A Novel Non-Metallic Photocatalyst: Phosphorus-Doped Sulfur Quantum Dots
Abstract
1. Introduction
2. Results and Discussion
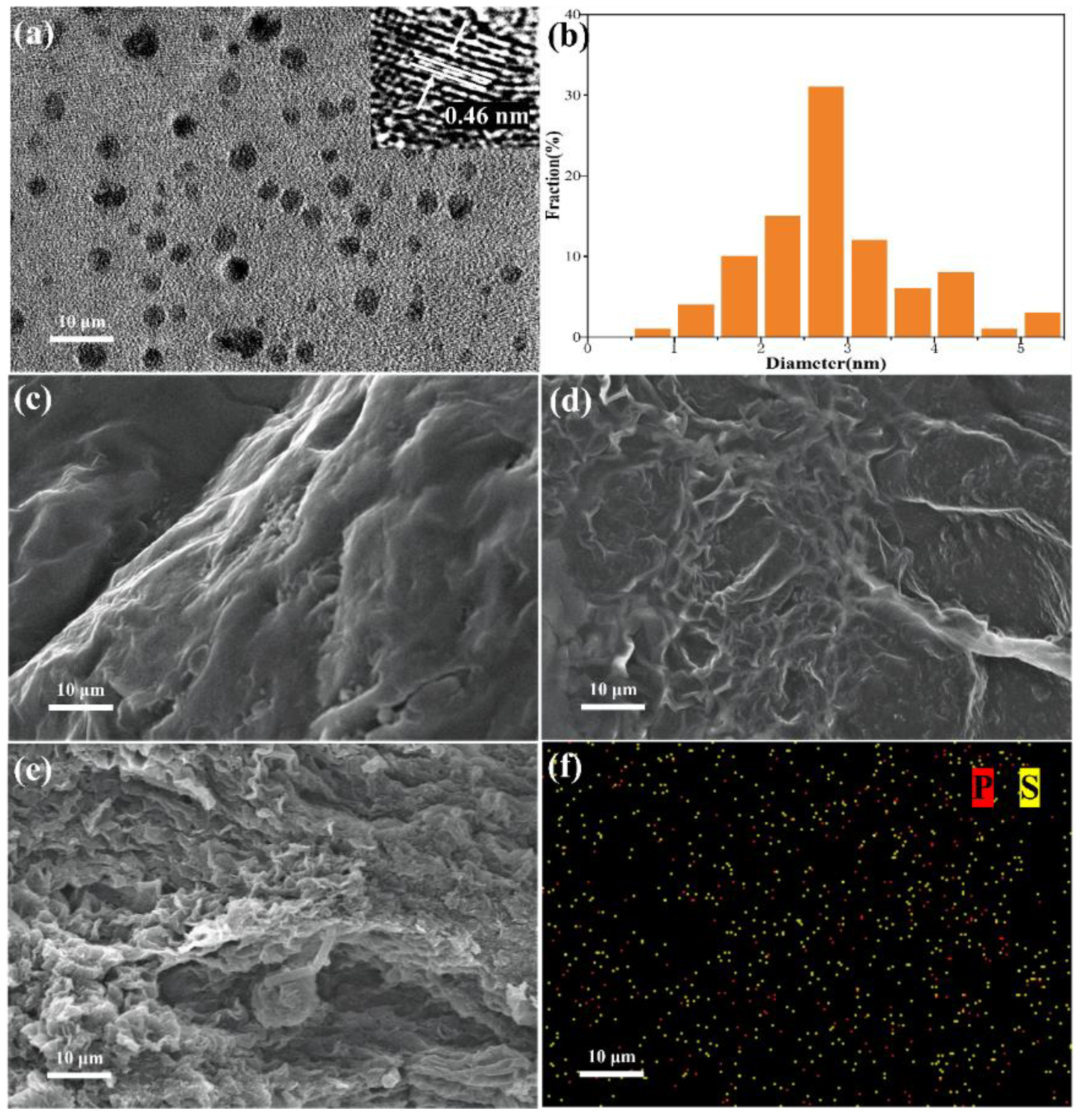
2.1. Morphological Structure Characterization
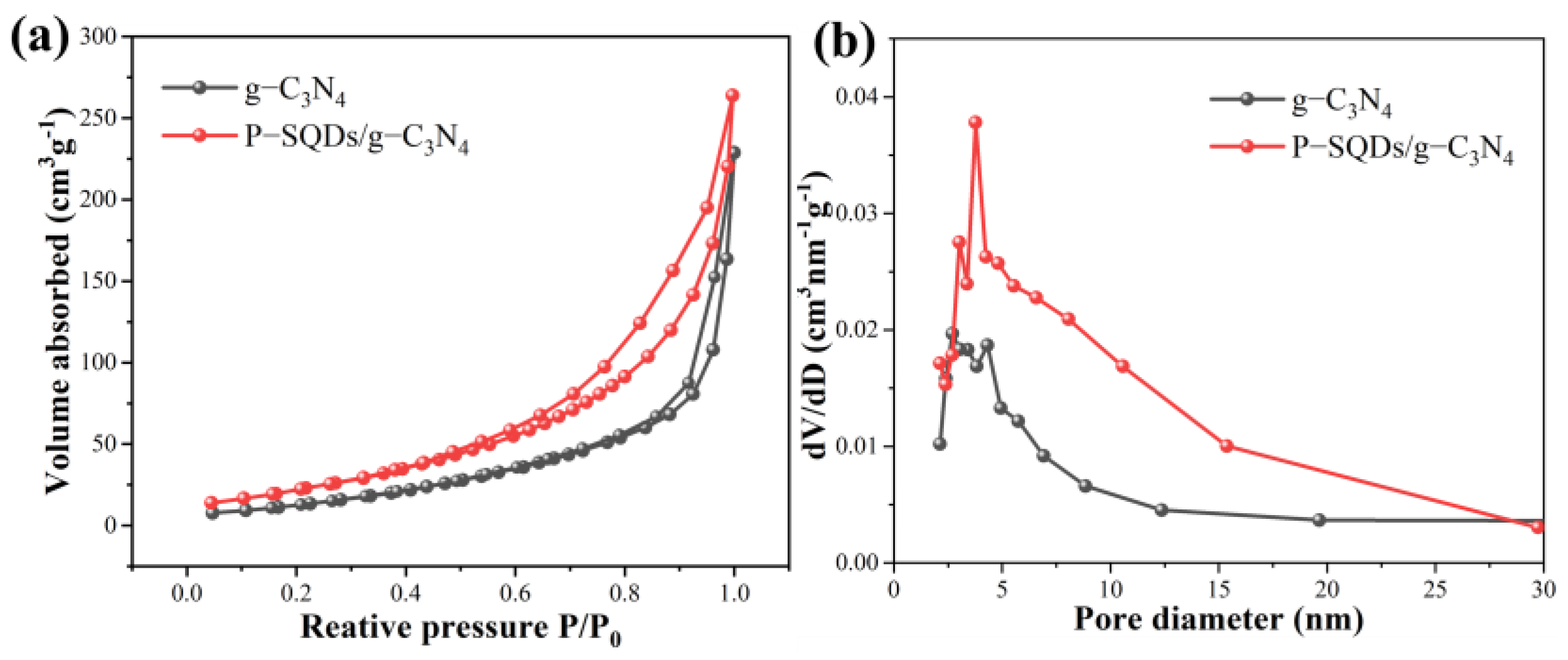
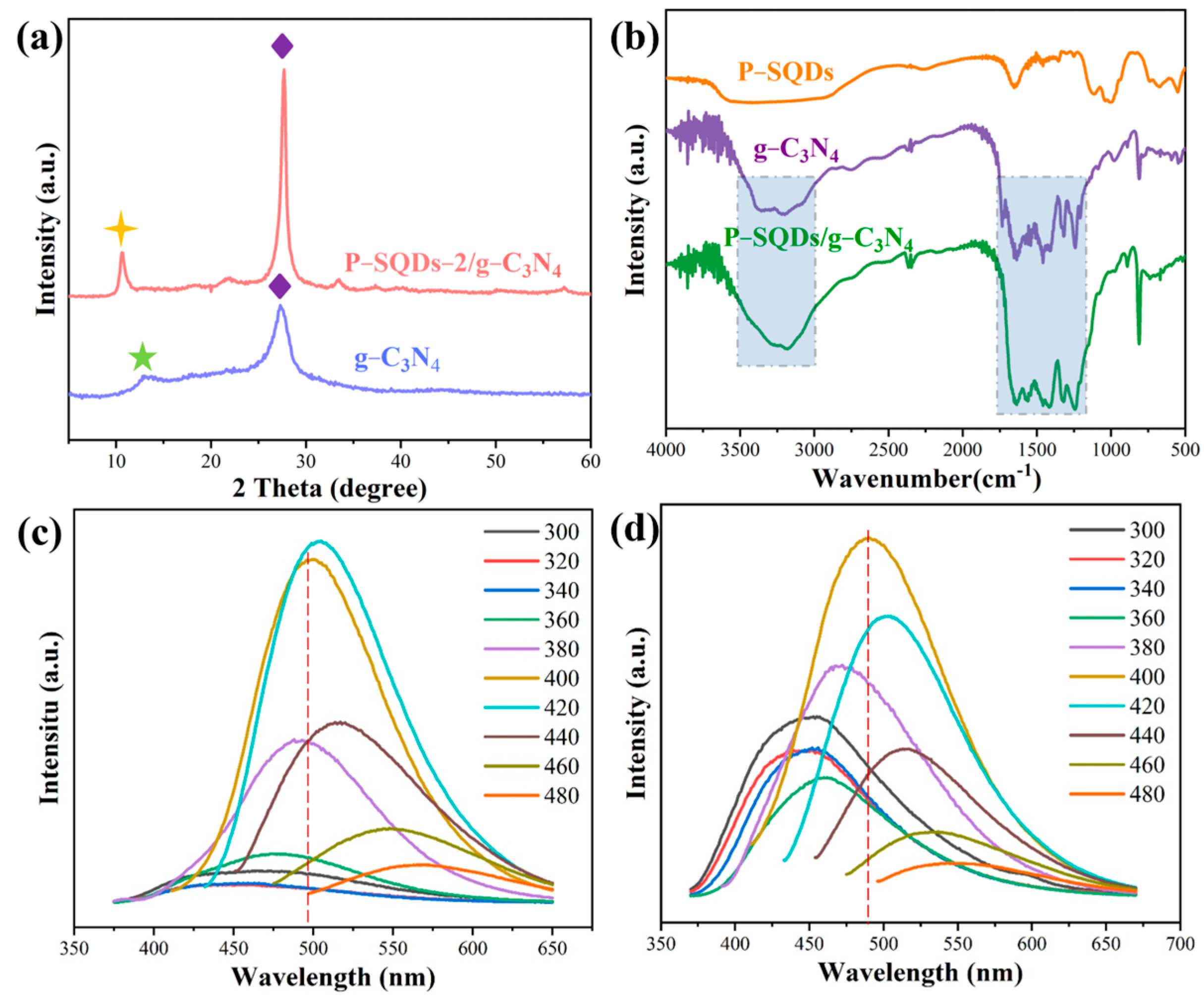
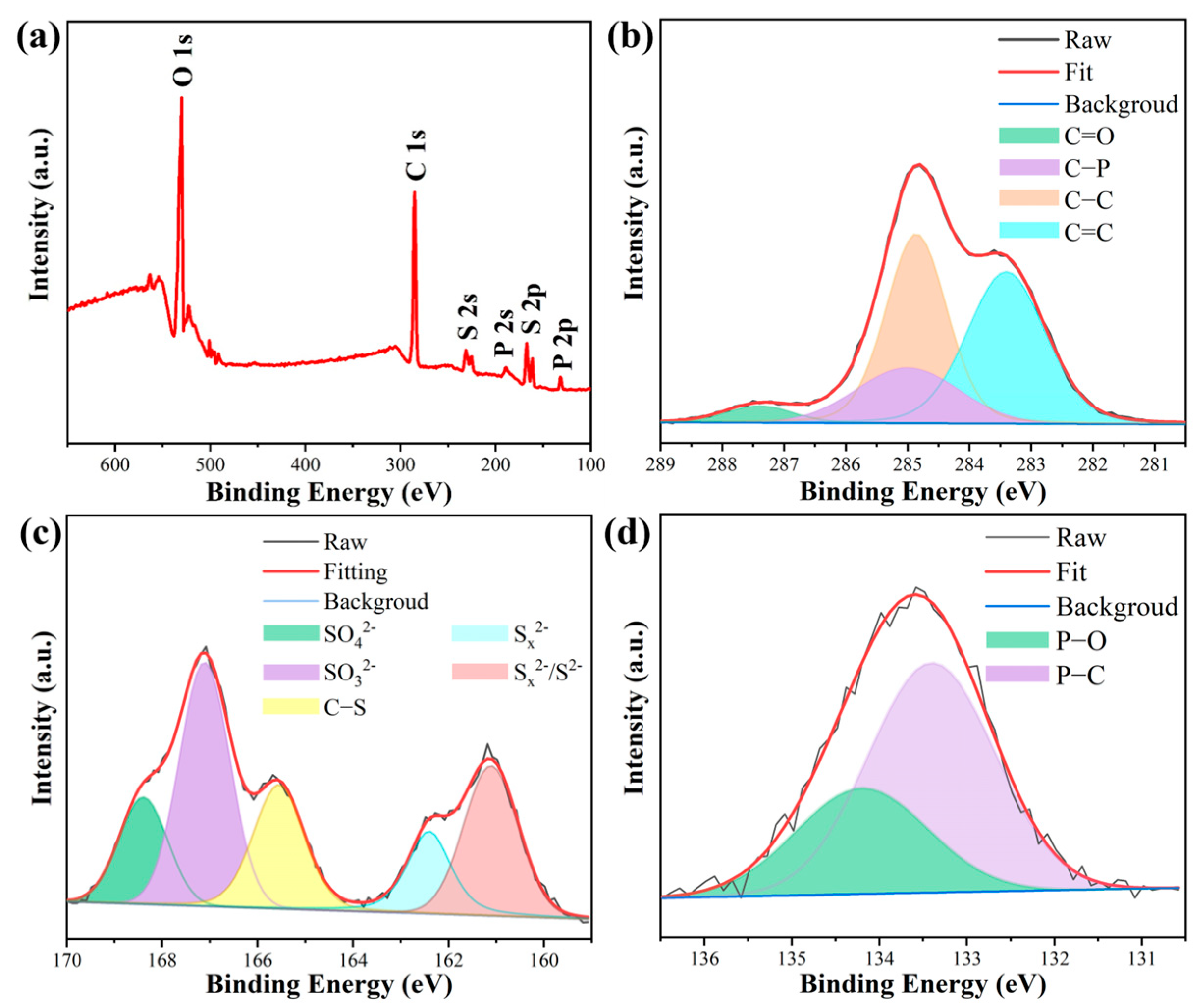
2.2. Nanostructure Investigation
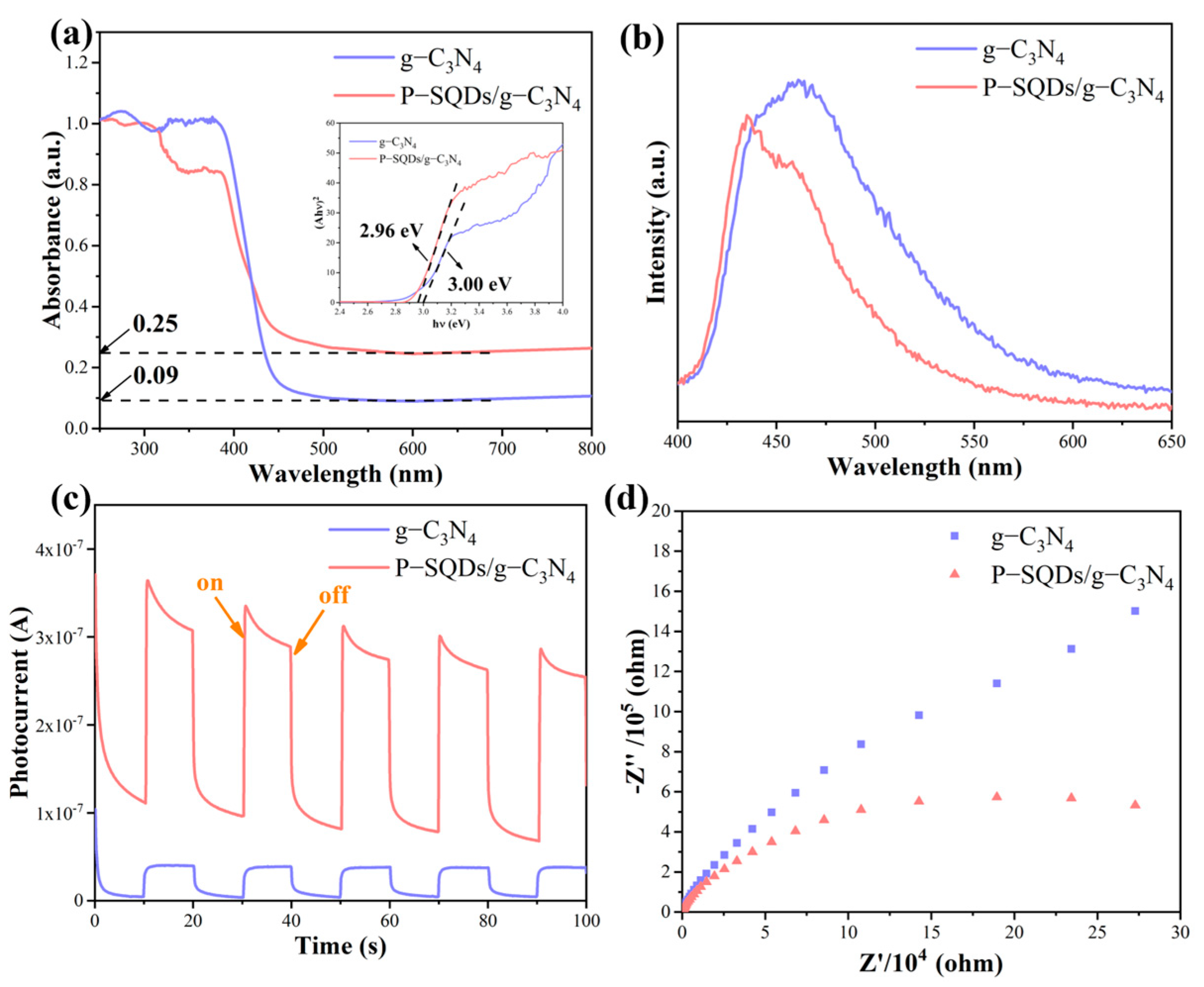
2.3. Band Structure and Photoelectric Properties
2.4. Photocatalytic Performance
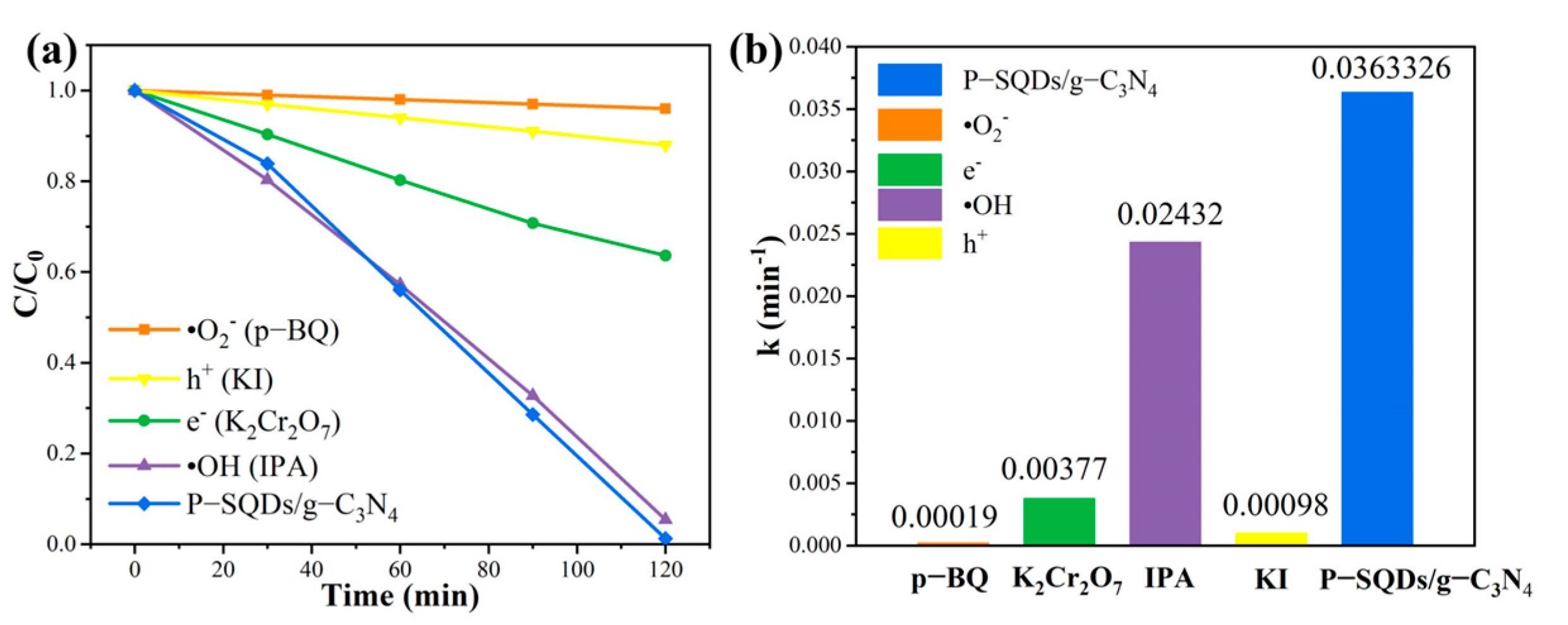
2.5. Analysis of the Photocatalytic Mechanism
3. Experimental Section
3.1. Material
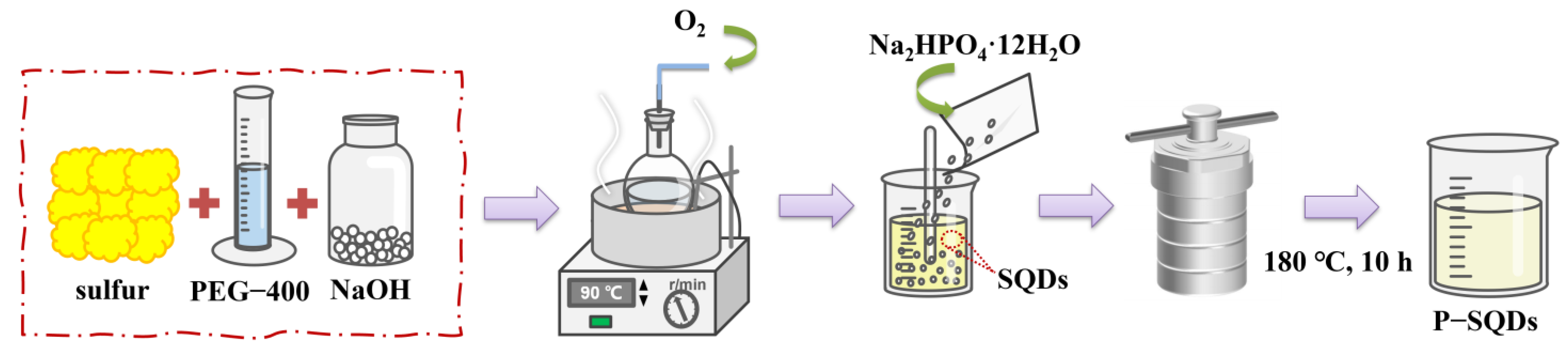
3.2. Preparation of P-SQDs
3.3. Preparation of P-SQDs/g-C3N4
3.4. Characterization
3.5. Electrochemical Measurements
3.6. Photocatalytic Degradation Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 20. [Google Scholar] [CrossRef]
- Cai, M.; Bai, D.; Li, H. Optimal planning of water pollution control. J. Exp. Bot. 2003, 54, 61. [Google Scholar]
- Zhou, Z.; Liu, J.; Zhou, N.; Zhang, T.; Zeng, H. Does the “10-point water plan” reduce the intensity of industrial water pollution? Quasi-experimental evidence from China. J. Environ. Manag. 2021, 295, 113048. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi, A.A.; Jafer, R.; Li, X.; Zhang, P. A new heterojunction in photocatalysis: S-scheme heterojunction. Chin. J. Catal. 2021, 42, 667–669. [Google Scholar] [CrossRef]
