Abstract
Pyrrole-ligated 1,3,4-oxadiazole is a very important pharmacophore which exhibits broad therapeutic effects such as anti-tuberculosis, anti-epileptic, anti-HIV, anti-cancer, anti-inflammatory, antioxidant, and antibacterial activities. A one-pot Maillard reaction between D-Ribose and an L-amino methyl ester in DMSO with oxalic acid at 2.5 atm and 80 °C expeditiously produced pyrrole-2-carbaldehyde platform chemicals in reasonable yields, which were utilized for the synthesis of pyrrole-ligated 1,3,4-oxadiazoles. Benzohydrazide reacted with the formyl group of the pyrrole platforms to provide the corresponding imine intermediates, which underwent I2-mediated oxidative cyclization to the pyrrole-ligated 1,3,4-oxadiazole skeleton. The structure and activity relationship (SAR) of the target compounds with varying alkyl or aryl substituents of the amino acids and electron-withdrawing or electron-donating substituents on the phenyl ring of benzohydrazide were evaluated for antibacterial activity against Escherichia coli, Staphylococcus aureus, and Acinetobacter baumannii as representative Gram(–) and Gram(+) bacteria. Branched alkyl groups from the amino acid showed better antibacterial activities. Absolutely superior activities were observed for 5f-1 with an iodophenol substituent against A. baumannii (MIC < 2 μg/mL), a bacterial pathogen that displays a high resistance to commonly used antibiotics.
1. Introduction
Oxadiazole is a five-membered heterocyclic aromatic compound composed of four structural isomers depending on the positions of two nitrogen atoms relative to an oxygen atom [1]. Among them, 1,3,4-oxadiazole has received intensive attention in the field of medicinal chemistry due to its broad metabolic profile [2,3,4] and in the field of material science for its excellent optoelectronic properties [5,6,7,8]. As an isostere of an amide and an ester, 1,3,4-oxadiazole serves as a promising pharmacophore for the discovery of new drugs exhibiting antimicrobial, anticonvulsant, anti-inflammatory, analgesic, antitumor, antiviral, antihypertensive, and enzyme inhibitory activities [9]. There have been extensive literature reviews on the specific synthetic methods and diverse biological activities of 1,3,4-oxadiazole derivatives [10,11,12].
Motivated by Raltegravir [13,14], an antiretroviral drug used to treat HIV/AIDS, and Zibotentan [15,16], an anti-cancer drug candidate, a number of poly heterocyclic compounds containing a 1,3,4-oxadiazole pharmacophore were constructed, and their biological activities were investigated. Indole-ligated 1,3,4-oxadiazoles [A] were synthesized and evaluated to show antimicrobial, anti-inflammatory, and antiproliferative activities (Figure 1) [17,18]. Their biological importance was also identified by their antioxidants and acetylcholinesterase inhibition properties [19]. Likewise, various 2-benzofuranyl-1,3,4-oxadiazoles [B] were synthesized [20,21], demonstrating their biological activities in α-Glucosidase inhibition as well as the inhibition of glycogen synthase kinase 3β for treating diabetes and Alzheimer’s disease, respectively [22,23,24].
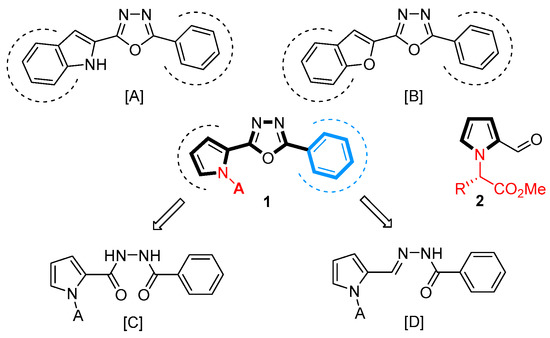
Figure 1.
Heterocycle-ligated 1,3,4-oxadiazoles [A] and [B], and retrosynthesis of 1 from pyrrole 2 through the cyclization using key precursors [C] and [D].
Pyrrole is a very important structural motif in drug discovery projects because of the wide presence of natural, biologically active pyrrole alkaloid products [25,26]; thus, pyrrole-ligated 1,3,4-oxadiazole would be a perfect base structure for the development of potential lead compounds [27]. Considering the efficacy of the procedures of constructing a 1,3,4-oxadiazole ring, 2-pyrrolyl-5-phenyl-1,3,4-oxadizole 1 would be an ideal core structure, achieved either through dehydration from aroylhydrazide [C] or by cyclization from aroylhydrazone [D]. In the benzene ring, the substituent effects of the core structure 1 on antibacterial activity have been reported for the cases of 4,5-dibromopyrrole [28,29] and 4-nitropyrrole [30], respectively.
Pyrrole-2-carbaldehydes 2, derived from the conversion of D-ribose with L-amino acids [31], were demonstrated to be useful, sustainable platform chemicals for the construction of highly functionalized poly heterocyclic compounds [32]. Since natural amino acids themselves demonstrate specific biological activities [33,34,35], it was envisioned that 1,3,4-oxadiazoles 1 from pyrrole-2-carbaldehydes 2 with the N-amino acid moiety would be very interesting core structures for the investigation of their biological activities. The effect of the amino acid moiety of 1 on antimicrobial activities was screened first, and the substituent effects on the benzene ring were then investigated for 5 and 6 with some selected amino acid moieties. We found a marginal size effect of the alkyl groups from amino acids (Val and Ile, etc.) and superior antibacterial activity of the iodophenol substituents of pyrrole-ligated 1,3,4-oxadiazoles 5 and 6 against S. aureus and A. baumannii. All the syntheses of pyrrole-ligated 1,3,4-oxadiazoles 1, 5, and 6 and their antibacterial activities are closely described herein.
2. Results and Discussion
Structure and activity relationships (SARs) for pyrrole-ligated 1,3,4-oxadiazoles 1 were generally studied by changing substituent groups in the aromatic rings [28,29,30]. We were interested in the SAR of 1 by N-alkyl substituents because pyrrole-2-carbaldehydes 2, the starting materials for 1,3,4-oxadiazoles 1, are easily prepared from L-amino acids, and each amino acid has its own biological activity. Ten L-amino acids with hydrogen, alkyl, aralkyl, ester, and sulfide substituents R were selected to assess the size effect (linear or branched) or potential electronic effect. Pyrrole-2-carbaldehydes 2 were efficiently prepared by a one-pot ribose conversion with an L-amino methyl ester in the presence of oxalic acid in DMSO, following an improved procedure under 2.5 atm argon at 80 °C [32]. The corresponding pyrrole platform chemicals 2a–2j with the N-amino acid moiety were prepared in yields of 32~63% (Scheme 1 and Table 1).
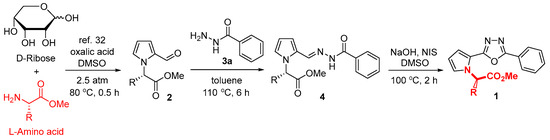
Scheme 1.
Preparations of pyrrole-2-carbaldehydes 2, N-benzoylhydrazones 4, and 1,3,4-oxadiazoles 1 from D-ribose conversion with L-amino acids.

Table 1.
Yields of pyrrole-2-carbaldehydes 2, N-benzoylhydrazones 4, and 1,3,4-oxadiazoles 1 from D-ribose conversion with L-amino acids in Scheme 1. .
.
 .
.
Two representative procedures are generally utilized for the construction of the 1,3,4-oxadizole core, as depicted in Figure 1 [12]. The cyclodehydration route from diacylhydrazine [C] is suitable for pyrrole-2-carboxylic acids [36], whereas the oxidative cyclization route from N-acylhydrazone [D] is widely used for pyrrole-2-carbaldehydes as starting materials [37]. There were various cyclization conditions for 1,3,4-oxadiazoles reported for each conversion [12]. We adopted the oxidative cyclization route of N-acylhydrazones 4, which can be obtained from pyrrole-2-carbaldehydes 2 by condensation with benzohydrazide 3a. The corresponding N-benzoylhydrazones 4a–4j were obtained in decent yields (70~96%) at the reflux temperature of toluene. Oxidative cyclization conditions were then screened using NBS, NIS, and I2 under K2CO3, DBU, Et3N, and NaOH as a base. The condition using NIS/NaOH in DMSO at 100 °C was optimal for providing pyrrole-ligated 1,3,4-oxadiazoles 1 in yields of 80~98%. It is noteworthy that the NIS-mediated further cyclization of the methylsulfide chain on the pyrrole ring occurred partly for 1j derived from methionine to produce 1k (at a yield of 49%), which explains the lower yield of 1j (50%). All eleven pyrrole-ligated oxadiazoles 1a–1j were rapidly assembled from pyrrole platform chemicals 2 with different amino acid residues and ready for antibacterial assays against Escherichia coli and Staphylococcus aureus as two representative Gram(–) and Gram(+) bacteria (Table 2).

Table 2.
Minimum inhibition activity (MIC) of 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 1 against E. coli and S. aureus.
There was a definite size effect of the alkyl substituent R on antibacterial activity in 1,3,4-oxadiazoles 1. The highest MIC value was required for 1a from glycine (R = H), and it decreased as the size (branch) of the alkyl group increased from alanine (R = Me) to isoleucine (R = s-Bu) (entries 1–5, Table 2). A benzene ring seemed be unimportant, judging from the cases of the benzyl and homobenzyl substituents (entries 6–7). There was no functional group effect for the ester and sulfide, reflecting a lack of electronic interactions between the substituent R and the bacterial enzymes. A comparison of the MIC values for 1j and its cyclized derivative 1k confirmed the importance of the size (or rigidity) effect of R on antibacterial activity (entries 10 and 11). An additional point to mention is that the MIC values for 1 were not much different between Gram(–) and Gram(+) bacteria, indicating that there would be no transport barriers through membranes for these small molecules.
The electronic effects of the substituents on the phenyl ring against antibacterial activities were then investigated for 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 5 and 6 with the maximum size effect in the series, derived from valine (R = isopropyl) and isoleucine (R = sec-butyl), respectively. Commercial benzohydrazides 3 with a substituent X of a different electronic nature (e.g., F, Cl, OH, and OMe) were utilized in the synthesis of 5 and 6 (Scheme 2 and Table 3). The condensation reaction of pyrrole-2-carbaldehyde 2c (R = i-Pr) and 2e (R = s-Bu) with various benzohydrazides 3 produced the corresponding N-benzoylhydrazone intermediates in refluxing toluene, which underwent an oxidative cyclization reaction (without purification) to afford 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 5 (R = i-Pr) and 6 (R = s-Bu) with various electronic substituents X on the phenyl ring.

Scheme 2.
Two-step preparation of various 1,3,4-oxadiazoles 5 and 6 with various electronic substituents X, derived from valine and isoleucine.

Table 3.
Yields of various 1,3,4-oxadiazoles 5 and 6 with various electronic substituents X, derived from valine and isoleucine in Scheme 2.
