Green Synthesis of Aromatic Nitrogen-Containing Heterocycles by Catalytic and Non-Traditional Activation Methods
Abstract
1. Introduction
2. Five-Membered Rings
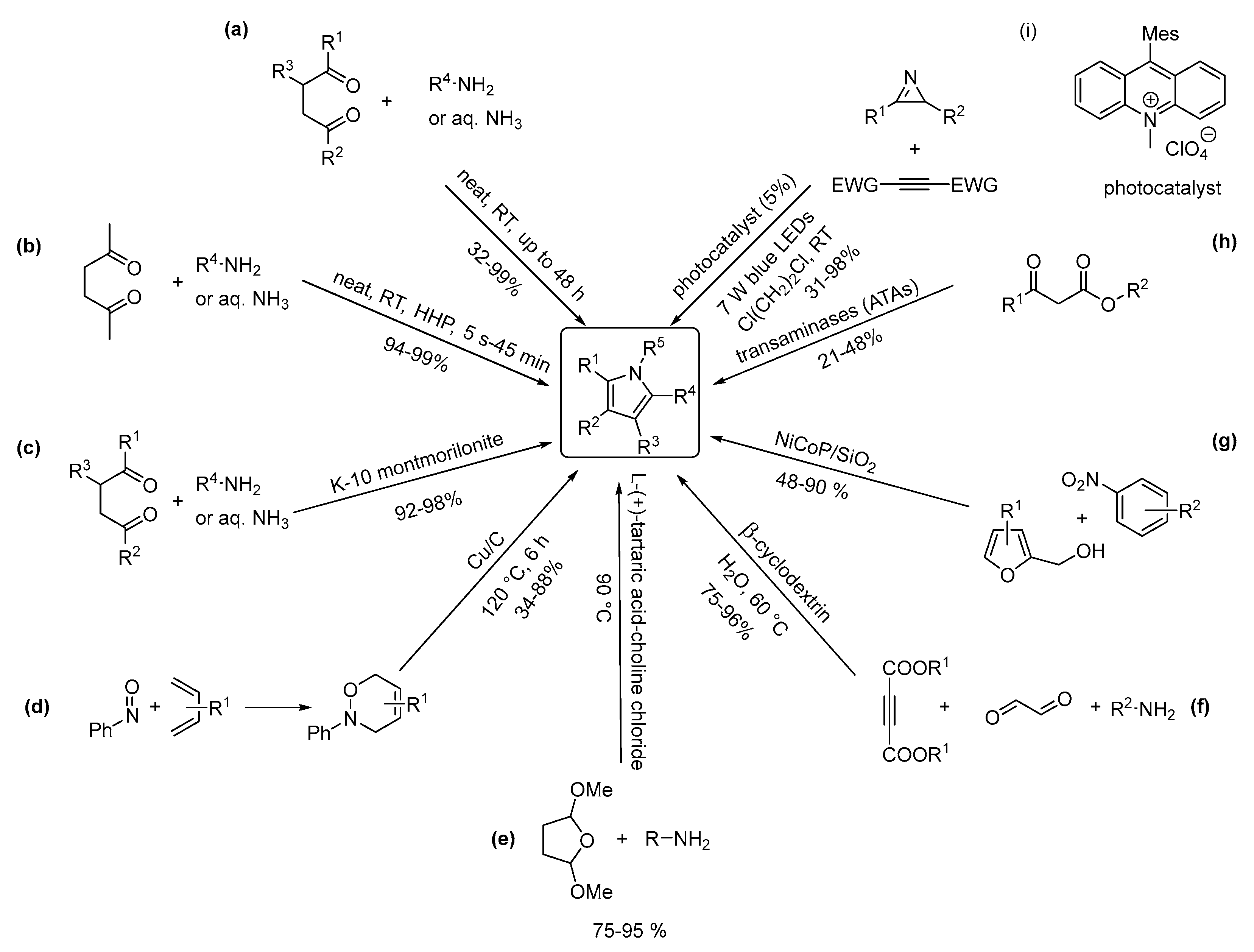
2.1. One-Nitrogen-Containing Heterocycles: Pyrroles
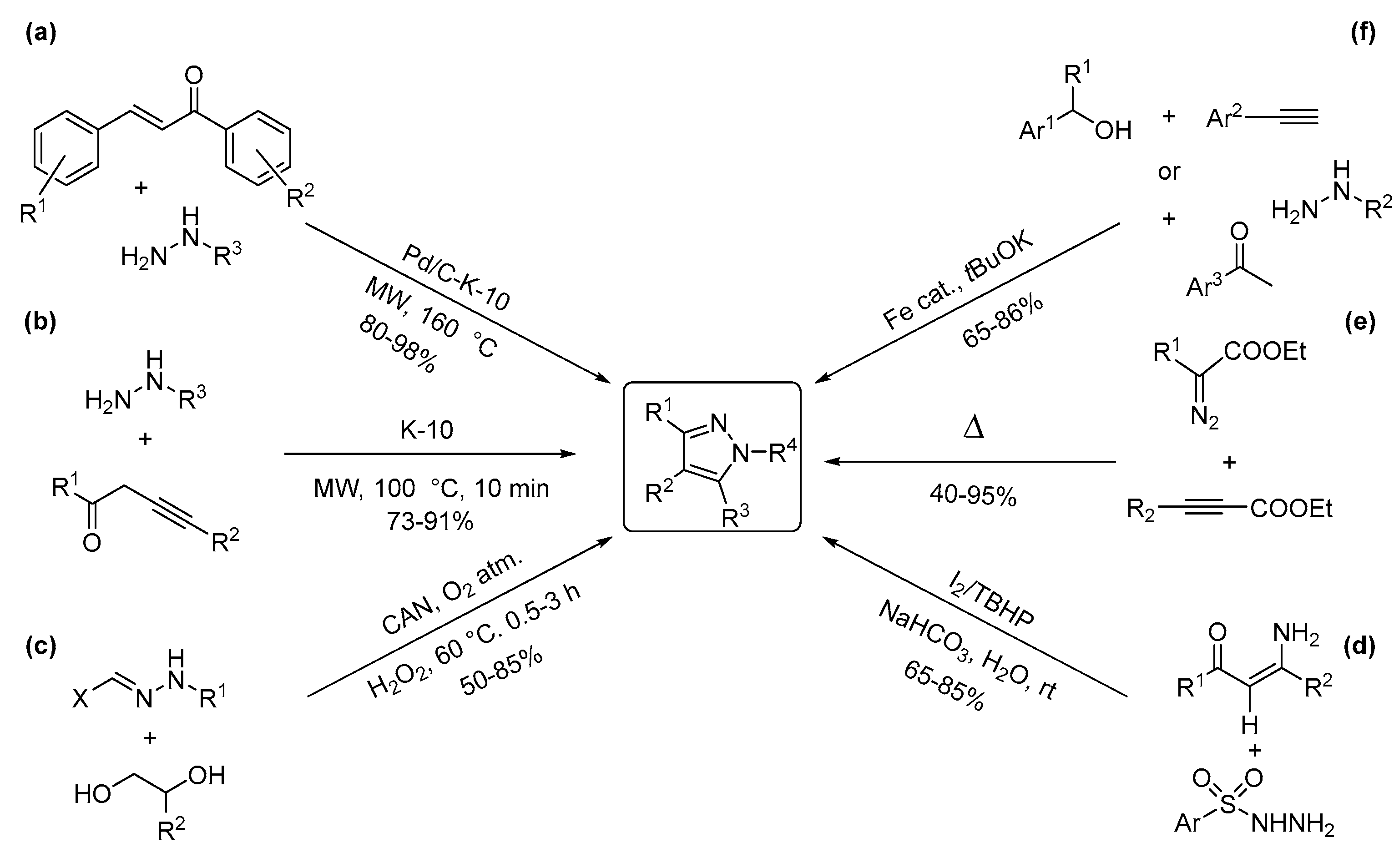
2.2. Two-Nitrogen-Containing Heterocycles: Pyrazoles and Imidazoles
2.3. Three-Nitrogen-Containing Heterocycles: Triazoles
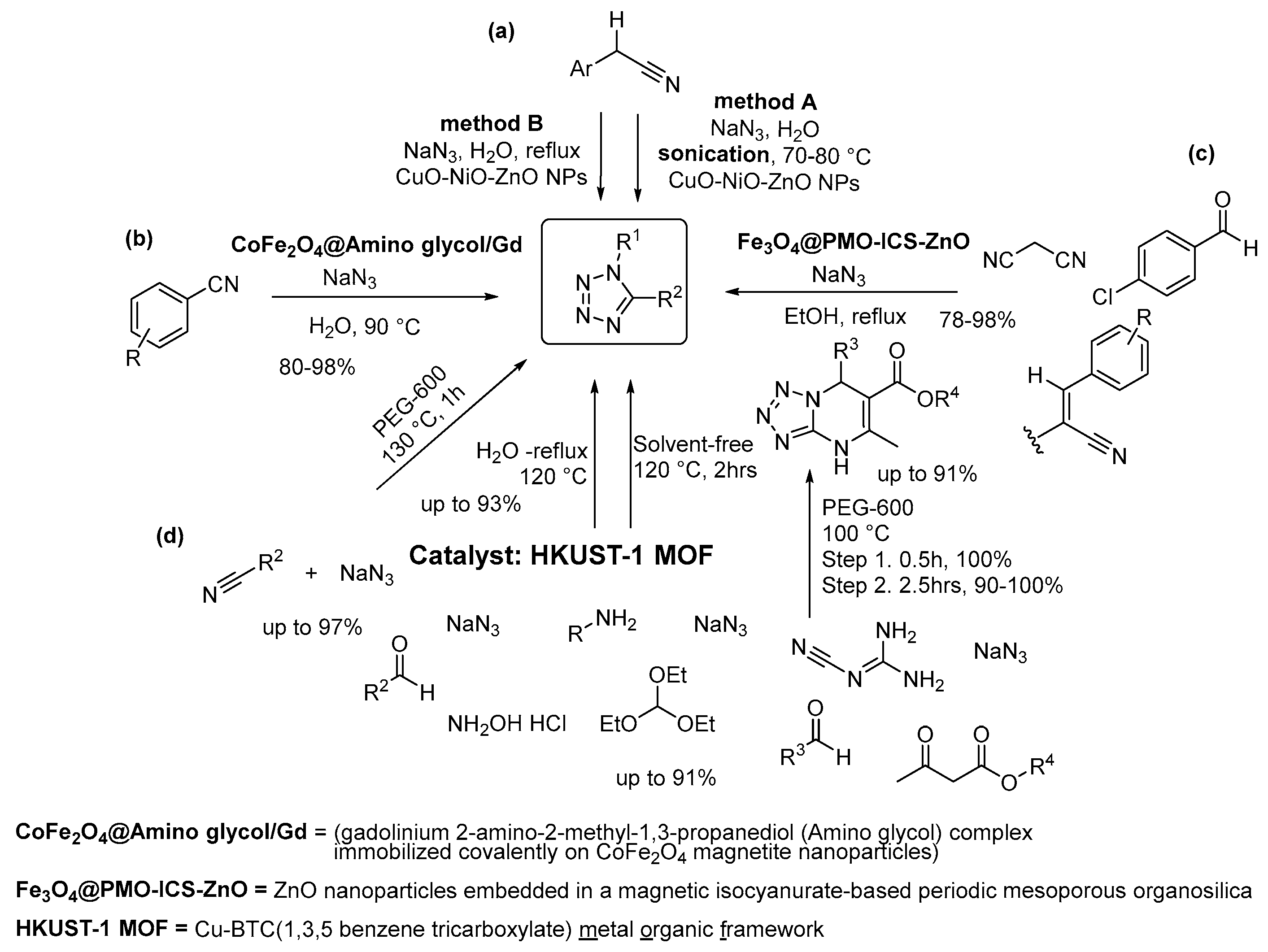
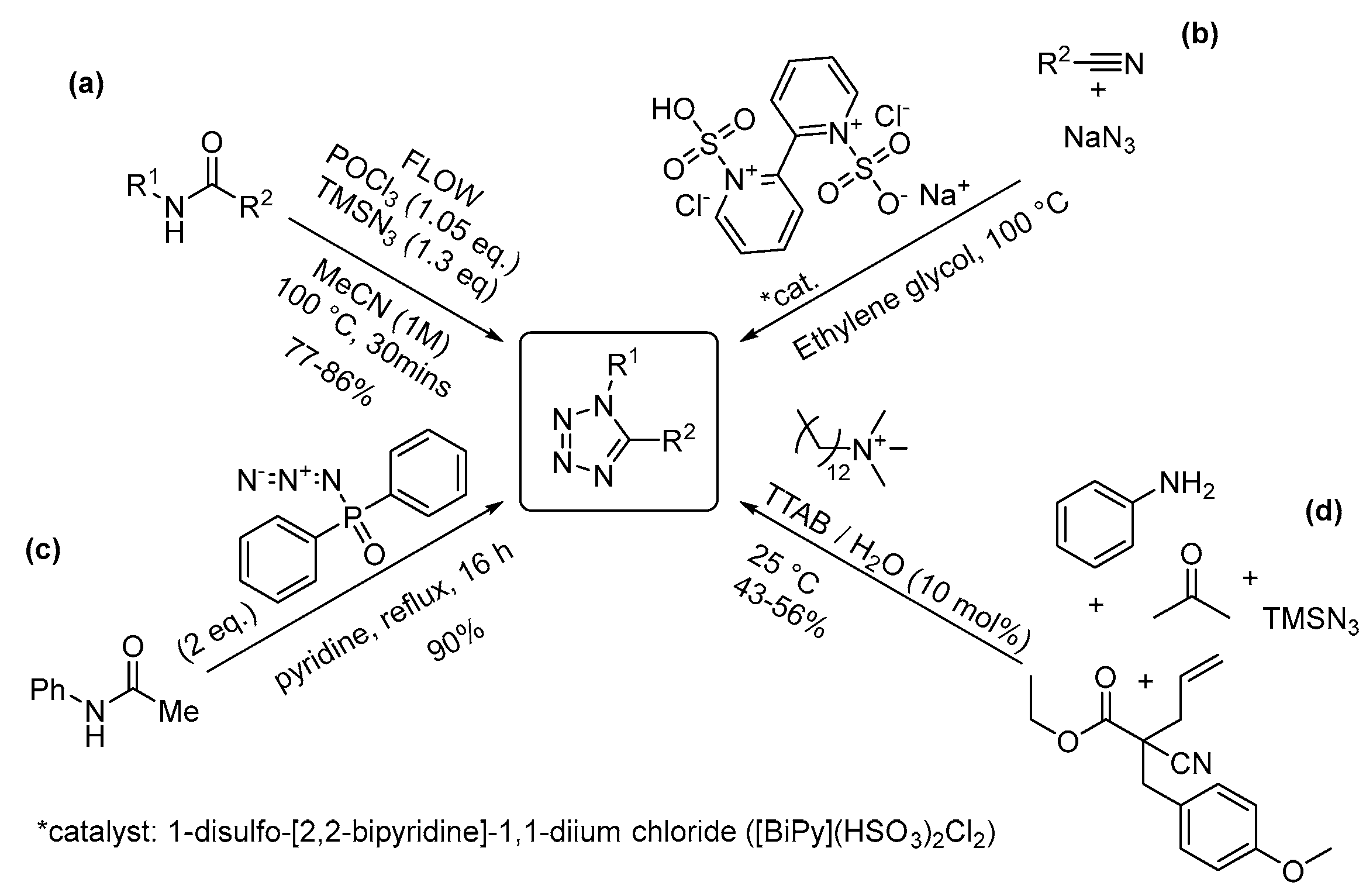
2.4. Four-Nitrogen-Containing Heterocycles: Tetrazoles
3. Six-Membered Rings
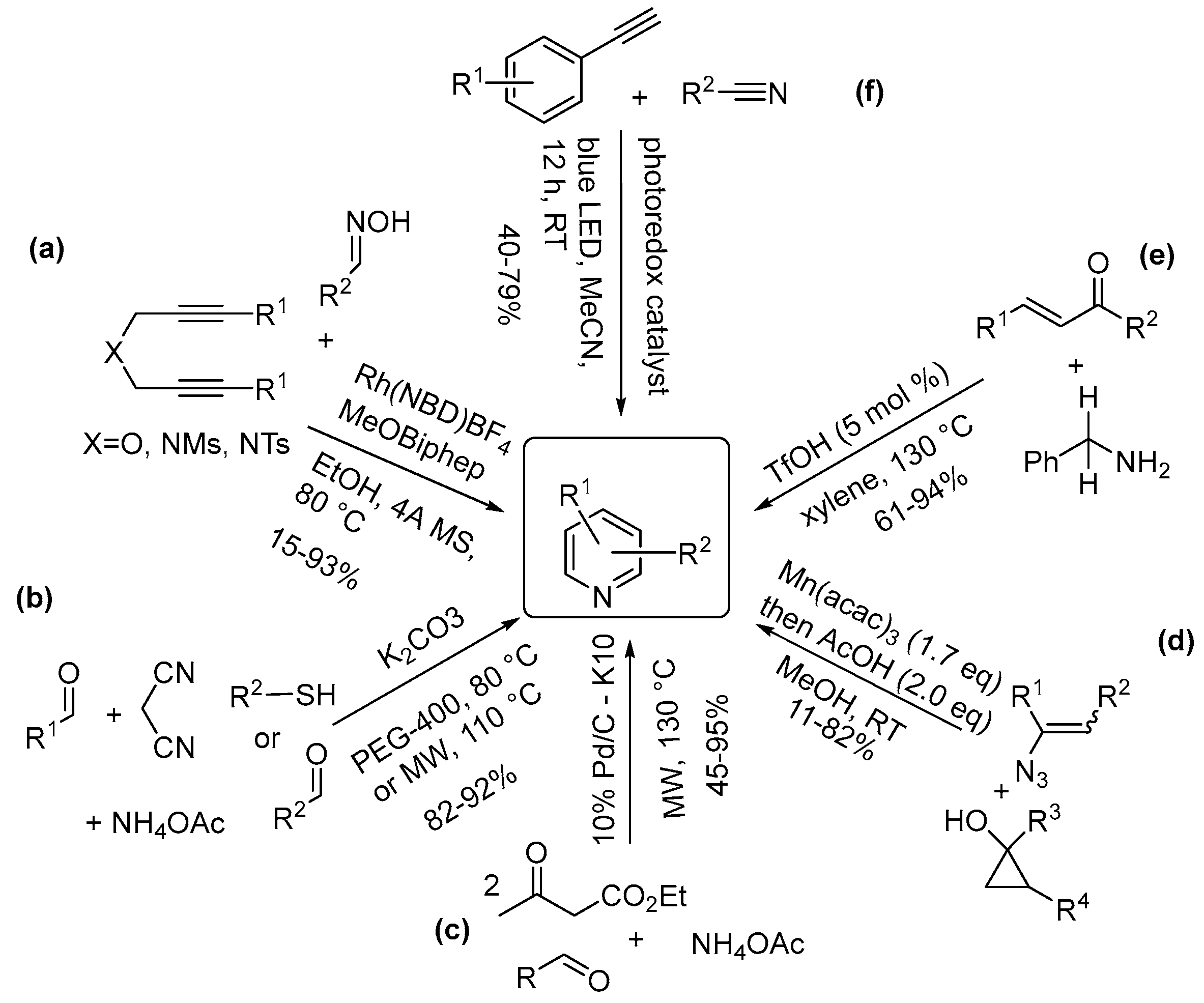
3.1. One-Nitrogen-Containing Heterocycles: Pyridines
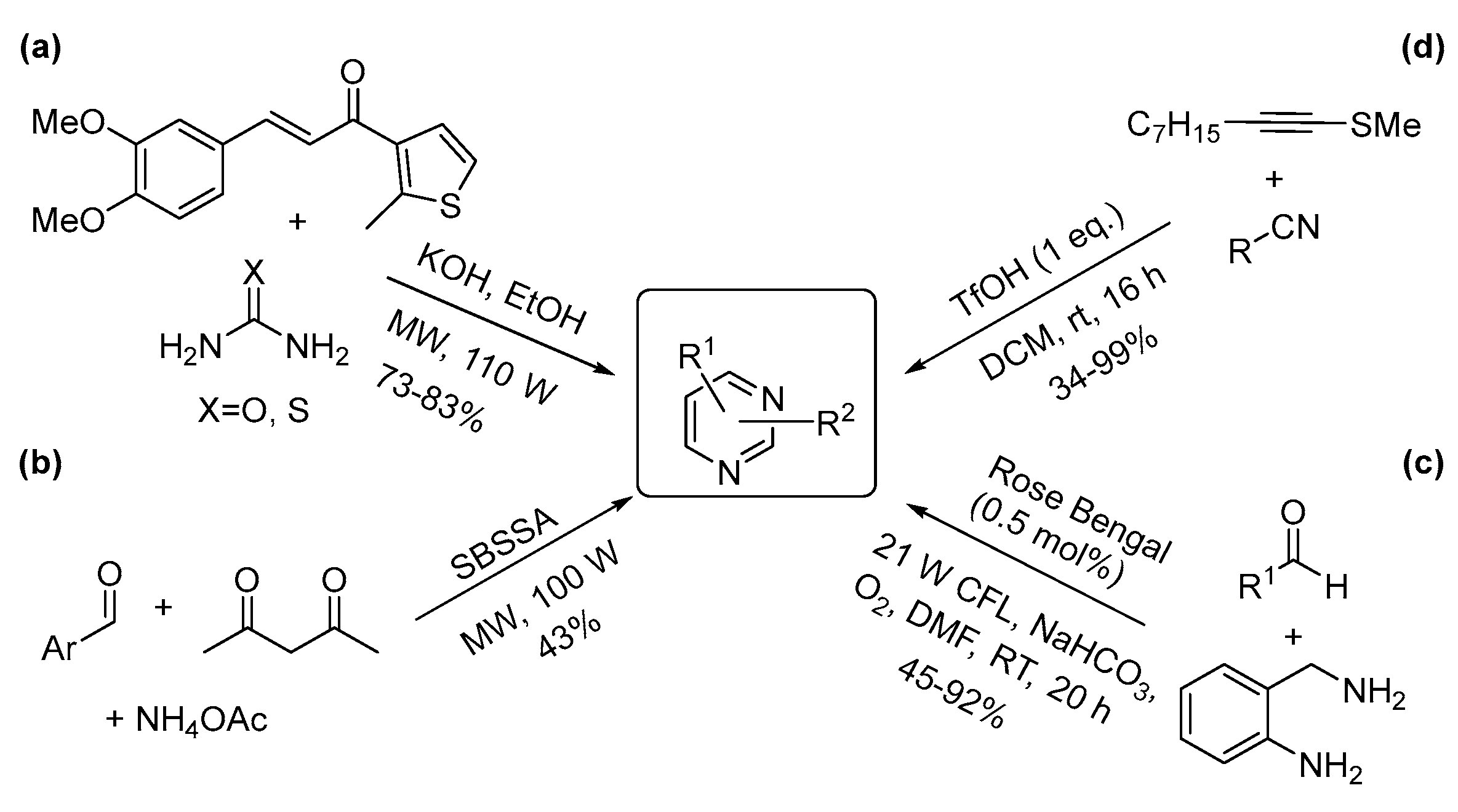
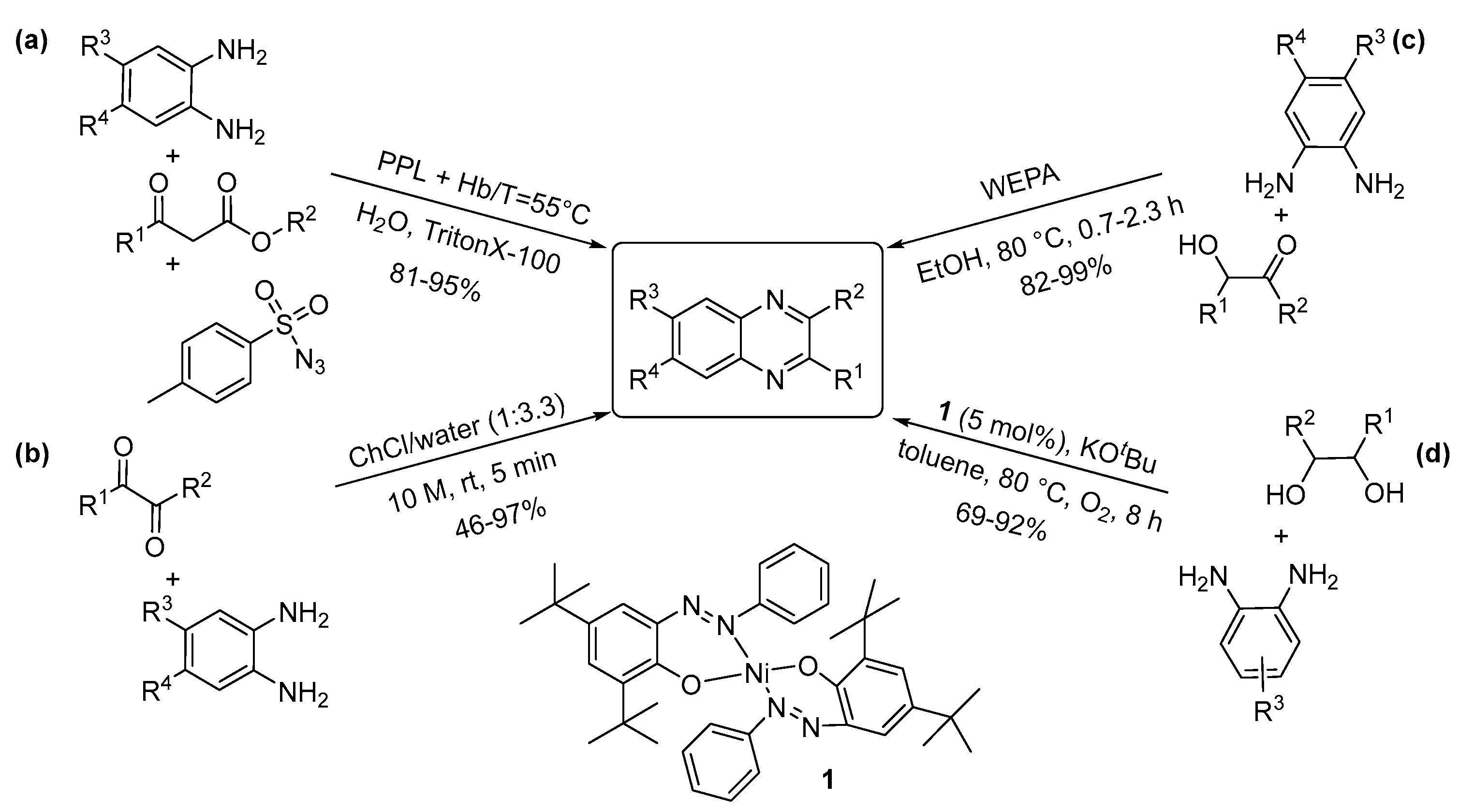
3.2. Two-Nitrogen-Containing Heterocycles: Pyrimidines, Pyrazines
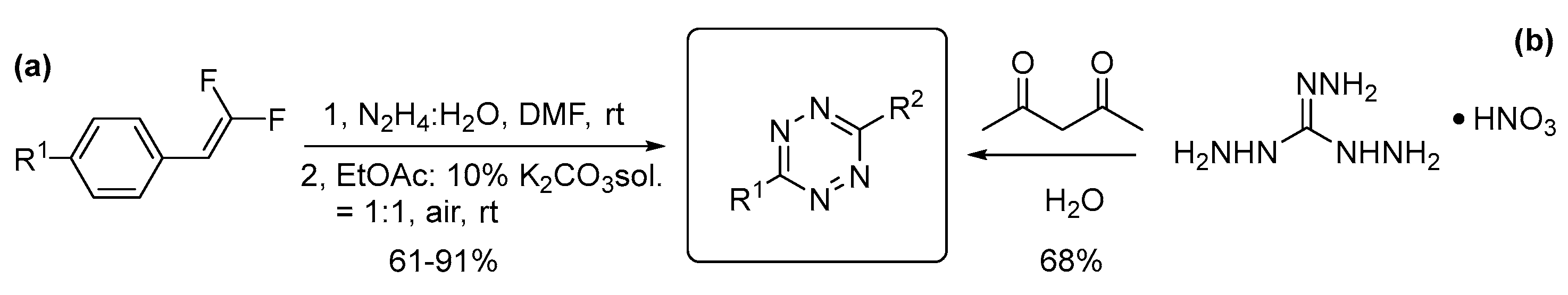
3.3. Three- and Four- Nitrogen-Containing Heterocycles: Triazines and Tetrazines
4. One-Nitrogen-Containing Condensed Heterocycles
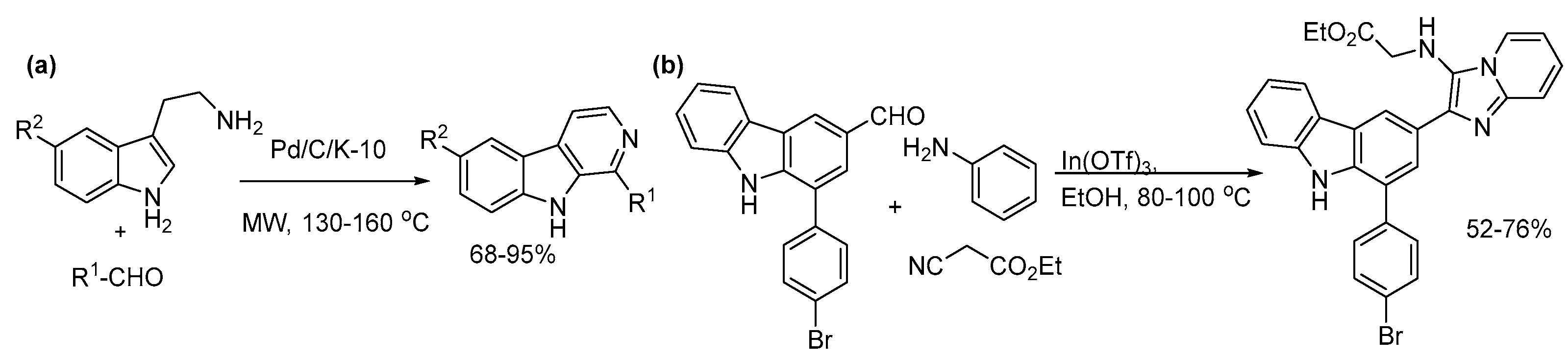
4.1. Indoles
4.2. Indolizines
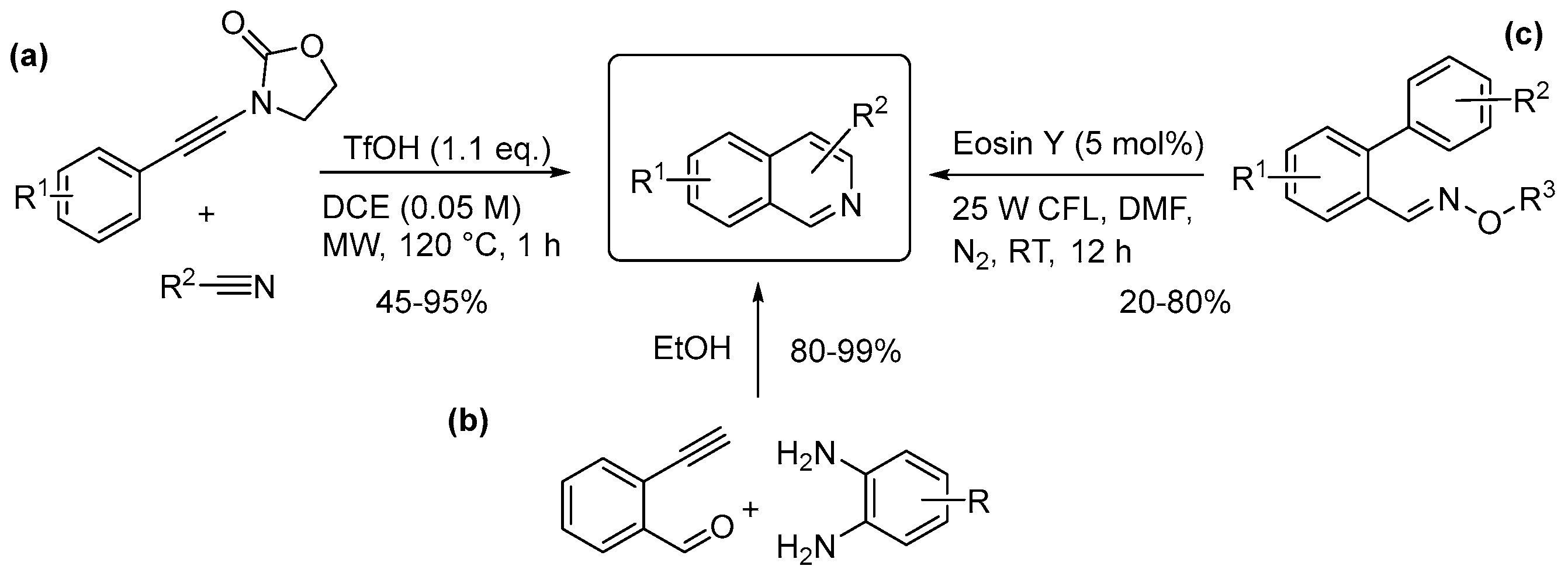
4.3. Quinolines, Isoquinolines
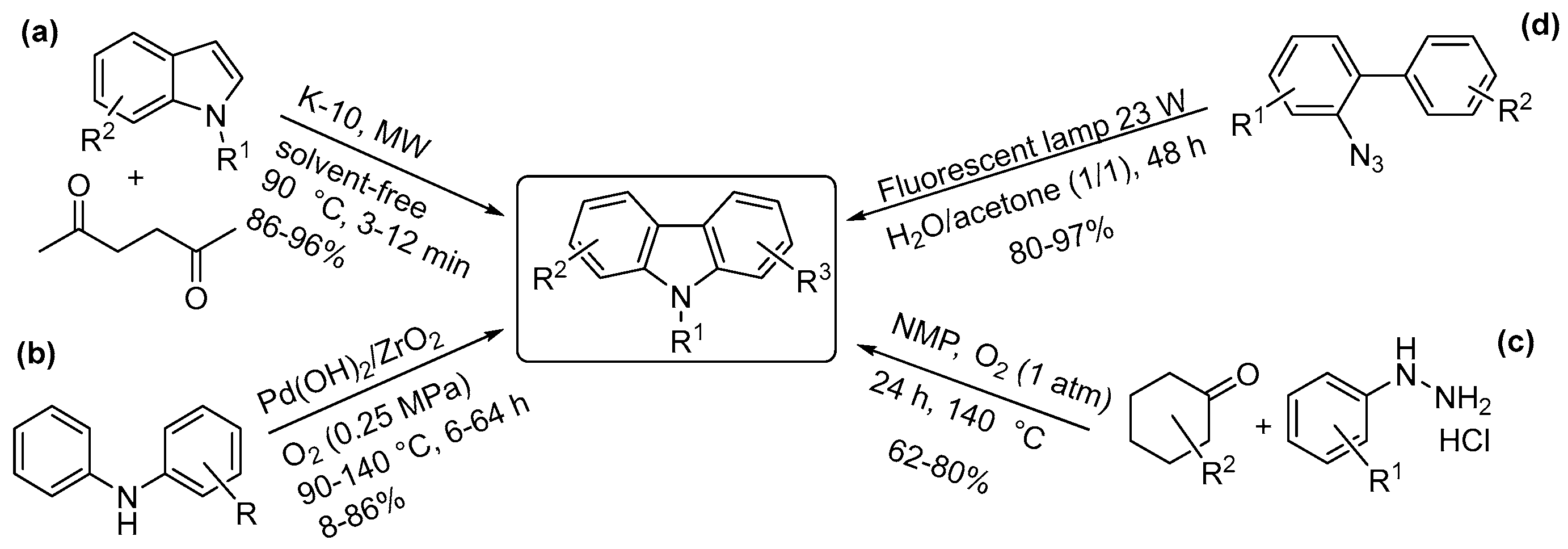
4.4. Carbazoles
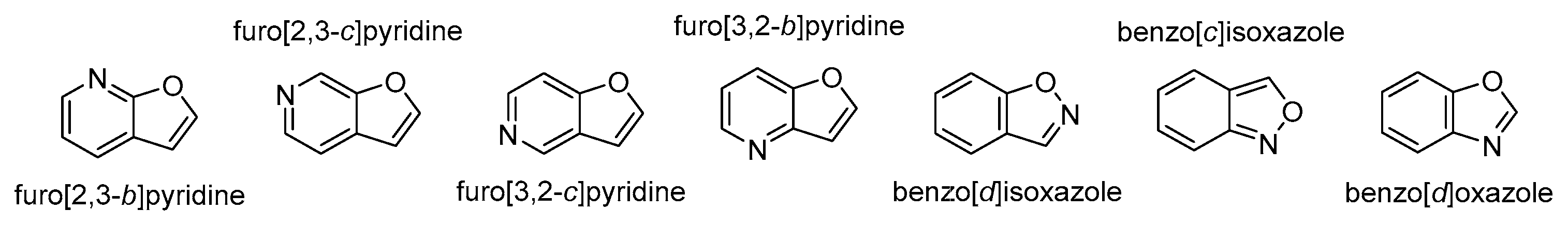
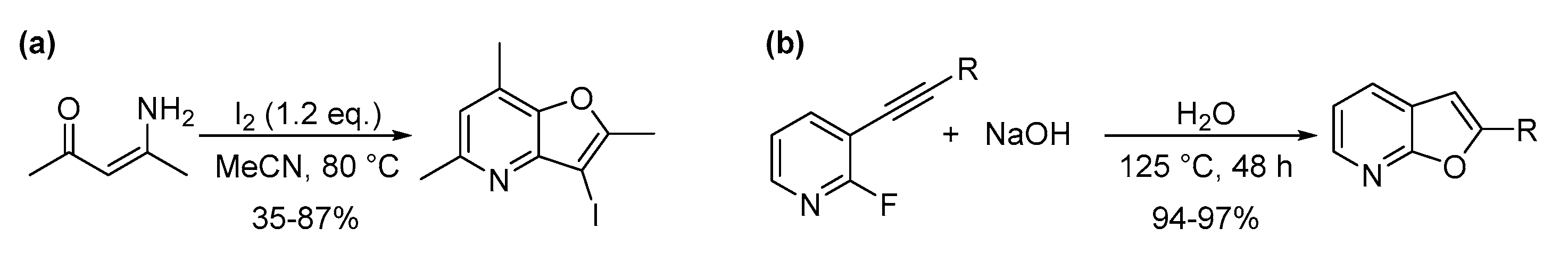
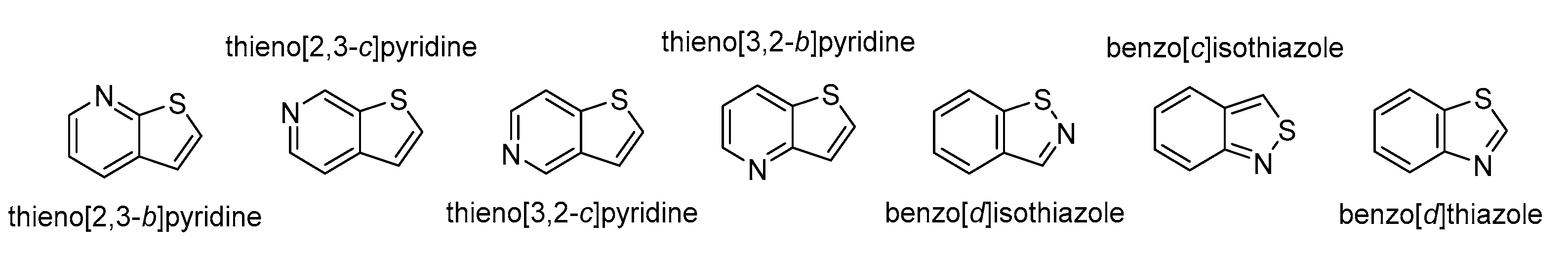
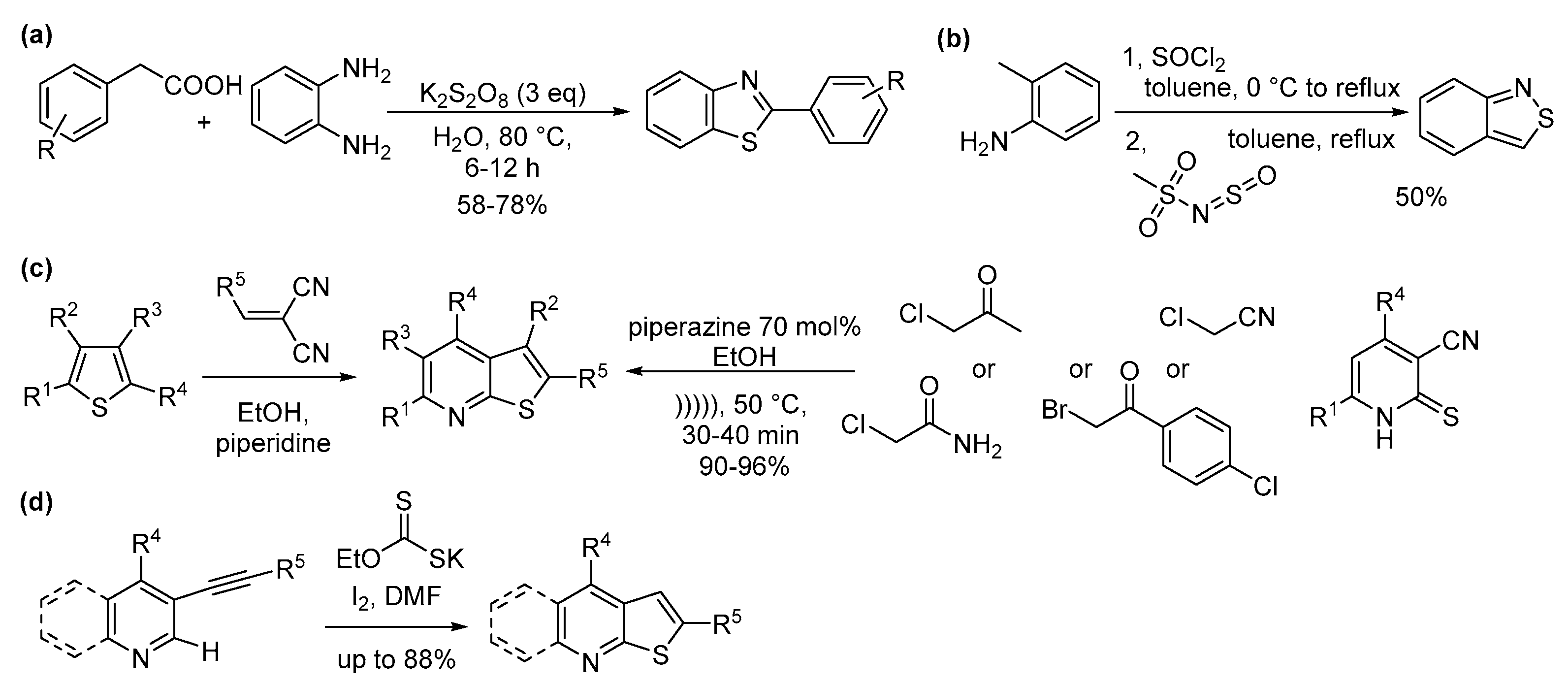
4.5. N and Other Heteroatom
5. Two-Nitrogen-Containing-Condensed Heterocycles with Multiple N Atoms
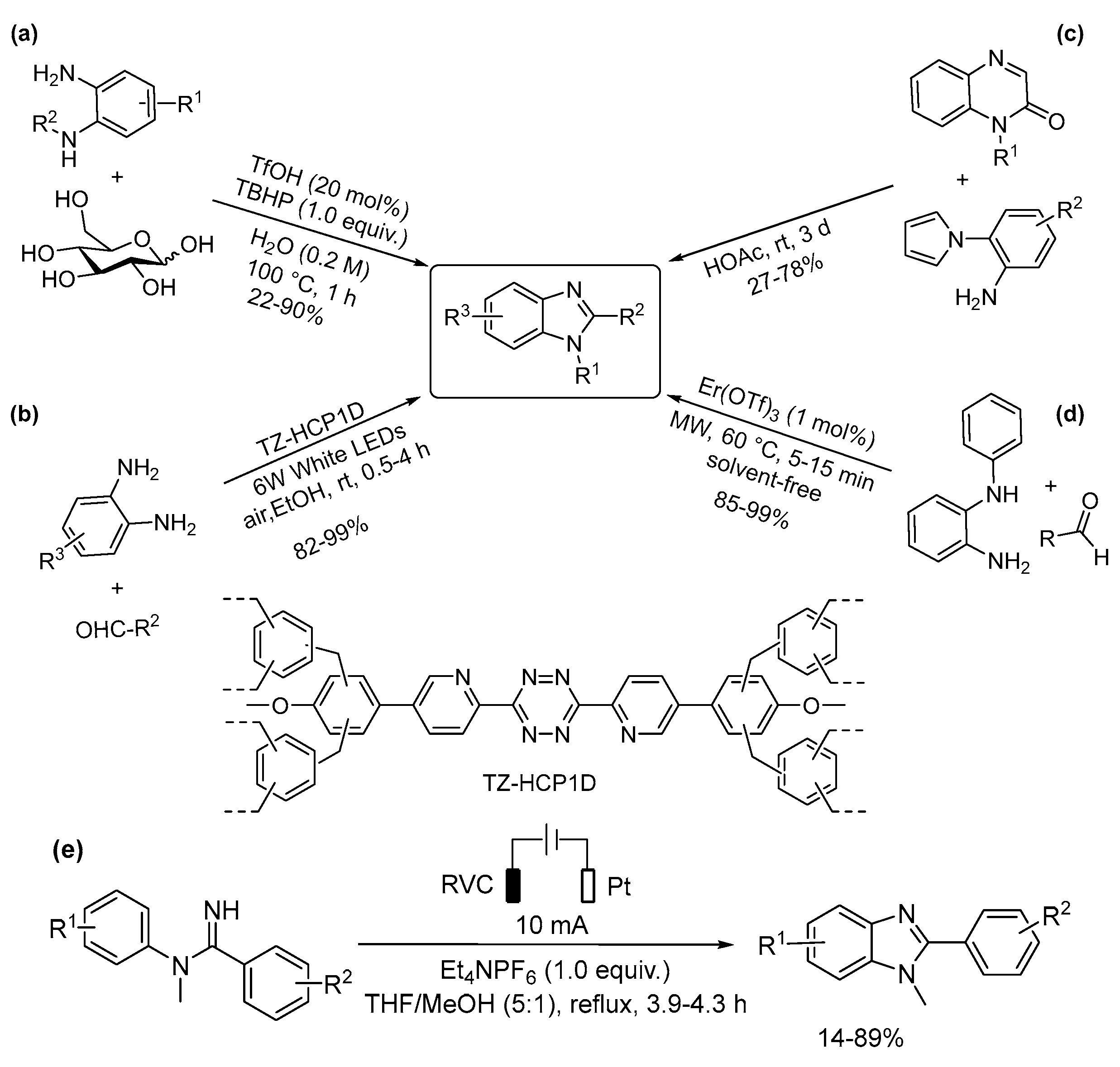
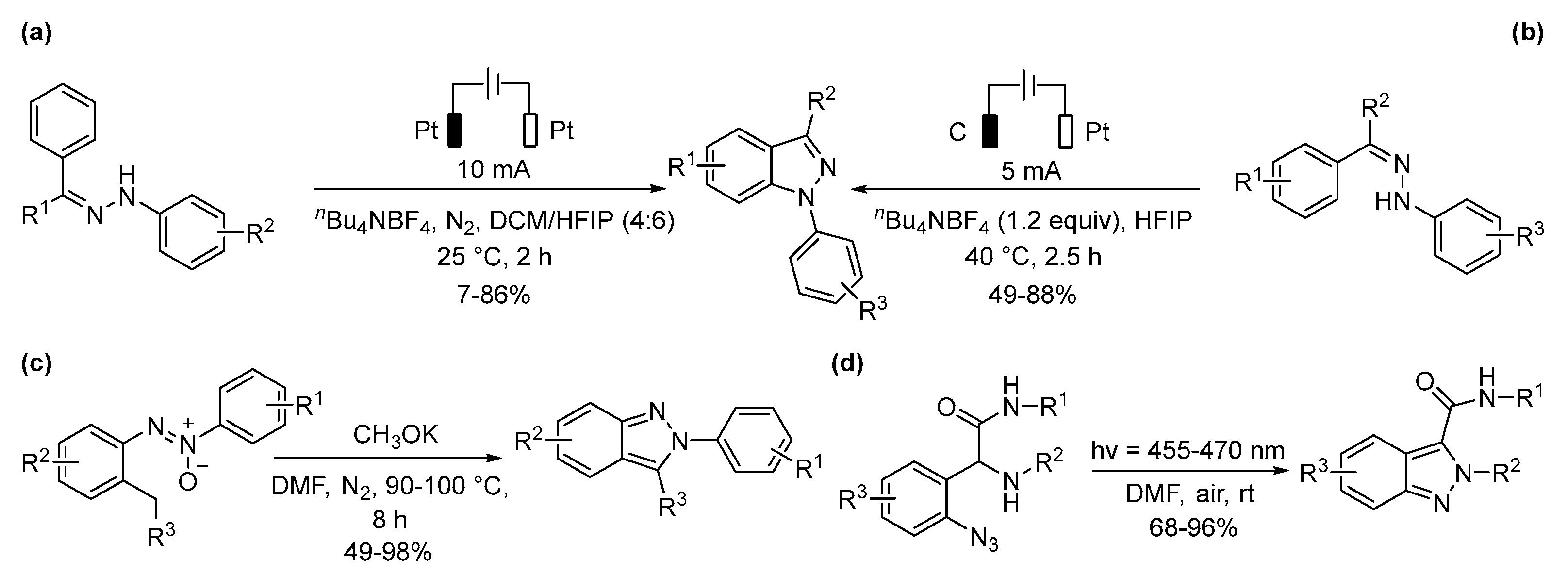
5.1. Benzimidazoles and Indazoles
5.2. Carbolines
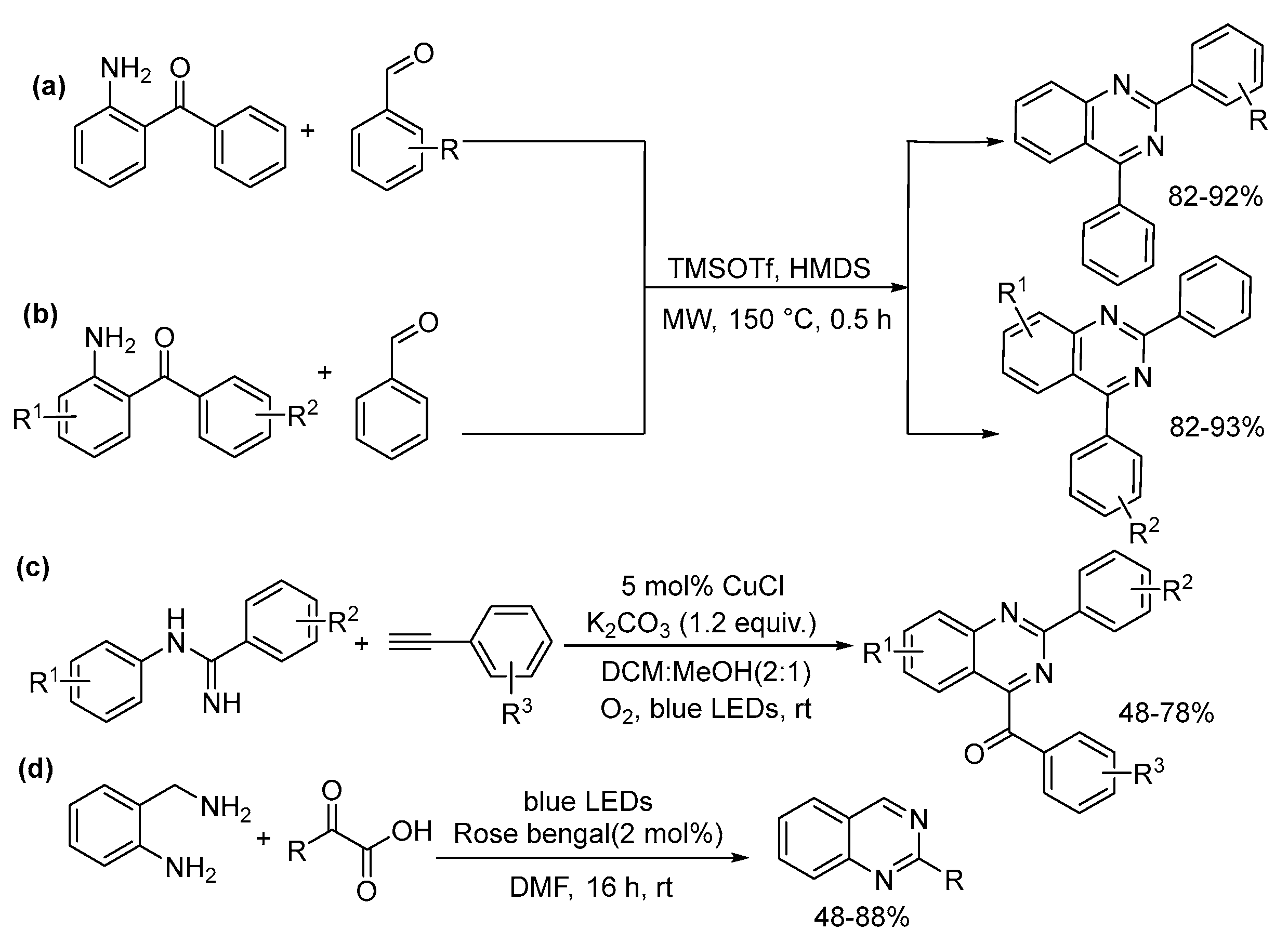
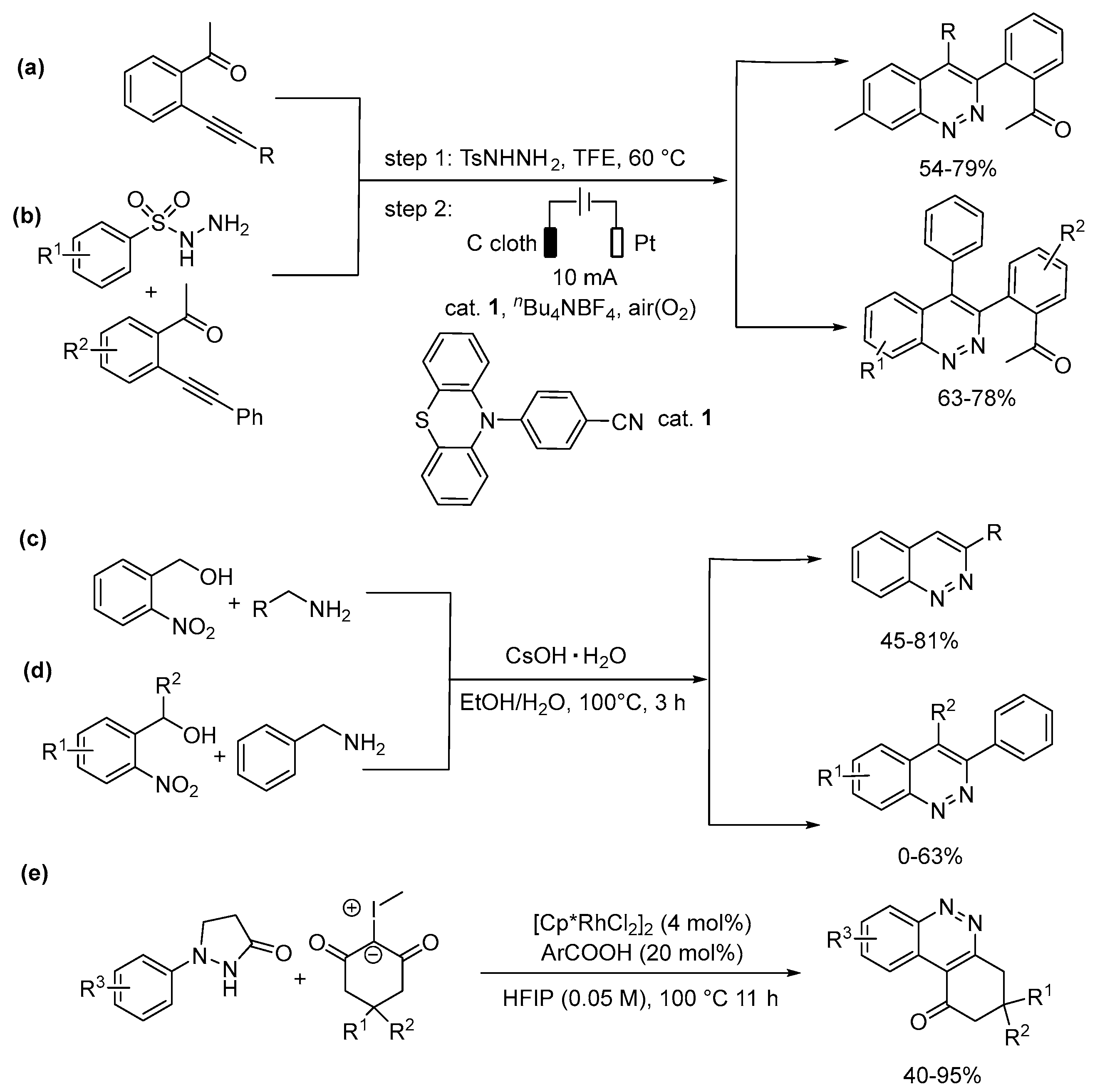
5.3. Quinazoline, Quinoxaline, Cinnoline
5.4. N and Other Heteroatoms
6. Three- and Four-N-containing Condensed Heterocycles
Purine, 3H-imidazo[4,5-b]pyridine, Imidazo[1,2-c]pyrimidine
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schäfer, C.; Cho, H.; Vlocskó, R.B.; Xie, G.; Török, B. Recent Advances in the Green Synthesis of Heterocycles: From Building Blocks to Biologically Active Compounds. Curr. Org. Synth. 2022, 19, 426–462. [Google Scholar] [CrossRef]
- Brahmachari, G. Catalyst-Free Organic Synthesis; RSC: London, UK, 2018. [Google Scholar]
- Gilchrist, T.L. Heterocyclic Chemistry, 3rd ed; Addison-Wesley-Longman, Ltd.: London, UK, 1997. [Google Scholar]
- Török, B.; Dransfield, T. (Eds.) Green Chemistry: An Inclusive Approach; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2018. [Google Scholar]
- Török, M. (Ed.) Contemporary Chemical Approaches for Green and Sustainable Drugs; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2022. [Google Scholar]
- Siddiki, S.M.A.H.; Toyao, T.; Shimizu, K. Acceptorless dehydrogenative coupling reactions with alcohols over heterogeneous catalysts. Green Chem. 2018, 20, 2933–2952. [Google Scholar] [CrossRef]
- Török, B.; Schäfer, C.; Kokel, A. Multicomponent reactions. In Heterogeneous Catalysis in Sustainable Synthesis; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021; Chapter 3.7; pp. 443–490. [Google Scholar]
- Török, B.; Schäfer, C.; Kokel, A. Ring transformations by heterogeneous catalysis. In Heterogeneous Catalysis in Sustainable Synthesis; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021; Chapter 3.8; pp. 491–542. [Google Scholar]
- Török, B.; Schäfer, C. (Eds.) Non-Traditional Activation Methods in Green and Sustainable Applications: Microwaves, Ultrasounds, Photo, Electro and Mechanochemistry and High Hydrostatic Pressure; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021. [Google Scholar]
- Knorr, L. Synthese von Furfuranderivaten aus dem Diacetbernsteinsäureester. Ber. Dtsch. Chem. Ges. 1884, 17, 2863–2870. [Google Scholar] [CrossRef]
- Paal, C. Ueber die Derivate des Acetophenonacetessigesters und des Acetonylacetessigesters. Ber. Dtsch. Chem. Ges. 1884, 17, 2756–2767. [Google Scholar] [CrossRef]
- Balakrishna, A.; Aguiar, A.; Sobral, P.J.M.; Wani, M.Y.; Almeida, J.; Sobral, S.A.J.N.F. Paal–Knorr synthesis of pyrroles: From conventional to green synthesis. Catal. Rev. Sci. Eng. 2019, 61, 84–110. [Google Scholar] [CrossRef]
- Cho, H.; Madden, R.; Nisanci, B.; Török, B. The Paal-Knorr reaction revisited. A catalyst and solvent-free synthesis of underivatized and N-substituted pyrroles. Green Chem. 2015, 17, 1088–1099. [Google Scholar] [CrossRef]
- Xie, G.; Lazarev, A.; Török, B. High Pressure Initiated Solvent and Catalyst-Free Instant Paal-Knorr Reactions. Green Chem. 2023, 25, 1582–1587. [Google Scholar] [CrossRef]
- Akbaslar, D.; Demirkol, O.; Giray, S. Paal-Knorr pyrrole synthesis in water. Synth. Commun. 2014, 44, 1323–1332. [Google Scholar] [CrossRef]
- Guan, Z.-H.; Li, L.; Ren, Z.-H.; Li, J.; Zhao, M.N. A facile and efficient synthesis of multisubstituted pyrroles from enaminoesters and nitroolefins. Green Chem. 2011, 13, 1664–1668. [Google Scholar] [CrossRef]
- Abid, M.; Spaeth, A.; Török, B. Solvent-Free Solid Acid Catalyzed Electrophilic Annelations: A New Green Approach for the Synthesis of Substituted Five-Membered N-Heterocycles. Adv. Synth. Catal. 2006, 348, 2191–2196. [Google Scholar] [CrossRef]
- Abid, M.; Landge, S.M.; Török, B. An Efficient and Rapid Synthesis of N-substituted Pyrroles by Microwave Assisted Solid Acid Catalysis. Org. Prep. Proced. Int. 2006, 35, 495–500. [Google Scholar] [CrossRef]
- Abid, M.; Teixeira, L.; Török, B. Triflic acid controlled successive annelation of aromatic sulfonamides: An efficient one-pot synthesis of N-sulfonyl pyrroles, indoles and carbazoles. Tetrahedron Lett. 2007, 48, 4047–4050. [Google Scholar] [CrossRef]
- Daştan, A.; Kulkarni, A.; Török, B. Environmentally Benign Synthesis of Heterocyclic Compounds by Combined Microwave-Assisted Heterogeneous Catalytic Approaches. Green Chem. 2012, 14, 17–37. [Google Scholar] [CrossRef]
- Kokel, A.; Schäfer, C.; Török, B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Yasukawa, N.; Kuwata, M.; Imai, T.; Monguchi, Y.; Sajiki, H.; Sawama, Y. Copper-catalyzed pyrrole synthesis from 3,6-dihydro-1,2-oxazines. Green Chem. 2018, 20, 4409–4413. [Google Scholar] [CrossRef]
- Wang, P.; Ma, F.-P.; Zhang, Z.-H. L-(+)-Tartaric acid and choline chloride based deep eutectic solvent: An efficient and reusable medium for synthesis of N-substituted pyrroles via Clauson-Kaas reaction. J. Mol. Liq. 2014, 198, 259–262. [Google Scholar] [CrossRef]
- Naeimi, H.; Dadaei, M. Efficient and Green Synthesis of N-aryl Pyrroles Catalyzed by Ionic Liquid [H-NMP][HSO4] in Water at Room Temperature. J. Chin. Chem. Soc. 2014, 61, 1127–1132. [Google Scholar] [CrossRef]
- Singh, S.B.; Verma, P.K.; Tiwari, K.; Srivastava, M.; Ankit, P.; Singh, M.; Singh, J.; Tiwari, K.P. Supramolecular catalysis in the synthesis of polyfunctionalised pyrroles. Supramol. Chem. 2014, 26, 882–889. [Google Scholar] [CrossRef]
