Unearthing of the Antidiabetic Potential of Aqueous Extract of Solanum betaceum Cav. Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compound Profile
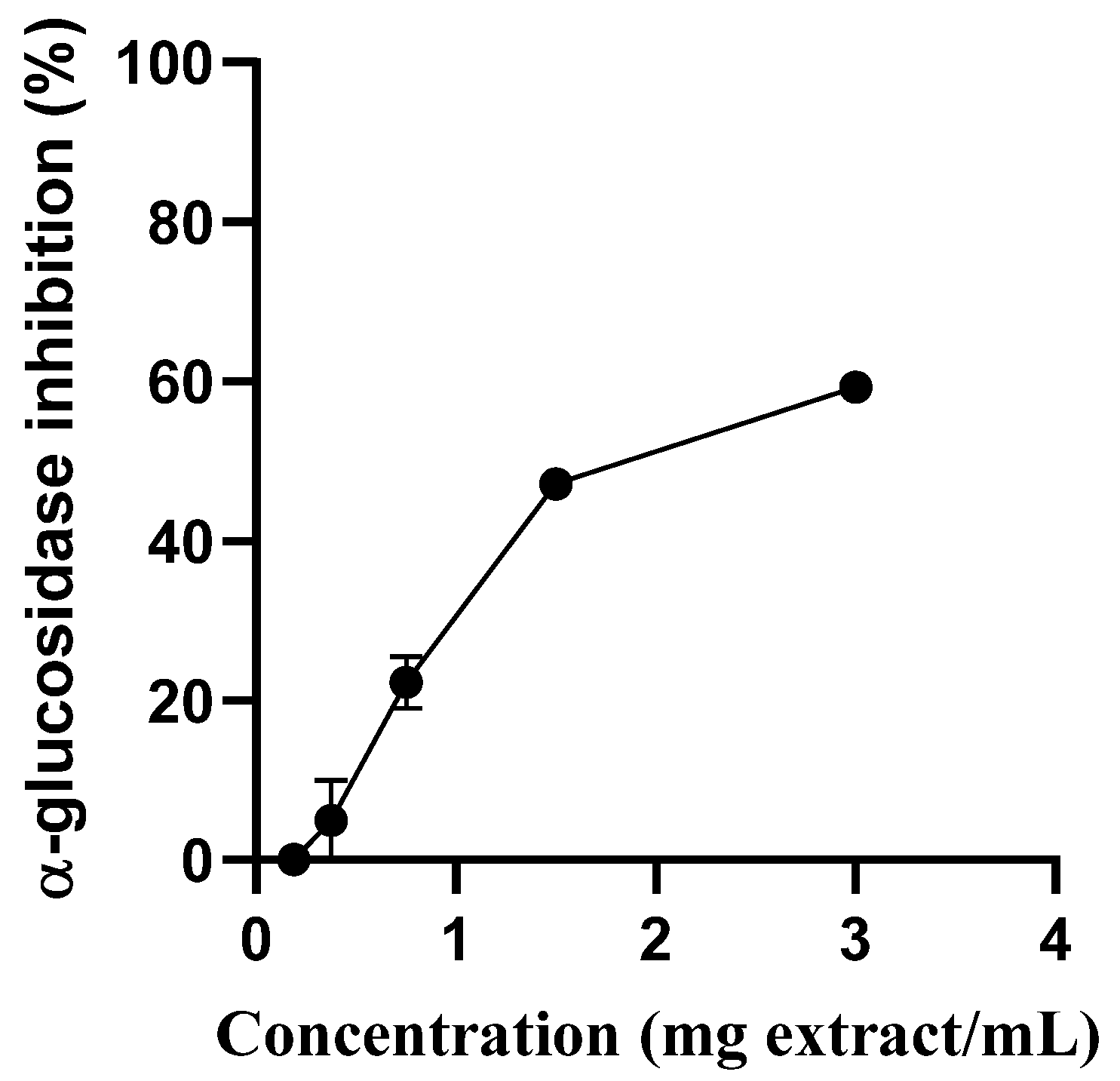
2.2. S. betaceum Leaves Extract in Glycaemic Control
2.3. S. betaceum Leaf Aqueous Extract in the Management of Diabetes-Related Complications
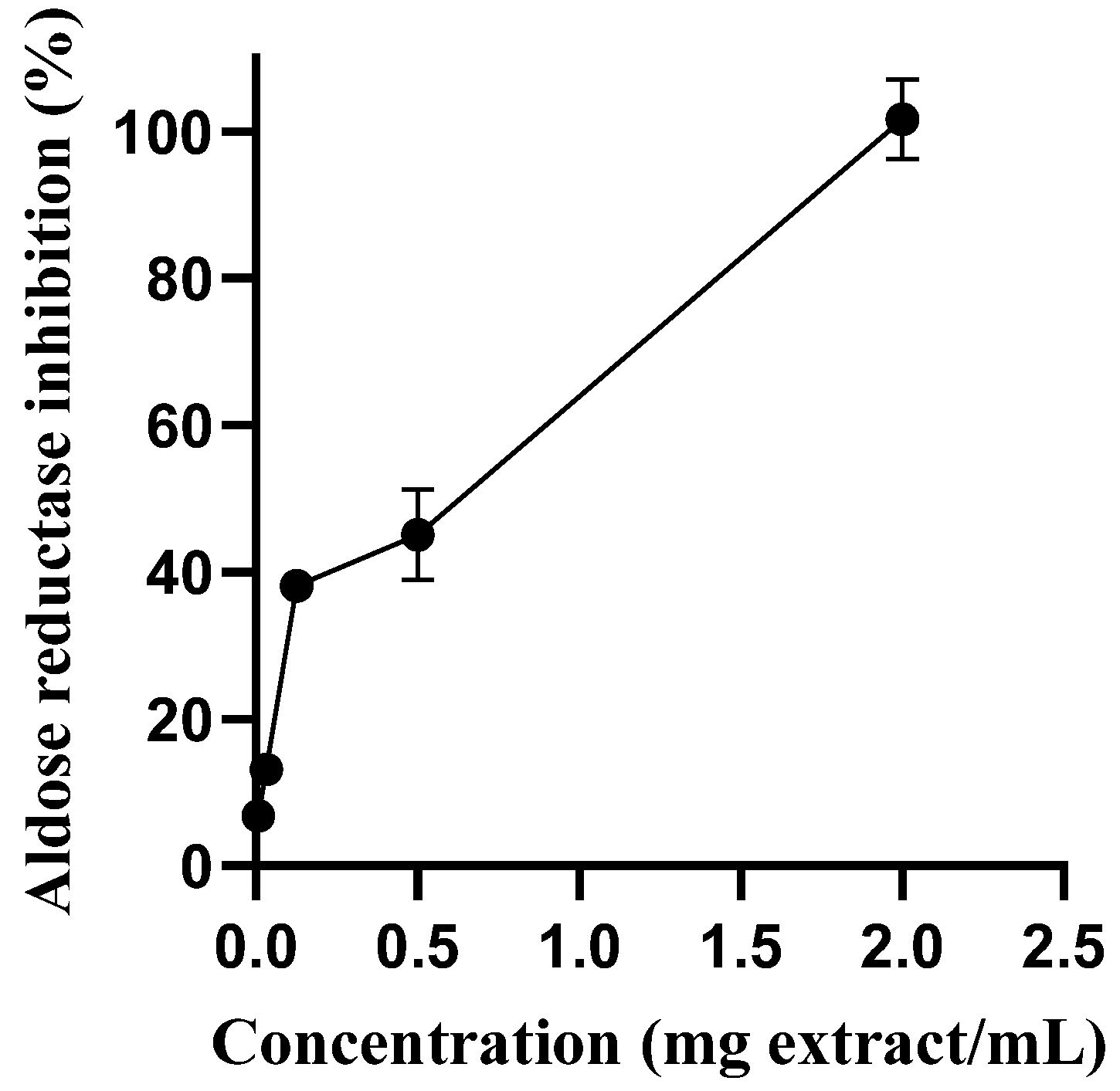
2.3.1. Aldose Reductase Inhibition
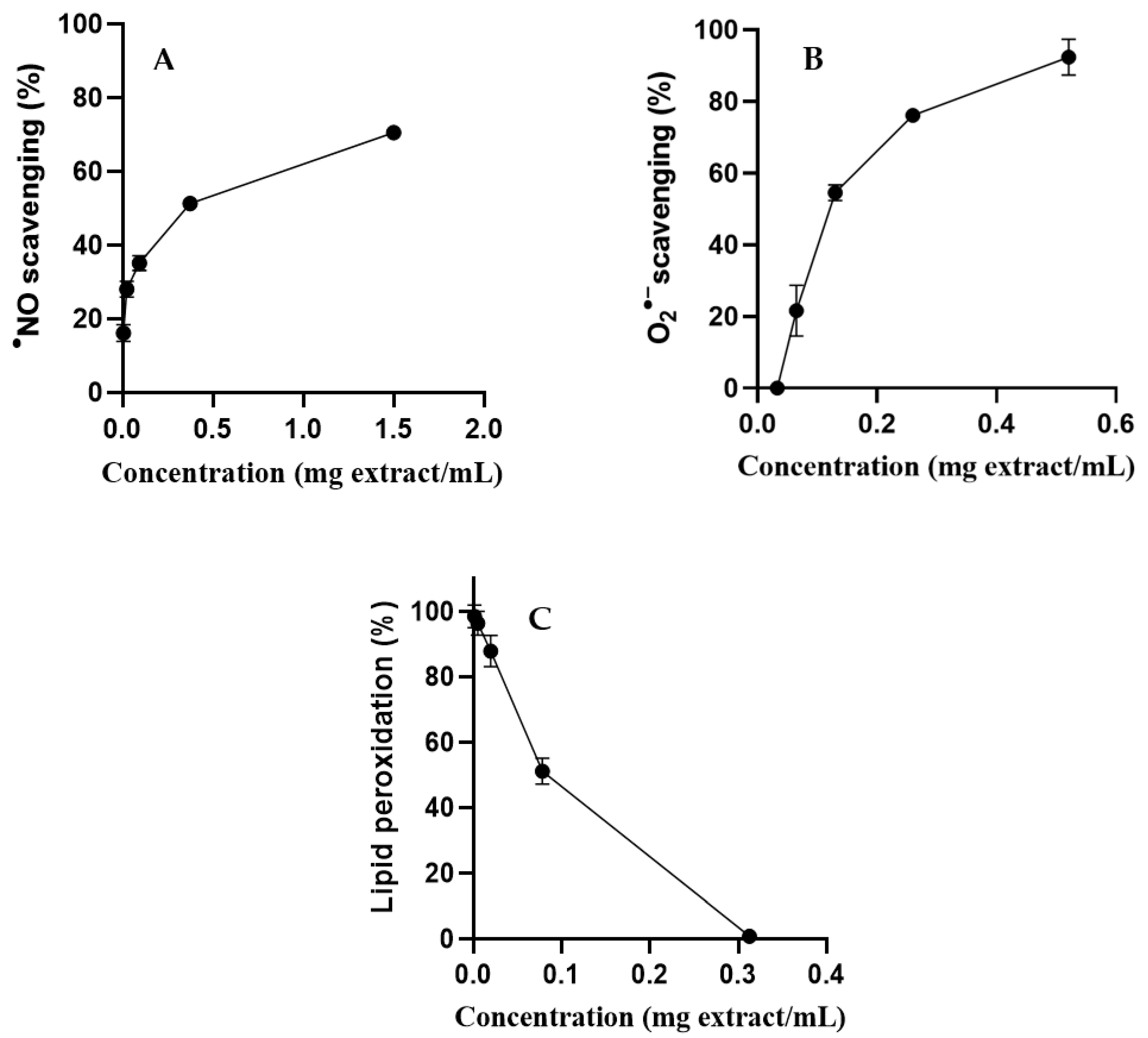
2.3.2. Oxidative Stress
3. Materials and Methods
3.1. Standards and Reagents
3.2. S. betaceum Leaves’ Collection
3.3. Aqueous Extraction
3.4. Phenolic Compounds Profiling
3.4.1. HPLC-DAD Analysis
Linearity