A milder oxidative cyclization condition was required in these cases, for which I2/K2CO3 in 1,4-dioxane at 85 °C was optimal for the production of 5 in yields of 40~85% and 6 in yields of 62~89% in two steps [38]. Serendipitously, we found extra iodination reactions under the oxidative cyclization conditions for 1,3,4-oxadiazoles 5e, 5f, 6e, and 6f with phenol substituents, obtaining the corresponding di-iodination or tetra-iodination products 5e-1, 5f-1, 6e-1, and 6f-1, respectively in yields of 32~45% (Figure 2). It was very fortunate to achieve further iodination on the phenol rings so that we were able to find the superior Iodophenol antibacterial effects for these oxadiazole derivatives (vide infra). The corresponding deiodination (originally intended) products were prepared by reduction using Zn dust in AcOH to produce 5f (X = p-OH) in a yield of 97%, 6e (X = o-OH) in a yield of 50%, and 6f (X = p-OH) in a yield of 42%.

Figure 2.
Extra iodination products from 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 5 and 6.
To assess the “iodine effect” on antibacterial activity, separate iodination reactions (I2 in DMSO at 85 °C) were intentionally carried out for the 1,3,4-oxadiazoles 5g, 5h, and 6h with the electron-rich anisole substituent in which 3-mono-iodination or 3,4-di-iodination reactions proceeded on the pyrrole ring (not on the anisole ring) to provide the corresponding 5g-1 (X = o-OMe) in a yield of 61%, 5h-1 (X = p-OMe) in a yield of 69% as a 1.3:1 mixture of di- and 3’-mono-iodination products, and 6h-1 (X = p-OMe) in a yield of 83%, respectively. All twenty-two pyrrole-ligated oxadiazoles 5 and 6 were rapidly assembled from pyrrole-2-carbaldehydes 2c (R = i-Pr) and 2e (R = s-Bu) with diversely X-substituted benzohydrazide 3 and ready for antibacterial assays against Escherichia coli, Staphylococcus aureus, and Acinetobacter baumannii together with Vancomycin and Erythromycin as positive controls (Table 4).

Table 4.
Minimum inhibition concentration (MIC) of 1,3,4-oxadiazoles 5 and 6 against E. coli, S. aureus, and A. baumannii.
SARs of the phenyl substituent X of 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 5 and 6 can be deduced from the MIC (μg/mL) in Table 4. ortho-F substitutions provided generally better antibacterial activities than the meta- and para-F counterparts (entries 1–3 and 12–14), and chloride was better than fluoride (entries 4 and 15 versus 3 and 14). Iodophenol substituents exhibited superior antibacterial activities against A. baumannii and S. aureus regardless of the position of the hydroxyl substituent (entries 5, 6, 16, and 18). The MIC values of <2 μg/mL for 5f-1 and 8 μg/mL for 6f-1 against A. baumannii were much lower than those of the positive controls (>1024 μg/mL for vancomycin and 128 μg/mL for erythromycin). The “iodophenol effect” on antibacterial activity is obvious when compared with the cases of deiodination products 5f and 6f, the MIC values for which were significantly increased to 128 μg/mL and 512 μg/mL, respectively (entries 7 and 19). The mechanism of the “iodophenol effect” is not clear at present, but it is reasonable to explain that iodide or molecular I2 may be liberated by the neighboring OH group [39]. No effects or only slight improvements in antibacterial activities were observed for the pyrrole iodination products 5g-1 and 5h-1 from 5g and 5h (entries 8–11), whereas the reverse effect was clear for the pyrrole iodination product 6h-1 from 6h (entries 21 and 22).
3. Materials and Methods
3.1. Experimental
3.1.1. General Chemical Syntheses
1H- and 13C-NMR spectra were recorded on 400 MHz and 100 MHz NMR spectrometers, respectively, in a deuterated solvent (notified in parenthesis) with tetramethylsilane (TMS) as an internal reference. The column chromatography was performed using the method of Still with silica gel 60 and a 70–230 mesh ASTM, using a gradient mixture of EtOAc/hexanes. Reactions were performed in a well-dried flask under an argon atmosphere unless mentioned otherwise.
3.1.2. General Procedure for the Preparation of 1
Formation of Hydrazone 4 from pyrrole-2-carbaldehyde (Step-1): The solution of pyrraline 2 (~1.00 g, 1 equiv.) and benzohydrazide 3a (1 equiv.) in toluene (10 mL) was heated at 110 °C for 6 h. The reaction mixture was cooled to room temperature, diluted with EtOAc, washed with brine and H2O, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography to obtain the corresponding benzohydrazone 4.
2-pyrrolyl-5-phenyl-1,3,4-oxadiazole 1 from hydrazone 4 (Step-2): The mixture of benzohydrazone 4 (1 equiv.), NaOH (2 equiv.), and N-iodosuccinimide (1 equiv.) in DMSO (10 mL) was heated at 110 °C for 2 h. The reaction mixture was cooled to room temperature, diluted with EtOAc, washed with brine and H2O, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography to obtain the corresponding 2-pyrrolyl-5-phenyl-1,3,4-oxadiazole 1.
Methyl 2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)acetate (1a). Data for 4a: white solid in a yield of 96% (1.64 g, 5.76 mmol); 1H-NMR (DMSO-d6) δ = 3.69 (s, 3H), 5.21 (s, 2H), 6.16 (dd, J = 3.6, 2.8 Hz, 1H), 6.53 (dd, J = 3.6, 1.6 Hz, 1H), 7.02 (dd, J = 2.8, 1.6 Hz, 1H), 7.48–7.54 (m, 2H), 7.54–7.60 (m, 1H), 7.86–7.90 (m, 2H), 8.28 (s, 1H), 11.49 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 51.1, 53.1, 109.9, 117.2, 128.4, 128.7, 129.7, 129.9, 132.7, 134.9, 142.0, 163.7, 170.7 ppm; IR (CH2Cl2) ν = 3237, 3063, 3006, 2954, 2848, 1750, 1649, 1616, 1552, 1494, 1468, 1433, 1345, 1322, 1279, 1216, 1188, 1140, 1086, 1031, 1001, 914, 801, 754, 690 cm−1.
Data for 1a: light-yellow solid in a yield of 80% (0.60 g, 2.13 mmol); 1H-NMR (acetone-d6) δ = 3.73 (s, 3H), 5.39 (s, 2H), 6.32 (dd, J = 4.0, 2.4 Hz, 1H), 7.06 (dd, J = 4.0, 1.6 Hz, 1H), 7.18 (dd, J = 2.4, 1.6 Hz, 1H), 7.57–7.65 (m, 3H), 8.08–8.14 (m, 2H) ppm; 13C-NMR (acetone-d6) δ = 50.1, 51.6, 109.2, 114.3, 117.5, 124.0, 126.5, 129.1, 129.2, 131.6, 159.1, 162.4, 168.9 ppm; IR ν = 3119, 3069, 2957, 2927, 2857, 1752, 1713, 1614, 1556, 1501, 1490, 1455, 1424, 1401, 1376, 1328, 1304, 1297, 1264, 1213, 1106, 1081, 995, 956, 787, 773, 760, 725, 693, 583 cm−1; HRMS (ESI) calcd for C15H13N3O3+Na, 306.0849: found 306.0851.
Methyl (S)-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)propanoate (1b). Data for 4b: white solid in a yield of 88% (1.45 g, 4.85 mmol); 1H-NMR (DMSO-d6) δ = 1.70 (d, J = 7.2 Hz, 3H), 3.67 (s, 3H), 6.02 (q, J = 7.2 Hz, 1H), 6.20 (dd, J = 3.6, 2.8 Hz, 1H), 6.54 (dd, J = 3.6, 1.6 Hz, 1H), 7.17 (dd, J = 2.8, 1.6 Hz, 1H), 7.48–7.61 (m, 3H), 7.86–7.92 (m, 2H), 8.32 (s, 1H), 11.51 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 19.1, 53.4, 56.1, 110.2, 117.5, 126.6, 128.2, 128.7, 129.6, 132.7, 134.9, 142.4, 163.7, 172.8 ppm; IR ν = 3232, 3065, 2954, 1748, 1716, 1644, 1612, 1556, 1494, 1461, 1426, 1359, 1286, 1225, 1146, 1091, 1063, 1031, 962, 910, 887, 857, 802, 714, 695 cm−1; HRMS (ESI) calcd for C16H17N3O3+Na, 322.1162: found 322.1164.
Data for 1b: light-yellow solid in a yield of 91% (0.70 g, 2.35 mmol); 1H-NMR (CD3OD) δ = 1.82 (d, J = 7.2 Hz, 3H), 3.71 (s, 3H), 6.00 (q, J = 7.2 Hz, 1H), 6.33 (dd, J = 4.0, 2.8 Hz, 1H), 7.02 (dd, J = 4.0, 1.6 Hz, 1H), 7.23 (dd, J = 2.8, 1.6 Hz, 1H), 7.50–7.59 (m, 3H), 8.00–8.04 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 19.5, 54.5, 58.5, 112.2, 117.6, 119.7, 126.0, 128.1, 129.1, 131.7, 134.4, 162.1, 165.5, 174.7 ppm; IR ν = 3129, 3003, 2955, 2848, 1751, 1663, 1608, 1553, 1505, 1450, 1384, 1336, 1289, 1224, 1203, 1110, 1091, 1062, 1015, 981, 962, 853, 813, 776, 729, 691, 610 cm−1; HRMS (ESI) calcd for C16H15N3O3+Na, 320.1006: found 320.1007.
Methyl (S)-3-methyl-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)butanoate (1c). Data for 4c: white solid in a yield of 83% (1.30 g, 3.98 mmol); 1H-NMR (DMSO-d6) δ = 1.03 (d, J = 6.8 Hz, 3H), 1.35 (d, J = 6.8 Hz, 3H), 2.76 (m, 1H), 4.04 (s, 3H), 6.35 (d, J = 8.8 Hz, 1H), 6.57 (dd, J = 3.6, 2.8 Hz, 1H), 6.87 (dd, J = 3.6, 1.6 Hz, 1H), 7.51 (dd, J = 2.8, 1.6 Hz, 1H), 7.82–7.97 (m, 3H), 8.21–8.30 (m, 2H), 8.73 (s, 1H), 11.90 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 17.6, 18.4, 31.3, 51.4, 62.8, 108.8, 114.6, 124.7, 126.6, 126.7, 127.6, 130.7, 132.8, 140.3, 161.7, 169.9 ppm; IR ν = 3241, 3071, 2975, 2878, 1750, 1644, 1615, 1557, 1495, 1459, 1433, 1392, 1356, 1286, 1211, 1160, 1133, 1083, 1057, 1023, 1005, 952, 915, 890, 835, 800, 755, 715, 695, 617 cm−1.
