Research Progress of Ferula ferulaeoides: A Review
Abstract
1. Introduction
2. Chemical Constituents of F. ferulaeoides
3. Pharmacological Effects of F. ferulaeoides
3.1. Antimicrobial Activity
3.2. Antitumor Effect
3.3. Anti-Inflammatory Effect
3.4. Insecticidal Activity
3.5. Toxic Effect
4. Research on Quality Control
4.1. Traits and Microstructure
4.2. Studies on Fingerprint
4.3. Content Determination
5. Prospect: Application of F. ferulaeoides in Food Industry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amalraj, A.; Gopi, S. Biological Activities and Medicinal Properties of Asafoetida: A Review. J. Tradit. Complement. Med. 2017, 7, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Sarker, S.D.; Nahar, L.; Akbarzadeh, A. The Genus Ferula: Ethnobotany, Phytochemistry and Bioactivities—A Review. Ind. Crops Prod. 2019, 129, 350–394. [Google Scholar] [CrossRef]
- Yaqoob, D.; Nawchoo, I.A. Distribution and Taxonomy of Ferula L.: A Review. Res. Rev. J. Bot. Sci. 2016, 5, 15–23. [Google Scholar]
- Kan, M.M.; Wang, H.; Kang, D.J.; Yu, F.H. Study on chemical constituents of Ferula syreitschikowii in Xinjiang. J. Shihezi Univ. 2017, 35, 132. [Google Scholar] [CrossRef]
- Salehi, M.; Naghavi, M.R.; Bahmankar, M. A Review of Ferula Species: Biochemical Characteristics, Pharmaceutical and Industrial Applications, and Suggestions for Biotechnologists. Ind. Crops Prod. 2019, 139, 111511. [Google Scholar] [CrossRef]
- Liu, J. Study on Chemical Constituents and Quality Control Method of Ferula ferulaeoides. Master’s Thesis, Inner Mongolia Medical University, Hohhot, China, 2019. [Google Scholar]
- Wang, S. Study on Fingerprint of Active Site of Gastric Cancer In Vitro of Ferula sinkiangensis. Master’s Thesis, Xinjiang Medical University, Ürümqi, China, 2015. [Google Scholar]
- Yang, M.H.; Tang, D.P.; Sheng, P. Research Progress of Ferula ferulaeoides. China Mod. Appl. Pharmacol. 2020, 37, 2031–2041. [Google Scholar] [CrossRef]
- Wang, L.; Sun, R.; Xu, M.; Shi, Y.Y. Research on the Development of Chemical Components, Pharmacological Activities and Toxicity of Resina Ferulae. World Tradit. Chin. Med. 2020, 15, 3887–3894. [Google Scholar]
- Liu, H.D.; Li, G.F.; Niu, L.Z.; Zhuang, L.; Shi, Y.X. Research of the extraction process and determination of total flavonoids in Uygur medicine Ferula ferulaeoides. Chin. J. Tradit. Chin. Med. 2016, 31, 310–312. [Google Scholar]
- Tan, Y.; Gao, T.; Kan, M.M.; Wang, H.; Yu, F.H. Determination of polysaccharide in five kinds of Ferula in Xinjiang from different parts. Food Sci. Technol. 2016, 41, 262–265. [Google Scholar] [CrossRef]
- Feng, X.; Wang, L.; Liu, H.D.; Niu, L.Z.; Li, Y.X. Study on optimized extraction technology of ferulic acid from Ferula ferulaeoides (Steud.) Korov by an orthogonal experiment method. Heilongjiang Anim. Husb. Vet. Med. 2016, 5, 196–199. [Google Scholar] [CrossRef]