- Yang, B.; Dai, J.; Zhao, Y.; Wu, J.; Ji, C.; Zhang, Y. Advances in preparation, application in contaminant removal, and environmental risks of biochar-based catalysts: A review. Biochar 2022, 4, 51. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhou, F.; Zhao, X.; Lu, P.; Sun, G.; Song, Y.; Li, Y.; Liu, X.; Dai, H. Graphitic carbon nitride-based photocatalysts in the applications of environmental catalysis. J. Environ. Sci. 2023, 124, 570–590. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic metal oxide-based photocatalysts with integrated silver for water treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 photocatalysts for degradation of organic dyes and pharmaceuticals without oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Song, B.; Yi, H.; et al. In situ grown single-atom cobalt on polymeric carbon nitride with bidentate ligand for efficient photocatalytic degradation of refractory antibiotics. Small 2020, 16, 2001634. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Y.; Liu, Y.; Vequizo, J.J.M.; Sun, H.; Yang, C.; Yamakata, A.; Fan, F.; Lin, W.; Wang, X.; et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride. Angew. Chem. Int. Ed. 2020, 59, 16209–16217. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Zhang, G.; Li, K.; Huang, Z.; Wang, X.; Guo, Y.; Hou, J.; Song, C.; Guo, X. Self-Supporting 3D carbon nitride with tunable n -> pi* electronic transition for enhanced solar hydrogen production. Adv. Mater. 2021, 33, 2104361. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Dong, F.; Reshak, A.H.; Ye, L.; Pinna, N.; Zeng, C.; Zhang, T.; Huang, H. Chlorine intercalation in graphitic carbon nitride for efficient photocatalysis. Appl. Catal. B 2017, 203, 465–474. [Google Scholar] [CrossRef]
- Hu, C.; Chen, F.; Wang, Y.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Exceptional cocatalyst-free photo-enhanced piezocatalytic hydrogen evolution of carbon nitride nanosheets from strong in-plane polarization. Adv. Mater. 2021, 33, 2101751. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, H.; Li, M.; Tang, T.; Wen, J.; Li, X. Phosphorus-doped graphene quantum dots loaded on TiO2 for enhanced photodegradation. Appl. Surf. Sci. 2020, 526, 146724. [Google Scholar] [CrossRef]