Data for 1c: light-yellow solid in a yield of 98% (0.15 g, 0.46 mmol); 1H-NMR (CD3OD) δ = 0.81 (d, J = 7.2 Hz, 3H), 1.08 (d, J = 7.2 Hz, 3H), 2.53 (m, 1H), 3.76 (s, 3H), 5.99 (d, J = 10.0 Hz, 1H), 6.38 (dd, J = 4.0, 2.8 Hz, 1H), 7.03 (dd, J = 4.0, 1.6 Hz, 1H), 7.36 (dd, J = 2.8, 1.6 Hz, 1H), 7.54–7.64 (m, 3H), 8.06–8.12 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 20.4, 21.1, 35.2, 54.1, 67.1, 112.3, 119.0, 128.5, 130.0, 130.5, 131.1, 134.4, 136.0, 145.0, 167.8, 174.2 ppm; IR ν = 2958, 2927, 2851, 1744, 1669, 1609, 1560, 1500, 1454, 1376, 1260, 1221, 1105, 1076, 1013, 775, 727, 691 cm−1; HRMS (ESI) calcd for C18H19N3O3+Na, 348.1319: found 348.1319.
Methyl (S)-4-methyl-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)pentanoate (1d). Data for 4d: white solid in a yield of 77% (1.18 g, 3.46 mmol); 1H-NMR (DMSO-d6) δ = 0.86 (d, J = 6.4 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H), 1.92 (dd of A of ABq, JAB = 14.0, Jd = 9.2, 4.8 Hz, 1H), 2.12 (dd of B of ABq, JAB = 14.0, Jd = 11.6, 4.4 Hz, 1H), 3.67 (s, 3H), 6.21 (dd, J = 3.6, 2.8 Hz, 1H), 6.30 (dd, J = 9.2, 4.4 Hz, 1H), 6.52 (dd, J = 3.6, 1.6 Hz, 1H), 7.19 (dd, J = 2.8, 1.6 Hz, 1H), 7.48–7.62 (m, 3H), 7.86–7.92 (m, 2H), 8.34 (s, 1H), 11.52 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 22.6, 24.1, 25.7, 42.1, 53.5, 58.5, 110.6, 117.6, 127.2, 128.4, 128.7, 129.6, 132.7, 134.8, 142.5, 163.6, 172.8 ppm; IR ν = 3234, 3065, 2956, 2874, 1743, 1646, 1614, 1556, 1495, 1459, 1427, 1348, 1278, 1240, 1203, 1174, 1086, 1034, 998, 953, 928, 903, 795, 754 cm−1.
Data for 1d: light-yellow solid in 87% yield (1.35g, 4.01 mmol); 1H-NMR (acetone-d6) δ = 0.94 (d, J = 6.4 Hz, 3H), 0.95 (d, J = 6.4 Hz, 3H), 1.49 (m, 1H), 2.02–2.16 (m, 1H), 2.16–2.30 (m, 1H), 3.71 (s, 3H), 6.38 (dd, J = 3.6, 2.8 Hz, 1H), 7.06 (dd, J = 3.6, 1.6 Hz, 1H), 7.34 (dd, J = 2.8, 1.6 Hz, 1H), 7.56–7.65 (m, 3H), 8.08–8.16 (m, 2H) ppm; 13C-NMR (acetone-d6) δ = 20.9, 22.3, 24.7, 41.0, 51.9, 58.0, 109.8, 114.4, 117.6, 123.9, 126.1, 126.6, 129.2, 131.6, 159.3, 162.3, 171.2 ppm; IR ν = 3119, 3069, 2957, 2867, 1750, 1704, 1664, 1609, 1557, 1504, 1454, 1412, 1369, 1333, 1273, 1240, 1197, 1176, 1133, 1106, 1080, 1021, 1000, 964, 925, 880, 836, 811, 730, 690, 613 cm−1; HRMS (ESI) calcd for C19H21N3O3+Na, 362.1475: found 362.1474.
Methyl (2S,3S)-3-methyl-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)pentanoate (1e). Data for 4e: white solid in a yield of 86% (0.91 g, 2.67 mmol); 1H-NMR (DMSO-d6) δ = 0.79 (t, J = 7.2 Hz, 3H), 0.96 (d, J = 6.4 Hz, 3H), 1.01–1.20 (m 2H), 2.15–2.26 (m 2H), 3.69 (s, 3H), 6.03 (d, J = 6.8 Hz, 1H), 6.22 (dd, J = 3.6, 2.8 Hz, 1H), 6.52 (dd, J = 3.6, 1.6 Hz, 1H), 7.18 (dd, J = 2.8, 1.6 Hz, 1H), 7.49–7.62 (m, 3H), 7.88–7.95 (m, 2H), 8.39 (s, 1H), 11.55 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 10.7, 15.5, 24.3, 38.1, 52.2, 62.8, 109.7, 115.5, 125.4, 127.5, 127.6, 128.4, 131.6, 133.6, 141.1, 162.5, 170.9 ppm.
Data for 1e: light-yellow solid in 90% yield (0.42 g, 1.23 mmol, a 2:1 mixture of stereoisomers); 1H-NMR (major isomer, CDCl3) δ = 0.84 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.06–1.28 (m, 2H), 2.27–2.40 (m, 1H), 3.75 (s, 3H), 6.18 (d, J = 10.4 Hz, 1H), 6.39 (dd, J = 4.0, 2.8 Hz, 1H), 7.04 (dd, J = 4.0, 2.0 Hz, 1H), 7.40 (dd, J = 2.8, 2.0 Hz, 1H), 7.58–7.66 (m, 3H), 8.10–8.16 (m, 2H) ppm; 13C-NMR (major isomer, CDCl3) δ = 10.1, 15.0, 24.7, 38.8, 51.7, 63.7, 110.1, 114.0, 114.1, 123.9, 126.0, 126.6, 129.2, 131.6, 159.3, 162.4, 170.7 ppm; IR ν = 3144, 3124, 3066, 2970, 2932, 2879, 1747, 1704, 1606, 1552, 1501, 1492, 1449, 1414, 1387, 1334, 1284, 1236, 1196, 1178, 1100, 1077, 1020, 964, 926, 883, 727, 694, 618 cm−1; HRMS (ESI) calcd for C19H21N3O3+Na, 362.1475: found 362.1475.
Methyl (S)-3-phenyl-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)propanoate (1f). Data for 4f: white solid in a yield of 83% (0.94 g, 2.51 mmol); 1H-NMR (DMSO-d6) δ = 3.42 (d of A of ABq, JAB = 14.4, Jd = 10.0 Hz, 1H), 3.49 (d of B of ABq, JAB = 14.4, Jd = 6.4 Hz, 1H), 3.67 (s, 3H), 6.11 (dd, J = 3.6, 2.8 Hz, 1H), 6.43 (dd, J = 3.6, 1.6 Hz, 1H), 6.49 (dd, J = 10.0, 6.4 Hz, 1H), 7.10–7.25 (m, 6H), 7.50–7.62 (m, 3H), 7.88–7.93 (m, 2H), 8.23 (s, 1H), 11.50 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 39.1, 53.6, 61.1, 110.4, 117.6, 127.5, 127.7, 128.2, 128.7, 129.3, 129.7, 130.4, 132.8, 134.9, 137.9, 142.2, 163.8, 171.7 ppm; IR ν = 3236, 3064, 3033, 2957, 2843, 1743, 1645, 1613, 1555, 1497, 1455, 1436, 1348, 1280, 1220, 1185, 1164, 1076, 1032, 1008, 904, 843, 802, 753, 699 cm−1.
Data for 1f: light-yellow solid in a yield of 86% (2.31 g, 6.19 mmol); 1H-NMR (CD3OD) δ = 3.38 (d of A of ABq, JAB = 14.0, Jd = 10.0 Hz, 1H), 3.59 (d of B of ABq, JAB = 14.0, Jd = 5.2 Hz, 1H), 3.75 (s, 3H), 6.29 (dd, J = 4.0, 2.8 Hz, 1H), 6.39 (dd, J = 10.0, 5.2 Hz, 1H), 6.90 (dd, J = 4.0, 1.6 Hz, 1H), 7.02–7.14 (m, 5H), 7.24 (dd, J = 2.8, 1.6 Hz, 1H), 7.54–7.62 (m, 3H), 8.01–8.05 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 41.4, 54.6, 63.9, 112.5, 117.3, 119.8, 126.0, 129.2, 129.2, 129.4, 130.7, 131.5, 131.8, 134.5, 139.0, 162.1, 165.5, 173.4 ppm; IR ν = 3068, 3033, 3004, 2956, 2848, 1747, 1703, 1665, 1606, 1559, 1505, 1449, 1412, 1372, 1337, 1274, 1230, 1201, 1174, 1107, 1083, 1012, 982, 926, 884, 778, 728, 699, 615 cm−1; HRMS (ESI) calcd for C22H19N3O3+Na 396.1319, found 396.1321.
Methyl (S)-4-phenyl-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)butanoate (1g). Data for 4g: white solid in a yield of 79% (1.31 g, 3.48 mmol); 1H-NMR (DMSO-d6) δ = 2.36–2.60 (m, 4H), 3.68 (s, 3H), 6.00–6.07 (m, 1H), 6.26 (dd, J = 3.6, 2.8 Hz, 1H), 6.58 (dd, J = 3.6, 1.2 Hz, 1H), 7.11–7.28 (m, 6H), 7.50–7.61 (m, 3H), 7.87–7.92 (m, 2H), 8.34 (s, 1H), 11.52 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 32.8, 35.1, 53.5, 60.1, 110.6, 117.2, 127.2, 128.5, 128.7, 129.5, 129.6, 129.6, 129.6, 132.7, 134.8, 141.8, 142.3, 163.7, 172.1 ppm; IR ν = 3227, 3063, 3030, 2951, 2866, 1744, 1646, 1610, 1558, 1494, 1457, 1431, 1325, 1287, 1217, 1164, 1079, 1055, 1037, 1029, 1004, 952, 913, 755, 698 cm−1.
Data for 1g: light-yellow solid in 86% yield (0.42 g, 1.13 mmol); 1H-NMR (CD3OD) δ = 2.40–2.67 (m, 4H), 3.71 (s, 3H), 5.92 (dd, J = 10.4, 4.0 Hz, 1H), 6.43 (dd, J = 4.0, 2.8 Hz, 1H), 6.99–7.05 (m, 3H), 7.06 (dd, J = 4.0, 1.6 Hz, 1H), 7.08–7.16 (m, 2H), 7.31 (dd, J = 2.8, 1.6 Hz, 1H), 7.53–7.62 (m, 3H), 8.00–8.06 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 31.4, 33.3, 51.7, 58.9, 110.0, 114.5, 117.3, 123.2, 125.8, 126.0, 126.4, 128.0, 128.0, 129.0, 131.7, 139.8, 159.2, 162.7, 171.3 ppm; IR ν = 3064, 3026, 2956, 2931, 2854, 1745, 1662, 1606, 1557, 1504, 1450, 1415, 1254, 1233, 1198, 1177, 1082, 1012, 979, 814, 772, 725, 698, 605 cm−1; HRMS (ESI) calcd for C23H21N3O3+Na, 410.1475: found 410.1477.