- Eshbakova, K.A.; Saidkhodzhaev, A.I.; Vdovin, A.D.; Abdullaev, N.D. Terpenoid Coumarins from Ferula ferulaeoides. Chem. Nat. Compd. 2009, 45, 708. [Google Scholar] [CrossRef]
- Liu, T.; Wang, S.; Xu, L.; Fu, W.; Gibbons, S.; Mu, Q. Sesquiterpenoids with Anti-MDR Staphylococcus Aureus Activities from Ferula Ferulioides. Chem. Biodivers. 2015, 12, 599–614. [Google Scholar] [CrossRef]
- Meng, H.; Li, G.; Huang, J.; Zhang, K.; Wei, X.; Ma, Y.; Zhang, C.; Wang, J. Sesquiterpenoid Derivatives from Ferula ferulaeoides (Steud.) Korov. Phytochemistry 2013, 86, 151–158. [Google Scholar] [CrossRef]
- Isaka, K.; Nagatsu, A.; Ondognii, P.; Zevgeegiin, O.; Gombosurengyin, P.; Davgiin, K.; Kojima, K.; Ogihara, Y. Sesquiterpenoid Derivatives from Ferula Ferulaeioides. V. Chem. Pharm. Bull. 2001, 49, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Osman, K.; Kaatz, G.W.; Gibbons, S.; Mu, Q. Antibacterial Sesquiterpenoid Derivatives from Ferula ferulaeoides. Planta Med. 2013, 79, 701–706. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.-D.; Li, G.-Y.; Li, N.; Zuo, W.-J.; Zeng, Y.-M.; Meng, H.; Li, X.; Wang, J.-H. Two Novel Sesquiterpenoids from the Roots of Ferula ferulaeoides (Steud.) Korov. Helv. Chim. Acta 2010, 93, 1019–1024. [Google Scholar] [CrossRef]
- Yang, X.W. Bioactive Material Basis of Medicinal Plants in Genus Ferula. Chin. Mod. Tradit. Chin. Med. 2018, 20, 123–144. [Google Scholar] [CrossRef]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in Disguise: A Review of Phytochemical Composition and Antimicrobial Activity of Plants Belonging to the Genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 53. [Google Scholar] [CrossRef]
- Bhatnager, R.; Rani, R.; Dang, A.S. Antibacterial Activity of Ferula Asafoetida: A Comparison of Red and White Type. J. Appl. Biol. Biotechnol. 2015, 3, 018–021. [Google Scholar] [CrossRef]
- Gao, T.T.; Yu, F.H.; Tan, Y. Study on Antimicrobial Activity in vitro of Extracts from Three Species of Ferula Root. Beifang Yuanyi 2013, 24, 156–158. [Google Scholar]
- Boghrati, Z.; Iranshahi, M. Ferula Species: A Rich Source of Antimicrobial Compounds. J. Herb. Med. 2019, 16, 100244. [Google Scholar] [CrossRef]
- Khoury, M.; El Beyrouthy, M.; Eparvier, V.; Ouaini, N.; Stien, D. Chemical Diversity and Antimicrobial Activity of the Essential Oils of Four Apiaceae Species Growing Wild in Lebanon. J. Essent. Oil Res. 2018, 30, 25–31. [Google Scholar] [CrossRef]
- Baccari, W.; Znati, M.; Zardi-Bergaoui, A.; Chaieb, I.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Composition and Insecticide Potential against Tribolium Castaneum of the Fractionated Essential Oil from the Flowers of the Tunisian Endemic Plant Ferula Tunetana Pomel Ex Batt. Ind. Crops Prod. 2020, 143, 111888. [Google Scholar] [CrossRef]
