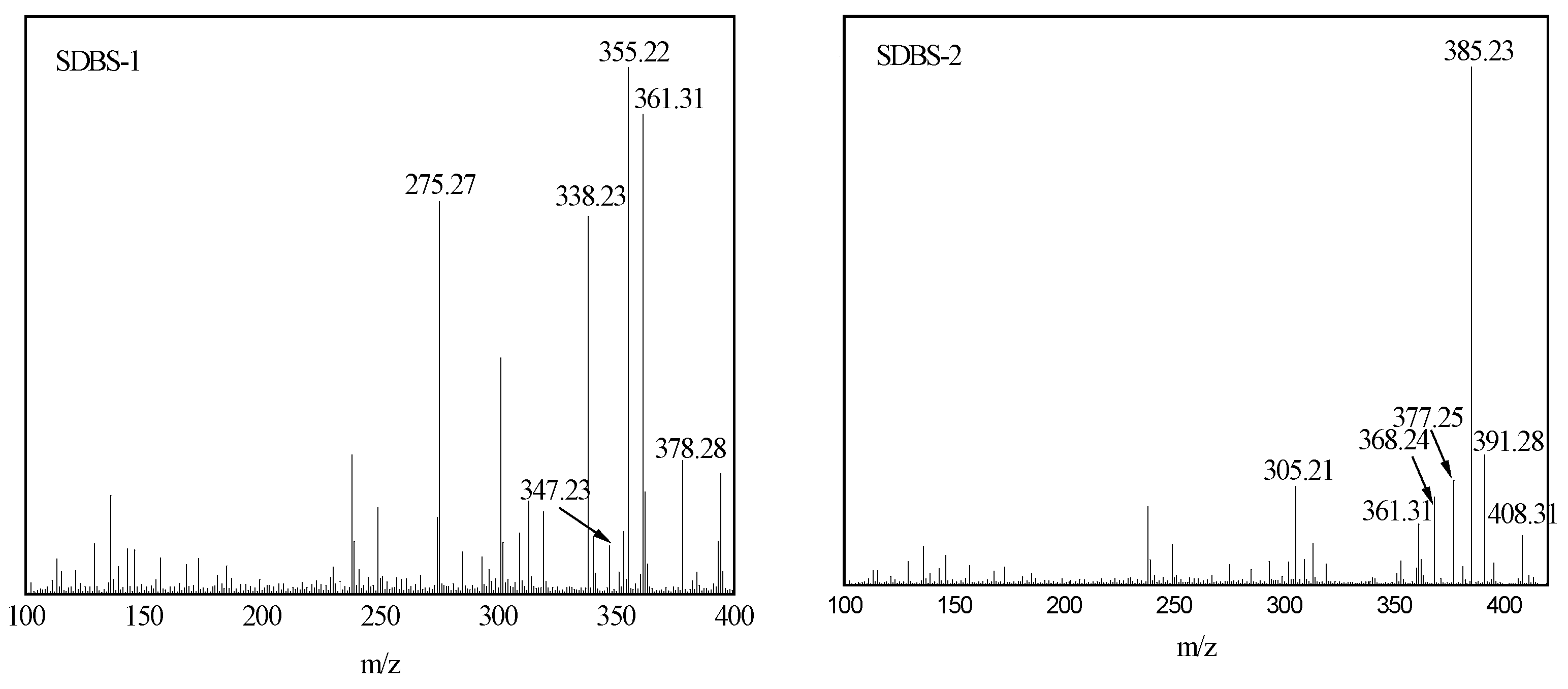
2.4. Mass Spectrum
2.5. Surface-Tension Evaluation
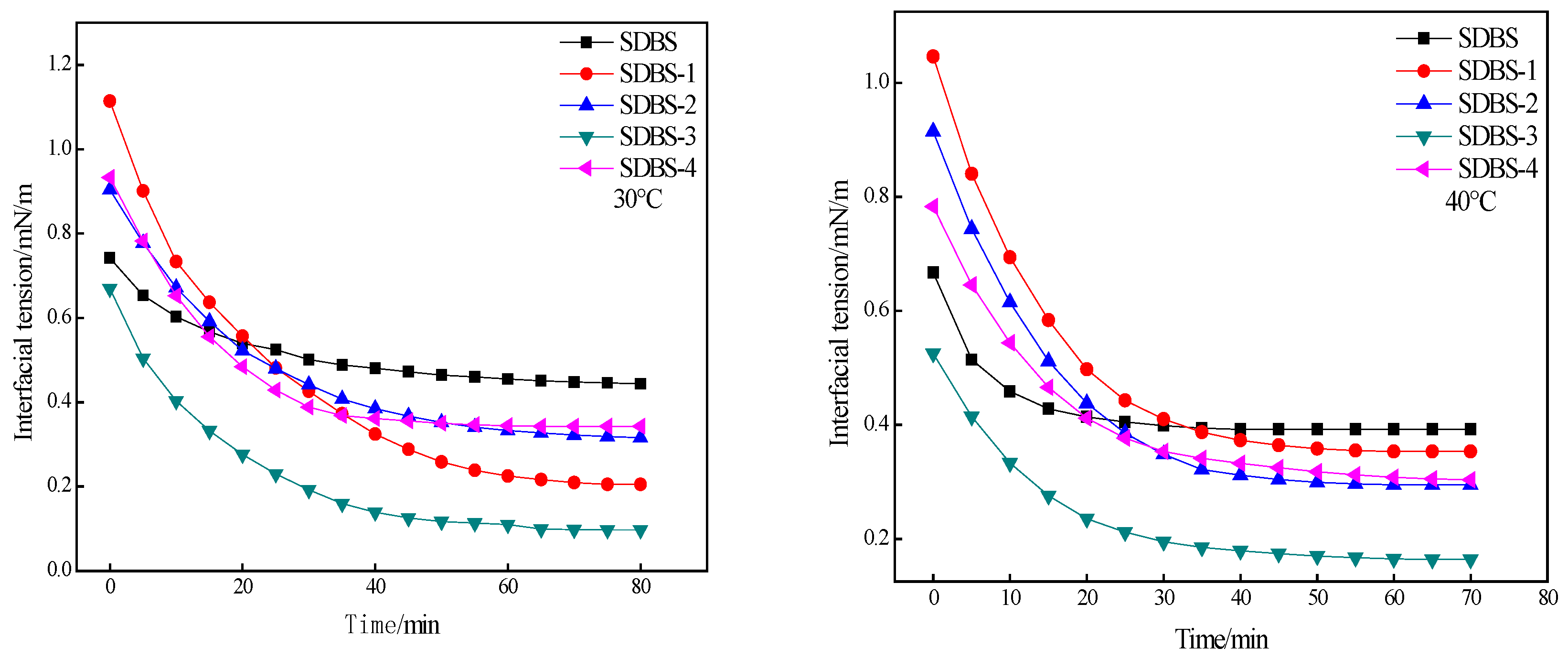
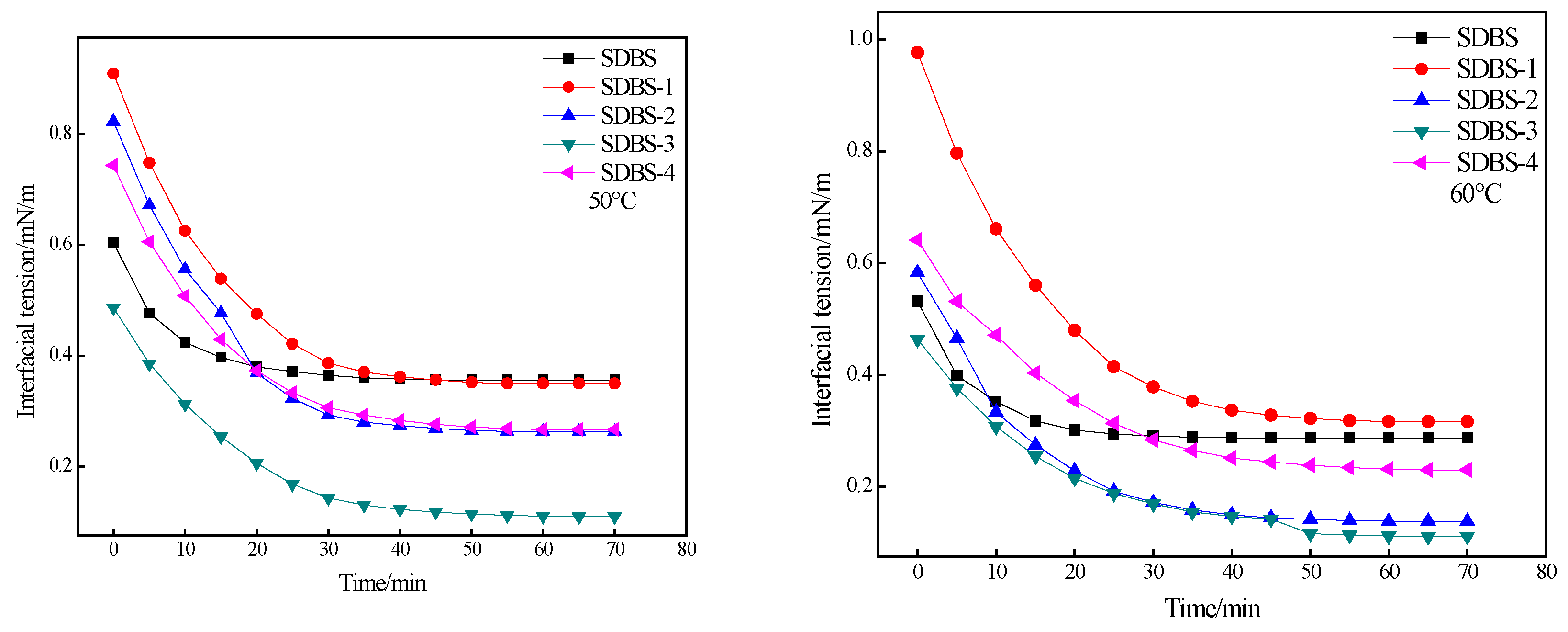
2.6. Interfacial-Tension Evaluation
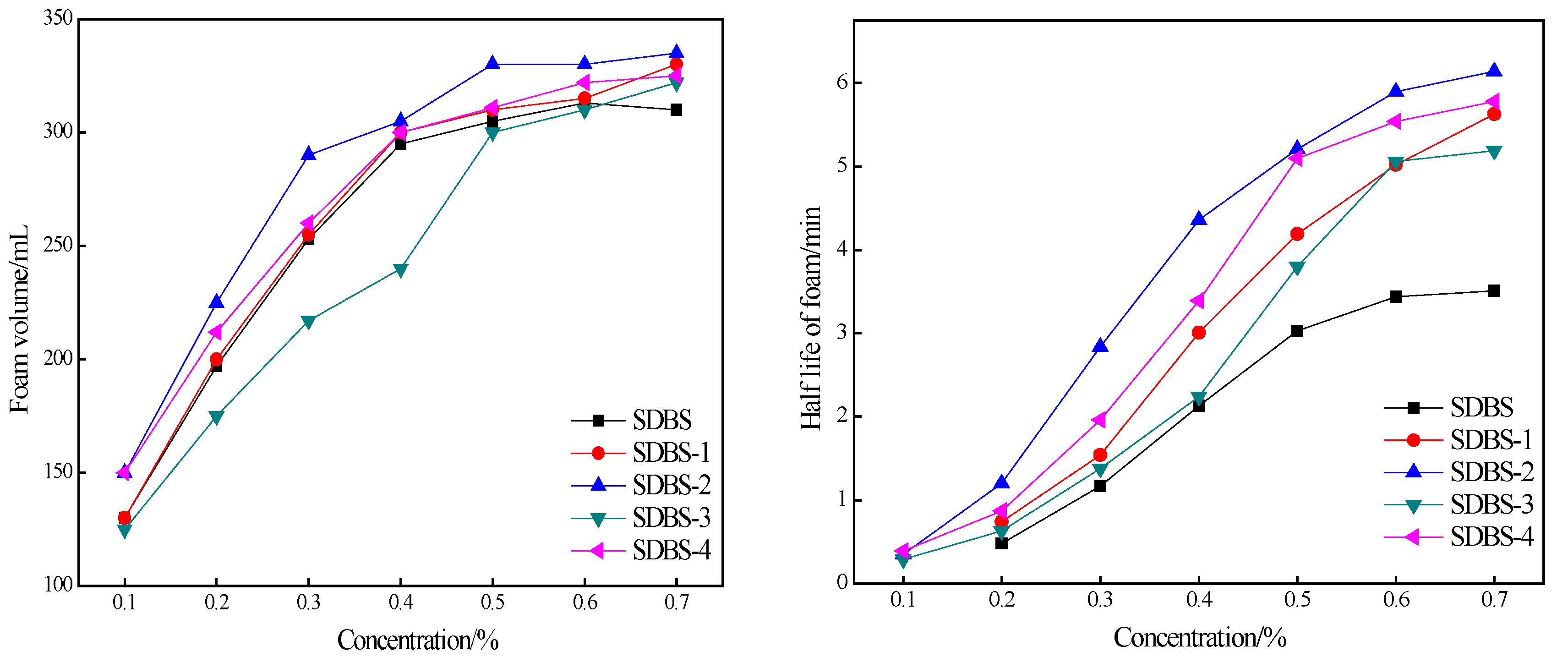
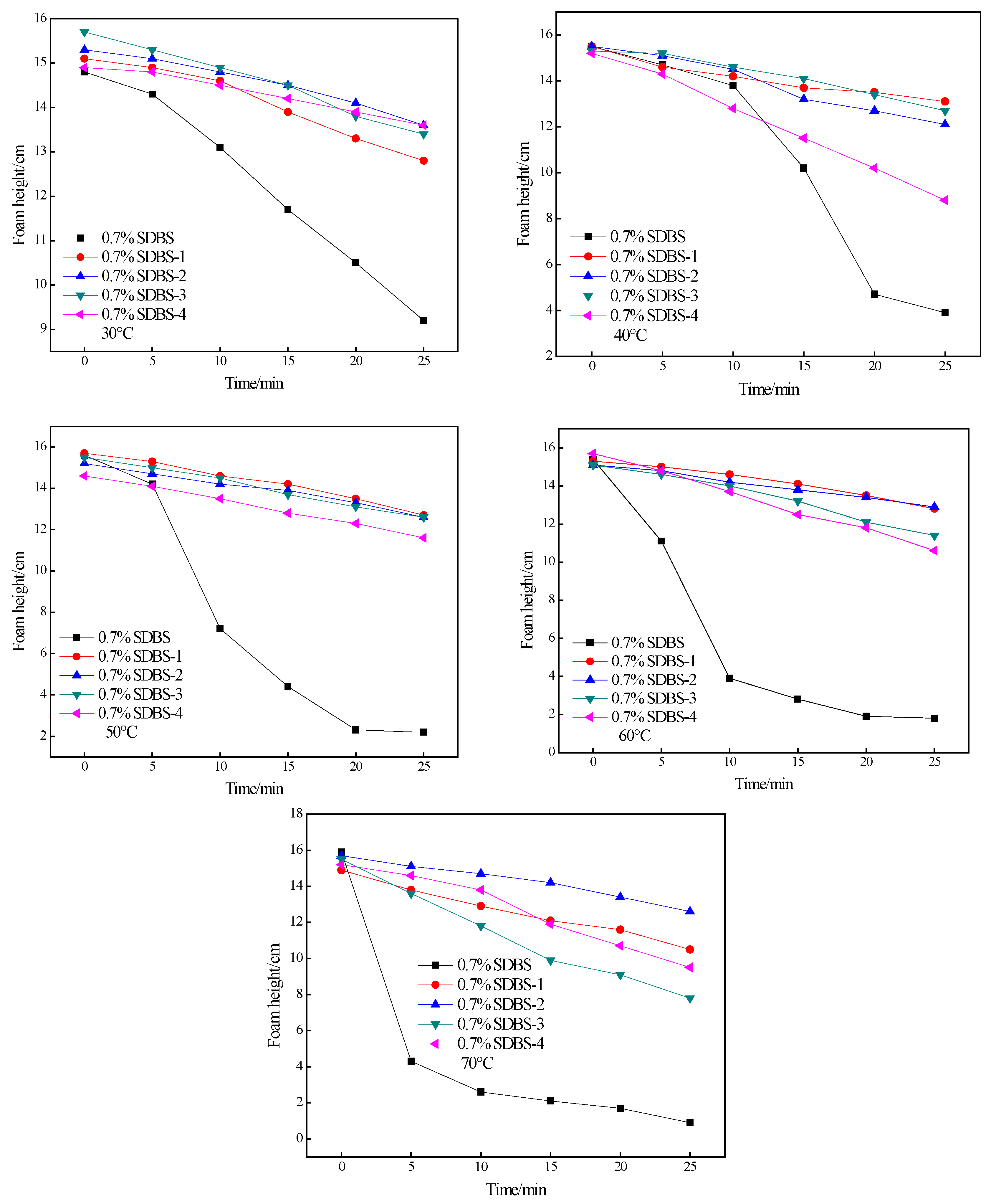
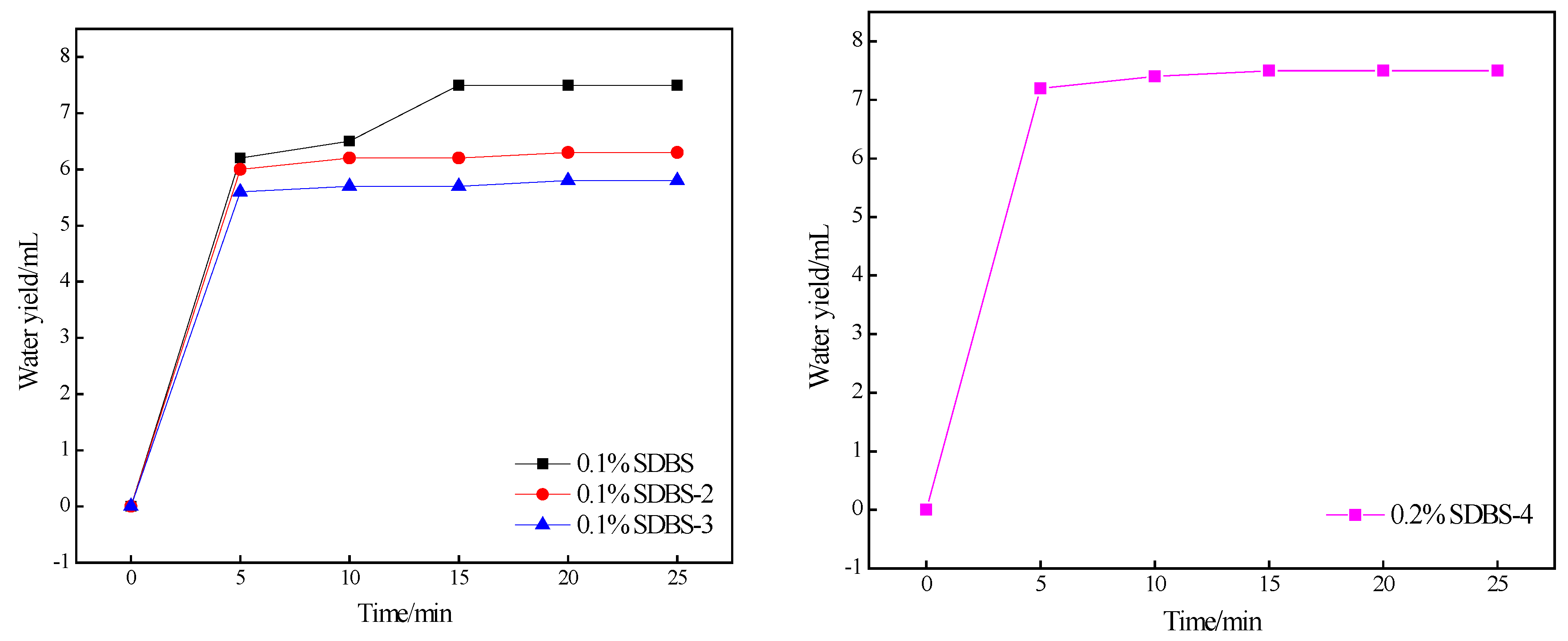
2.7. Foaming-Ability Test
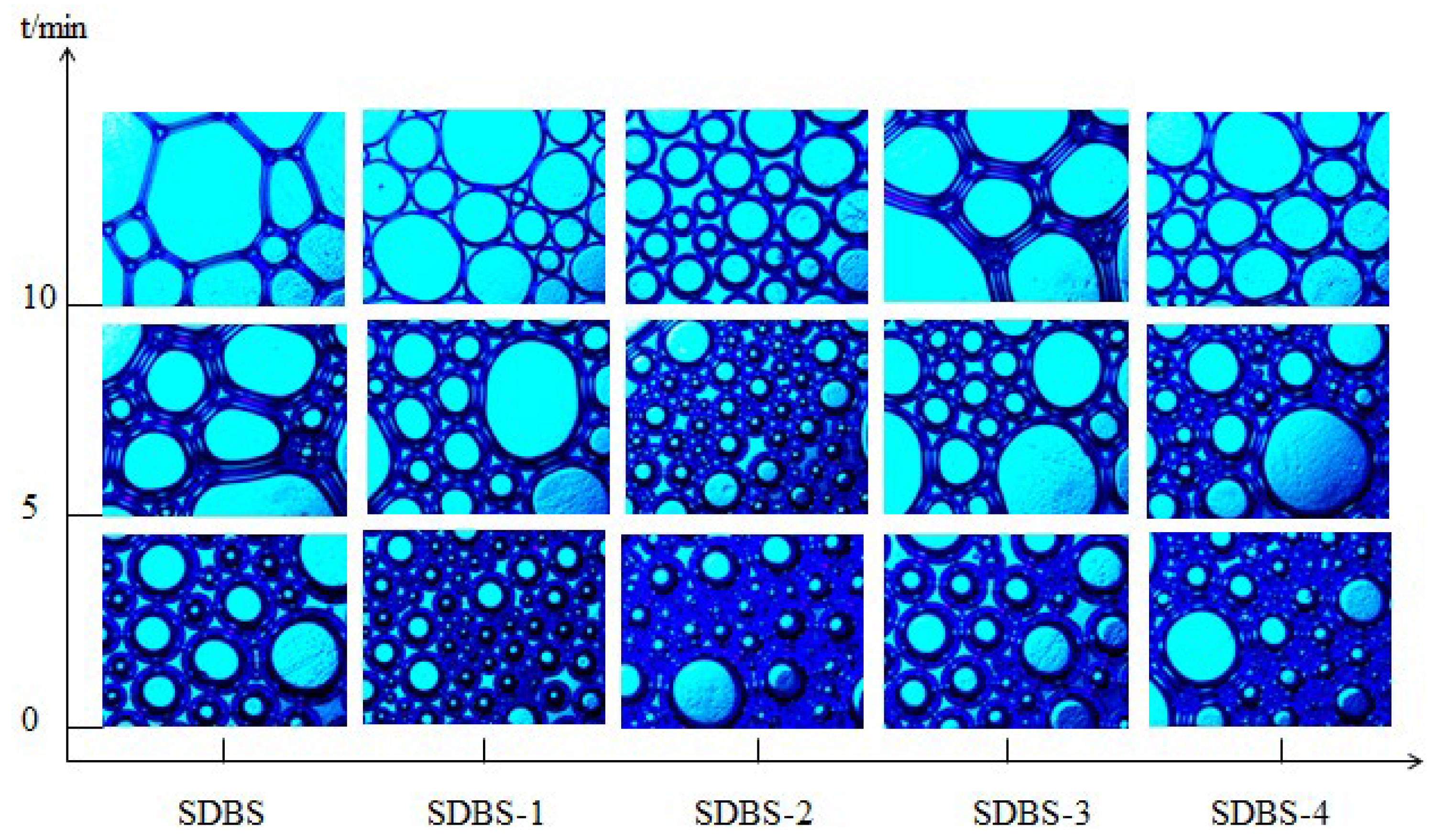
2.8. Microstructure Analysis of Foam
2.9. Emulsification Test
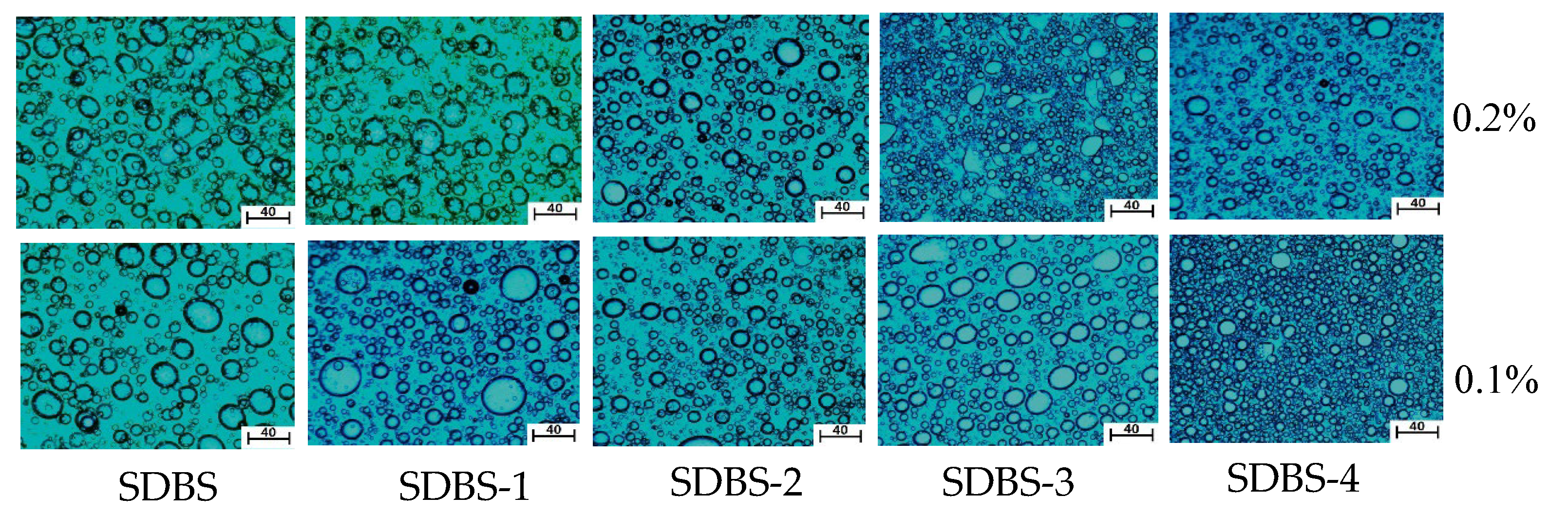
2.10. Microstructure of Emulsion
2.11. Oil-Displacement Ability
3. Materials and Methods
3.1. Materials
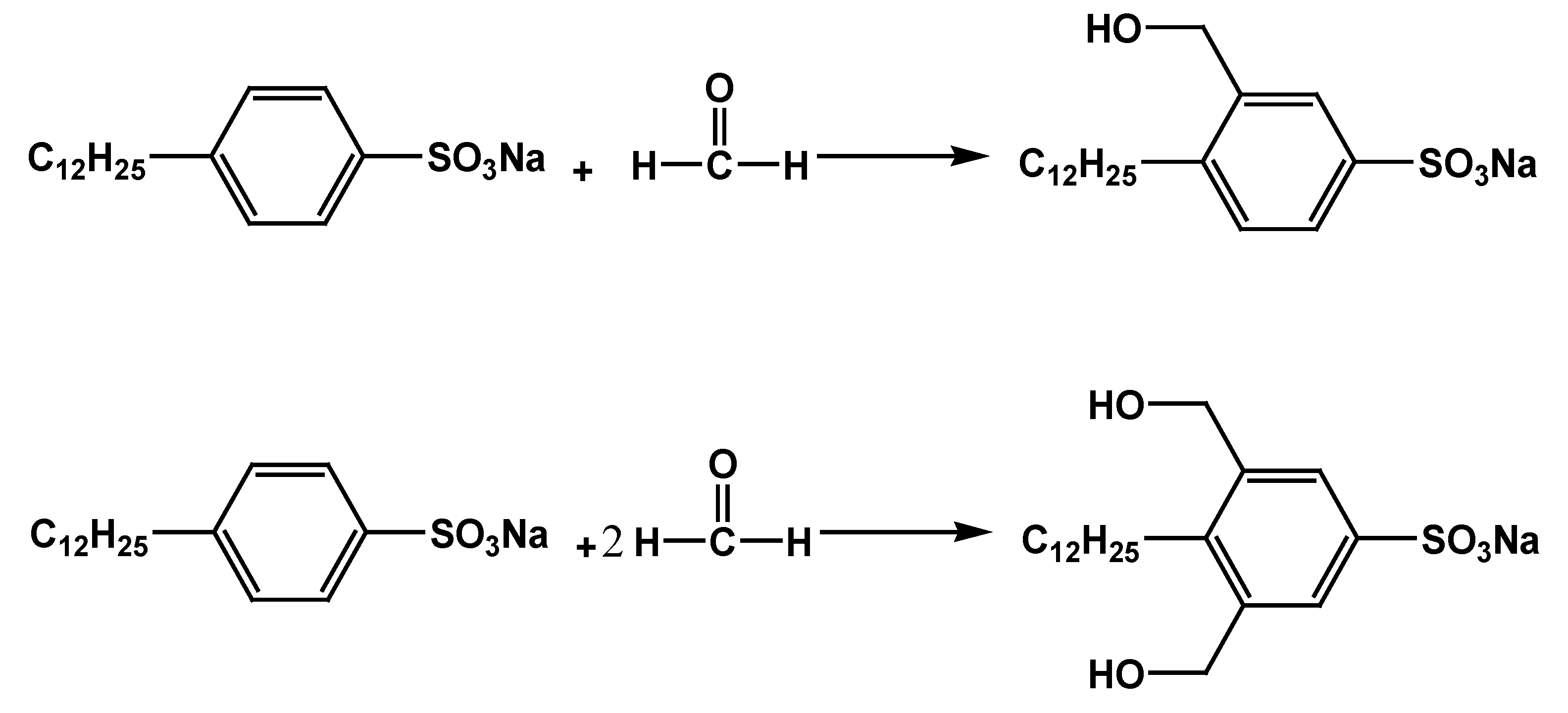
3.2. Preparation of Multifunctional Surfactant
3.3. Thermogravimetric-Analysis Conditions
3.4. Infrared-Analysis Conditions
3.5. Ultraviolet-Analysis Conditions
3.6. Mass-Spectrometric-Analysis Conditions
3.7. Surface-Tension Test Conditions
3.8. Test Conditions for Interfacial Tension
3.9. Test Conditions for Foaming Capacity
3.10. Microstructure Analysis of Foam
3.11. Test Conditions for Emulsifying Ability
3.12. Microstructure Analysis of Emulsion
3.13. Test Conditions for Oil-Displacement Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, Z.; Dong, S.; Zhang, X.; Zhang, J.; Chen, G. Synthesis of multi-alkylpolyamine and performance in crude oil as flow improver. Tenside Surfact. Deterg. 