- Mhadgut, S.C.; Palaniappan, K.; Thimmaiah, M.; Hackney, S.A.; Török, B.; Liu, J. A Metal Nanoparticle-Based Supramolecular Approach for Aqueous Biphasic Reactions. Chem. Commun. 2005, 3207–3209. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wu, Z.; Chen, S.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S.; Deng, Q. Breaking binary competitive adsorption in the domino synthesis of pyrroles from furan alcohols and nitroarenes over metal phosphide. Appl. Catal. B Environ. 2022, 316, 21665. [Google Scholar] [CrossRef]
- Xu, J.; Green, A.P.; Turner, N.J. Chemo-Enzymatic Synthesis of Pyrazines and Pyrroles. Angew. Chem. Int. Ed. 2018, 57, 16760–16763. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 2021, 1, 523–598. [Google Scholar] [CrossRef]
- Lenardon, G.V.A.; Nicchio, L.; Fagnoni, M. Photogenerated electrophilic radicals for the umpolung of enolate chemistry. J. Photochem. Photobiol. C Photochem. Rev. 2021, 46, 100387. [Google Scholar] [CrossRef]
- Pawlowski, R.; Stanek, F.; Stodulski, M. Recent Advances on Metal-Free, Visible-Light-Induced Catalysis for Assembling Nitrogen- and Oxygen-Based Heterocyclic Scaffolds. Molecules 2019, 24, 1533. [Google Scholar] [CrossRef]
- Xuan, J.; Xia, X.-D.; Zeng, T.-T.; Feng, Z.-J.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Visible-Light-Induced Formal [3 + 2] Cycloaddition for Pyrrole Synthesis under Metal-Free Conditions. Angew. Chem. Int. Ed. 2014, 53, 5653–5656. [Google Scholar] [CrossRef]
- Li, X.T.; Liu, Y.H.; Liu, X.; Zhang, Z.H. Meglumine Catalyzed One-Pot, Three-Component Combinatorial Synthesis of Pyrazoles Bearing a Coumarin Unit. RSC Adv. 2015, 5, 25625–25633. [Google Scholar] [CrossRef]
- Schmitt, D.C.; Taylor, A.P.; Flick, A.C.; Kyne, R.E. Synthesis of Pyrazoles from 1,3-Diols via Hydrogen Transfer Catalysis. Org. Lett. 2015, 17, 1405–1408. [Google Scholar] [CrossRef]
- Matcha, K.; Antonchick, A.P. Cascade Multicomponent Synthesis of Indoles, Pyrazoles and Pyridazinones by Functionalization of Alkenes. Agnew. Chem. Int. Ed. 2014, 53, 11960–11964. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Shiri, L.; Khani, Z. Recent advances in the synthesis of pyrazole scaffolds via nanoparticles: A review. Tetrahedron 2022, 110, 132688. [Google Scholar] [CrossRef]
- Dias, D.; Pacheco, B.S.; Cunico, W.; Pizzuti, L.; Pereira, C.M.P. Recent Advances on the Green Synthesis and Antioxidant Activities of Pyrazoles. Mini-Rev. Med. Chem. 2014, 14, 1078–1092. [Google Scholar] [CrossRef]
- Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Ilamanova, M.; Mastyugin, M.; Schäfer, C.; Kokel, A.; Török, B. Heterogeneous Metal Catalysis for the Environmentally Benign Synthesis of Medicinally Important Scaffolds, Intermediates and Building Blocks. Curr. Org. Chem. 2021, 25, 2304–2330. [Google Scholar] [CrossRef]
- Landge, S.M.; Schmidt, A.; Outerbridge, V.; Török, B. Synthesis of Pyrazoles by a One-Pot Tandem Cyclization-Dehydrogenation Approach on Pd/C/K-10 Catalyst. Synlett 2007, 1600–1604. [Google Scholar] [CrossRef]
- Borkin, D.A.; Puscau, M.; Carlson, A.; Solan, A.; Wheeler, K.A.; Török, B.; Dembinski, R. Synthesis of diversely 1,3,5-trisubstituted pyrazoles via 5-exo-dig cyclization. Org. Biomol. Chem. 2012, 10, 4505–4508. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pal, C.; Kumar Jena, A. Ce-catalyzed regioselective synthesis of pyrazoles from 1,2-diols via tandem oxidation and C–C/C–N bond formation. Org. Biomol. Chem. 2023, 21, 59–64. [Google Scholar]
- Guo, Y.; Wang, G.; Wei, L.; Wan, J.-P. Domino C-H Sulfonylation and Pyrazole Annulation for Fully Substituted Pyrazole Synthesis in Water Using Hydrophilic Enaminones. J. Org. Chem. 2019, 84, 2984–2990. [Google Scholar] [CrossRef]
- Vuluga, D.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Synthesis of Pyrazoles through Catalyst-Free Cycloaddition of Diazo Compounds to Alkynes. Green Chem. 2009, 11, 156–159. [Google Scholar] [CrossRef]
- Mondal, R.; Kumar Guin, A.; Pal, S.; Mondal, S.; Paul, N.D. Sustainable synthesis of pyrazoles using alcohols as the primary feedstock by an iron catalyzed tandem C–C and C–N coupling approach. Org. Chem. Front. 2022, 9, 5246–5258. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, M.-M.; Qin, P.; Xue, X.-S.; Zhang, F.-G.; Ma, J.-A. Silver-catalysed [3+2] annulation reaction of aryldiazonium salts with allenes enabled by boronate direction. Org. Chem. Front. 2023, 10, 74–82. [Google Scholar] [CrossRef]
- Cardinale, L.; Neumeier, M.; Majek, M.; von Wangelin, A.J. Aryl Pyrazoles from Photocatalytic Cycloadditions of Arenediazonium. Org. Lett. 2020, 22, 7219–7224. [Google Scholar] [CrossRef]
- Deng, Q.-H.; Zou, Y.-Q.; Lu, L.-Q.; Tang, Z.-L.; Chen, J.-R.; Xiao, W.-J. De Novo Synthesis of Imidazoles by Visible-Light-Induced Photocatalytic Aerobic Oxidation/[3+2] Cycloaddition/Aromatization Cascade. Chem. Asian J. 2014, 9, 2432–2435. [Google Scholar] [CrossRef]
- Takeda, A.; Okai, H.; Watabe, K.; Iida, H. Metal-Free Atom-Economical Synthesis of Tetra-Substituted Imidazoles via Flavin-Iodine Catalyzed Aerobic Cross-Dehydrogenative Coupling of Amidines and Chalcones. J. Org. Chem. 2022, 87, 10372–10376. [Google Scholar] [CrossRef]
- Kafi-Ahmadi, L.; Khademinia, S.; Poursattar, A.; Nozad, M.; Nozad, E. Microwave-assisted preparation of polysubstituted imidazoles using Zingiber extract synthesized green Cr2O3 nanoparticles. Sci. Rep. 2022, 12, 19942. [Google Scholar] [CrossRef]
- Ghanbari, N.; Ghafuri, H. Design and preparation of nanoarchitectonics of LDH/polymer composite with particularmorphology as catalyst for greensynthesis of imidazole derivatives. Sci. Rep. 2022, 12, 11288. [Google Scholar] [CrossRef]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: A brief review. Future J. Pharm. Sci. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef] [PubMed]
- Tratrat, C. 1,2,4-Triazole: A Privileged Scaffold for the Development of Potent Antifungal Agents—A Brief Review. Curr. Top. Med. Chem. 2020, 20, 2235–2258. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.; Hadda, T.B.; Almalk, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2020, 9, 864286. [Google Scholar] [CrossRef]
- Brunel, D.; Dumur, F. Recent advances in organic dyes and fluorophores comprising a 1,2,3-triazole moiety. New J. Chem. 2020, 44, 3546–3561. [Google Scholar] [CrossRef]
- Kaya, M.; Menteşe, E. Synthesis and solvent-dependent photophysics of a novel fluorescent triazole-coumarin-based dye. J. Heterocycl. Chem. 2020, 57, 1714–1719. [Google Scholar] [CrossRef]