3.5. Biological Assays
3.5.1. α-Amylase Inhibition
3.5.2. α-Glucosidase Inhibition
3.5.3. Aldose Reductase Inhibition
3.5.4. Lipid Peroxidation
3.5.5. Superoxide Anion Radical Scavenging
3.5.6. Nitric Oxide Radical Scavenging
3.5.7. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Board of Trustees of the Royal Botanic Gardens Kew. Solanum beyaceum Cav. 2022. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:306169-2 (accessed on 1 December 2022).
- Isla, M.I.; Orqueda, M.E.; Moreno, M.A.; Torres, S.; Zampini, I.C. Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications. Foods 2022, 11, 3363. [Google Scholar] [CrossRef]
- Elizalde-Romero, C.A.; Montoya-Inzunza, L.A.; Contreras-Angulo, L.A.; Heredia, J.B.; Gutierrez-Grijalva, E.P. Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and Their Relationship with Their Health-Promoting Effects. Front. Nutr. 2021, 8, 790582. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical composition, biological properties, and product innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S.; Gravett, I.M. Seasonal accumulation of mineral nutrients by tamarillo. 1. Leaves. Sci. Horticult. 1989, 40, 119–131. [Google Scholar] [CrossRef]
- WHO Global Report on Diabetes. 2016. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 21 February 2023).