2022, 59, 104–110. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, Q.; Liu, Z.; Tang, L.; Liang, L.; Zhang, C.; Li, Q.; Xu, N.; Sun, J.; et al. Achieving the super gas-wetting alteration by functionalized nano-silica for improving fluid flowing capacity in gas condensate reservoirs. ACS Appl. Mater. Interfaces 2021, 13, 10996–11006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.; Chu, Z.; He, S.; Zhang, J.; Feng, Y. Thermally induced structural transitions from fluids to hydrogels with pH-switchable anionic wormlike micelles. Colloid Interface Sci. 2013, 394, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Slaný, M.; Guo, R.; Du, W.; Li, Y.; Chen, G. Study on synergistic catalysis of ex-situ catalyst and in-situ clay in aquathermolysis of water-heavy oil-ethanol at low temperature. Chem. Eng. J. 2023, 453, 139872. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, Q.; Tang, L.; Yuan, L.; Wang, G.; Zhang, R. Optimization the synthesis parameters of gas-wetting alteration agent and evaluation its performance. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 438–445. [Google Scholar] [CrossRef]
- Xia, P.; Wang, J.; Yang, X.; Yuan, Y.; Zhang, Y. Direct determination of linear alkyl benzene sulfonate anionic surfactants in environmental water by high performance liquid chromatography. Environ. Monit. China 2022, 38, 242–247. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, Q.; Liang, L.; Tang, L.; Zhang, C.; Lan, J.; Meng, L.; Jiang, B. Design of fluorine-modified nanocrystalline cellulose achieving super gas-wetting alteration of reservoir cores. J. Mol. Liq. 2021, 333, 115933. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Z.; Yang, H.; Li, X.; Qu, H.; Xie, Y.; Zeng, S. Lab-scale investigation of slickwater impact on methane desorption on longmaxi gas shale. Chem. Eng. Technol. 2023, 46, 350–356. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Li, G.; Zhao, Z.; Wu, X.; Yang, Y.; Gao, Z.; Zhou, M. Screening and evaluation of surfactant flooding system in low temperature, high salt and low permeability reservoirs. Oilfield Chem. 2022, 39, 682–687. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, C.; Cheng, P. Mechanism and application of surfactants in oil and gas field development. J. Prog. Fine Petrochem. Ind. 2022, 23, 46–54. [Google Scholar] [CrossRef]
- Qu, W. Synthesis and properties of alkyl benzene sulfonate surfactants. Oil Refin. Chem. Ind. 2013, 24, 7–69. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Dong, S.; Li, J.; Wang, M.; Wu, Y.; Pu, C.; Chen, G. The effect of halide counter ions and methanol on the foaming activity of cationic surfactants and a mechanism study. Tenside Surfactants Deterg. 