- Guo, Z.S.; Ni, S.N.; Wu, H.; Wen, J.F.; Li, X.Y.; Tang, T.; Li, M.; Liu, M. Designing nitrogen and phosphorus co-doped graphene quantum dots/g-C3N4 heterojunction composites to enhance visible and ultraviolet photocatalytic activity. Appl. Surf. Sci. 2021, 548, 149211. [Google Scholar] [CrossRef]
- Li, F.; Li, M.; Luo, Y.; Li, M.; Li, X.Y.; Zhang, J.Y.; Wang, L. The synergistic effect of pyridinic nitrogen and graphitic nitrogen of nitrogen-doped graphene quantum dots for enhanced TiO2 nanocomposites’ photocatalytic performance. Catalysts 2018, 8, 438. [Google Scholar] [CrossRef]
- Li, F.; Sun, L.; Luo, Y.; Li, M.; Xu, Y.J.; Hu, G.H.; Li, X.Y.; Wang, L. Effect of thiophene S on the enhanced ORR electrocatalytic performance of sulfur-doped graphene quantum dot/reduced graphene oxide nanocomposites. RSC Adv. 2018, 8, 19635–19641. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, applications, and prospects of graphene quantum dots: A comprehensive review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef]
- Gao, P.; Wang, G.; Zhou, L. Luminescent sulfur quantum dots: Synthesis, properties and potential applications. Chemphotochem 2020, 4, 5235–5244. [Google Scholar] [CrossRef]