- Safiri, S.; Karamzad, N.; Kaufman, J.S.; Bell, A.W.; Nejadghaderi, S.A.; Sullman, M.J.M.; Moradi-Lakeh, M.; Collins, G.; Kolahi, A.A. Prevalence, Deaths and Disability-Adjusted-Life-Years (DALYs) Due to Type 2 Diabetes and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019: Results from the Global Burden of Disease Study 2019. Front. Endocrinol. 2022, 13, 838027. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Barbosa, M.; Fernandes, F.; Carlos, M.J.; Valentao, P.; Andrade, P.B. Adding value to marine invaders by exploring the potential of Sargassum muticum (Yendo) Fensholt phlorotannin extract on targets underlying metabolic changes in diabetes. Algal Res. Biomass Biofuels Bioprod. 2021, 59, 102455. [Google Scholar] [CrossRef]
- Espin, S.; Gonzalez-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Durán, B.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential alpha-amylase/alpha-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Clinical Practice Recommendations for ManagingType 2 Diabetes in Primary Care. 2017. Available online: https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html (accessed on 21 February 2023).
- Ferreres, F.; Andrade, C.; Gomes, N.G.M.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of kitul, an overlooked food plant: Phenolic profiling of fruits and inflorescences and assessment of their effects on diabetes-related targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Yang, H.; Peng, X.; Yang, X.; Liu, X.; Sun, L. Caffeoyl substitution decreased the binding and inhibitory activity of quinic acid against α-amylase: The reason why chlorogenic acid is a relatively weak enzyme inhibitor. Food Chem. 2022, 371, 131278. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crop Prod. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.D.; Kwon, Y.S.; Jin, S.E.; Son, Y.K.; Park, J.J.; Sohn, H.S.; Choi, J.S. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem. Toxicol. 2011, 49, 376–384. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, J.H.; Lee, M.-H.; Lee, B.W.; Kwon, H.S.; Park, C.-H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Salem, H.H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Shati, A.A.; El-Askary, H. Flavonol Glycosides: In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules 2020, 25, 5864. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Cosentino, F.; Hishikawa, K.; Katusic, Z.S.; Lüscher, T.F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997, 96, 25–28. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Bernardo, J.; Malheiro, I.; Videira, R.A.; Valentao, P.; Santos, A.C.; Veiga, F.; Andrade, P.B. Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. J. Ethnopharmacol. 2021, 271, 113865. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to control hyperglycemia and diabetes-related vascular complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Effects of 5α,8α-Epidioxycholest-6-en-3β-ol, a Steroidal Endoperoxide Isolated from Aplysia depilans, Based on Bioguided Fractionation and NMR Analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef] [PubMed]
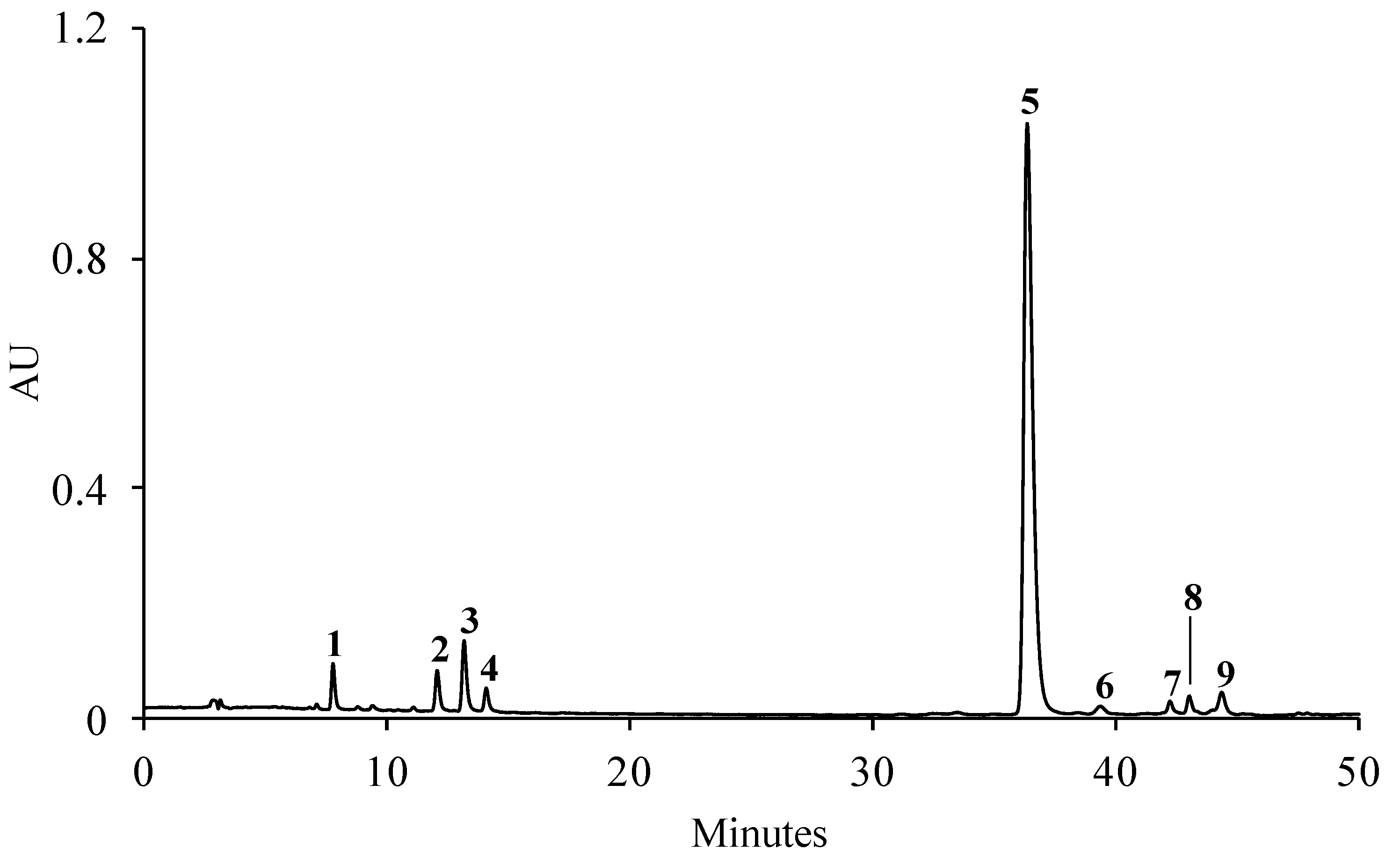
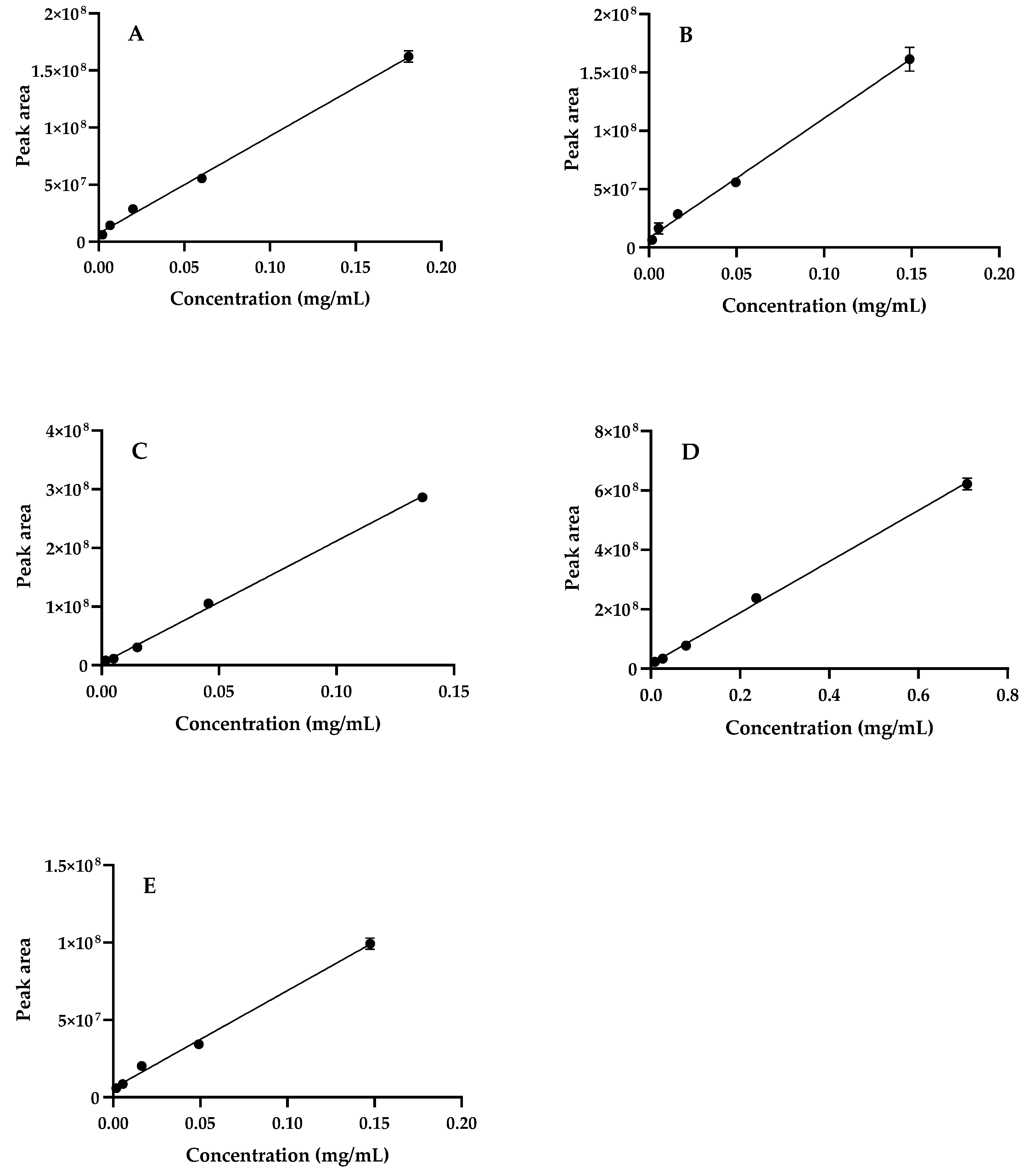
| Peak | Compound | Regression Equation (mg/mL) | r2 | Linearity (mg/mL) | S. betaceum Leaves (mg/g Dry Extract) 1 |