- Elghwaji, W.; El-Sayed, A.M.; El-Deeb, K.S.; ElSayed, A.M. Chemical Composition, Antimicrobial and Antitumor Potentiality of Essential Oil of Ferula Tingitana L. Apiaceae Grow in Libya. Pharmacogn. Mag. 2017, 13, S446–S451. [Google Scholar] [CrossRef]
- Jin, B.; Liu, H.; Feng, J.; Yang, X.G.; Zhou, J.H. Application and Prospect of Traditional Chinese Medicine in Cancer Treatment. Wisdom Health 2019, 5, 50–51. [Google Scholar] [CrossRef]
- Yang, M.; Luo, J.Y.; Qiao, M.L.; Sheng, P.; Yang, M.H. Mechanism of Uygur Medicine Root of Ferula ferulaeoides in Resisting Gastric Cancer Activity and Inducing Cell Apoptosis and Cell Cycle Arrest in vitro. Chin. J. Exp. Formul. 2018, 24, 112–122. [Google Scholar]
- Li, X.X.; Zhang, S.J.; Chiu, A.P.; Lo, L.H.; Huang, J.; Rowlands, D.K.; Wang, J.; Keng, V.W. Targeting of AKT/ERK/CTNNB1 by DAW22 as a Potential Therapeutic Compound for Malignant Peripheral Nerve Sheath Tumor. Cancer Med. 2018, 7, 4791–4800. [Google Scholar] [CrossRef]
- Yang, Y.; He, P.Y.; Zhang, Y.; Li, N. Natural Products Targeting the Mitochondria in Cancers. Molecules 2020, 26, 92. [Google Scholar] [CrossRef]
- Ma, R.T.; Sun, J.N.; Zhao, S.J.; Zhang, H.Y. Study on anti-cervical cancer cell activity of 4 Ferula ethanol extracts from Xinjiang. J. Xinjiang Med. Univ. 2022, 45, 1341–1347. [Google Scholar]
- Motai, T.; Daikonya, A.; Kitanaka, S. Sesquiterpene Coumarins from Ferula Fukanensis and Their Pro-Inflammatory Cytokine Gene Expression Inhibitory Effects. Chem. Pharm. Bull. 2013, 61, 618–623. [Google Scholar] [CrossRef]
- Ye, E.B.; Liu, F.; Xiong, Y.J.; Chen, G.; Yang, X.Z.; Gao, Y. Anti-inflammatory and immunosuppressive effects of three kinds of Xinjiang Ferula. Northwest J. Pharm. 1993, 8, 72–75. [Google Scholar]
- Li, G.Y. A Group of Sesquiterpene Coumarins, Sesquiterpene Chromogenic Ketones. CN201310023821.2, 16 September 2015. [Google Scholar]
- Sun, J.N.; Ma, R.T.; Zhao, S.J.; Zhang, H.Y. Study on the spectrum-effect relationship of anti-inflammatory activity of different polar parts of three kinds of Ferula. Chem. Biol. Eng. 2022, 39, 24–34. [Google Scholar]
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Al-Mutairi, K.A.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Ahmed, N. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In Global Decline of Insects; Books on Demand: Paris, France, 2021; pp. 1–19. [Google Scholar]
- Liu, T. Chemical Constituents from Two Plants of Apiaceae and Their Bioligocal Activities. Ph.D. Thesis, Fudan University, Shanghai, China, 2013. [Google Scholar]
- Li, J.W.; Zhao, H.M. Study on killing snails and schistosome miracidia by Ferula sinkiangensis. Tradit. Chin. Vet. Med. 2007, 4, 17–19. [Google Scholar] [CrossRef]
- Fu, K.; Zhao, H.M. Study on the molluscicidal mechanism of Ferulic guaiol against Oncomelania. Tradit. Chin. Vet. Med. 2015, 34, 56–58. [Google Scholar] [CrossRef]