Dimethyl (S)-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)succinate (1h). Data for 4h: white solid in a yield of 81% (1.01g, 2.84 mmol); 1H-NMR (CD3OD) δ = 3.27 (d of A of ABq, JAB = 16.8, Jd = 8.0 Hz, 1H), 3.37 (d of B of ABq, JAB = 16.8, Jd = 6.0 Hz, 1H), 3.63 (s, 3H), 3.73 (s, 3H), 6.20 (dd, J = 3.6, 2.8 Hz, 1H), 6.32 (dd, J = 8.0, 6.0 Hz, 1H), 6.58 (dd, J = 3.6, 1.6 Hz, 1H), 7.02 (dd, J = 2.8, 1.6 Hz, 1H), 7.46–7.60 (m, 3H), 7.86–7.91 (m, 2H), 8.24 (s, 1H) ppm; 13C-NMR (CD3OD) δ = 39.4, 53.8, 54.6, 59.5, 112.0, 120.3, 129.4, 129.9, 130.0, 131.1, 134.4, 136.0, 144.6, 167.8, 173.2, 173.8 ppm; IR ν = 3411, 2958, 1737, 1644, 1613, 1581, 1554, 1495, 1444, 1414, 1348, 1285, 1238, 1177, 1123, 1084, 1008, 979, 908, 803, 786, 712 cm−1.
Data for 1h: light-yellow solid in 88% yield (0.79 g, 2.22 mmol); 1H-NMR (CD3OD) δ = 3.25 (d of A of ABq, JAB = 16.8, Jd = 8.0 Hz, 1H), 3.41 (d of B of ABq, JAB = 16.8, Jd = 6.0 Hz, 1H), 3.63 (s, 3H), 3.72 (s, 3H), 6.32 (dd, J = 3.6, 2.8 Hz, 1H), 6.39 (dd, J = 8.0, 6.0 Hz, 1H), 7.03 (dd, J = 3.6, 1.6 Hz, 1H), 7.17 (dd, J = 2.8, 1.6 Hz, 1H), 7.50–7.60 (m, 3H), 8.00–8.07 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 36.4, 51.2, 52.0, 56.8, 109.8, 115.0, 117.0, 123.2, 126.4, 126.7, 129.0, 131.7, 159.1, 162.8, 169.9, 170.5 ppm; IR ν = 3123, 3006, 2958, 2854, 1741, 1662, 1605, 1553, 1505, 1485, 1439, 1417, 1371, 1271, 1224, 1173, 1083, 1010, 985, 860, 819, 781, 726, 692, 672, 611 cm−1; HRMS (ESI) calcd for C18H17N3O5+Na, 378.1060: found 378.1062.
Dimethyl (S)-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)pentanedioate (1i). Data for 4i: white solid in a yield of 70% (1.52 g, 4.08 mmol), 1H-NMR (DMSO-d6) δ = 2.10–2.21 (m, 1H), 2.24–2.42 (m, 2H), 2.44–2.53 (m, 1H), 3.55 (s, 3H), 3.70 (s, 3H), 6.01–6.09 (m, 1H), 6.22 (dd, J = 3.6, 2.8 Hz, 1H), 6.55 (dd, J = 3.6, 1.6 Hz, 1H), 7.12 (dd, J = 2.8, 1.6 Hz, 1H), 7.49–7.61 (m, 3H), 7.87–7.92 (m, 2H), 8.32 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 28.7, 31.3, 52.7, 53.6, 59.7, 110.7, 117.5, 127.6, 128.5, 128.7, 129.7, 132.8, 134.8, 142.2, 163.7, 171.7, 173.5 ppm; IR ν = 3234, 3020, 2954, 1738, 1650, 1615, 1558, 1458, 1437, 1346, 1280, 1218, 1177, 1075, 1029, 913, 888, 801, 753 cm−1.
Data for 1i: light-yellow solid in a yield of 95% (1.04 g, 2.82 mmol); 1H-NMR (acetone-d6) δ = 2.24–2.39 (m, 2H), 2.44–2.54 (m, 1H), 2.63–2.73 (m, 1H), 3.57 (s, 3H), 3.73 (s, 3H), 6.24 (dd, J = 10.8, 5.2 Hz, 1H), 6.39 (dd, J = 3.6, 2.8 Hz, 1H), 7.06 (dd, J = 3.6, 1.6 Hz, 1H), 7.30 (dd, J = 2.8, 1.6 Hz, 1H), 7.58–7.65 (m, 3H), 8.10–8.16 (m, 2H) ppm; 13C-NMR (acetone-d6) δ = 29.4, 31.4, 52.6, 53.7, 60.7, 111.8, 116.2, 119.4, 125.6, 128.0, 128.3, 130.9, 133.3, 160.9, 164.1, 172.0, 173.7 ppm; IR ν = 3120, 3005, 2956, 2923, 2853, 1741, 1664, 1607, 1552, 1504, 1490, 1450, 1371, 1240, 1202, 1174, 1106, 1078, 1012, 989, 964, 930, 882, 847, 821, 727, 694, 612 cm−1; HRMS (ESI) calcd for C19H19N3O5+Na, 392.1217: found 392.1220.
Methyl (S)-4-(methylthio)-2-(2-(5-phenyl-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)butanoate (1j) and methyl (S)-6-(5-phenyl-1,3,4-oxadiazol-2-yl)-3,4-dihydro-2H-pyrrolo[2,1-b][1,3]thiazine-4-carboxylate (1k). Data for 4j: white solid in a yield of 82% (0.77 g, 2.14 mmol); 1H-NMR (DMSO-d6) δ = 2.02 (s, 3H), 2.24–2.46 (m, 4H), 3.69 (s, 3H), 5.97–6.05 (m, 1H), 6.22 (dd, J = 3.6, 2.8 Hz, 1H), 6.55 (dd, J = 3.6, 1.2 Hz, 1H), 7.15 (dd, J = 2.8, 1.2 Hz, 1H), 7.48–7.61 (m, 3H), 7.86–8.02 (m, 2H), 8.33 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 15.8, 30.8, 32.9, 53.6, 59.7, 110.6, 117.3, 127.4, 128.5, 128.7, 129.7, 132.8, 134.8, 142.1, 163.7, 171.8 ppm; IR ν = 3230, 3006, 2957, 12918, 2849, 1743, 1649, 1614, 1556, 1491, 1459, 1431, 1350, 1288, 1230, 1209, 1144, 1091, 1033, 1001, 955, 909, 888, 798, 756, 613 cm−1.
Data for 1j: light-yellow solid in a yield of 50% (0.31 g, 0.86 mmol); 1H-NMR (CD3OD) δ = 2.03 (s, 3H), 2.28–2.36 (m, 1H), 2.43–2.63 (m, 3H), 3.74 (s, 3H), 6.18 (dd, J = 10.0, 4.8 Hz, 1H), 6.39 (dd, J = 4.0, 2.8 Hz, 1H), 7.07 (dd, J = 4.0, 2.0 Hz, 1H), 7.25 (dd, J = 2.8, 2.0 Hz, 1H), 7.55–7.63 (m, 3H), 8.06–8.10 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 13.7, 29.6, 31.1, 51.8, 58.7, 109.8, 114.8, 117.2, 123.2, 126.4, 129.0, 131.7, 159.2, 162.8, 171.0, 179.6 ppm; IR ν = 2956, 2925, 2857, 1750, 1667, 1603, 1554, 1505, 1452, 1381, 1274, 1243, 1090, 1016, 961, 883, 817, 774, 732, 691 cm−1; HRMS (ESI) calcd for C18H19N3O3S+Na, 380.1039: found 380.1040.
Data for 1k: light-yellow solid in 49% yield (0.31g, 0.72 mmol); 1H-NMR (CD3OD) δ = 2.39–2.49 (m, 1H), 2.86–3.02 (m, 3H), 3.73 (s, 3H), 5.94 (dd, J = 5.2, 3.2 Hz, 1H), 6.06 (d, J = 4.0 Hz, 1H), 7.01 (d, J = 4.0 Hz, 1H), 7.49–7.57 (m, 3H), 7.97–8.01 (m, 2H) ppm; 13C-NMR (CD3OD) δ = 22.0, 28.0, 53.3, 58.2, 108.5, 116.1, 119.0, 124.7, 127.7, 128.7, 130.3, 132.9, 160.2, 163.6, 172.5 ppm; IR ν = 3008, 2948, 2852, 1751, 1648, 1604, 1552, 1497, 1439, 1401, 1355, 1292, 1257, 1214, 1176, 1145, 1125, 1085, 1070, 1023, 974, 929, 898, 854, 758, 727, 696 cm−1; HRMS (ESI) calcd for C17H15N3O3S+Na, 364.0726: found 364.0729.
3.1.3. General Procedure for the Preparation of 5 (R = i-Pr) and 6 (R = s-Bu)
Step-1: At 25 °C, under an argon atmosphere, benzohydrazide 3 (1.1 equiv.) was added to a stirred solution of pyrrole-2-carbaldehyde 2 (~1.0 g, 1 equiv.) in toluene/DMSO (v:v = 15:1). The mixture was heated at 110 °C for 12 h and cooled to room temperature. The solvent was removed under reduced pressure in a rotary evaporator, and the crude product was filtered through a short pad of SiO2 (EtOAc eluent) and concentrated under reduced pressure.
Step-2: I2 (1.2 equiv.) and K2CO3 (3 equiv.) were added to a stirred solution of the above imine in 1,4-dioxane (20 mL). The mixture was heated at 85 °C for 6h under an argon atmosphere and cooled to room temperature. The mixture was diluted with CH2Cl2, washed with a 10% Na2S2O3 solution, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The product was purified by SiO2 flash column chromatography to obtain pyrrole-fused 1,3,4-oxadiazole 5 or 6.
Methyl (S)-2-(2-(5-(2-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5a). Orange oil, 40% yield (663 mg, 1.93 mmol); Rf = 0.45 (4:1 hexane/EtOAc); Data for 5a: 1H-NMR (CDCl3) δ = 0.83 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H), 2.48 (m, 1H), 3.75 (s, 3H), 6.15 (d, J = 10.0 Hz, 1H), 6.36 (dd, J = 4.0, 3.2 Hz, 1H), 6.98 (dd, J = 4.0, 2.0 Hz, 1H), 7.23–7.28 (m, 1H), 7.28–7.34 (m, 1H), 7.31 (dd, J = 3.2, 2.0 Hz, 1H), 7.50–7.57 (m, 1H), 8.08–8.13 (m, 1H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.2, 52.3, 64.6, 110.4, 114.5, 117.0 (d, J = 20.5 Hz), 117.8, 124.6 (d, J = 3.1 Hz), 126.0, 129.6 (d, J = 1.5 Hz), 133.3 (d, J = 8.4 Hz), 158.8, 159.4, (d, J = 5.4 Hz), 159.7 (d, J = 1.5 Hz), 161.3, 171.3 ppm; IR ν = 2970, 2880, 1748, 1605, 1500, 1475, 1450, 1273, 1239, 1219, 1200, 1185, 1170, 1102, 1078, 1009, 827, 742, 669, 617 cm−1; HRMS (ESI) calcd for C18H18FN3O3+Na 366.1224: found 366.1225.