- Song, S.; Ko, Y.-G.; Lee, H.; Wi, D.; Ree, B.J.; Li, Y.; Michinobu, T.; Ree, M. High-performance triazole-containing brush polymers via azide–alkyne click chemistry: A new functional polymer platform for electrical memory devices. NPG Asia Mater. 2015, 7, 228. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Marzi, M.; Farjam, M.; Kazeminejad, Z.; Shiroudi, A.; Kouhpayeh, A.; Zarenezhad, E. A Recent Overview of 1,2,3-Triazole-Containing Hybrids as Novel Antifungal Agents: Focusing on Synthesis, Mechanism of Action, and Structure-Activity Relationship (SAR). J. Chem. 2022, 2022, 7884316. [Google Scholar] [CrossRef]
- Nino, A.D.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef]
- Kim, W.G.; Kang, M.E.; Lee, J.B.; Jeon, M.H.; Lee, S.; Lee, J.; Choi, B.; Cal, P.M.S.D.; Kang, S.; Kee, J.-M.; et al. Nickel-Catalyzed Azide-Alkyne Cycloaddition to Access 1,5-Disubstituted 1,2,3-Triazoles in Air and Water. J. Am. Chem. Soc. 2017, 139, 12121–12124. [Google Scholar] [CrossRef]
- Tripathi, A.; Rode, C.V.; Llop, J.; Chavan, S.P.; Joshi, S.M. An enolate-mediated regioselective synthesis of 1,2,3-triazoles via azidealdehydes or ketones [3+2]-cycloaddition reactions in aqueous phase. Tetrahedron Lett. 2020, 61, 151662. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Lv, L.; Gao, G.; Luo, Y.; Mao, K.; Li, Z. Three-Component Reactions of α-CF3 Carbonyls, NaN3, and Amines for the Synthesis of NH-1,2,3-Triazoles. J. Org. Chem. 2021, 86, 17197–17212. [Google Scholar] [CrossRef] [PubMed]
- Bubyrev, A.; Malkova, K.; Kantin, G.; Dar’in, D.; Krasavin, M. Metal-Free Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. J. Org. Chem. 2021, 86, 17516–17522. [Google Scholar] [CrossRef]
- Deng, L.; Cao, X.; Liu, Y.; Wan, J.-P. In-Water Synthesis of 5-Thiolated 1,2,3-Triazoles from β-Thioenaminones by Diazo Transfer Reaction. J. Org. Chem. 2019, 84, 14179–14186. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, M.; Naimi-Jamal, M.R. Copper-doped functionalized β-cyclodextrin as an efficient green nanocatalyst for synthesis of 1,2,3-triazoles in water. Sci. Rep. 2022, 12, 4948. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-G.; Liao, X.-J.; Yuan, L.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Visible-Light-Mediated Click Chemistry for Highly Regioselective Azide–Alkyne Cycloaddition by a Photoredox Electron-Transfer Strategy. Chem. Eur. J. 2020, 26, 5694–5700. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owensab, E.A.; Cravotto, G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sarma, D.; Ali, A.A. Microwave assisted metal-free approach to access 1,2,3-triazoles through multicomponent synthesis. Curr. Res. Green Sustain. Chem. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Ye, Z.; Ding, M.; Wu, Y.; Li, Y.; Huaa, W.; Zhang, F. Electrochemical synthesis of 1,2,4-triazole-fused heterocycles. Green Chem. 2018, 20, 1732. [Google Scholar] [CrossRef]
- Zhao, Z.; He, Y.; Li, M.; Xu, J.; Li, X.; Zhang, L.; Gu, L. An electrochemical multicomponent [3+1+1] annulations to synthesize polysubstituted 1,2,4-triazoles. Tetrahedron 2021, 87, 132111. [Google Scholar] [CrossRef]
- Rodríguez, D.F.; Durán-Osorio, F.; Duarte, Y.; Olivares, P.; Moglie, Y.; Dua, K.; Zacconi, F.Z. Green by Design: Convergent Synthesis, Computational Analyses, and Activity Evaluation of New FXa Inhibitors Bearing Peptide Triazole Linking Units. Pharmaceutics 2022, 14, 33. [Google Scholar] [CrossRef]
- Kiranmye, T.; Vadivelu, M.; Sampath, S.; Muthu, K.; Karthikeyan, K. Ultrasound-assisted catalyst free synthesis of 1,4-/1,5-disubstituted-1,2,3-triazoles in aqueous medium. Sustain. Chem. Pharm. 2021, 19, 100358. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Bravo, N.-F.; Tamayo, L.-V.; Mestizo, P.-D.; Hurtado, J.; Macías, M.; Portilla, J. Water-Compatible Synthesis of 1,2,3-Triazoles under Ultrasonic Conditions by a Cu(I) Complex-Mediated Click Reaction. ACS Omega 2020, 5, 30148–30159. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.N.S.; Behrouz, S.; Mohammadtaghi-Nezhad, J.; Zarenezhad, E.; Agholi, M. Silica-tethered cuprous acetophenone thiosemicarbazone (STCATSC) as a novel hybrid nano-catalyst for highly efficient synthesis of new 1,2,3-triazolyl-based metronidazole hybrid analogues having potent antigiardial activity. Appl. Organomet. Chem. 2019, 33, e4799. [Google Scholar]
- Zarenezhad, E.; Rad, M.N.S.; Behrouz, S.; Esmaielzadeh, S.; Farjam, M. Immobilized [Cu(cdsalMeen)] on silicagel: A highly efficient heterogeneouscatalyst for ‘Click’ [3+2] Huisgencycloaddition. J. Iran. Chem. Soc. 2017, 14, 509–519. [Google Scholar] [CrossRef]
- Lv, F.; Liu, Y.; Zou, J.; Zhang, D.; Yao, Z. Synthesis of the Novel Photographic DIAR Couplers. Dyes Pigm. 2006, 68, 211–216. [Google Scholar] [CrossRef]
- Manzoor, S.; Tariq, Q.; Yin, X.; Zhang, J.-G. Nitro-tetrazole based high performing explosives: Recent overview of synthesis and energetic properties. Def. Technol. 2021, 17, 1995–2010. [Google Scholar] [CrossRef]
- Wei, C.-X.; Bian, M.; Gong, G.-H. Tetrazolium Compounds: Synthesis and Applications in Medicine. Molecules 2015, 20, 5528–5553. [Google Scholar] [CrossRef]
- Myznikov, L.V.; Hrabalek, A.; Koldobskii, G.I. Drugs in the Tetrazole Series. Chem. Heterocycl. Compd. 2007, 43, 1–9. [Google Scholar] [CrossRef]
- Ataroda, M.; Safaria, J.; Tebyanianb, H. Ultrasound irradiation and green synthesized CuO-NiO-ZnO mixed metal oxide: An efficient sono/nano-catalytic system toward a regioselective synthesis of 1-aryl-5-amino-1H-tetrazoles. Synth. Commun. 2020, 50, 1993–2006. [Google Scholar] [CrossRef]
- Molaei, S.; Moeini, N.; Ghadermazi, M. Synthesis of CoFe2O4@Amino glycol/Gd nanocomposite as a high-efficiency and reusable nanocatalyst for green oxidation of sulfides and synthesis of 5-substituted 1H-tetrazoles. J. Organomet. Chem. 2022, 977, 122459. [Google Scholar] [CrossRef]
- Safapoor, S.; Dekamin, M.G.; Akbari, A.; Naimi-Jamal, M.R. Synthesis of (E)-2-(1H-tetrazole-5-yl)-3-phenylacrylenenitrile derivatives catalyzed by new ZnO nanoparticles embedded in a thermally stable magnetic periodic mesoporous organosilica under green conditions. Sci. Rep. 2022, 12, 10723. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T.; Maleki, A.; Tarlani, A.; Soroush, M.R. Synthesis and Characterization of Ultrapure HKUST-1 MOFs as Reusable Heterogeneous Catalysts for the Green Synthesis of Tetrazole Derivatives. ChemistrySelect 2020, 5, 3164–3172. [Google Scholar] [CrossRef]
- Afsarian, M.H.; Farjam, M.; Zarenezhad, E.; Behrouz, S.; Rad, M.N.S. Synthesis, Antifungal Evaluation and Molecular Docking Studies of Some Tetrazole Derivatives. Acta Chim. Slov. 2019, 66, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, P.; Kaldre, D.; Sedelmeier, J.; Moessner, C.; Püntener, K.; Kummli, D.; Williams, J.D.; Kappe, C.O. Intensified Continuous Flow Synthesis and Workup of 1,5-Disubstituted Tetrazoles Enhanced by Real-Time Process Analytics. Org. Process Res. Dev. 2021, 25, 1206–1214. [Google Scholar] [CrossRef]
- Aali, E.; Gholizadeh, M.; Noroozi-Shad, N. 1-Disulfo-[2,2-bipyridine]-1,1-diium chloride ionic liquid as an efficient catalyst for the green synthesis of 5-substituted 1 H -tetrazoles. J. Mol. Struct. 2022, 1247, 131289. [Google Scholar] [CrossRef]