- Lin, N. Insecticidal and Antifungal Activities of Volatile oil from Ferula ferulaeoidis. Master’s Thesis, Shihezi University, Shihezi, China, 2008. [Google Scholar]
- Luo, X.; Ma, G.Z.; Shan, M.; Wang, J.; Yu, F.S.; Teng, L.; Wang, C.H. Comparative study on acute toxicity and chemical constituents of volatile oil from two kinds of Ferulae. Chin. Pat. Med. 2015, 37, 1130–1135. [Google Scholar]
- Wang, C.X.; Xu, F.; Chen, Y.; Tang, W.; He, J.; Zhao, J.; Zhang, Y.F. Identification study of three commonly used medicinal herbs. Xinjiang Tradit. Chin. Med. 2011, 29, 50–52. [Google Scholar]
- Ding, X.; Tan, Y.; Yan, L.; Cheng, Y.H.; Zhao, W.B. Study on the identification of Ferula ferulaeoides. Chin. Med. Mater. 2011, 34, 879–881. [Google Scholar]
- Liu, M.; Zhao, Y.; Ma, Y.; Liu, S.; Yao, J.; Chi, Y.; Li, H.; Liao, K.; Zhu, Y. The Study of Schizogenous Formation of Secretory Ducts in Ferula ferulaeoides (Steud.) Korov. Protoplasma 2022, 259, 679–689. [Google Scholar] [CrossRef]
- Xu, S.; Xu, W.F.; Kuang, Y.M.; Jin, P.F. Research progressin quality evaluation of Chinese herbal medicine Scutellaria barbata Herbal. Northwest J. Pharm. 2023, 38, 222–226. [Google Scholar]
- Miao, L.J. Fingerprints Study on Ferula ferulaeoides (Steud.) Korov. from National Medicine in Xinjiang. Master’s Thesis, Xinjiang Medical University, Ürümqi, China, 2014. [Google Scholar]
- Sheng, P.; Tang, D.P.; Miao, L.J.; Zhan, W.J.; Sun, Y.; Wang, Y.J.; Wang, G.P. Fingerprints study on Ferula ferulaeoides (Steud.) Korov. from nztional medicine in Xinjiang. China Mod. Appl. Pharmacol. 2015, 32, 30–37. [Google Scholar] [CrossRef]
- Wang, S.; Sheng, P.; Yao, L.; Du, B.J. GC-MS fingerprint of in vitro anti-gastric cancer active parts from roots of Uygur medicine Ferula ferulaeoides. Chin. Herb. Med. 2015, 46, 2874–2879. [Google Scholar]
- Luo, X. Preliminary Study on Chemical Fingerprint and Antiulcer Activity of Two Species of ferula. Master’s Thesis, Xinjiang Medical University, Ürümqi, China, 2015. [Google Scholar]
- Zhu, Y.; Zhang, K.; Liang, X.; Tang, Y.; Huang, J.; Wang, J.H. Content Determination of Sesquiterpene Coumarin (DAW22) in Ferula ferulaeoides by UPLC. Chin. J. Exp. Prescr. 2016, 22, 63–65. [Google Scholar] [CrossRef]
- Jin, W.H.; Zhang, J.L.; Gao, L.; Wang, X.G.; Wang, X.S. Evaluation of antioidative properties of ferulic acid amides with different dosages in flying system of soybean oil. China Fats Oils 2021, 46, 89–92+116. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M.; Sabbagh, F. Simulating Release Model and Antimicrobial Efficiency of LDPE Film Carrying Ferula Asafetida Leaf and Gum Extracts. Polym. Bull. 2022, 79, 1151–1174. [Google Scholar] [CrossRef]
- Valinezhad, N.; Talebi, A.F.; Alamdari, S. Preparation, Characterization, and Antibacterial Effects of Ferula Gummosa Essential Oil–Chitosan (CS-FEO) Nanocomposite. Nanochem. Res. 2022, 7, 85–92. [Google Scholar]