Methyl (S)-2-(2-(5-(3-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5b). Orange oil, 70% yield (759 mg, 2.21 mmol); Rf = 0.59 (4:1 hexane/EtOAc); Data for 5b: 1H-NMR (CDCl3) δ = 0.83 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H), 2.49 (m, 1H), 3.75 (s, 3H), 6.14 (d, J = 10.0 Hz, 1H), 6.37 (dd, J = 4.0, 2.8 Hz, 1H), 6.97 (dd, J = 4.0, 2.0 Hz, 1H), 7.20–7.27 (m, 1H), 7.32 (dd, J = 2.8, 2.0 Hz, 1H), 7.47–7.54 (m, 1H), 7.76–7.81 (m, 1H), 7.87–7.91 (m, 1H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.1, 52.3, 64.6, 110.4, 113.8 (d, J = 24.2 Hz), 114.4 117.7, 118.6 (d, J = 21.2 Hz), 122.5 (d, J = 3.0 Hz), 125.7 (d, J = 9.1 Hz), 126.1, 130.9 (d, J = 8.3 Hz), 159.7, 161.6, (d, J = 2.3 Hz), 164.0, 171.2 ppm; IR ν = 2970, 2880, 1750, 1600, 1565, 1500, 1495, 1450, 1410, 1375, 1307, 1275, 1240, 1205, 1181, 1105, 1080, 1010, 995, 870, 800, 735, 680, 615 cm−1; HRMS (ESI) calcd for C18H18FN3O3+Na 366.1224: found 366.1226.
Methyl (S)-2-(2-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5c). Yellow oil, 54% yield (721 mg, 2.10 mmol); Rf = 0.65 (4:1 hexane/EtOAc); Data for 5c: 1H-NMR (CDCl3) δ = 0.83 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H), 2.48 (m, 1H), 3.75 (s, 3H), 6.14 (d, J = 10.0 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.94 (dd, J = 4.0, 2.0 Hz, 1H), 7.18–7.25 (m, 2H), 7.31 (dd, J = 2.8, 2.0 Hz, 1H), 8.07–8.13 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.1, 52.3, 64.6, 110.3, 114.1, 116.4 (d, J = 22.0 Hz), 120.2 (d, J = 3.0 Hz), 125.9, 129.0 (d, J = 9.1 Hz), 159.4, 161.8, 163.4, 165.9, 171.2 ppm; IR ν = 2970, 2880, 1750, 1605, 1500, 1470, 1455, 1435, 1415, 1270, 1235, 1215, 1200, 1160, 1100, 1075, 1015, 965, 845, 740, 625 cm−1; HRMS (ESI) calcd for C18H18FN3O3+Na 366.1224, found 366.1228.
Methyl (S)-2-(2-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5d). Brown solid, 62% yield (533 mg, 1.48 mmol); Rf = 0.61 (4:1 hexane/EtOAc); Data for 5d: 1H-NMR (CDCl3) δ = 0.82 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H), 2.48 (m, 1H), 3.75 (s, 3H), 6.14 (d, J = 10.0 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.95 (dd, J = 4.0, 2.0 Hz, 1H), 7.31 (dd, J = 2.8, 2.0 Hz, 1H), 7.48–7.53 (m, 2H), 8.01–8.06 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.2, 52.3, 64.6, 110.3, 114.3, 117.8, 122.3, 126.0, 128.1, 129.5, 137.8, 159.6, 161.8, 171.2 ppm; IR ν = 2970, 2880, 1750, 1605, 1500, 1485, 1455, 1410, 1270, 1237, 1200, 1180, 1100, 1080, 1015, 970, 840, 740 cm−1; HRMS (ESI) calcd for C18H18ClN3O3+Na 382.0929, found 382.0933.
Methyl (S)-2-(2-(5-(2-hydroxy-3,5-diiodophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5e-1). White solid, 33% yield (748 mg, 1.26 mmol); Rf = 0.54 (4:1 hexane/EtOAc); Data for 5e-1: 1H-NMR (CDCl3) δ = 0.82 (d, J = 6.4 Hz, 3H), 1.09 (d, J = 6.4 Hz, 3H), 2.49 (m, 1H), 3.77 (s, 3H), 5.99 (d, J = 9.6 Hz, 1H), 6.40 (dd, J = 4.0, 2.8 Hz, 1H), 7.05 (dd, J = 4.0, 2.0 Hz, 1H), 7.36 (dd, J = 2.8, 2.0 Hz, 1H), 8.06 (d, J = 2.0 Hz, 1H), 8.18 (d, J = 2.0 Hz, 1H) 11.02 (s, 1H) ppm; 13C-NMR (CDCl3) δ = 18.6, 19.3, 33.2, 52.4, 64.9, 81.3, 86.7, 109.8, 110.8, 115.7, 116.9, 127.0, 134.7, 149.8, 156.2, 158.7, 160.1, 171.0 ppm; IR ν = 2970, 2890, 1750, 1600, 1565, 1535, 1500, 1445, 1415, 1380, 1255, 1240, 1220, 1185, 1105, 1080, 1015, 1000, 915, 870, 740, 660, 615, 600 cm−1; HRMS (ESI) calcd for C18H17I2N3O4+Na 615.9201: found 615.9203.
Methyl (S)-2-(2-(5-(4-hydroxy-2,3,5,6-tetraiodophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5f-1): Yellow solid, 32% yield (1.33 g, 1.57 mmol); Rf = 0.55 (4:1 hexane/EtOAc); Data for 5f-1. 1H-NMR (CDCl3) δ = 0.81 (d, J = 6.4 Hz, 3H), 1.07 (d, J = 6.4 Hz, 3H), 2.41–2.53 (m, 1H), 3.75 (s, 3H), 6.09 (d, J = 10.0 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.96 (dd, J = 4.0, 1.6 Hz, 1H), 7.31 (dd, J = 2.8, 1.6 Hz, 1H), 8.40 (s, 1H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.1, 52.3, 64.6, 82.4, 110.4, 114.5, 117.7, 119.9, 126.1, 137.6, 156.2, 159.5, 159.6, 171.2 ppm; IR ν = 3450, 3145, 3125, 2970, 2875, 1745, 1610, 1595, 1500, 1450, 1395, 1300, 1270, 1235, 1220, 1200, 1180, 1160, 1135, 1105, 1075, 1010, 995, 965, 910, 890, 810, 735, 710, 685, 650, 615 cm−1; HRMS (ESI) calcd for [C18H15I4N3O4 –I2+H2]+Na 615.9201: found 615.9213.
Methyl (S)-2-(2-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5g). Red oil, 85% yield (1.03 g, 2.89 mmol); Rf = 0.66 (3:2 hexane/EtOAc); Data for 5g: 1H-NMR (CDCl3) δ = 0.83 (d, J = 6.4 Hz, 3H), 1.07 (d, J = 6.4 Hz, 3H), 2.48 (m, 1H), 3.74 (s, 3H), 4.00 (s, 3H), 6.19 (d, J = 10.0 Hz, 1H), 6.35 (dd, J = 4.0, 2.8 Hz, 1H), 6.94 (dd, J = 4.0, 2.0 Hz, 1H), 7.06–7.12 (m, 2H), 7.29 (dd, J = 2.8, 2.0 Hz, 1H), 7.48–7.53 (m, 1H), 7.99 (dd, J = 7.6, 2.0 Hz, 1H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.2, 52.2, 56.0, 64.5, 110.1, 112.0, 112.9, 113.9, 118.2, 120.7, 125.5, 130.2, 132.8, 158.0, 159.0, 161.3, 171.4 ppm; IR ν = 2970, 2875, 2840, 1750, 1600, 1545, 1500, 1470, 1455, 1440, 1270, 1260, 1235, 1215, 1185, 1170, 1130, 1100, 1075, 1025, 750, 735, 675, 616 cm−1; HRMS (ESI) calcd for C19H21N3O4+Na 378.1424, found 378.1428.
Methyl (S)-2-(2-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5h). Ivory solid, 70% yield (1.12 g, 3.15 mmol); Rf = 0.70 (3:2 hexane/EtOAc); Data for 5h: 1H-NMR (CDCl3) δ = 0.82 (d, J = 6.4 Hz, 3H), 1.07 (d, J = 6.4 Hz, 3H), 2.48 (m, 1H), 3.74 (s, 3H), 3.89 (s, 3H), 6.15 (d, J = 10.0 Hz, 1H), 6.35 (dd, J = 4.0, 2.8 Hz, 1H), 6.92 (dd, J = 4.0, 2.0 Hz, 1H), 7.00–7.05 (m, 2H), 7.29 (dd, J = 2.8, 2.0 Hz, 1H), 8.01–8.05 (m, 2H), 7.99 (dd, J = 7.6, 2.0 Hz, 1H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.1, 52.3, 55.5, 64.5, 110.1, 113.8, 114.5, 116.4, 118.2, 125.6, 128.6, 159.0, 162.2, 162.6, 171.3 ppm; IR ν = 2970, 2875, 1745, 1610, 1600, 1505, 1450, 1395, 1300, 1270, 1240, 1220, 1200, 1180, 1160, 1135, 1100, 1080, 1015, 995, 910, 890, 735, 715, 685, 650, 615 cm−1; HRMS (ESI) calcd for C19H21N3O4+Na 378.1424: found 378.1426.
Methyl (2S,3S)-2-(2-(5-(2-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6a). Orange-red oil, 89% yield (1.03 g, 2.89 mmol); Rf = 0.47 (4:1 hexane/EtOAc); Data for 6a: 1H-NMR (CDCl3) δ = 0.84 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.06–1.27 (m, 2H), 2.21–2.32 (m, 1H), 3.74 (s, 3H), 6.20 (d, J = 9.6 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.98 (dd, J = 4.0, 2.0 Hz, 1H), 7.23–7.29 (m, 1H), 7.30–7.34 (m, 1H), 7.31 (dd, J = 2.8, 2.0 Hz, 1H), 7.50–7.57 (m, 1H), 8.08–8.13 (m, 1H) ppm; 13C-NMR (CDCl3) δ = 11.0, 15.6, 24.8, 39.2, 52.3, 63.8, 110.3, 114.6, 117.0 (d, J = 21.3 Hz), 117.9, 124.6 (d, J = 3.8 Hz), 126.0, 129.6 (d, J = 0.5 Hz), 133.3 (d, J = 8.4 Hz), 158.8, 159.4, (d, J = 5.4 Hz), 159.6 (d, J = 1.5 Hz), 161.3, 171.4 ppm; IR ν = 2970, 2937, 2880, 1750, 1600, 1500, 1475, 1450, 1400, 1260, 1235, 1198, 1180, 1100, 1080, 1026, 1000, 910, 825, 767, 735, 698, 670, 615 cm−1; HRMS (ESI) calcd for C19H20FN3O3+Na 380.1381:found 380.1386.
