A MD Simulation Prediction for Regulation of N-Terminal Modification on Binding of CD47 to CD172a in a Force-Dependent Manner
Abstract
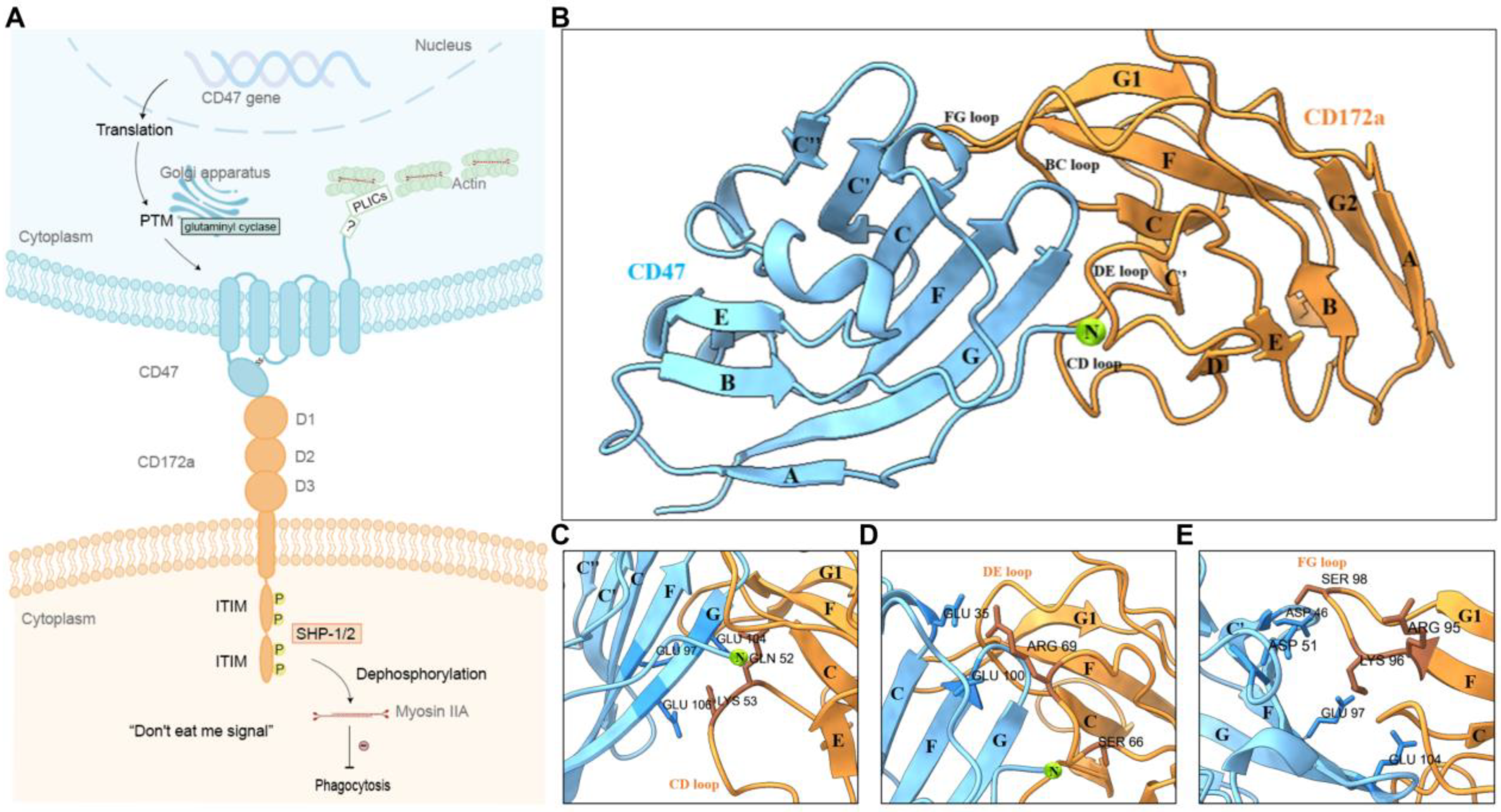
1. Introduction
2. Results
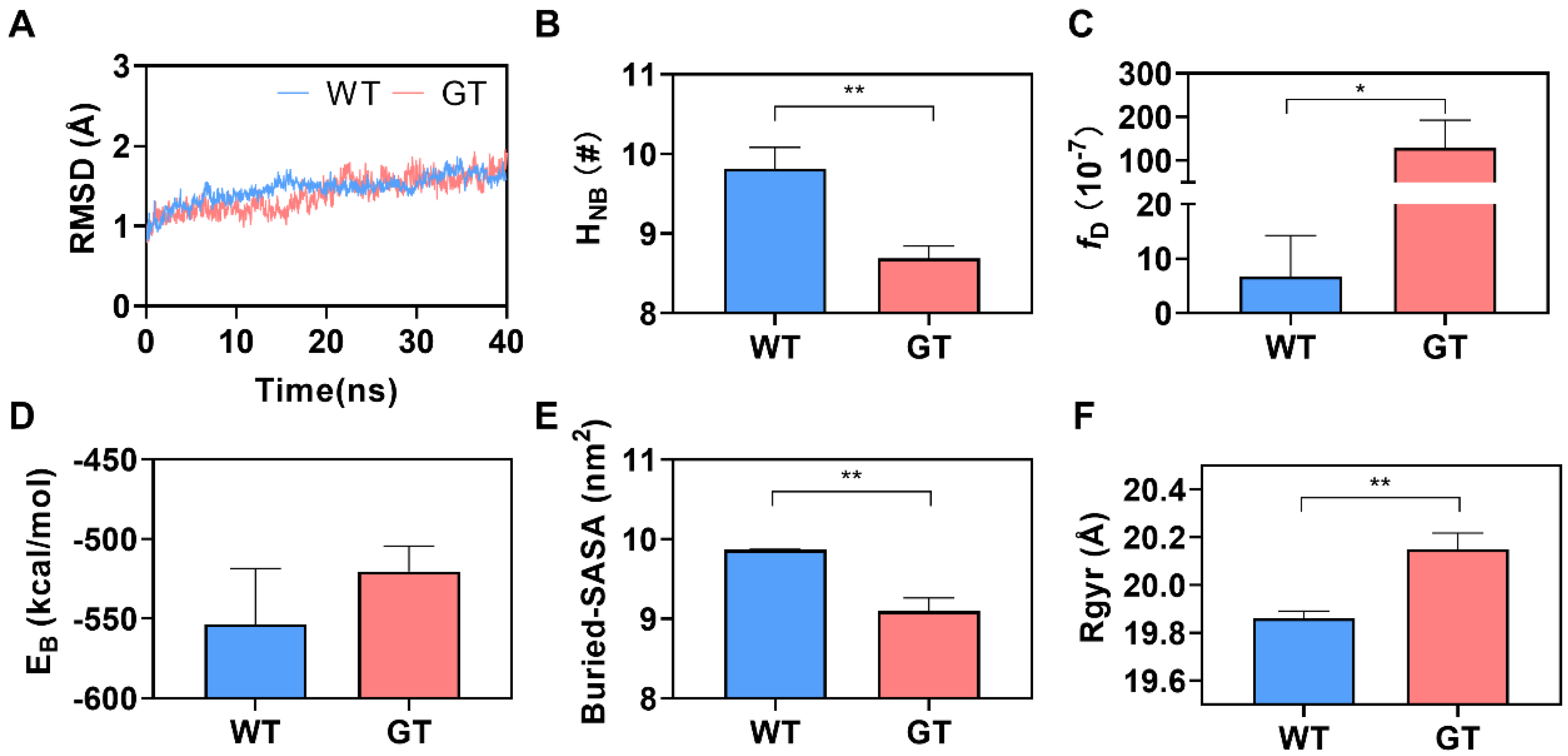
2.1. N-Terminal Post-Translational Modification Improved CD47 Affinity to CD172a
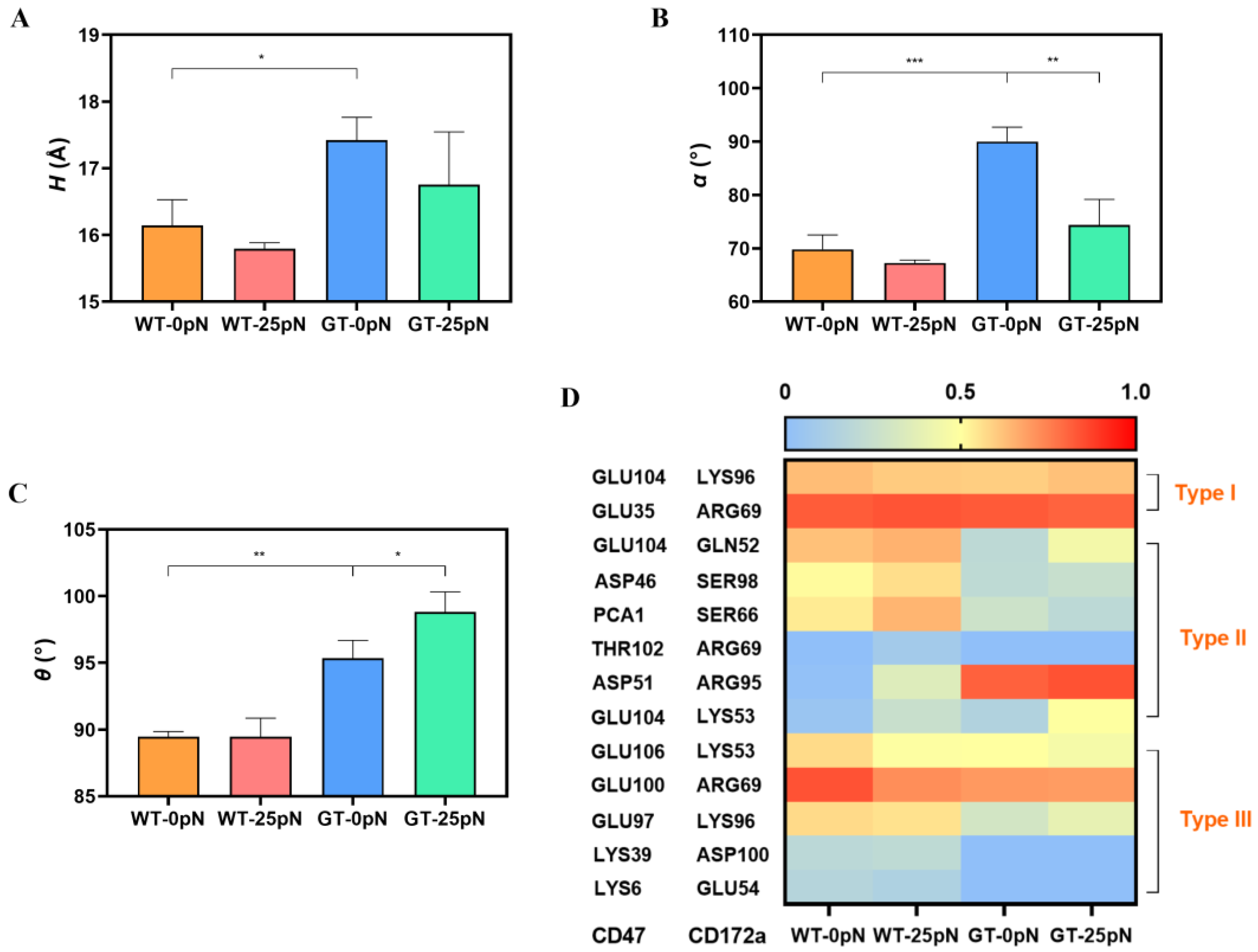
2.2. PTM Enhanced Interfacial H-Bonding and Induced Contraction of Binding Pocket of Bound CD172a
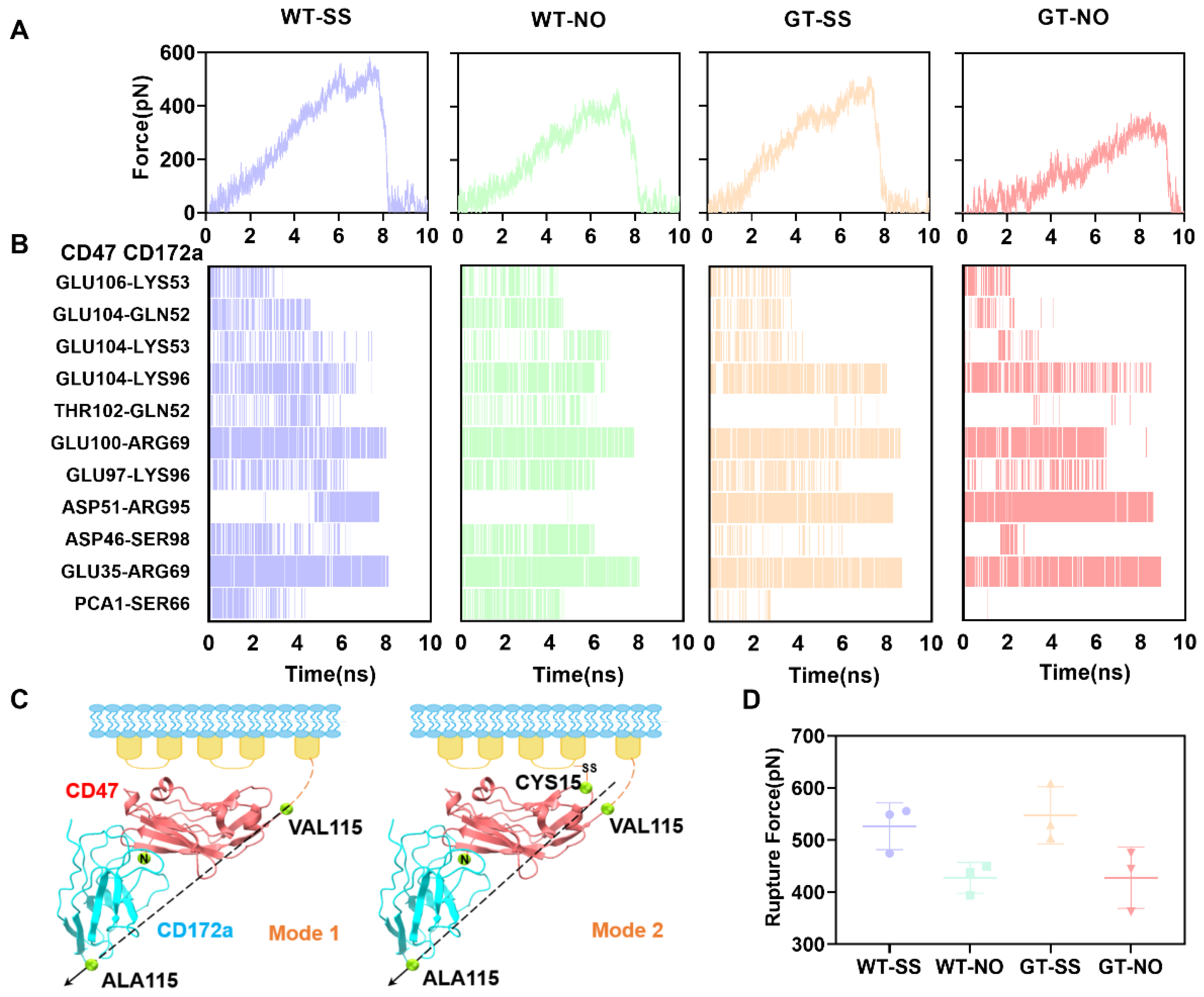
2.3. Mechanical Constraint on CYS15 of CD47, Instead of N-Terminal PTM, Might Improve Tensile Strength of CD172a/CD47 Complex
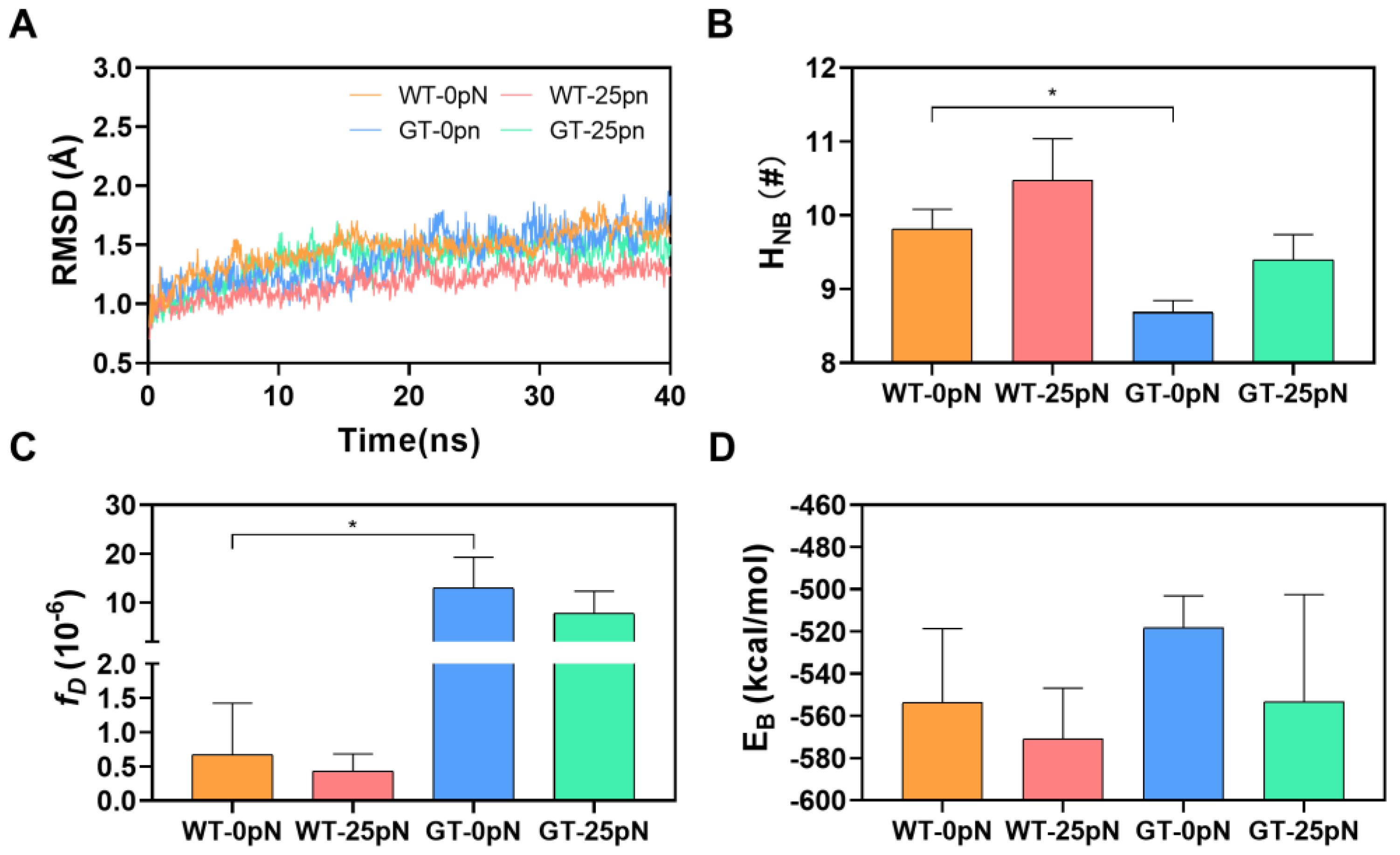
2.4. Catch Bond Mechanism Mediated the PTM-Enhanced CD172a-CD47 Interaction by Making the CD172a Binding Pocket Contract Further
3. Discussion
4. Materials and Methods
4.1. System Setup
4.2. Molecular Dynamics Simulation
4.3. Data Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, S.; Jee, S.; Paik, S.S.; Jang, K. Clinicopathological Significance of CD47 Expression in Hepatocellular Carcinoma. J. Clin. Pathol. 