- Li, T.T.; Zhu, Y.F.; Li, H.J.; Shi, S.L.; Yu, D. Research progress of functional foods. Mod. Food 2022, 28, 79–81. [Google Scholar] [CrossRef]
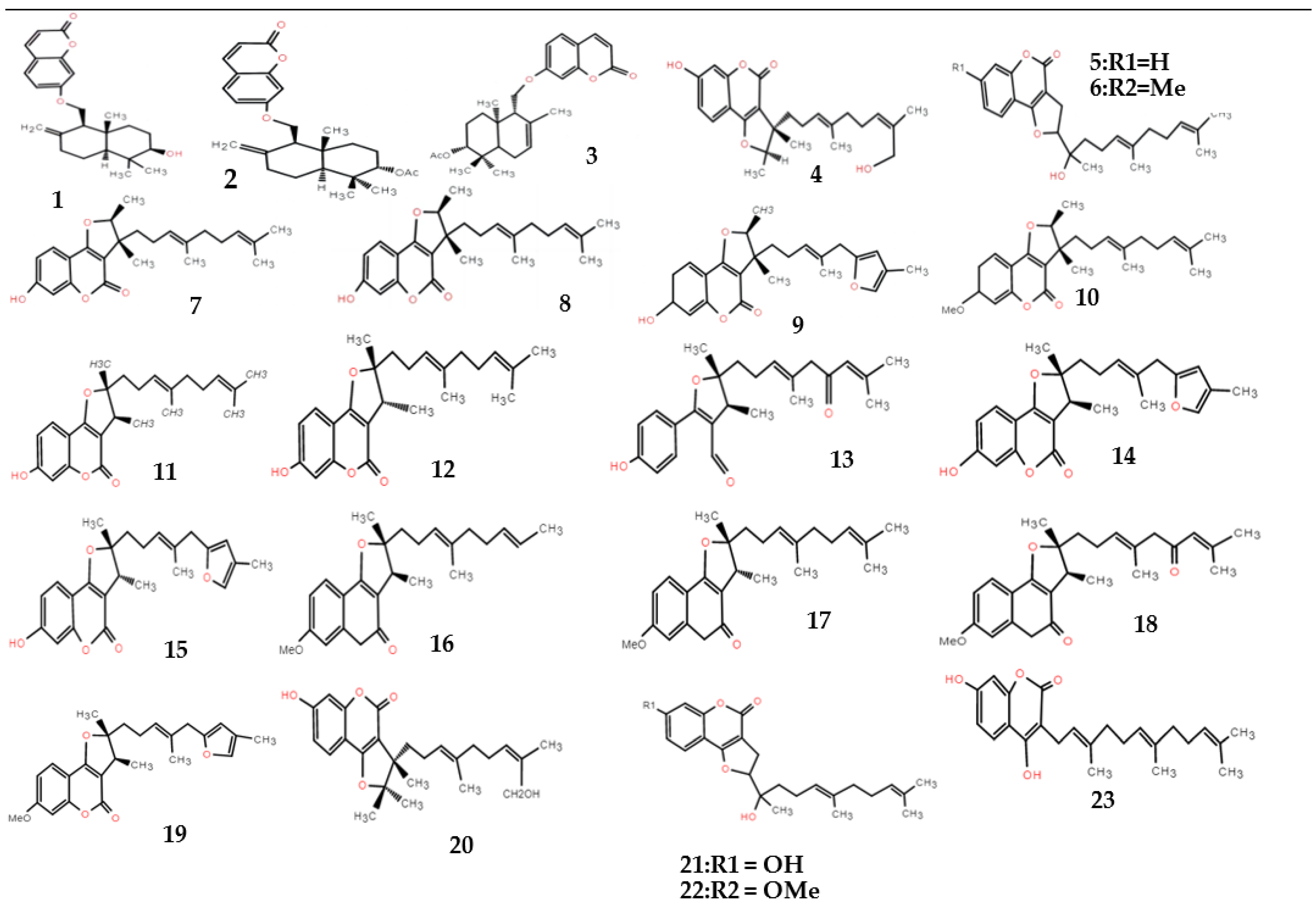
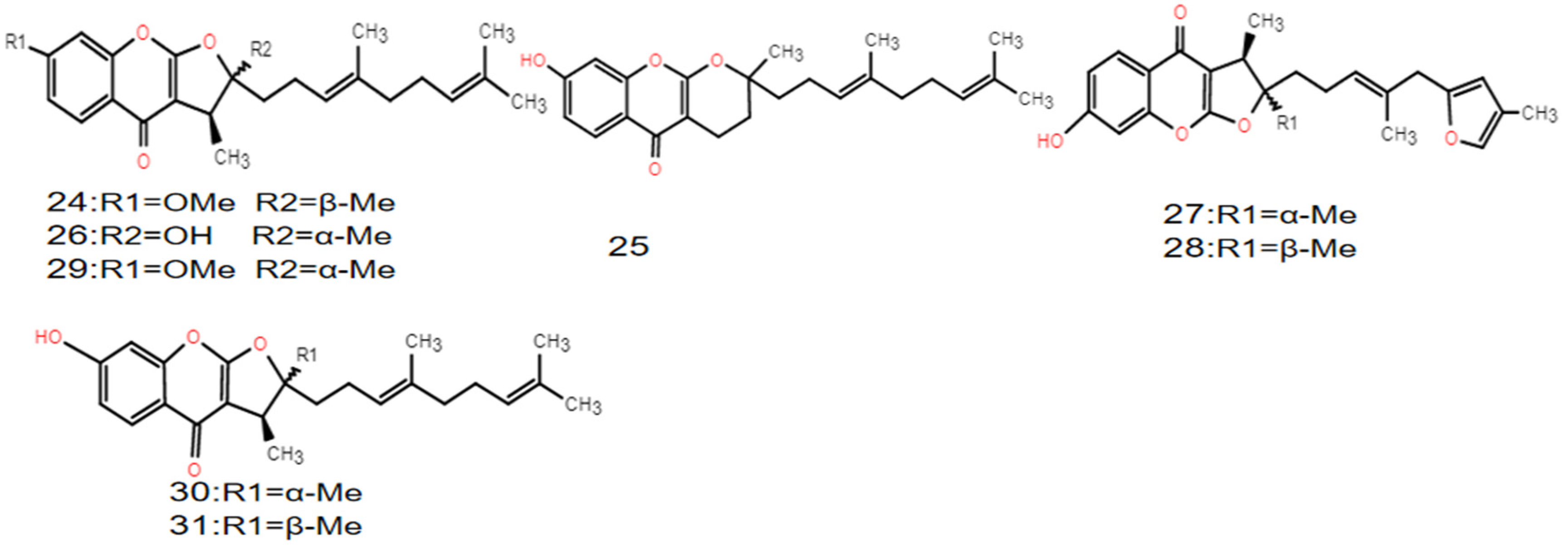
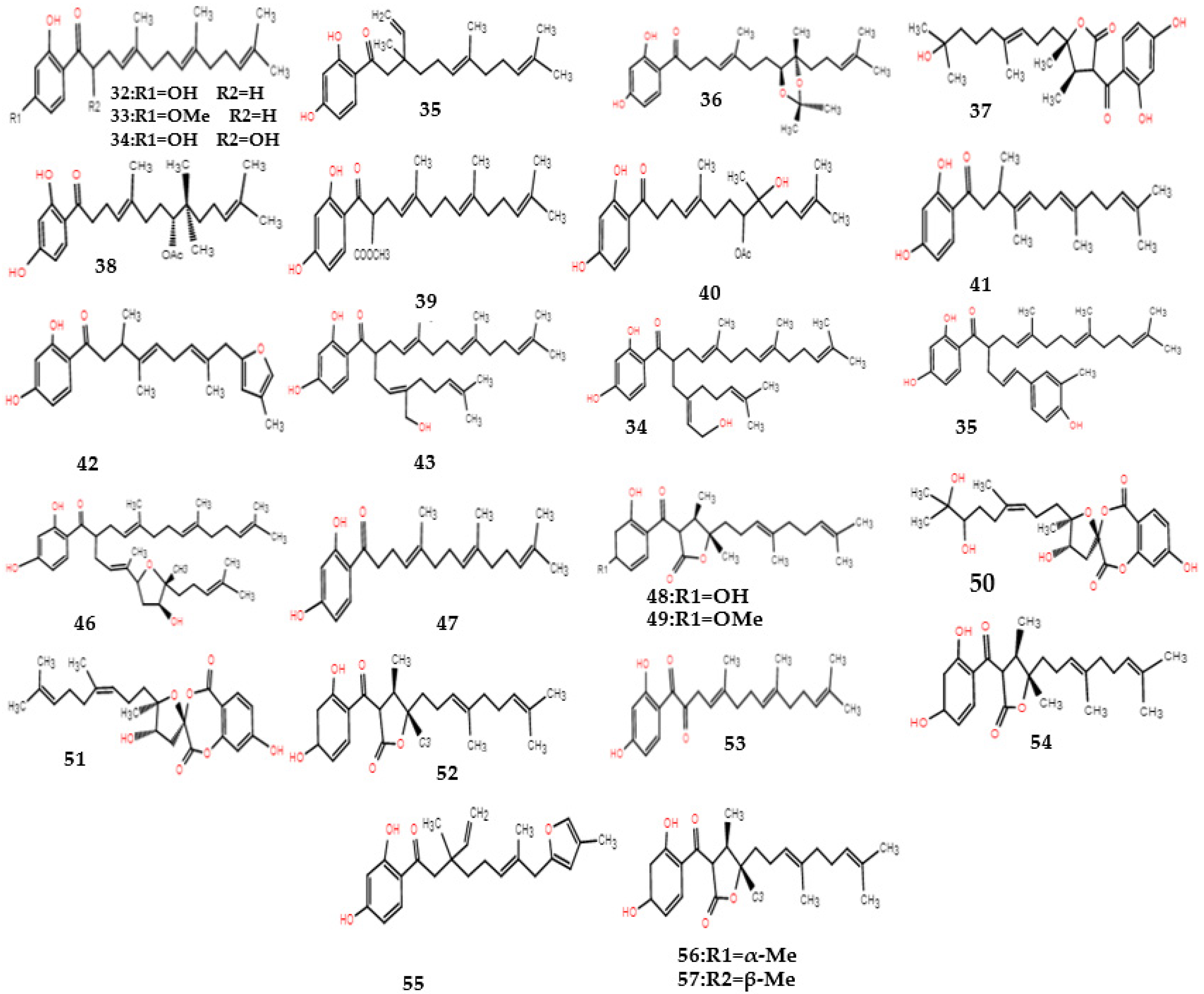
| Type | No. | Compound | Formula | Molecular Weight | References |
|---|---|---|---|---|---|
| Sesquiterpene–coumarins | 1 | Badrakemin | C24H30O4 | 382 | [13] |
| 2 | Badrakemin acetate | C26H32O5 | 424 | [13] | |
| 3 | Conferol acetates | C26H32O5 | 424 | [13] | |
| 4 | Ferulin A | C25H33O5 | 413 | [14] | |
| 5 | Ferulin B | C24H32O5 | 400 | [15] | |
| 6 | Ferulin C | C25H34O5 | 414 | [14] | |
| 7 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-3-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C24H30O4 | 382 | [14] | |
| 8 | 2,3-dihydro-7-hydroxy-2R*,3R*-dimethyl-3-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C24H30O4 | 382 | [14,16] | |
| 9 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-3-[4-methyl-5-(4-methyl-2-furyl)-3(E)-pentenyl]-furo [3,2-c] coumarin | C24H26O5 | 394 | [14] | |
| 10 | 2,3-dihydro-7-methoxy-2S*,3R*-dimethyl-3-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C25H32O4 | 396 | [15] | |
| 11 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C24H30O4 | 382 | [15] | |
| 12 | 2,3-dihydro-7-hydroxy-2R*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C24H30O4 | 382 | [15] | |
| 13 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadien-6-onyl]-furo [3,2-c] coumarin | C25H32O4 | 396 | [15] | |
| 14 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4-methyl-5-(4-methyl-2-furyl)-3(E)-pentenyl]-furo [3,2-c] coumarin | C24H26O5 | 394 | [14] | |
| 15 | 2,3-dihydro-7-hydroxy-2R*,3R*-dimethyl-2-[4-methyl-5-(4-methyl-2-furyl)-3(E)-pentenyl]-furo [3,2-c] coumarin | C24H26O5 | 394 | [14] | |
| 16 | 2,3-dihydro-7-methoxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C25H32O4 | 396 | [15] | |
| 17 | 2,3-dihydro-7-methoxy-2R*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-c] coumarin | C25H32O4 | 396 | [15] | |
| 18 | 2,3-dihydro-7-methoxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadien-6-onyl]-furo [3,2-c] coumarin | C25H30O5 | 410 | [15] | |
| 19 | 2,3-dihydro-7-methoxy-2S*,3R*-dimethyl-2-[4-methyl-5-(4-methyl-2-furyl)-3(E)-pentenyl]-furo [3,2-c] coumarin | C25H28O5 | 408 | [15] | |
| 20 | (trans)-2,3-dimethyl-3-[9-hydroxymethyl-4-methyl-3E,7Z-nonadienyl]-7-hydroxy-2(3H)-furo [3,2-c] coumarin | C24H31O5 | 399 | [15] | |
| 21 | 7-hydroxy-2-[1-hydroxy-1,5,9-trimethyl-4E,8-decadienyl]-2(3H)-furo [3,2-c] coumarin | C24H31O5 | 399 | [15] | |
| 22 | 2-[1-hydroxy-1,5,9-trimethyl-4E,8-decadienyl]-7-methoxy-2(3H)-furo [3,2-c] coumarin | C25H33O5 | 408 | [15] | |
| 23 | 4,7-dihydroxy-3-[3,7,11-trimethyl-2(E),6(E),10-dodecatrienyl] coumarin | C24H30O4 | 382 | [14] | |