Methyl (2S,3S)-2-(2-(5-(3-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6b). Orange-red oil, 88% yield (0.99 g, 2.77 mmol); Rf = 0.47 (4:1 hexane/EtOAc); Data for 6b: 1H-NMR (CDCl3) δ = 0.84 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.06–1.28 (m, 2H), 2.20–2.32 (m, 1H), 3.75 (s, 3H), 6.19 (d, J = 10.0 Hz, 1H), 6.36 (dd, J = 3.6, 2.8 Hz, 1H), 6.97 (dd, J = 3.6, 2.0 Hz, 1H), 7.20–7.27 (m, 1H), 7.33 (dd, J = 2.8, 2.0 Hz, 1H), 7.47–7.53 (m, 1H), 7.76–7.81 (m, 1H), 7.87–7.91 (m, 1H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 39.1, 52.3, 63.8, 110.4, 113.8 (d, J = 24.2 Hz), 114.5, 117.7, 118.6 (d, J = 21.3 Hz), 122.5 (d, J = 3.0 Hz), 125.7 (d, J = 8.3 Hz), 126.1, 130.9 (d, J = 8.4 Hz), 159.6, 161.6 (d, J = 3.1 Hz), 164.0, 171.3 ppm; IR ν = 2970, 2940, 2880, 1750, 1595, 1560, 1505, 1490, 1454, 1415, 1245, 1205, 1178, 1105, 1080, 995, 915, 870, 795, 735, 680, 615 cm−1; HRMS (ESI) calcd for C19H20FN3O3+Na 380.1381, found 380.1384.
Methyl (2S,3S)-2-(2-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6c). Yellow oil, 62% yield (768 mg, 2.15 mmol); Rf = 0.58 (4:1 hexane/EtOAc); Data for 6c: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.4 Hz, 3H), 1.05–1.27 (m, 2H), 2.22–2.32 (m, 1H), 3.74 (s, 3H), 6.19 (d, J = 10.4 Hz, 1H), 6.36 (dd, J = 3.6, 2.8 Hz, 1H), 6.94 (dd, J = 3.6, 2.0 Hz, 1H), 7.18–7.25 (m, 2H), 7.31 (dd, J = 2.8, 2.0 Hz, 1H), 8.07–8.13 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 39.1, 52.3, 63.7, 110.3, 114.2, 116.4 (d, J = 22.8 Hz), 117.9, 126.0, 129.1 (d, J = 8.3 Hz), 159.4, 161.8, 163.4, 165.9, 171.4 ppm; IR ν = 2970, 2940, 2880, 1750, 1670, 1605, 1500, 1455, 1415, 1240, 1200, 1180, 1160, 1080, 1015, 1000, 850, 740, 620 cm−1; HRMS (ESI) calcd for C19H20FN3O3+Na 380.1381: found 380.1387.
Methyl (2S,3S)-2-(2-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6d). Orange oil, 79% yield (254 mg, 0.68 mmol); Rf = 0.65 (4:1 hexane/EtOAc); Data for 6d: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.4 Hz, 3H), 1.06–1.27 (m, 2H), 2.20–2.32 (m, 1H), 3.74 (s, 3H), 6.19 (d, J = 10.4 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.95 (dd, J = 4.0, 1.6 Hz, 1H), 7.32 (dd, J = 2.8, 1.6 Hz, 1H), 7.48–7.52 (m, 2H), 8.01–8.05 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 39.1, 52.3, 63.8, 110.3, 114.4, 117.8, 122.3, 126.1, 128.1, 129.4, 137.8, 159.5, 161.8, 171.3 ppm; IR ν = 2970, 2935, 2880, 1750, 1605, 1505, 1485, 1455, 1410, 1255, 1235, 1200, 1178, 1095, 1080, 1015, 840, 735 cm−1; HRMS (ESI) calcd for C19H20ClN3O3+Na 396.1085: found 396.1089.
Methyl (2S,3S)-2-(2-(5-(2-hydroxy-3,5-diiodophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6e-1). Green solid, 45% yield (893 mg, 1.47 mmol); Rf = 0.50 (4:1 hexane/EtOAc); Data for 6e-1: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.02–1.22 (m, 2H), 2.20–2.33 (m, 1H), 3.77 (s, 3H), 6.04 (d, J = 9.6 Hz, 1H), 6.39 (dd, J = 4.0, 2.8 Hz, 1H), 7.04 (dd, J = 4.0, 1.6 Hz, 1H), 7.37 (dd, J = 2.8, 1.6 Hz, 1H), 8.06 (d, J = 2.0 Hz, 1H), 8.17 (d, J = 2.0 Hz, 1H), 11.02 (s, 1H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 38.1, 52.4, 64.0, 81.3, 86.7, 109.8, 110.8, 115.7, 116.9, 127.0, 134.7, 149.8, 156.2, 158.7, 160.1, 171.1 ppm; IR ν = 3415, 2970, 2880, 1750, 1605, 1565, 1530, 1500, 1455, 1415, 1380, 1255, 1235, 1190, 1180, 1100, 1080, 990, 915, 875, 758, 740, 665, 598 cm−1; HRMS (ESI) calcd for C19H19I2N3O4+Na 629.9357: found 629.9358.
Methyl (2S,3S)-2-(2-(5-(4-hydroxy-3,5-diiodophenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6f-1). Yellow oil, 43% yield (935 mg, 1.54 mmol); Rf = 0.45 (4:1 hexane/EtOAc); Data for 6f-1: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.4 Hz, 3H), 1.05–1.24 (m, 2H), 2.20–2.32 (m, 1H), 3.75 (s, 3H), 6.14 (d, J = 10.0 Hz, 1H), 6.21 (br s, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.96 (dd, J = 4.0, 2.0 Hz, 1H), 7.32 (dd, J = 2.8, 2.0 Hz, 1H), 8.40 (s, 2H), 8.17 (d, J = 2.0 Hz, 1H), 11.02 (s, 1H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 39.1, 52.3, 63.8, 82.5, 110.4, 114.5, 117.7, 119.9, 126.1, 137.6, 156.2, 159.4, 159.6, 171.3 ppm; IR ν = 3450, 3145, 3125, 3070, 2970, 2880, 1745, 1608, 1595, 1500, 1450, 1395, 1300, 1235, 1195, 1178, 1155, 1105, 1075, 990, 890, 775, 736, 710, 685, 615 cm−1; HRMS (ESI) calcd for C19H19I2N3O4+Na 629.9357: found 629.9360.
Methyl (2S,3S)-2-(2-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6g). Orange-red oil, 80% yield (768 mg, 2.08 mmol); Rf = 0.19 (4:1 hexane/EtOAc); Data for 6g: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.4 Hz, 3H), 1.05–1.27 (m, 2H), 2.20–2.32 (m, 1H), 3.73 (s, 3H), 3.99 (s, 3H), 6.24 (d, J = 9.6 Hz, 1H), 6.34 (dd, J = 3.6, 2.8 Hz, 1H), 6.94 (dd, J = 3.6, 1.6 Hz, 1H), 7.05–7.11 (m, 2H), 7.30 (dd, J = 2.8, 1.6 Hz, 1H), 7.47–7.53 (m, 1H), 7.98 (dd, J = 7.6, 2.0 Hz, 1H) ppm; 13C-NMR (CDCl3) δ = 11.0, 15.5, 24.8, 39.2, 52.2, 56.0, 63.7, 110.1, 112.0, 112.9, 114.0, 118.3, 120.7, 125.6, 130.2, 132.8, 157.9, 159.0, 161.3, 171.5 ppm; IR ν = 2970, 2942, 2882, 2845, 1750, 1605, 1550, 1500, 1485, 1455, 1440, 1415, 1255, 1240, 1200, 1180, 1100, 1080, 1050, 1025, 915, 730, 678, 650, 620 cm−1; HRMS (ESI) calcd for C20H23N3O4+Na 392.1581: found 392.1585.
Methyl (2S,3S)-2-(2-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6h). Orange oil; 79% yield (739 mg, 2.00 mmol); Rf = 0.24 (4:1 hexane/EtOAc); Data for 6h: 1H-NMR (CDCl3) δ = 0.83 (t, J = 7.2 Hz, 3H), 1.03 (d, J = 6.4 Hz, 3H), 1.05–1.23 (m, 2H), 2.20–2.30 (m, 1H), 3.74 (s, 3H), 3.88 (s, 3H), 6.20 (d, J = 9.6 Hz, 1H), 6.34 (dd, J = 3.6, 2.8 Hz, 1H), 6.92 (dd, J = 3.6, 1.6 Hz, 1H), 6.99–7.03 (m, 2H), 7.29 (dd, J = 2.8, 1.6 Hz, 1H), 8.00–8.04 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 11.0, 15.5, 24.8, 39.1, 52.2, 55.4, 63.7, 110.1, 113.8, 114.5, 116.4, 118.2, 125.6, 128.6, 158.9, 162.2, 162.6, 171.4 ppm; IR ν = 2970, 2940, 2880, 2840, 1750, 1610, 1500, 1460, 1445, 1310, 1255, 1180, 1100, 1080, 1030, 1000, 915, 840, 730, 700, 625, 610 cm−1; HRMS (ESI) calcd for C20H23N3O4+Na 392.1581: found 392.1584.
3.2. General Procedure for Deiodination Reaction on the Phenol Ring
Zn dust (2~5 equiv.) and acetic acid (5 equiv.) were added to a stirred solution of 2-pyrrolyl-5-(iodophenolic)-1,3,4-oxadiazole 5f-1 or 6e-1 (~0.5–1.0 g, 1 equiv.) in THF (30mL). The mixture was stirred at 25 °C for 1~6 h under an argon atmosphere. The mixture was quenched with saturated NaHCO3 solution, extracted with Et2O, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The product was purified by SiO2 flash column chromatography to produce the deiodination product.
Methyl (S)-2-(2-(5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5f). White solid, 97% yield (270 mg, 0.79 mmol); Rf = 0.58 (3:2 hexane/EtOAc); Data for 5f: 1H-NMR (CDCl3) δ = 0.83 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H), 2.43–2.54 (m, 1H), 3.75 (s, 3H), 6.13 (d, J = 9.6 Hz, 1H), 6.36 (dd, J = 4.0, 2.8 Hz, 1H), 6.93 (dd, J = 4.0, 1.6 Hz, 1H), 7.01 (d, J = 8.4 Hz, 2H), 7.30 (dd, J = 2.8, 1.6 Hz, 1H), 7.99 (d, J = 8.4 Hz, 2H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.3, 33.1, 52.3, 64.6, 110.2, 114.0, 116.0, 116.2, 118.0, 125.7, 128.8, 159.0, 159.1, 162.7, 171.3 ppm; IR ν = 3120, 2970, 2940, 2880, 1750, 1610, 1595, 1500, 1440, 1394, 1375, 1335, 1285, 1270, 1240, 1200, 1105, 1090, 1075, 1010, 995, 915, 840, 775, 735, 719, 635 cm−1; HRMS (ESI) calcd for C18H19N3O4+Na 364.1268: found 365.1271.