|---|---|---|---|---|---|
| 1 | 3-O-Caffeoylquinic acid | y = 8.5 × 108x + 7,222,443.7 | 0.997 | 0.002–0.181 | 2.91 ± 0.21 |
| 2 | 4-O-Caffeoylquinic acid | y = 8.5 × 108x + 7,222,443.7 | 0.997 | 0.002–0.181 | 3.57 ± 0.10 |
| 3 | 5-O-Caffeoylquinic acid | y = 1.0 × 109x + 8,240,529.4 | 0.997 | 0.002–0.149 | 5.09 ± 0.01 |
| 4 | Caffeic acid | y = 2.1 × 109x + 3,056,465.2 | 0.999 | 0.002–0.136 | 0.81 ± 0.01 |
| 5 | Rosmarinic acid | y = 8.6 × 108x + 16,145,014.1 | 0.998 | 0.009–0.709 | 99.62 ± 1.02 |
| 6 + 9 | Unknown kaempferol glycoside deriv. | y = 6.3 × 108x + 5,924,781.1 | 0.996 | 0.002–0.148 | 7.75 ± 0.22 |
| 7 + 8 | Unknown hydroxycinnamic acid deriv. | y = 1.0 × 109x + 8,240,529.4 | 0.997 | 0.002–0.149 | 1.82 ± 0.05 |
| Total | 121.57 ± 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.; Fernandes, F.; Valentão, P. Unearthing of the Antidiabetic Potential of Aqueous Extract of Solanum betaceum Cav. Leaves. Molecules 2023, 28, 3291. https://doi.org/10.3390/molecules28083291
Martins R, Fernandes F, Valentão P. Unearthing of the Antidiabetic Potential of Aqueous Extract of Solanum betaceum Cav. Leaves. Molecules. 2023; 28(8):3291. https://doi.org/10.3390/molecules28083291
Chicago/Turabian StyleMartins, Raquel, Fátima Fernandes, and Patrícia Valentão. 2023. "Unearthing of the Antidiabetic Potential of Aqueous Extract of Solanum betaceum Cav. Leaves" Molecules 28, no. 8: 3291. https://doi.org/10.3390/molecules28083291
APA StyleMartins, R., Fernandes, F., & Valentão, P. (2023). Unearthing of the Antidiabetic Potential of Aqueous Extract of Solanum betaceum Cav. Leaves. Molecules, 28(8), 3291. https://doi.org/10.3390/molecules28083291