| Sesquiterpene–heteroketone compounds | 24 | Ferulin D | C25H32O4 | 396 | [15] |
| 25 | Ferulin E | C24H31O4 | 383 | [14] | |
| 26 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-b] chromone | C24H30O4 | 382 | [14] | |
| 27 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4-methyl-5-(4-methyl-2-furyl)-3(E),7-pentenyl]-furo [2,3-b] chromone | C24H26O5 | 394 | [15] | |
| 28 | 2,3-dihydro-7-hydroxy-2R*,3R*-dimethyl-2-[4-methyl-5-(4-methyl-2-furyl)-3(E),7-pentenyl]-furo [2,3-b] chromone | C24H26O5 | 394 | [15] | |
| 29 | 4-hydro-7-hydroxy-2-methyl-2-[4,8-dimethyl-3E,7-nonadienyl]-2(3H)-pyro [2,3-b] chromone | C24H31O4 | 383 | [14] | |
| 30 | 2,3-dihydro-7-hydroxy-2S*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo [3,2-b] chromone | C24H30O4 | 382 | [15] | |
| 31 | 2,3-dihydro-7-hydroxy-2R*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo[3,2-b] chromone | C24H30O4 | 382 | [15] | |
| Sesquiterpene–phenolic compounds | 32 | Dshamirone (secoammoresinol) | C23H33O3 | 356 | [15] |
| 33 | (4E,8E)-1-(2,4-dihydroxyphenyl)-5,9,13-trimethyl-tetradeca-4,8,12-trien-1-one | C23H32O3 | 356 | [14] | |
| 34 | (4E,8E)-1-(2,4-dihydroxyphenyl)-2-methoxycar-bonyl-5,9,13-trimethyltetradeca-4,8,12-trien-1-one | C25H34O5 | 414 | [14] | |
| 35 | 1-(2,4-dihydroxyphenyl)-3,7,11-trimethyl-3-vinyl-6(E),10-dodecadiene-1-one | C23H32O3 | 356 | [15] | |
| 36 | 8,9-oxoisopropanyldshami-rone | C22H30O5 | 374 | [17] | |
| 37 | Ferulaeolactone A | C24H34O6 | 418 | [17] | |
| 38 | 8-acetoxy-9-hydroxydshmirone | C24H34O5 | 402 | [17] | |
| 39 | Ferulaeone A | C25H33O5 | 413 | [15] | |
| 40 | Ferulaeone B | C25H37O6 | 433 | [15] | |
| 41 | Ferulaeone C | C23H31O3 | 355 | [15] | |
| 42 | Ferulaeone D | C23H28O4 | 369 | [15] | |
| 43 | Ferulaeone E | C33H49O4 | 509 | [15] | |
| 44 | Ferulaeone F | C33H49O4 | 509 | [15] | |
| 45 | Ferulaeone G | C33H43O5 | 519 | [15] | |
| 46 | Ferulaeone H | C38H55O5 | 591 | [15] | |
| 47 | 3-(2,4-dihydroxybenzoyl)-4S*,5R*-dimethyl-5-[4,8-dimethyl-3(E),7(E)-nonadien-1-yl] tetrahydro-2-furanone | C24H32O5 | 400 | [15] | |
| 48 | 3-(2-hydroxyl-4-methoxybenzoyl)-4S*,5R*-dimethyl-5-[4,8-dimethyl-3(E),7(E)-nonadien-1-yl] tetrahydro-2-furanone | C25H34O5 | 414 | [15] | |
| 49 | 3-(2,4-dihydroxybenzoyl)-4R*,5R*-dimethyl-5-[4,8-dimethyl-3(E),7(E)-nonadien-1-yl] tetra-hydro-2-furanone | C24H32O5 | 400 | [15] | |
| 50 | Ferulactone A | C24H32O9 | 463 | [18] | |
| 51 | Ferulactone B | C24H30O7 | 429 | [18] | |
| 52 | 3S*-(2,4-dihydroxybenzoyl)-4R*,5R*-dimethyl-5-(4,8-dimethyl-3(E),7(E)-nonadien-1-yl) tetrahydro-2-furanone | C24H32O5 | 400 | [19] | |
| 53 | 1-(2,4-dihydroxyphenyl)-2-hydroxy-5,9,13-trimethy1-4(E),8(E),12-tetradecatrien-1-one | C23H32O4 | 372 | [19] | |
| 54 | 1-(2,4-dihydroxyphenyl)-3,7,11-trimethyl-3-vinyl-6(E),10-dodecadiene-1,9-dione | C23H30O4 | 370 | [19] | |