- Ishihara, K.; Ishihara, K.; Tanaka, Y.; Shioiri, T.; Matsugi, M. Practical synthesis of tetrazoles from amides and phosphorazidates in the presence of aromatic bases. Tetrahedron 2022, 108, 132642. [Google Scholar] [CrossRef]
- Abdessalam, M.; Sidhoum, M.A.; Zradni, F.-Z.; Ilikti, H. Synthesis of 1,5-Disubstituted Tetrazoles in Aqueous Micelles at Room Temperature. Molbank 2021, 2021, M1194. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-Free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.; Wang, H.; Li, X.; Wan, B. Eco-friendly synthesis of pyridines via rhodium-catalyzed cyclization of diynes with oximes. Green Chem. 2015, 17, 799–803. [Google Scholar] [CrossRef]
- Yi, Y.K.; Zhao, M.N.; Ren, Z.H.; Wang, Y.Y.; Guan, Z.H. Synthesis of symmetrical pyridines by iron-catalyzed cyclization of ketoxime acetates and aldehydes. Green Chem. 2017, 19, 1023–1027. [Google Scholar] [CrossRef]
- Kidwai, M.; Chauhan, R. K2CO3 catalyzed green and rapid access to 2-amino-3,5-dicarbonitrile-6-thio-pyridines. J. Iran. Chem. Soc. 2014, 11, 1005–1013. [Google Scholar] [CrossRef]
- Shaikh, Y.I.; Shaikh, A.A.; Nazeruddin, G.M. Ammonia solution catalyzed one-pot synthesis of highly functionalized pyridine derivatives. J. Chem. Pharm. Res. 2012, 4, 4953–4956. [Google Scholar]
- Yang, X.-H.; Zhou, Y.-H.; Zhang, P.-H.; Zhang, L.Y. An effective, one-pot synthesis of fully substituted pyridines under microwave irradiation in the absence of solvent. J. Heterocycl. Chem. 2013, 50, 1346–1350. [Google Scholar] [CrossRef]
- Yin, G.; Liu, Q.; Ma, J. Solvent- and catalyst-free synthesis of new hydroxylated trisubstituted pyridines under microwave irradiation. Green Chem. 2012, 14, 1796–1798. [Google Scholar] [CrossRef]
- De Paolis, O.; Baffoe, J.; Landge, S.M.; Török, B. Multicomponent Domino Cyclization-Oxidative Aromatization on a Bifunctional Pd/C/K-10 Catalyst: An Environmentally Benign Approach toward the Synthesis of Pyridines. Synthesis 2008, 21, 3423–3428. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chiba, S. Mn(III)-Mediated Reactions of Cyclopropanols with Vinyl Azides: Synthesis of Pyridine and 2-Azabicyclo[3.3.1]non-2-en-1-ol Derivatives. J. Am. Chem. Soc. 2009, 131, 12570–12572. [Google Scholar] [CrossRef]
- Mao, Z.Y.; Liao, X.Y.; Wang, H.S.; Wang, C.G.; Huang, K.B.; Pan, Y.M. Acid-catalyzed tandem reaction for the synthesis of pyridine derivatives via C=C/C(sp3)–N bond cleavage of enones and primary amines. RSC Adv. 2017, 7, 13123–13129. [Google Scholar] [CrossRef]
- Wang, K.; Meng, L.G.; Wang, L. Visible-Light-Promoted [2+2+2] Cyclization of Alkynes with Nitriles to Pyridines Using Pyrylium Salts as Photoredox Catalysts. Org. Lett. 2017, 19, 1958–1961. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, J. Pyrimidines. In Heterocyclic Chemistry in Drug Discovery; Jie, J.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 569–613. [Google Scholar]
- Zarenezhad, E.; Farjam, M.; Iraji, A. Synthesis and biological activity of pyrimidines-containing hybrids: Focusing on pharmacological application. J. Mol. Struct. 2021, 1230, 129833. [Google Scholar] [CrossRef]
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent. Today 2013, 5, 148–152. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Kumar, S.; Sharma, K. Green synthesis, antibacterial activity and computational study of pyrazoline and pyrimidine derivatives from 3-(3,4-dimethoxy-phenyl-1-(2,5-dimethylthiophen-3-yl)-propenone. Eur. J. Chem. 2014, 5, 85–90. [Google Scholar] [CrossRef]
- Ezhilarasi, M.R.; Prabha, B.; Prabakaran, S. Microwave assisted synthesis and spectral studies of thiophenyl pyrimidine derivatives. J. Appl. Chem. 2014, 3, 1929–1935. [Google Scholar]
- Babu, K.R.; Paul, V.L.; Rao, V.M. Substituted pyridine catalysed domino synthesis of pyrazolines and pyrimidines. World J. Pharm. Res. 2014, 3, 389–398. [Google Scholar]
- Sivagamisundari, G.; Pushpalatha, A.M.; Ranee, S.J. Silica bonded S-sulphonic acid as a green catalyst in the synthesis of functionalized pyrimidine under solvent-free microwave irradiation conditions. Int. J. Sci. Eng. Technol. 2014, 3, 852–855. [Google Scholar]
- Yamaguchi, T.; Sugiura, Y.; Yamaguchi, E.; Tada, N.; Itoh, A. Synthetic Method for the Preparation of Quinazolines by the Oxidation of Amines Using Singlet Oxygen. Asian J. Org. Chem. 2017, 6, 432–435. [Google Scholar] [CrossRef]
- Xie, L.G.; Niyomchon, S.; Mota, A.J.; Maulide, N. Metal-free intermolecular formal cycloadditions enable an orthogonal access to nitrogen heterocycles. Nat. Commun. 2016, 7, 10914. [Google Scholar] [CrossRef]
- Liu, K.; Song, C.; Wu, J. Electrochemical Oxidation Synergizing with Brønsted-Acid Cataly-sis Leads to [4+2] Annulation for the Synthesis of Pyrazines. Green Chem. 2019, 21, 765–769. [Google Scholar] [CrossRef]
- Daw, P.; Ben-David, Y.; Milstein, D. Acceptorless Dehydrogenative Coupling using Ammonia: Direct Synthesis of N-Heteroaromatics from Diols Catalyzed by Ruthenium. J. Am. Chem. Soc. 2018, 140, 11931–11934. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Sivaramakrishna, A. Functionalized Triazines and Tetrazines: Synthesis and Applications. Top. Curr. Chem. 2022, 380, 34. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xue, Q.; Zhang, C.; Wu, H.; Feng, P. Derivatization based on tetrazine scaffolds: Synthesis of tetrazine derivatives and their biomedical applications. Org. Chem. Front. 2022, 9, 481. [Google Scholar] [CrossRef]
- Choi, S.-K.; Kim, J.; Kim, E. Overview of Syntheses and Molecular-Design Strategies forTetrazine-Based Fluorogenic Probes. Molecules 2021, 26, 1868. [Google Scholar] [CrossRef] [PubMed]
- Kędziaa, A.; Kudelkoa, A.; Świątkowskib, M.; Kruszyński, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dyes Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Lambert, W.D.; Fang, Y.; Mahapatra, S.; Huang, Z.; Am Ende, C.W.; Fox, J.F. Installation of Minimal Tetrazines through Silver-Mediated Liebeskind−Srogl Coupling with Arylboronic Acids. J. Am. Chem. Soc. 2019, 141, 17068–17074. [Google Scholar] [CrossRef]
- Xie, Y.; Fang, Y.; Huang, Z.; Tallon, A.M.; Am Ende, C.W.; Fox, J.M. Divergent Synthesis of Monosubstituted and Unsymmetrical 3,6-Disubstituted Tetrazines from Carboxylic Ester Precursors. Angew. Chem. Int. Ed. 2020, 59, 16967–16973. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Hu, W.-L.; Liu, D.-Y.; Yua, C.-Y.; Hu, X.-G. Synthesis of tetrazines from gem-difluoroalkenes under aerobic conditions at room temperature. Green Chem. 2017, 19, 1299. [Google Scholar] [CrossRef]
- Tang, J.; Chen, D.; Zhang, G.; Yang, H.; Cheng, G. A “Green” Primary Explosive: Design, Synthesis, and Testing. Synlett 2019, 30, 885–892. [Google Scholar]