- Pal, A.; Arshad, F.; Sk, M.P. Emergence of sulfur quantum dots: Unfolding their synthesis, properties, and applications. Adv. Colloid Interface Sci. 2020, 285, 102274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Chen, D.J.; Zheng, F.Y.; Zhou, H.F.; Jiang, S.X.; Wu, Y.J. Water-soluble and lowly toxic sulphur quantum dots. Adv. Funct. Mater. 2014, 24, 7133–7138. [Google Scholar] [CrossRef]
- Shen, L.H.; Wang, H.N.; Liu, S.N.; Bai, Z.W.; Zhang, S.C.; Zhang, X.R.; Zhang, C.X. Assembling of sulfur quantum dots in fission of sublimed sulfur. J. Am. Chem. Soc. 2018, 140, 7878–7884. [Google Scholar] [CrossRef]
- Song, Y.H.; Tan, J.S.; Wang, G.; Gao, P.X.; Lei, J.H.; Zhou, L. Oxygen accelerated scalable synthesis of highly fluorescent sulfur quantum dots. Chem. Sci. 2020, 11, 772–777. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Yin, L.; Cheng, H.-M. Alpha-sulfur crystals as a visible-light-active photocatalyst. J. Am. Chem. Soc. 2012, 134, 9070–9073. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Lei, L.; Wu, X.; Wang, W.; Ma, L. Generation mechanism of the defects in g-C3N4 synthesized in N2 atmosphere and the method for improving photocatalysis activity. Catalysts 2023, 13, 269. [Google Scholar] [CrossRef]