| 55 | 1-(2,4-dihydroxyphenyl)-3,7-dimethyl-3-vinyl-8-(4-methyl-2-furyl)-6(E)-octen-l-one | C23H28O4 | 368 | [15] | |
| 56 | 3S*-(2,4-dihydroxybenzoyl)-4R*,5R*-dimethyl-5-[4-methyl-5-(4-methyl-2-furyl)-3(E)-penten-1-yl] tetrahydro-2-furanone | C24H28O6 | 412 | [15] | |
| 57 | 3S*-(2,4-dihydroxybenzoyl)-4R*,5S*-dimethyl-5-[4-methyl-5-(4-methyl-2-furyl)-3(E)-penten-l-yl] tetrahydro-2-furanone | C24H28O6 | 421 | [15] |
| Type | Compound | References |
|---|---|---|
| Monoterpenoids | α-pinene, β-pinene, camphene, δ-3-carene, limonene, D-limonene, L-limonene, α-phellandrene, β-thujene, γ-terpinene, ocimene, β-ocimene, terpinolene, α-terpinolene, myrcene, β-myrcene, sabinene, fenchene, fenchol, borneol, (R)-camphor, citronellol, P-cymen-8-ol, α-terpined, 1,7,7-trimethyl-exo-bicyclo[2.2.1]heptan-2-ol, tanacetone, Z-3-pnen-2-ol, isogeraniol), 3-methoxy-p-cymene, dihydrocarvyl aetate, thymylether methyl, 2-camphanol acetate, exobornyl acetate, α-fenchyl acetate, [1,3,6-octatriene,3,7-dimethyl-,(Z)-], 3-isopropylidene-5-methyl-hex-4-en-2-one) | [8] |
| Sesquiterpene | Farnesene, α-guaiene, α-farnesene, β-farnesene, α-curcumene, β-curcumene, α-elemene, β-elemene, valencene, (−)-alloaromadendrene, trans-caryophyllene, aristolene, α-gurjunene, α-cedrene, β-cedrene, hujopsenet, α-himachalene, β-himachalene, α-bulnesene, isoledene, (Z,E)-α-farnesene, 1.2,3,4,4A,5,6,8A-octa-hydro-naphthalene, bergamotene, γ-selinene, α-bergamotene, zingiberene, isocaryophyllene, ledene, β-bisabolene, τ-guaiene, guaiol, α-guaiol, δ-guaiol, bulnesol, 10-O-γ-eudesmol, α-eudesmol, τ-eudesmol, β-eudesmol, elemiol, cedrol, hinesol, agarospirol, ginsenol, calareneexoide, eudesm-7(11)-en-4-ol, β-bisabolol, nerolidol, trans-nerolidol, 1,2- propanediol,3-methoxy-, 2,6,6-trimethyl-1- methylen-cyclohex-2-ene, 5,9-undecadien- 2-one,6,10-dimethyl-,(Z)-, 1,1-dimethyl-2,4-di(1-propenyl)cyclohexane, (Z)-2,6,10- trimethyl-1,5,9-undecatriee, 5-β-H,7-β, 10-α-selina-4(19),11-diethy, 4-(1E)-1,3-butadien-1-yl-3,5,5-trimethyl] | [8] |
| Aromatic | Toluene, resorcinol, O-cymene, benzene, 2-methoxy-4-vinylphenol, 1-methyl-4-(1-methylethyl) benzene, 4-(1-methylethyl)-benzene-methanol, 1-1,5-dimethyl-4- hexenyl-4-methyl benzene | [8] |
| Alcohol esters | cyclopentanol,2-methyl acetate, neryl acetate, butane-2,3-diol, (2S,3S)-(+)-2,3-butane diol, 4,8-dimethyl-3,7-nonadien-2-ol, 4-terpinyl acetate, α-terpinyl acetate, ethyl palmitate, ethyl linoleate | [8] |
| Type | No. | Compound | Formula | Molecular Weight | References |
|---|---|---|---|---|---|
| Phenylpropanoids | 1 | myristicin | C11H12O3 | 192 | [19] |
| Phenols, phenolic acids | 2 | 2,4-dihydroxyacetophenone | C8H8O3 | 152 | [19] |
| 3 | 2-hydroxy-4-methoxyacetophenone | C9H10O3 | 166 | [19] | |
| 4 | 2,4-dihydroxybenzoic acid | C7H6O4 | 154 | [19] | |