2021, 74, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Ticchioni, M.; Rouquette-Jazdanian, A.K.; Samson, M.; Deckert, M.; Greenberg, A.H.; Bernard, A. CD47 and the 19 KDa Interacting Protein-3 (BNIP3) in T Cell Apoptosis. J. Biol. Chem. 2003, 278, 23915–23921. [Google Scholar] [CrossRef] [PubMed]
- Legrand, N.; Huntington, N.D.; Nagasawa, M.; Bakker, A.Q.; Schotte, R.; Strick-Marchand, H.; de Geus, S.J.; Pouw, S.M.; Böhne, M.; Voordouw, A.; et al. Functional CD47/Signal Regulatory Protein Alpha (SIRPα) Interaction Is Required for Optimal Human T- and Natural Killer- (NK) Cell Homeostasis in Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 13224–13229. [Google Scholar] [CrossRef]
- Olsson, M.; Bruhns, P.; Frazier, W.A.; Ravetch, J.V.; Oldenborg, P.-A. Platelet Homeostasis Is Regulated by Platelet Expression of CD47 under Normal Conditions and in Passive Immune Thrombocytopenia. Blood 2005, 105, 3577–3582. [Google Scholar] [CrossRef]
- Brittain, J.E.; Han, J.; Ataga, K.I.; Orringer, E.P.; Parise, L.V. Mechanism of CD47-Induced A4β1 Integrin Activation and Adhesion in Sickle Reticulocytes. J. Biol. Chem. 2004, 279, 42393–42402. [Google Scholar] [CrossRef]