- Dong, G.; Wen, Y.; Fan, H.; Wang, C.; Cheng, Z.; Zhang, M.; Ma, J.; Zhang, S. Graphitic carbon nitride with thermally-induced nitrogen defects: An efficient process to enhance photocatalytic H2 production performance. RSC Adv. 2020, 10, 18632–18638. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.S.; Hu, S.N.; Yao, Y.X.; Lin, X.G.; Du, H.W.; Yuan, Y.P. Engineering graphitic carbon nitride with expanded interlayer distance for boosting photocatalytic hydrogen evolution. Chin. J. Catal. 2021, 42, 217–224. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B 2022, 303, 120904. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; Xie, Y.; Li, F.; Fan, Z.; Wang, F.; Wang, Q.; Wang, Y.; Kang, X.; Wu, G. Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions. Dalton Trans. 2015, 44, 20889–20897. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. Pi-pi stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Miao, H.; Li, W.L.; Li, H.Q.; Zhu, Y.F. Designed synthesis of a p-Ag2S/n-PDI self-assembled supramolecular heterojunction for enhanced full-spectrum photocatalytic activity. J. Mater. Chem. A 2019, 7, 6482–6490. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Wang, Z.; Zhu, Y. Enhanced visible photocatalytic oxidation activity of perylene diimide/g-C3N4 n-n heterojunction via π-π interaction and interfacial charge separation. Appl. Catal. B 2020, 271, 118933. [Google Scholar] [CrossRef]