- Chaurasia, S.R.; Dange, R.; Bhanage, B.M. Graphene oxide as a carbo-catalyst for the synthesis of tri-substituted 1,3,5-triazines using biguanides and alcohols. Catal. Commun. 2020, 137, 105933. [Google Scholar] [CrossRef]
- Poly, S.S.; Hashiguchi, Y.; Nakamura, I.; Fujitani, T.; Siddiki SM, A.H. Direct synthesis of triazines from alcohols and amidines using supported Pt nanoparticle catalysts via the acceptorless dehydrogenative methodology. Catal. Sci. Technol. 2022, 12, 4679. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Xu, Y.; Zhao, Z.; Su, J.; Luo, C.; Ning, Y.; Li, Z.; Li, C.; Wang, L. Efficient synthesis of unsymmetrical trisubstituted 1,3,5-triazines catalyzed by hemoglobin. Mol. Catal. 2021, 505, 111519. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, M.; Zhang, L.; Xu, P.; Wang, H.; Sun ZIs Fang, L.; Duan, Y.; Bai, S.; Zhou, X.; Han, M.; et al. Open-Air Dual-Diamination of Aromatic Aldehydes: Direct Synthesis of Azolo-Fused 1,3,5-Triazines Facilitated by Ammonium Iodide. J. Org. Chem. 2021, 86, 17265–17273. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Low, S.T.; Ho, E.L.K.; Halcovitch, N.R.; Tiekink, E.R.T.; Dolzhenko, A.V. A multicomponent reaction of 2-aminoimidazoles: Microwave-assisted synthesis of novel 5-aza-7-deaza-adenines. RSC Adv. 2017, 7, 51062–51068. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, Y.X.; Xun, Z.; Huang, Z.B.; Shi, D.Q. Microwave-Assisted Regioselective Synthesis of 3-Functionalized Indole Derivatives via Three-Component Domino Reaction. ACS Comb. Sci. 2017, 19, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.H.; Zheng, H.X.; Yang, B.; Tie, L.; Fu, J.L.; Qu, J.P.; Kang, Y.B. Copper-catalyzed oxidative benzylic C-H cyclization via iminyl radical from intermolecular anion-radical redox relay. Nat. Commun. 2019, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Qi, L.; Hu, K.; Gong, J.L.; Cheng, T.X.; Wang, Q.Z.; Chen, J.X.; Wu, H.Y. The Development of a Palladium-Catalyzed Tandem Addition/Cyclization for the Construction of Indole Skeletons. J. Org. Chem. 2017, 82, 3631–3638. [Google Scholar] [CrossRef]
- Xu, D.; Yang, W.; Luo, S.; Wang, B.; Wu, J.; Xu, Z. Fischer indole synthesis in Brønsted acidic ionic liquids: A green, mild, and regiospecific reaction system. Eur. J. Org. Chem. 2007, 1007–1012. [Google Scholar] [CrossRef]
- Xu, D.; Wu, J.; Luo, S.; Zhang, J.; Wu, J.; Du, X.; Xu, Z. Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water. Green Chem. 2009, 11, 1239–1246. [Google Scholar] [CrossRef]
- Tambe, S.D.; Rohokale, R.S.; Kshirsagar, U.A. Visible-Light-Mediated Eosin Y Photoredox-Catalyzed Vicinal Thioamination of Alkynes: Radical Cascade Annulation Strategy for 2-Substituted-3-sulfenylindoles. Eur. J. Org. Chem. 2018, 18, 2117–2121. [Google Scholar] [CrossRef]
- Jana, S.; Verma, A.; Kadu, R.; Kumar, S. Visible-light-induced oxidant and metal-free dehydrogenative cascade trifluoromethylation and oxidation of 1,6-enynes with water. Chem. Sci. 2017, 8, 6633–6644. [Google Scholar] [CrossRef]
- Sadowski, B.; Klajn, J.; Gryko, D.T. Recent advances in the synthesis of indolizines and their π-expanded analogues. Org. Biomol. Chem. 2016, 14, 7804–7828. [Google Scholar] [CrossRef]
- Dinica, R.; Furdui, B.; Ghinea, I.; Bahrim, G.; Bonte, S.; Demeunynck, M. Novel one-pot green synthesis of indolizines biocatalysed by Candida antarctica lipases. Mar. Drugs 2013, 11, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yu, Y.; Wu, Y. One-Pot Regiospecific Synthesis of Indolizines: A Solvent-Free, Metal-Free, Three-Component Reaction of 2-(Pyridin-2-yl)acetates, Ynals, and Alcohols or Thiols. Org. Lett. 2018, 20, 2477–2480. [Google Scholar] [CrossRef]
- Zeng, K.; Mei, R.; Dechert, S.; Ackermann, L.; Zhang, K. A recyclable stereoauxiliary aminocatalyzed strategy for one-pot synthesis of indolizine-2-carbaldehydes. Nat. Commun. Chem. 2023, 6, 40. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Deng, J.; Huang, Y.S.; Pan, Z.H.; Yu, Y.; Cao, H. Electrochemical Diselenylation of Indolizines via Intermolecular C-Se Formation of 2-Methylpyridines, α-Bromoketones with Diselenides. Chem. Commun. 2019, 56, 735–738. [Google Scholar] [CrossRef]
- Török, B.; Schäfer, C.; Kokel, A. Heterogeneous Catalysis in Sustainable Synthesis; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021. [Google Scholar]
- Cheng, C.C.; Yan, S.J. The Friedländer Synthesis of Quinolines; Organic Reactions; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Arcadi, A.; Chiarini, M.; Giuseppe, S.D.; Marinelli, F. A new approach to the Friedländer synthesis of quinolines. Synlett 2003, 203–206. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–448. [Google Scholar] [CrossRef]
- Motokura, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Multifunctional catalysis of a ruthenium-grafted hydrotalcite: One-pot synthesis of quinolines from 2-aminobenzyl alcohol and various carbonyl compounds via aerobic oxidation and aldol reaction. Tetrahedron Lett. 2004, 45, 6029–6032. [Google Scholar] [CrossRef]
- Akbari, J.; Heydari, A.; Kalhor, H.R.; Kohan, S.A. Sulfonic acid functionalized ionic liquid in combinatorial approach, a recyclable and water tolerant-acidic catalyst for one-pot Friedländer quinoline synthesis. J. Comb. Chem. 2010, 12, 137–140. [Google Scholar] [CrossRef]
- Kulkarni, A.; Török, B. Microwave-assisted multicomponent domino cyclization–aromatization: An efficient approach for the synthesis of substituted quinolines. Green Chem. 2010, 12, 875–878. [Google Scholar] [CrossRef]
- Reddy, T.R.; Reddy, L.S.; Reddy, G.R.; Yarbagi, K.; Lingappa, Y.; Rambabu, D.; Krishna, G.R.; Reddy, C.M.; Kumar, K.S.; Pal, M. Construction of quinoline ring via a 3-component reaction in water: Crystal structure analysis and H-bonding patterns of a 2-aryl quinoline. Green Chem. 2012, 14, 1870–1872. [Google Scholar] [CrossRef]
- He, L.; Wang, J.Q.; Gong, Y.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Titania-Supported Iridium Subnanoclusters as an Efficient Heterogeneous Catalyst for Direct Synthesis of Quinolines from Nitroarenes and Aliphatic Alcohols. Angew. Chem. Int. Ed. 2011, 50, 10216–10220. [Google Scholar] [CrossRef]
- Sun, D.; Yin, K.; Zhang, R. Visible-light-induced multicomponent cascade cycloaddition involving N-propargyl aromatic amines, diaryliodonium salts and sulfur dioxide: Rapid access to 3-arylsulfonylquinolines. Chem. Commun. 2018, 54, 1335–1338. [Google Scholar] [CrossRef]
- Mishra, M.; Twardy, D.; Ellstrom, C.; Wheeler, K.A.; Dembinski, R.; Török, B. Catalyst-free ambient temperature synthesis of isoquinoline-fused benzimidazoles from 2-alkynylbenzaldehydes via alkyne hydroamination. Green Chem. 2019, 21, 99–108. [Google Scholar] [CrossRef]
- Liu, X.; Qing, Z.; Cheng, P.; Zheng, X.; Zeng, J.; Xie, H. Metal-Free Photoredox Catalyzed Cyclization of O-(2,4-Dinitrophenyl)oximes to Phenanthridines. Molecules 2016, 21, 1690. [Google Scholar] [CrossRef]