2021, 58, 278–286. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Y.; Ge, Z.; Zhou, Z.; Chai, C.; Wang, H.; Zhang, L.; Bo, T. Viscoelastic surfactant fracturing fluids for use in coal seams: Effects of surfactant composition and formulation. Chem. Eng. Sci. 2020, 215, 115370. [Google Scholar] [CrossRef]
- Fan, W.; Wu, G.; Zhang, T.; He, K.; He, X.; He, W.; Zhou, W. Study on the preparation and membrane sulfonation synthesis of petroleum sulfonate raw oil. J. Xi’an Shiyou Univ. (Nat. Sci. Ed.) 2023, 38, 102–107. [Google Scholar]
- Liu, Q.; Bai, Y.; Dong, S.; Li, J.; Song, Z.; Chen, S.; Zhang, J.; Chen, G. Preparation and the foaming activity study of hydroxyme thyl cetyltrimethyl ammonium chloride: Herstellung von hydroxymethylcetyltrimethylammoniumchlorid und untersuchung seines schaumvermögens. Tenside Surfactants Deterg. 2021, 58, 153–160. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Weng, R.; Luo, Y. Simultaneous determination of sodium laurate and sodium dodecyl benzene sulfonate by HPLC. Oilfield Chem. 2004, 21, 381–384. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, F.; Fan, H.L. Study of oil displacement performance of hydroxyl sulfobetaine surfactant. Adv. Mater. Res. 2014, 853, 223–228. [Google Scholar] [CrossRef]