- Hu, T.; Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Zhang, Y.; Song, Y.; Hu, J.; He, X.; et al. Tumor-Intrinsic CD47 Signal Regulates Glycolysis and Promotes Colorectal Cancer Cell Growth and Metastasis. Theranostics 2020, 10, 4056–4072. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 Antibody–Mediated Phagocytosis of Cancer by Macrophages Primes an Effective Antitumor T-Cell Response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPα Signaling Axis as an Innate Immune Checkpoint in Cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Li, S.; Lin, W.; Wang, Z.; Wang, S.; Chen, W.; Shi, W.; Chen, T.; Zhou, H.; et al. Metabolic Control of CD47 Expression through LAT2-Mediated Amino Acid Uptake Promotes Tumor Immune Evasion. Nat. Commun. 2022, 13, 6308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, S.; Ma, J.; Yan, Z.; Xie, H.; Feng, Y.; Che, S. CD47 as a Promising Therapeutic Target in Oncology. Front. Immunol. 2022, 13, 757480. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhao, H.; Xu, J.; Shen, C. CD47: The next Checkpoint Target for Cancer Immunotherapy. Crit. Rev. Oncol. Hematol. 2020, 152, 103014. [Google Scholar] [CrossRef] [PubMed]
- Rebres, R.A.; Vaz, L.E.; Green, J.M.; Brown, E.J. Normal Ligand Binding and Signaling by CD47 (Integrin-Associated Protein) Requires a Long Range Disulfide Bond between the Extracellular and Membrane-Spanning Domains. J. Biol. Chem. 2001, 276, 34607–34616. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, F.P.; Gresham, H.D.; Schwarz, E.; Brown, E.J. Molecular Cloning of Integrin-Associated Protein: An Immunoglobulin Family Member with Multiple Membrane-Spanning Domains Implicated in Alpha v Beta 3-Dependent Ligand Binding. J. Cell Biol. 1993, 123, 485–496. [Google Scholar] [CrossRef]
- Reinhold, M.I.; Lindberg, F.P.; Plas, D.; Reynolds, S.; Peters, M.G.; Brown, E.J. In Vivo Expression of Alternatively Spliced Forms of Integrin-Associated Protein (CD47). J. Cell Sci. 1995, 108, 3419–3425. [Google Scholar] [CrossRef]
- Hatherley, D.; Graham, S.C.; Turner, J.; Harlos, K.; Stuart, D.I.; Barclay, A.N. Paired Receptor Specificity Explained by Structures of Signal Regulatory Proteins Alone and Complexed with CD47. Mol. Cell 2008, 31, 266–277. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, F.; Liu, Y.; Jiang, J.; Yang, Y.-G.; Wang, H. Studying the Mechanism of CD47–SIRPα Interactions on Red Blood Cells by Single Molecule Force Spectroscopy. Nanoscale 2014, 6, 9951–9954. [Google Scholar] [CrossRef]