- Abid, M.; DePaolis, O.; Török, B. A Novel One-Pot Synthesis of N-Acylindoles from Primary Aromatic Amides. Synlett 2008, 410–413. [Google Scholar] [CrossRef]
- Kulkarni, A.; Zhou, W.-H.; Török, B. Heterogeneous catalytic hydrogenation of unprotected indoles in water: A green solution to a long-standing challenge. Org. Lett. 2011, 13, 5124–5127. [Google Scholar] [CrossRef]
- Ishida, T.; Tsunoda, R.; Zhang, Z.; Hamasaki, A.; Honma, T.; Ohashi, H.; Yokoyama, T.; Tokunaga, M. Supported palladium hydroxide-catalyzed intramolecular double C-H bond functionalization for synthesis of carbazoles and dibenzofurans. Appl. Catal. B Environ. 2014, 150–151, 523–531. [Google Scholar] [CrossRef]
- Xiao, F.; Liao, Y.; Wu, M.; Deng, G.J. One-pot synthesis of carbazoles from cyclohexanones and arylhydrazine hydrochlorides under metal-free conditions. Green Chem. 2012, 14, 3277–3280. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zou, X.; Lu, H.J.; Li, G.G. Visible-Light-Promoted Intramolecular C–H Amination in Aqueous Solution: Synthesis of Carbazoles. Green Chem. 2018, 20, 1362–1366. [Google Scholar] [CrossRef]
- Ishihara, Y.; Montero, A.; Baran, P.S. The Portable Chemist’s Consultant; Apple Publishing Group: New York, NY, USA, 2013. [Google Scholar]
- Yan, R.; Li, X.; Yang, X.; Kang, X.; Xiang, L.; Huang, G. A novel one-pot method for the synthesis of substituted furopyridines: Iodine-mediated oxidation of enaminones by tandem metal-free cyclization. Chem. Commun. 2015, 51, 2573. [Google Scholar] [CrossRef]
- Hudson, R.; Bizier, N.P.; Esdalea, K.N.; Katz, J.L. Synthesis of indoles, benzofurans, and related heterocycles via an acetylene-activated SNAr/intramolecular cyclization cascade sequence in water or DMSO. Org. Biomol. Chem. 2015, 13, 2273. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Nagasawa, H. Synthesis of 2-Arylbenzoxazoles by Copper-Catalyzed Intramolecular Oxidative C-O Coupling of Benzanilides. Angew. Chem. Int. Ed. 2008, 47, 6411–6413. [Google Scholar] [CrossRef]
- Evindar, G.; Batey, R.A. Parallel Synthesis of a Library of Benzoxazoles and Benzothiazoles Using Ligand-Accelerated Copper-Catalyzed Cyclizations of ortho-Halobenzanilides. J. Org. Chem. 2006, 71, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, F.; Zhao, Y.; Liu, Y.; Zhou, Y.; Chen, T.; Yin, S.F. Copper-Catalyzed Aerobic Oxidative C(aryl)-OH Bond Functionalization of Catechols with Amines Affording Benzoxazoles. Adv. Synth. Catal. 2015, 357, 2924–2930. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Wang, Y.; Chen, B.; Jing, Z.; Chen, G.; Zhao, P. OMS-2-Supported Cu Hydroxide-Catalyzed Benzoxazoles Synthesis from Catechols and Amines via Domino Oxidation Process at Room Temperature. J. Org.Chem. 2017, 82, 6922–6931. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cao, Y.; Ruan, F.; Li, F.; Jin, Y.; Ha, M.N.; Han, X.; Ke, Q. New Approach for Controllable Synthesis of N-MnOx Microflowers and Their Superior Catalytic Performance for Benzoxazole Synthesis. Ind. Eng. Chem. Res. 2020, 59, 9408–9413. [Google Scholar] [CrossRef]
- Sharghi, H.; Aboonajmi, J.; Aberi, M. One-Pot Multicomponent Reaction of Catechols, Ammonium Acetate, and Aldehydes for the Synthesis of Benzoxazole Derivatives Using the Fe(III)−Salen Complex. J. Org. Chem. 2020, 85, 6567–6577. [Google Scholar] [CrossRef]
- Laha, J.K.; Gulati, U.; Saima; Gupta, A.; Indurthi, H.K. Improved, gram-scale synthesis of sildenafil in water using arylacetic acid as the acyl source in the pyrazolo[4,3-d]pyrimidin-7-one ring formation. New J. Chem. 2021, 45, 2643–2648. [Google Scholar] [CrossRef]
- Agios Pharmaceuticals Inc. Therapeutic Compounds and Compositions; WO2014/139144; Agios Pharmaceuticals Inc.: Cambridge, MA, USA, 2014. [Google Scholar]
- Alamshany, Z.M.; Tashkandi, N.Y.; Othman, I.M.M.; Anwar, M.M.; Nossier, E.S. New thiophene, thienopyridine and thiazoline-based derivatives: Design, synthesis and biological evaluation as antiproliferative agents and multitargeting kinase inhibitors. Bioorg. Chem. 2022, 127, 105964. [Google Scholar] [CrossRef]
- Sanad, S.M.H.; Mekky, A.E.M. Novel Nicotinonitriles and Thieno[2,3-b]pyridines as Potent Biofilm and COX-2 Inhibitors: Synthesis, In Vitro and In Silico Studies. ChemistrySelect 2020, 5, 8494–8503. [Google Scholar] [CrossRef]
- He, R.; Liu, Y.; Feng, Y.; Chen, L.; Huang, Y.; Xie, F.; Li, Y. Access to Thienopyridine and Thienoquinoline Derivatives via Site-Selective C–H Bond Functionalization and Annulation. Org. Lett. 2022, 24, 3167–3172. [Google Scholar] [CrossRef]
- Chai, K.; Shi, Y.; Wang, Y.; Zou, P.; Yuan, Q.; Xu, W.; Zhang, P. Visible light-driven oxidative coupling of dibenzylamine and substituted anilines with a 2D WSe2 nanomesh material. Nanoscale 2020, 12, 21869. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dai, W.; Saravanamurugan, S.; Li, H.; Yang, S. Endogenous X–C=O species enable catalyst-free formylation prerequisite for CO2 reductive upgrading. Green Chem. 2020, 22, 5822. [Google Scholar] [CrossRef]
- Rong, B.; Xu, G.; Yan, H.; Zhang, S.; Wu, Q.; Zhu, N.; Fang, Z.; Duan, J.; Guo, K. Synthesis of benzofuro- and benzothieno[2,3-c] pyridines via copper-catalyzed [4+2] annulation of ketoxime acetates with acetoacetanilide. Org. Chem. Front. 2021, 8, 2939–2943. [Google Scholar] [CrossRef]
- Morais, G.R.; Palma, E.; Marques, F.; Gano, L.; Oliveira, M.C.; Abrunhosa, A.; Miranda, H.V.; Outeiro, T.F.; Santos, I.; Paulo, A. Synthesis and biological evaluation of novel 2-aryl benzimidazoles as chemotherapeutic agents. J. Heterocycl. Chem. 2015, 54, 255–267. [Google Scholar] [CrossRef]
- Kankate, R.S.; Pagare, A.H.; Kakad, R.D.; Gide, P.S.V.C.; Nathe, V.C. Nathe Synthesis and biological evaluation of benzimidazolyl biphenyl derivatives as antihypertensive agents. Int. J. Chem. Concepts. 2016, 2, 111–119. [Google Scholar]
- Sethi, P.; Bansal, Y.; Bansal, G. Synthesis and pass-assisted evaluation of coumarin–benzimidazole derivatives as potential anti-inflammatory and anthelmintic agents. Med. Chem. Res. 2017, 27, 61–71. [Google Scholar] [CrossRef]
- Ajani, O.; Aderohunmu, D.; Olorunshola, S.; Ikpo, C.; Olanrewaju, I. Facile synthesis, characterization and antimicrobial activity of 2-alkanamino benzimidazole derivatives. Orient. J. Chem. 2016, 32, 109–120. [Google Scholar] [CrossRef]
- Bellam, M.; Gundluru, M.; Sarva, S.; Chadive, S.; Netala, V.R.; Tartte, V.; Cirandur, S.R. Synthesis and antioxidant activity of some new n-alkylated pyrazole-containing benzimidazoles. Chem. Heterocycl. Comp. 2017, 53, 173–178. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Y.; Gao, X.; Gao, Y. Synthesis and anticoagulant bioactivity evaluation of 1,2,5-trisubstituted benzimidazole fluorinated derivatives. Chem. Res. Chin. Univ. 2016, 32, 973–978. [Google Scholar] [CrossRef]