Methyl (2S,3S)-2-(2-(5-(2-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6e). White solid, 50% yield (210 mg, 0.59 mmol); Rf = 0.54 (3:2 hexane/EtOAc); Data for 6e: 1H-NMR (DMSO-d6) δ = 0.79 (t, J = 7.2 Hz, 3H), 0.96 (d, J = 6.4 Hz, 3H), 0.98–1.19 (m, 2H), 2.16–2.26 (m, 1H), 3.69 (s, 3H), 6.02 (d, J = 9.6 Hz, 1H), 6.24 (dd, J = 3.6, 2.8 Hz, 1H), 6.56 (dd, J = 3.6, 1.6 Hz, 1H), 6.92–6.99 (m, 2H), 7.20 (dd, J = 2.8, 1.6 Hz, 1H), 7.41–7.46 (m, 1H), 7.86–7.90 (m, 1H) 8.39 (s, 1H) ppm; 13C-NMR (DMSO-d6) δ = 11.1, 16.0, 24.8, 38.5, 52.7, 63.3, 110.3, 115.9, 116.5, 117.8, 119.3, 126.2, 127.8, 128.6, 134.2, 142.5, 159.8, 164.9, 171.3 ppm; IR ν = 3240, 3080, 2970, 2935, 2880, 1750, 1640, 1600, 1570, 1540, 1495, 1465, 1425, 1360, 1335, 1315, 1258, 1230, 1200, 1175, 1155, 1105, 1075, 1060, 1040, 995, 950, 895, 825, 757, 740, 670, 618 cm−1; HRMS (ESI) calcd for C19H23N3O4+Na 380.1581: found 380.1586.
Methyl (2S,3S)-2-(2-(5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6f). White solid, 42% yield (98 mg, 0.28 mmol); Rf = 0.67 (3:2 hexane/EtOAc); Data for 6f: 1H-NMR (CDCl3) δ = 0.77 (t, J = 7.2 Hz, 3H), 0.98 (d, J = 6.8 Hz, 3H), 0.98–1.18 (m, 2H), 2.26–2.35 (m, 1H), 3.70 (s, 3H), 5.96 (d, J = 10.0 Hz, 1H), 6.38 (dd, J = 3.6, 2.8 Hz, 1H), 6.97 (d, J = 8.4 Hz, 2H), 6.98 (dd, J = 3.6, 1.6 Hz, 1H), 7.40 (dd, J = 2.8, 1.6 Hz, 1H), 7.90 (d, J = 8.4 Hz, 2H), 10.34 (s, 1H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.5, 24.8, 39.1, 52.3, 63.7, 110.3, 114.2, 115.5, 117.9, 125.9, 129.0, 136.9, 157.7, 159.2, 161.1, 171.4 ppm; IR ν = 3137, 2965, 2875, 2838, 1742, 1610, 1579, 1558, 1497, 1464, 1466, 1438, 1338, 1307, 1287, 1254, 1224, 1202, 1173, 1092, 1060, 1027, 961, 938, 911, 837, 810, 798, 731, 696 cm−1; HRMS (ESI) calcd for C19H21N3O4+Na 378.1424: found 378.1427.
3.3. General Procedure for Iodination Reaction on the Pyrrole Ring
I2 (4 equiv.). was added to a stirred solution of 2-pyrrolyl-5-(anisyl)-1,3,4-oxadiazoles 5g, 5h, or 6h (~1.0 g, 1 equiv.) in DMSO (10 mL). The mixture was heated at 90 ℃ for 3~5 h under an argon atmosphere. After cooling to room temperature, the mixture was diluted with Et2O, washed with 10% Na2S2O3 solution, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The product was purified by SiO2 flash column chromatography to produce iodination products 5g-1, 5h-1, or 6h-1 on the pyrrole ring.
Methyl (S)-2-(3,4-diiodo-2-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5g-1). Orange oil, 61% yield (923 mg, 1.52 mmol); Rf = 0.60 (3:2 hexane/EtOAc); Data for 5g-1: 1H-NMR (CDCl3) δ = 0.86 (d, J = 6.4 Hz, 3H), 1.04 (d, J = 6.4 Hz, 3H), 2.42 (m, 1H), 3.73 (s, 3H), 4.03 (s, 3H), 6.12 (d, J = 10.0 Hz, 1H), 7.08–7.14 (m, 2H), 7.45 (s, 1H), 7.51–7.56 (m, 1H), 8.12 (dd, J = 7.6, 1.6 Hz, 1H) ppm; 13C-NMR (CDCl3) δ = 18.6, 19.2, 33.1, 52.5, 56.0, 65.6, 78.0, 80.4, 112.0, 112.3, 120.8, 121.4, 130.4, 130.5, 133.2, 156.7, 158.1, 162.1, 170.6 ppm; IR ν = 2970, 2937, 2880, 2840, 1750, 1665, 1605, 1590, 1545, 1500, 1475, 1470, 1435, 1390, 1285, 1265, 1220, 1205, 1185, 1165, 1130, 1060, 1050, 1025, 943, 915, 768, 755, 735, 677, 650 cm−1; HRMS (ESI) calcd for C19H19I2N3O4+Na 629.9357: found 629.9363.
Methyl (S)-2-(3,4-diiodo-2-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylbutanoate (5h-1). Yellow oil (a 1.3:1 mixture of di- and mono-iodide products); di-iodide, 39% yield (199 mg, 0.33 mmol, calcd), mono-iodide, 30% yield (121 mg, 0.25 mmol, calcd); Rf = 0.60 (3:2 hexane/EtOAc); Data for di-iodide at pyrrole: 1H-NMR (CDCl3) δ = 0.86 (d, J = 6.4 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 2.43 (m, 1H), 3.74 (s, 3H), 3.89 (s, 3H), 6.09 (d, J = 10.0 Hz, 1H), 7.05 (d, J = 8.8 Hz, 2H), 7.44 (s, 1H), 8.11 (d, J = 8.8 Hz, 2H) ppm; 13C-NMR (CDCl3) δ = 18.6, 19.1, 33.0, 52.5, 55.5, 65.7, 78.0, 80.4, 114.6, 116.0, 120.3, 128.9, 130.6, 156.7, 162.5, 163.4, 170.5 ppm; IR ν = 2970, 1750, 1615, 1590, 1560, 1500, 1485, 1460, 1440, 1390, 1335, 1255, 1205, 1175, 1160, 1065, 1030, 945, 840, 755, 745, 625 cm−1; HRMS (ESI) calcd for C19H19I2N3O4+Na 629.9357, found 629.9358. Data for mono-iodide at pyrrole (C-3): 1H-NMR (CDCl3) δ = 0.61 (d, J = 6.4 Hz, 3H), 1.06 (d, J = 6.4 Hz, 3H), 2.43 (m, 1H), 3.76 (s, 3H), 3.89 (s, 3H), 6.13 (d, J = 9.6 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H), 7.02 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 2.0 Hz, 1H), 8.02 (d, J = 8.4 Hz, 2H) ppm; 13C-NMR (CDCl3) δ = 18.5, 19.2, 33.3, 52.4, 61.3, 64.9, 77.2, 114.5, 116.0, 120.1, 121.5, 128.6, 130.1, 157.7, 162.4, 162.8, 170.8 ppm; HRMS (ESI) calcd for C19H20IN3O4+Na 504.0396: found 504.0387.
Methyl (2S,3S)-2-(3-iodo-2-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-1H-pyrrol-1-yl)-3-methylpentanoate (6h-1). Yellow oil, 83% yield (308 mg, 0.67 mmol); Rf = 0.40 (4:1 hexane/EtOAc); Data for 6h-1: 1H-NMR (CDCl3) δ = 0.84 (t, J = 7.2 Hz, 3H), 1.02 (d, J = 6.4 Hz, 3H), 1.06–1.26 (m, 2H), 2.16–2.28 (m, 1H), 3.75 (s, 3H), 3.89 (s, 3H), 6.18 (d, J = 10.0 Hz, 1H), 6.99–7.04 (m, 2H), 7.01 (d, J = 1.6 Hz, 1H), 7.35 (d, J = 1.6 Hz, 1H), 7.99–8.03 (m, 2H) ppm; 13C-NMR (CDCl3) δ = 10.9, 15.4, 24.8, 39.2, 52.4, 55.5, 64.1, 77.2, 114.6, 116.0, 120.1, 120.3, 128.7, 130.1, 157.6, 162.4, 162.9, 171.0 ppm; IR ν = 2970, 2935, 2880, 2840, 1750, 1607, 1498, 1464, 1440, 1310, 1260, 1176, 1100, 1065, 1030, 1000, 915, 840, 815, 745, 640, 625, 607 cm−1; HRMS (ESI) calcd for C20H22IN3O4+Na 518.0547: found 518.0548.
3.4. Biological Evaluation
The minimum inhibitory concentrations (MICs) were determined using the broth microdilution method in a 96-well plate [40,41]. The 96-well plates containing chemicals in two-fold serial dilutions (4 μg/mL to 2048 μg/mL for series 1; 2 μg/mL to 1024 μg/mL for series 5 and 6) were prepared in Luria–Bertani (LB) medium. E. coli, S. aureus, and A. baumannii cells were grown in LB broth to the exponential phase. A 10 μL volume of cells diluted with LB broth to a concentration of 108 cells/mL was inoculated on the plates. The MIC was determined after incubation at 37 °C for 16 h under aerobic conditions. The optical density was measured in triple at 600 nm (OD600) using a microplate reader (Bio-Rad, USA) at 20 h after treatment of the chemicals in concentrations of 2, 4, 8, 16, 32, 64, 128, 256, 512, and 1024 μg/mL. The average and standard deviation values of OD600 are reported in Table S1 and Table S2 of the Supporting Information. Vancomycin and Erythromycin were used as positive controls (see Table S2 for the average and standard deviation values of OD600). As the chemicals were dissolved in 100% DMSO, 100% DMSO and triple-distilled water were used as negative controls. The minimum inhibitory concentration (MIC) in Table 2 and Table 4 is defined as the lowest concentration of chemicals which provides an average OD600 value of less than 0.100.
4. Conclusions
We extended the synthetic utility of pyrrole platform chemicals 2, which can be readily prepared from the sustainable ribose conversion with amino acids, to the pyrrole-ligated 1,3,4-oxadiazole core structure 1 through the reaction with benzohydrazide 3a. The size effect of the R group from the amino acids clearly offered better antibacterial activities for 1,3,4-oxadiazoles 1c and 1e, which were derived from valine and isoleucine, respectively. Benzohydrazides 3 with various electronic X-substituents were utilized for the construction of 2-pyrrolyl-5-phenyl-1,3,4-oxadiazoles 5 and 6 with N-valine and N-isoleucine residues, respectively. Relationships of structure and antibacterial activity were deduced from MIC values for 1,3,4-oxadiazoles 5 and 6 against E. coli, S. aureus and A. baumannii. A positive ortho effect was marginally observed for fluoride substituents. Most importantly, a superior iodophenol effect was evident in the antibacterial activities of 1,3,4-oxadiazoles 5f-1 and 6f-1, which provided much lower MIC values against A. baumannii than those of the vancomycin and erythromycin as positive controls. These findings provide a guiding principle for the design of superior future antimicrobial agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083638/s1, (1) 1H/13C-NMR spectra; (2) MIC data against E. coli, S. aureus, and A. baumannii; (3) High-Resolution Mass Spectra for the entire 1,3,4-oxadiazoles synthesized in this paper. Table S1. Determination of MIC for 1 for E. coli and S. aureus by OD600 (20 h). Each value was obtained as an average of at least triple measurements. Table S2. Determination of MIC for 5 and 6 for E. coli, S. aureus, and A. baumannii by OD600 (20 h). Each value was obtained as an average of at least triple measurements (vancomycin and erythromycin as positive controls).