- Lee, W.Y.; Weber, D.A.; Laur, O.; Severson, E.A.; McCall, I.; Jen, R.P.; Chin, A.C.; Wu, T.; Gernet, K.M.; Parkos, C.A. Novel Structural Determinants on SIRPα That Mediate Binding to CD47. J. Immunol. 2007, 179, 7741–7750. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Chen, Z.; Sures, I.; Wang, H.; Schilling, J.; Ullrich, A. A Family of Proteins That Inhibit Signalling through Tyrosine Kinase Receptors. Nature 1997, 386, 181–186. [Google Scholar] [CrossRef]
- Barclay, A.N.; Brown, M.H. The SIRP Family of Receptors and Immune Regulation. Nat. Rev. Immunol. 2006, 6, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, M.E.W.; Jansen, J.H.M.; Raaben, M.; Toebes, M.; Franke, K.; Brandsma, A.M.; Matlung, H.L.; Fauster, A.; Gomez-Eerland, R.; Bakker, N.A.M.; et al. Glutaminyl Cyclase Is an Enzymatic Modifier of the CD47- SIRPα Axis and a Target for Cancer Immunotherapy. Nat. Med. 2019, 25, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Weng, L.; Zhang, T.; Tian, H.; Fang, L.; Teng, H.; Zhang, W.; Gao, J.; Hao, Y.; Li, Y.; et