- Raja, D.; Philips, A.; Palani, P.; Lin, W.-Y.; Devikala, S.; Senadi, G.C. Metal-free synthesis of benzimidazoles via oxidative cyclization of d-glucose with o-phenylenediamines in water. J. Org. Chem. 2020, 85, 11531–11540. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent development of hypercrosslinked microporous organic polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef]
- An, W.-K.; Zheng, S.-J.; Zhang, H.-X.; Shang, T.-T.; Wang, H.-R.; Xu, X.-J.; Jin, Q.; Qin, Y.; Ren, Y.; Jiang, S.; et al. s-tetrazine-functionalized hyper-crosslinked polymers for efficient photocatalytic synthesis of benzimidazoles. Green Chem. 2021, 23, 1292–1299. [Google Scholar] [CrossRef]
- Kalinin, A.A.; Mamedov, V.A.; Levin, Y.A. Unexpected quinoxalinobenzimidazole rearrangement. Chem. Heterocycl. Comp. 2000, 36, 882–883. [Google Scholar] [CrossRef]
- Li, S.; Feng, L.; Ma, C. Simple and green synthesis of benzimidazoles and pyrrolo[1,2-a]quinoxalines via mamedov heterocycle rearrangement. New J. Chem. 2021, 45, 9320–9323. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Herrera Cano, N.; Oliverio, M.; Procopio, A. The highly efficient synthesis of 1,2-disubstituted benzimidazoles using microwave irradiation. Molecules 2022, 27, 1751. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, Y.; Liu, H.; He, M.; Yang, M.; Liu, P.; Yu, B.; Dong, X.; Lei, A. Electrochemical oxidative C–H/N–H cross-coupling for C–N bond formation with hydrogen evolution. Chem. Commun. 2019, 55, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Han, X.; Wang, J.Y.; Zhou, M.; Wu, Y.; Ma, L.; Niu, L.; Gao, W.; Zhou, J.; Hu, W.; et al. Tunable electrochemical C−N versus N−N bond formation of nitrogen-centered radicals enabled by dehydrogenative dearomatization: Biological applications. Angew. Chem. Int. Ed. 2020, 132, 11680–11687. [Google Scholar] [CrossRef]
- Zhao, H.B.; Zhuang, J.L.; Xu, H.C. Electrochemical synthesis of benzimidazoles via dehydrogenative cyclization of amidines. ChemSusChem 2021, 14, 1692–1695. [Google Scholar] [CrossRef]
- Twardy, D.J.; Wheeler, K.A.; Török, B. Dembinski, R. Recent Advances in the Synthesis of Isoquinoline-fused Benzimidazoles. Molecules 2022, 27, 2062. [Google Scholar] [CrossRef]
- Cerecetto, H.; Gerpe, A.; Gonzalez, M.; Aran, V.; de Ocariz, C. Pharmacological properties of indazole derivatives: Recent developments. Mini-Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar] [CrossRef]
- Shrivastava, A.; Chakraborty, A.K.; Upmanyu, N.; Singh, A. Recent progress in chemistry and biology of indazole and its derivatives: A brief review. Austin J. Anal. Pharm. Chem. 2016, 3, 1076. [Google Scholar]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole derivatives and their therapeutic applications: A Patent Review (2013–2017). Expert Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Li, D.; Xia, H.; Yang, L.; Alhumade, H.; Yi, H.; Lei, A. Synthesis of 1h-indazoles by an electrochemical radical CSP2–h/N–H cyclization of arylhydrazones. Chem. Commun. 2022, 58, 665–668. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Z.; Chen, N.; Chen, Z.; Zhang, F. Electrochemical dehydrogenative C–N coupling of hydrazones for the synthesis of 1h-indazoles. Green Chem. 2022, 24, 1463–1468. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Yépez-Mulia, L.; González-Sánchez, I.; Palacios-Espinosa, J.; Soria-Arteche, O.; Sainz-Espuñes, T.; Cerbón, M.; Rodríguez-Villar, K.; Rodríguez-Vicente, A.; Cortés-Gines, M.; et al. Synthesis and biological evaluation of 2H-indazole derivatives: Towards antimicrobial and anti-inflammatory dual agents. Molecules 2017, 22, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Bergman, R.G.; Lavis, L.D.; Ellman, J.A. Rhodium(III)-catalyzed indazole synthesis by C–H bond functionalization and Cyclative Capture. J. Am. Chem. Soc. 2013, 135, 7122–7125. [Google Scholar] [CrossRef]
- Jin, G.-Q.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Efficient synthesis of 2-aryl-2h-indazoles by base-catalyzed benzyl C–H deprotonation and cyclization. Chem. Commun. 2020, 56, 14617–14620. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, N.; Yang, Q.; Wang, L. Visible-light-driven photocyclization reaction: Photocatalyst-free mediated intramolecular N–N coupling for the synthesis of 2h-indazole-3-carboxamides from aryl azides. Org. Chem. Front. 2021, 8, 5296–5302. [Google Scholar] [CrossRef]
- Kraemer, N.; Li, C.J.; Zhu, J.S.; Larach, J.M.; Tsui, K.Y.; Tantillo, D.J.; Haddadin, M.J.; Kurth, M.J. Davis–Beirut reaction: A photochemical Brønsted acid catalyzed route to n-aryl 2h-indazoles. Org. Lett. 2019, 21, 6058–6062. [Google Scholar] [CrossRef]
- Szabó, T.; Volk, B.; Milen, M. Recent Advances in the Synthesis of β-Carboline Alkaloids. Molecules 2021, 26, 663. [Google Scholar] [CrossRef]
- Horton, W.; Sood, A.; Peerannawar, S.; Kugyela, N.; Tulsan, R.; Tran, C.D.; Soule, J.; LeVine III, H.; Török, B.; Török, M. Synthesis and Application of β-Carbolines as Novel Multi-functional Anti-Alzheimer’s Disease Agents. Bioorg. Med. Chem. Lett. 2017, 27, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Gu, H.; Li, N.; Wang, J. Synthesis and biological evaluation of bivalent β-carbolines as potential anticancer agents. Med. Chem. Commun. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, G.; Guo, L.; Fan, V.; Ma, Q.; Zhang, X.; Du, R.; Cao, R. Synthesis and biological evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors. Eur. J. Med. Chem. 2016, 124, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Abid, M.; Török, B.; Huang, X. A direct synthesis of b-carbolines via a three-step one-pot domino approach with a bifunctional Pd/C/K-10 catalyst. Tetrahedron Lett. 2009, 50, 1791–1794. [Google Scholar] [CrossRef]
- Devi, N.; Singh, D.; Kaur, G.; Mor, S.; Kishore Putta VP, R.; Polina, S.; Malakard, C.C.; Singh, V. In(OTf)3 assisted synthesis of -carboline C-3 tethered imidazo[1,2-a]azine derivatives. New J. Chem. 2017, 41, 1082–1093. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Ahamed, A.J.; Thiruneelakandan, G. Synthesis, antimicrobial, and Anti-HIV1 activity of Quinazoline-4(3H)-one derivatives. J. Appl. Chem. 2013, 5, 387191. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Ganguly, S.; Veeramy, R.; Jan, B. Synthesis, antiviral and cytotoxic investigation of 2-phenyl-3-substituted quinazolin-4(3H)-ones. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 673–681. [Google Scholar]