- Gao, M.; Jia, Y.; Lv, S.; Dong, S.; Wang, M.; Zhu, S.; Zhang, J.; Chen, G. Synergistic effect of octadecyl ammonium oxide and oleate amide propyl betaine and development of a foam drainage reagent for natural gas production. C. R. Chim. 2021, 24, 11–20. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Li, Q.; Lv, K.; Wang, J.; Wang, Z. Mechanism of organosilicate polymer as high-temperature resistant inhibitor in water-Based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128489. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Q.; Du, W.; Qu, C.; Song, Z.; Li, J.; Zhang, J.; Chen, G. Synthesis and properties of octadecyl trimethyl ammonium polyacrylic surfactants. Tenside Surfactants Deterg. 2020, 57, 122–128. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Liu, Q.; Zhang, J.; Gu, X.; Li, H.; Qu, C.; Zhang, Y. Synthesis and interface activity of a series of dicarboxylic cationic surfactants and a structure–efficiency relationship study. Surfactants Deterg. 2019, 22, 691–698. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Zhang, F.; Zhang, X.; Dong, S.; Zhang, J.; Chen, G. Synthesis of barium alkylbenzene sulfonate and its behavior as a flow improver for crude oil. Comptes Rendus. Chimie 2021, 24, 83–89. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Z.; Shi, X.; Zhang, X.; Dong, S.; Zhang, J. Synthesis of alkylbenzenesulfonate and its behavior as flow improver in crude oil. Fuel 2021, 288, 119644. [Google Scholar] [CrossRef]
- Gao, M.; Tian, W.; Ma, Z.; Dong, S.; Zhu, S.; Zhang, J.; Chen, G. Research of a surfactant gel with potential application in oilfield. Tenside Surfactants Deterg. 2021, 58, 360–370. [Google Scholar] [CrossRef]
- Ma, X.; Mu, H.; Guo, S. Synthesis and properties of viscoelastic surfactants for fracturing fluids. Chin. J. Appl. Chem. 2021, 38, 923–932. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, C.; Zhang, J. Preparation and evaluation of surfactant combination system for high efficiency oil displacement. Contemp. Chem. Ind. 2022, 51, 2588–5188. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Hydrogel from crosslinked polyacrylamide/guar gum graft copolymer for sorption of hexavalent chromium ion. Carbohydr. Polym. 2011, 86, 1306–1312. [Google Scholar] [CrossRef]
- Pu, W.-F.; Du, D.-J.; Liu, R. Preparation and evaluation of supramolecular fracturing fluid of hydrophobically associative polymer and viscoelastic surfactant. J. Pet. Sci. Eng. 2018, 167, 568–576. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, B.; Wang, Y.; Qu, H.; Zeng, S. Spontaneous imbibition characteristics of slickwater and its components in longmaxi shale. J. Pet. Sci. Eng. 2021, 203, 108599. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Li, W.; Dou, M.; Ma, L.; Wang, Q.; Zhao, B.; Chen, G. Enhanced sorption for the oil spills by SDS-modified rice straw. Gels 2023, 9, 285. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, F.; Li, Y.; Zhang, J.; Chen, S.; Qu, C.; Chen, G. Investigation of cationic surfactants as clean flow improvers for crude oil and a mechanism study. J. Pet. Sci. Eng. 2018, 164, 87–90. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Dong, S.; Zhang, J.; Chen, G. Synthesis of aluminium alkylbenzene sulfonate and its behavior as a flow improver for crude oil. Tenside Surfactants Deterg. 2022, 59, 353–361. [Google Scholar] [CrossRef]



| Surfactant | CMC(%) | Surface Tension (0.5%) (mN/m) |
|---|---|---|
| SDBS-1 | 0.0600 | 31.392 |
| SDBS-2 | 0.0353 | 33.471 |
| SDBS-3 | 0.0529 | 32.359 |
| SDBS-4 | 0.0637 | 31.274 |
| Φ Value | 0 min | 10 min |
|---|---|---|
| SDBS | 0.670 | 0.867 |
| SDBS-1 | 0.673 | 0.871 |
| SDBS-2 | 0.683 | 0.878 |
| SDBS-3 | 0.671 | 0.870 |
| SDBS-4 | 0.678 | 0.873 |
| Surfactant | Water-Splitting Rate | |
|---|---|---|
| 0.1% | 0.2% | |
| SDBS | 75 | 0 |
| SDBS-1 | 0 | 0 |
| SDBS-2 | 63 | 0 |
| SDBS-3 | 58 | 0 |