| 5 | 2,4-dihydroxy-α-oxobenzeneacetic acid | C8H8O4 | 168 | [19] | |
| 6 | β-resorcylic acid | C7H6O4 | 154 | [19] | |
| 7 | Methoxyresorcylic acid | C8H8O4 | 168 | [19] | |
| 8 | Umbelliferone | C9H6O3 | 162 | [6] | |
| 9 | Lehmannmlone | C24H30O4 | 382 | [6] | |
| 10 | methyl 2,4-dihydroxybenzoate | C8H8O4 | 168 | [6] | |
| 11 | ethyl 2,4-dihydroxybenzoate | C9H10O4 | 182 | [6] | |
| Steroids | 12 | β-sitosterol | C29H50O | 414 | [19] |
| 13 | Daucossterol | C35H60O6 | 576 | [6] |
| Ferula Plants | Antibacterial Ingredients | Microorganism | References |
|---|---|---|---|
| F. lycia | essential oil | Haemophilus influenza T | [23] |
| F. glauca | essential oil | Streptococcus mutans, Enterococcus faecalis, Escherichia coli | [23] |
| F. heuffelii | essential oil | Micrococcus luteus, Staphylococcus epidermidis, B. subtilis,Micrococcus flavus | [23] |
| F. assafoetida | organic extractswater extracts | coli, S. aureus, E. faecalis, Shigella flexneri, Klebsiella pneumonia | [21,23] |
| F. gummosa | oleo-resin | E. coli, P. aeruginosa, S. aureus, Salmonella enteritidis, Listeria monocytogenes | [23] |
| essential oil | C. albicans, S. epidermidis, S. aureus, E. coli, B. cereus, E. faecalis, P. aeruginosa | ||
| F. communis | petroleum ether extract | S. aureus, B. subtilis, Streptococcus durans, E. faecalis | [23] |
| F. szovitsiana | essential oil | B. subtilis | [23] |
| F. hermonis | essential oil | S. typhi, P. aeruginosa, E. coli, S. aureus, S. fecalis | [23] |
| F. vesceritensis | essential oil | E. coli, K. pneumonia, S. aureus, P. aeruginosa | [23] |
| F. kuhistanica | essential oil | S. aureus | [23] |
| F. ferulaeoides | sesquiterpene derivatives | Drug-resistant S. aureus | [14][22] |
| alcohol extract | S. aureus, B. subtilis, Sarcina | ||
| F. elaeochytris | essential oil | S. aureus | [24] |
| F.pseudalliacea | sesquiterpene coumarins | S. aureus, Enterococcus faecium, B. cereus, E. coli, P. aeruginosa | [23] |
| F. tunetana | essential oil | Salmonella typhimurium, S. epidermidis, Micrococcus luteus, B. Cereus, B. subtilis | [25] |
| F. tingitana L. | essential oil | Bacillus subtilis, Neisseria gonorrhoeae | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhou, G.; Ma, S. Research Progress of Ferula ferulaeoides: A Review. Molecules 2023, 28, 3579. https://doi.org/10.3390/molecules28083579
Chen Z, Zhou G, Ma S. Research Progress of Ferula ferulaeoides: A Review. Molecules. 2023; 28(8):3579. https://doi.org/10.3390/molecules28083579
Chicago/Turabian StyleChen, Zhengqiong, Gang Zhou, and Shengjun Ma. 2023. "Research Progress of Ferula ferulaeoides: A Review" Molecules 28, no. 8: 3579. https://doi.org/10.3390/molecules28083579
APA StyleChen, Z., Zhou, G., & Ma, S. (2023). Research Progress of Ferula ferulaeoides: A Review. Molecules, 28(8), 3579. https://doi.org/10.3390/molecules28083579