- Jiang, S.; Zeng, Q.; Gettayacamin, M.; Tungtaeng, A.; Wannaying, S.; Lim, A.; Hansukjariya, P.; Okunji, C.O.; Zhu, S.; Fang, D. Antimalarial activities and therapeutic properties of Febrifugine analogs. Antimicr. Agents Chemother. 2005, 49, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, A.R.; Mohammadpoor-Baltork, I.; Ghorbankhani, H. Bi(TFA)3–[NBP]FeCl4: A new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives. Tetrahedron Lett. 2006, 47, 3561–3564. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lai, C.-Y.; Wang, C.-C. TMSOTF-catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions. Org. Biomol. Chem. 2020, 18, 7201–7212. [Google Scholar] [CrossRef]
- Charpe, V.P.; Ragupathi, A.; Sagadevan, A.; Hwang, K.C. Photoredox synthesis of functionalized quinazolines via copper-catalyzed aerobic oxidative Csp2–h annulation of amidines with terminal alkynes. Green Chem. 2021, 23, 5024–5030. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Z.; Jin, L.; Chen, X.; Le, Z. Synthesis of quinazoline by decarboxylation of 2-aminobenzylamine and α-keto acid under visible light catalysis. Org. Biomol. Chem. 2022, 20, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A state of the art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef]
- Dailey, S.; Feast, W.J.; Peace, R.J.; Sage, I.C.; Till, S.; Wood, E.L. Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J. Mater. Chem. 2001, 11, 2238–2243. [Google Scholar] [CrossRef]
- Podsiadły, R. Photoreaction and photopolymerization studies on Fluoflavin Dye–Pyridinium Salt Systems. J. Photochem. Photobiol. A Chem. 2008, 198, 60–68. [Google Scholar] [CrossRef]
- Gedefaw, D.; Prosa, M.; Bolognesi, M.; Seri, M.; Andersson, M.R. Recent development of quinoxaline based polymers/small molecules for organic photovoltaics. Adv. Energy Mater. 2017, 7, 1700575. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Xu, Y.; Wang, C.; Wang, Z.; Li, Z.; Wang, L. A dual-protein cascade reaction for the regioselective synthesis of quinoxalines. Org. Lett. 2020, 22, 3900–3904. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Lupidi, G.; Palmieri, A.; Petrini, M. Sustainable and fast synthesis of functionalized quinoxalines promoted by natural deep eutectic solvents (NADESs). Green Chem. 2022, 24, 3629–3633. [Google Scholar] [CrossRef]
- Queneau, Y.; Han, B. Biomass: Renewable carbon resource for chemical and Energy Industry. Innovation 2022, 3, 100184. [Google Scholar] [CrossRef]
- Bains, A.K.; Singh, V.; Adhikari, D. Homogeneous nickel-catalyzed sustainable synthesis of quinoline and quinoxaline under aerobic conditions. J. Org. Chem. 2020, 85, 14971–14979. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Mosslemin, M.H.; Alborzi, A.; Anaraki-Ardakani, H.; Shams, N.; Khoshnood, M.M.; Zarenezhad, A. Efficient synthesis of 3,4-dihydro-1H-quinoxalin-2-ones and 1H-quinolin-2-ones and evaluation of their anti-bacterial activity. J. Chem. Res. 2014, 38, 337–340. [Google Scholar] [CrossRef]
- Szumilak, M.; Stanczak, A. Cinnoline scaffold—A molecular heart of medicinal chemistry? Molecules 2019, 24, 2271. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lu, Y.; Yuan, C.; Fang, Z.; Yang, X.; Liu, C.; Guo, K. Organocatalytic electrosynthesis of cinnolines through Cascade Radical Cyclization and migration. ACS Sustain. Chem. Eng. 2021, 9, 16989–16996. [Google Scholar] [CrossRef]
- Sa, Y.; Cai, M.-X.; Lv, X.; Wu, A.-B.; Shu, W.-M.; Yu, W.-C. Intramolecular redox cyclization reaction access to cinnolines from 2-Nitrobenzyl Alcohol and benzylamine via intermediate 2-nitrosobenzaldehyde. RSC Adv. 2022, 12, 33260–33263. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.-X.; Xu, J.-H.; Gao, Y.; Hu, X.-Q. Divergent synthesis of fused N-heterocycles via rhodium-catalysed [4+2] cyclization of pyrazolidinones with iodonium ylides. Org. Chem. Front. 2022, 9, 2181–2186. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, M.; Mahalanabis, K.K. The synthesis of novel ethyl 4-(substituted amino)isothiazolo[5,4-B]-pyridine-5-carboxylates. J. Chem. Res. 2006, 437–439. [Google Scholar] [CrossRef]
- Cabrera-Afonso, M.J.; Cembellín, S.; Halima-Salem, A.; Berton, M.; Marzo, L.; Miloudi, A.; Maestro, M.C.; Alemán, J. Metal-free visible light-promoted synthesis of isothiazoles: A catalytic approach for N–S bond formation from IMINYL radicals under batch and flow conditions. Green Chem. 2020, 22, 6792–6797. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.; Cai, C.; Wang, L.; Gong, H. Synthesis of benzisothiazoles by a three-component reaction using elemental sulfur and ammonium as heteroatom components under transition metal-free conditions. Green Synth. Catal. 2022, 3, 168–174. [Google Scholar] [CrossRef]
- Jemili, R.; Campos, J.F.; Dumuis, N.; Rabat, H.; Semmar, N.; Berteina-Raboin, S. Laser synthesis: A solvent-free approach for the preparation of phenylthiazolo[5,4-b]pyridine derivatives. RSC Adv. 2021, 11, 5003–5007. [Google Scholar] [CrossRef]
- Cecchini, M.M.; Charnay, C.; De Angelis, F.; Lamaty, F.; Martinez, J.; Colacino, E. Poly(ethylene glycol)-based ionic liquids: Properties and uses as alternative solvents in organic synthesis and catalysis. ChemSusChem 2013, 7, 45–65. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Xia, Y.-G.; Jin, Y.; Lu, M. Efficient aerobic oxidative synthesis of benzimidazoles with Fe(III) based PEG1000 dicationic imidazolium ionic liquid/toluene temperature-dependent biphasic system. J. Chin. Chem. Soc. 2014, 62, 103–106. [Google Scholar] [CrossRef]
- Guo, W.; Xie, Z.; Cai, L.; Liu, G.; Deng, L.; Mei, W.; Zou, X.; Zhong, Y.; Zhuo, X.; Zheng, L.; et al. Synthesis of purine analogues: Photocatalyst-free visible-light-enhanced annulation approach to pyrazolo[1,5-a][1,3,5]triazine-2,4-diamines. J. Org. Chem. 2021, 86, 8365–8380. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Mondal, R.; Guin, A.K.; Paul, N.D. Nickel catalyzed sustainable synthesis of benzazoles and purines via acceptorless dehydrogenative coupling and borrowing hydrogen approach. Org. Biomol. Chem. 2021, 19, 7217–7233. [Google Scholar] [CrossRef] [PubMed]
















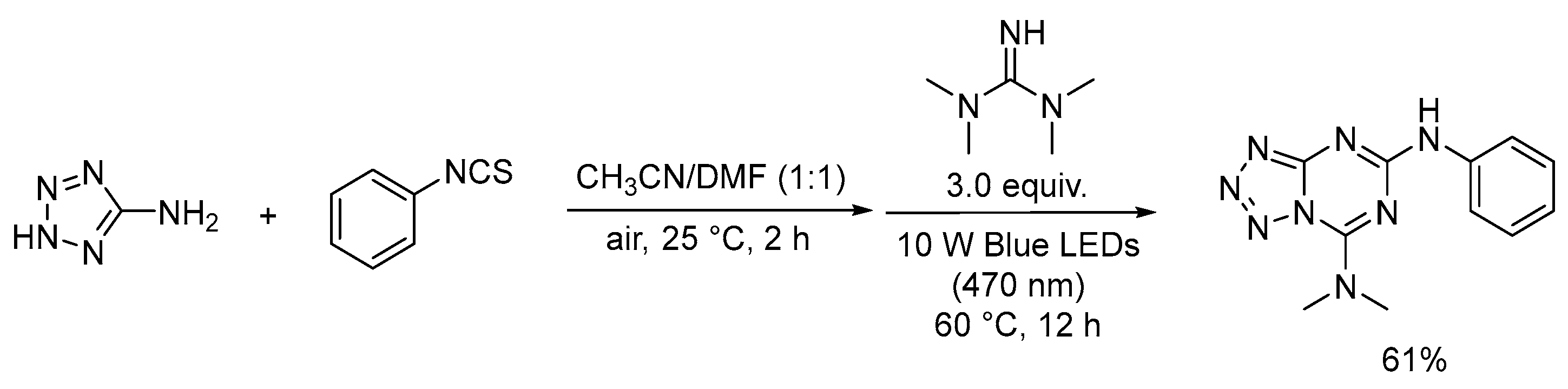
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlocskó, R.B.; Xie, G.; Török, B. Green Synthesis of Aromatic Nitrogen-Containing Heterocycles by Catalytic and Non-Traditional Activation Methods. Molecules 2023, 28, 4153. https://doi.org/10.3390/molecules28104153
Vlocskó RB, Xie G, Török B. Green Synthesis of Aromatic Nitrogen-Containing Heterocycles by Catalytic and Non-Traditional Activation Methods. Molecules. 2023; 28(10):4153. https://doi.org/10.3390/molecules28104153
Chicago/Turabian StyleVlocskó, R. Bernadett, Guoshu Xie, and Béla Török. 2023. "Green Synthesis of Aromatic Nitrogen-Containing Heterocycles by Catalytic and Non-Traditional Activation Methods" Molecules 28, no. 10: 4153. https://doi.org/10.3390/molecules28104153
APA StyleVlocskó, R. B., Xie, G., & Török, B. (2023). Green Synthesis of Aromatic Nitrogen-Containing Heterocycles by Catalytic and Non-Traditional Activation Methods. Molecules, 28(10), 4153. https://doi.org/10.3390/molecules28104153