| SDBS-4 | 0 | 75 |
| Surfactant | SDBS | SDBS-1 | SDBS-2 | SDBS-3 | SDBS-4 |
|---|---|---|---|---|---|
| Oil-Displacement Rate % | 10 | 15 | 25 | 15 | 20 |
| Reactant A | Reactant B | Raw-Material Ratio (n/n) | Solvent | Product Number |
|---|---|---|---|---|
| SDBS | Formaldehyde (40% solution) | 1:0 | Methanol | SDBS |
| 1:1 | SDBS-1 | |||
| 1:2 | SDBS-2 | |||
| 1:3 | SDBS-3 | |||
| 1:4 | SDBS-4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bai, Q.; Li, Q.; Huang, H.; Ni, W.; Wang, Q.; Xin, X.; Zhao, B.; Chen, G. Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules 2023, 28, 3640. https://doi.org/10.3390/molecules28083640
Li Y, Bai Q, Li Q, Huang H, Ni W, Wang Q, Xin X, Zhao B, Chen G. Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules. 2023; 28(8):3640. https://doi.org/10.3390/molecules28083640
Chicago/Turabian StyleLi, Yongfei, Quanzheng Bai, Qiang Li, Hai Huang, Weijun Ni, Qian Wang, Xin Xin, Bin Zhao, and Gang Chen. 2023. "Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry" Molecules 28, no. 8: 3640. https://doi.org/10.3390/molecules28083640
APA StyleLi, Y., Bai, Q., Li, Q., Huang, H., Ni, W., Wang, Q., Xin, X., Zhao, B., & Chen, G. (2023). Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules, 28(8), 3640. https://doi.org/10.3390/molecules28083640