- Sherpa, S.D.; Paniagua, S.A.; Levitin, G.; Marder, S.R.; Williams, M.D.; Hess, D.W. Photoelectron spectroscopy studies of plasma-fluorinated epitaxial graphene. J. Vac. Sci. Technol. 2012, 30, 03D102. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhou, H.Q.; Qiu, C.Y.; Yang, H.C.; Yu, F.; Sun, L.F. Layer-dependent fluorination and doping of graphene via plasma treatment. Nanotechnology 2012, 23, 115706. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Xiao, Q. Nitrogen, phosphorus and sulfur tri-doped carbon dots are specific and sensitive fluorescent probes for determination of chromium(VI) in water samples and in living cells. Mikrochim. ACTA 2019, 186, 851. [Google Scholar] [CrossRef]
- Liu, W.; Ning, C.; Sang, R.; Hou, Q.; Ni, Y. Lignin-derived graphene quantum dots from phosphous acid-assisted hydrothermal pretreatment and their application in photocatalysis. Ind. Crops Prod. 2021, 171, 113963. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for congo red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Li, T.; Zhang, L.; Hu, C. Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant. Appl. Catal. B 2020, 268, 118397. [Google Scholar] [CrossRef]
- Cui, L.F.; Song, J.L.; McGuire, A.F.; Kang, S.F.; Fang, X.Y.; Wang, J.J.; Yin, C.C.; Li, X.; Wang, Y.G.; Cui, B.X. Constructing highly uniform onion-ring-like graphitic carbon nitride for efficient visible-light-driven photocatalytic hydrogen evolution. ACS Nano 2018, 12, 5551–5558. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shan, C.; Wei, G.; Wen, J.; Jiang, L.; Hu, G.; Fang, Z.; Tang, T.; Li, M. A Novel Non-Metallic Photocatalyst: Phosphorus-Doped Sulfur Quantum Dots. Molecules 2023, 28, 3637. https://doi.org/10.3390/molecules28083637
Liu Z, Shan C, Wei G, Wen J, Jiang L, Hu G, Fang Z, Tang T, Li M. A Novel Non-Metallic Photocatalyst: Phosphorus-Doped Sulfur Quantum Dots. Molecules. 2023; 28(8):3637. https://doi.org/10.3390/molecules28083637
Chicago/Turabian StyleLiu, Ziyi, Chuanfu Shan, Guiyu Wei, Jianfeng Wen, Li Jiang, Guanghui Hu, Zhijie Fang, Tao Tang, and Ming Li. 2023. "A Novel Non-Metallic Photocatalyst: Phosphorus-Doped Sulfur Quantum Dots" Molecules 28, no. 8: 3637. https://doi.org/10.3390/molecules28083637
APA StyleLiu, Z., Shan, C., Wei, G., Wen, J., Jiang, L., Hu, G., Fang, Z., Tang, T., & Li, M. (2023). A Novel Non-Metallic Photocatalyst: Phosphorus-Doped Sulfur Quantum Dots. Molecules, 28(8), 3637. https://doi.org/10.3390/molecules28083637