Author Contributions
Conceptualization, S.K.; methodology, S.K., H.Y. (Haiyang Yu), and C.-R.L.; validation, S.K., H.Y. (Haiyang Yu), and C.-R.L.; formal analysis, H.K., L.G., and H.Y. (Huisu Yeo); investigation, H.K., L.G., H.Y. (Huisu Yeo), and U.C.; resources, S.K. and C.-R.L.; data curation, S.K. and C.-R.L.; writing—original draft preparation, S.K.; writing—review and editing, S.K. and C.-R.L.; visualization, S.K.; supervision, S.K.; project administration, S.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported under the framework of international cooperation program managed by the National Research Foundation of Korea (2021K2A9A1A01101863 and 2022K2A9A1A01097910) and partly by Basic Science Research Program funded by the Ministry of Education (2020R1A6A1A03038817).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romeo, G.; Chiacchio, U. Oxadiazoles. Mod. Heterocycle. Chem. 2011, 2, 1047–1252. [Google Scholar] [CrossRef]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-Oxadiazole and its derivatives: A review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Świątek, P. Antimicrobial activity of 1,3,4-oxadiazole derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Bruma, M.; Brehmer, L. Aromatic poly (1,3,4-oxadiazole)s as advanced materials. Adv. Mater. 1997, 9, 601–613. [Google Scholar] [CrossRef]
- Bruma, M.; Köpnik, T. Silicon-containing polyoxadiazoles—Synthesis and perspectives. Adv. Colloid Interface Sci. 2005, 116, 277–290. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef]
- Du, M.; Bu, X.-H. Angular Dipyridyl Ligands 2,5-Bis(4-pyridyl)-1,3,4-oxadiazole and Its 3-Pyridyl Analogue as Building Blocks for Coordination. Bull. Chem. Soc. Jpn. 2009, 82, 539–554. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; de Athayde-Filho, P.F. Synthetic approaches and pharmacological activity of 1,3,4-oxadaizoles: A review of the literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef]
- Salahuddin; Mazumder, A.; Yar, M.S.; Mazumder, R.; Chakraborthy, G.S.; Ahsan, M.J.; Rahman, M.U. Updates on synthesis and biological activities of 1,3,4-oxadiazole: A review. Synth. Commun. 2017, 47, 1805–1847. [Google Scholar] [CrossRef]
- Nayak, S.G.; Poojary, B. A Review on the preparation of 1,3,4-oxadiazoles from the dehydration of hydrazines and study of their biological roles. Chem. Afr. 2019, 2, 551–571. [Google Scholar] [CrossRef]
- Patel, K.D.; Prajapati, S.M.; Panchal, S.N.; Patel, H.D. Review of synthesis of 1,3,4-oxadiazole derivatives. Synth. Commun. 2014, 44, 1859–1875. [Google Scholar] [CrossRef]
- Deeks, S.G.; Kar, S.; Gubernick, S.I.; Kirkpatrick, P. Raltegravir. Nat. Rev. Drug Discov. 2008, 7, 117–118. [Google Scholar] [CrossRef]
- Croxtall, J.D.; Lyseng-Williamson, K.A.; Perry, C.M. Raltegravir. Drugs 2008, 68, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; Fizazi, K.; Miller, K.; Higano, C.; Moul, J.W.; Akaza, H.; Morris, T.; McIntosh, S.; Pemberton, K.; Gleave, M. Phase 3, randomized, placebo-controlled study of Zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer 2012, 118, 5709–5718. [Google Scholar] [CrossRef]
- Miller, K.; Moul, J.W.; Gleave, M.; Fizazi, K.; Nelson, J.B.; Morris, T.; Nathan, F.E.; McIntosh, S.; Pemberton, K.; Higano, C.S. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 187–192. [Google Scholar] [CrossRef]
- Narayana, B.; Ashalatha, B.V.; Raj, K.K.V.; Fernandes, J.; Sarojini, B.K. Synthesis of some new biologically active 1,3,4-oxadiazolyl nitroindoles and a modified Fischer indole synthesis of ethyl nitro indole-2-carboxylates. Bioorg. Med. Chem. 2005, 13, 4638–4644. [Google Scholar] [CrossRef]
- Narayana, B.; Ashalatha, B.V.; Raj, K.K.V.; Sarojni, B.K. Synthesis and studies on antimicrobial, anti-inflammatory and antiproliferative activities of heterocycles derived from 4-/5-/6-/7-nitro/5-fluoro/chloro/bromoindole-2-carbohydrazides. Indian J. Chem. 2009, 48B, 1794–1805. [Google Scholar]
- Bingul, M.; Saglam, M.F.; Kandemir, H.; Boga, M.; Sengul, I.F. Synthesis of indole-2-carbohydrazides and 2-(indol-2-yl)-1,3,4-oxadiazoles as antioxidants and their acetylcholinesterase inhibition properties. Monatsh. Chem. 2019, 150, 1553–1560. [Google Scholar] [CrossRef]
- Shekarchi, M.; Ellahiyan, F.; Akbarzadeh, T.; Shafiee, A. Syntheses of [1,2,3]Selenadiazolo[4,5-e]benzofuran or benzothiophene, [1,2,3]thiadiazolo[4,5-e]benzofuran or benzothiophene, and 2-benzofuranyl-1,3,4-oxodiazole derivatives. J. Heterocycl. Chem. 2003, 40, 427–433. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Li, P.; Wang, L. Highly efficient copper/palladium-catalyzed tandem Ullman reaction/arylation of azoles via C–H activation: Synthesis of benzofuranyl and indolyl azoles from 2-(gem-dibromovinyl)phenols(anilines) with azoles. Tetrahedron 2011, 67, 5913–5919. [Google Scholar] [CrossRef]
- Abedinifar, F.; Mohammadi-Khanaposhtani, M.; Asemanipoor, N.; Mojtabavi, S.; Faramarzi, M.A.; Mahdavi, M.; Biglar, M.; Larijani, B.; Hamedifar, H.; Hajimiri, M.H. Synthesis and biological evaluation of a new series of benzofuran-1,3,4-oxadiazole containing 1,2,3-triazoleacetamides as potential α-glucosidase inhibitors. J. Biochem. Mol. Toxicol. 2021, 35, e22688. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Iwashita, H.; Uno, Y.; Kunitomo, J.; Saitoh, M.; Kimura, E.; Fujita, H.; Uchiyama, N.; Kori, M.; Takizawa, M. A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S)-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer’s disease. J. Neurochem. 2011, 119, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Jun Kunitomo, J.; Kimura, E.; Iwashita, H.; Uno, Y.; Onishi, T.; Uchiyama, N.; Kawamoto, T.; Tanaka, T.; Mol, C.D.; et al. 2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3β with good brain permeability. J. Med. Chem. 2009, 52, 6270–6286. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef] [PubMed]
- Pinder, A.R. Azetidine, pyrrole, pyrrolidine, piperidine, and pyridine alkaloids. Nat. Prod. Rep. 1992, 9, 491–504. [Google Scholar] [CrossRef]
- Asgaonkar, K.D.; Mote, G.D.; Chitre, T.S. QSAR and molecular docking studies of oxadiazole-ligated pyrrole derivatives as enoyl-ACP (CoA) reductase inhibitors. Sci. Pharm. 2014, 82, 71–86. [Google Scholar] [CrossRef]
- Rane, R.A.; Karpoormath, R.; Naphade, S.S.; Bangalore, P.; Shaikh, M.; Hampannavar, G. Novel synthetic organic compounds inspired from antifeedant marine alkaloids as potent bacterial biofilm inhibitors. Bioorg. Chem. 2015, 61, 66–73. [Google Scholar] [CrossRef]
- Rane, R.A.; Gutte, S.D.; Sahu, N.U. Synthesis and evaluation of novel 1,3,4-oxadiazole derivatives of marine bromopyrrole alkaloids as antimicrobial agent. Bioorg. Med. Chem. Lett. 2012, 22, 6429–6432. [Google Scholar] [CrossRef]
- Rane, R.A.; Bangalore, P.; Borhade, S.D.; Khandare, P.K. Synthesis and evaluation of novel 4-nitropyrrole-based 1,3,4-oxadiazole derivatives as antimicrobial and anti-tubercular agents. Eur. J. Med. Chem. 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Das Adhikary, N.; Kwon, S.; Chung, W.-J.; Koo, S. One-pot conversion of carbohydrates into pyrrole-2-carbaldehydes as sustainable platform chemicals. J. Org. Chem. 2015, 80, 7693–7701. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Gu, L.; In, I.J.; Wu, B.; Lee, T.; Kim, H.; Koo, S. Ribose conversion with amino acids into pyrraline platform chemicals—Expeditious synthesis of diverse pyrrole-fused alkaloid compounds. RSC Adv. 2021, 11, 31511–31525. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.H.; Narayanan, A.; Hett, E.C. Understanding and applying tyrosine biochemical diversity. Mol. BioSyst. 2014, 10, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2015, 60, 134–146. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Smith, A.D.; Kozich, V.; Refsum, H. Cysteine and obesity. Obesity 2012, 20, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.K.; Samad, A.; Kumar, Y.; Shaharyar, M.; Khosa, R.L.; Jain, J.; Kumar, V.; Singh, P. Design, synthesis and biological evaluation of 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 4963–4967. [Google Scholar] [CrossRef]
- Yu, W.; Huang, G.; Zhang, Y.; Liu, H.; Dong, L.; Yu, X.; Li, Y.; Chang, J. I2-Mediated oxidative C−O bond formation for the synthesis of 1,3,4-oxadiazoles from aldehydes and hydrazides. J. Org. Chem. 2013, 78, 10337–10343. [Google Scholar] [CrossRef]
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. Synthesis of 2-amino-1,3,4-oxadiazoles and 2-amino-1,3,4-thiadiazoles via sequential condensation and I2-mediated oxidative C−O/C−S bond formation. J. Org. Chem. 2015, 80, 1018–1024. [Google Scholar] [CrossRef]
- Kubát, P.; Henke, P.; Mosinger, J. The effect of iodide and temperature on enhancing antibacterial properties of nanoparticles with an encapsulated photosensitizer. Colloids Surf. B 2019, 176, 334–340. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, 1–8. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, X.; Yoo, H.; Wang, T.; Sung, C.G.; Choi, U.; Lee, C.-R.; Yu, H.; Koo, S. Synthesis of Chalcone-derived heteroaromatics with antibacterial activities. ChemistrySelect 2020, 5, 12421–12424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).