al. Identification of Glutaminyl Cyclase Isoenzyme IsoQC as a Regulator of SIRPα-CD47 Axis. Cell Res. 2019, 29, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.L.; Jang, H.; Lee, J.; Ramachandran, S.; Kagan, B.L.; Nussinov, R.; Teran Arce, F. Activity and Architecture of Pyroglutamate-Modified Amyloid-β (Aβ pE3-42) Pores. J. Phys. Chem. B 2014, 118, 7335–7344. [Google Scholar] [CrossRef]
- Kuriyama, T.; Takenaka, K.; Kohno, K.; Yamauchi, T.; Daitoku, S.; Yoshimoto, G.; Kikushige, Y.; Kishimoto, J.; Abe, Y.; Harada, N.; et al. Engulfment of Hematopoietic Stem Cells Caused by Down-Regulation of CD47 Is Critical in the Pathogenesis of Hemophagocytic Lymphohistiocytosis. Blood 2012, 120, 4058–4067. [Google Scholar] [CrossRef]
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sézary Syndrome with SIRPαFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, C.C.; Yan, X.G.; Tseng, H.-Y.; Wang, C.Y.; Zhang, Y.Y.; Yari, H.; La, T.; Farrelly, M.; Guo, S.T.; et al. BRAF/MEK Inhibitors Promote CD47 Expression That Is Reversible by ERK Inhibition in Melanoma. Oncotarget 2017, 8, 69477–69492. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokobori, T.; Tanaka, N.; Sakai, M.; Sano, A.; Inose, T.; Sohda, M.; Nakajima, M.; Miyazaki, T.; Kato, H.; et al. CD47 Expression Regulated by the MiR-133a Tumor Suppressor Is a Novel Prognostic Marker in Esophageal Squamous Cell Carcinoma. Oncol. Rep. 2012, 28, 465–472. [Google Scholar] [CrossRef]
- Casey, S.C.; Baylot, V.; Felsher, D.W. The MYC Oncogene Is a Global Regulator of the Immune Response. Blood 2018, 131, 2007–2015. [Google Scholar] [CrossRef]
- Burger, P.; Hilarius-Stokman, P.; de Korte, D.; van den Berg, T.K.; van Bruggen, R. CD47 Functions as a Molecular Switch for Erythrocyte Phagocytosis. Blood 2012, 119, 5512–5521. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, W.; Lou, J.; Rittase, W.; Li, K. Mechanosensing through Immunoreceptors. Nat. Immunol. 2019, 20, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Orazizadeh, M.; Lee, H.; Groenendijk, B.; Sadler, S.J.; Wright, M.O.; Lindberg, F.P.; Salter, D.M. CD47 Associates with Alpha 5 Integrin and Regulates Responses of Human Articular Chondrocytes to Mechanical Stimulation in an in Vitro Model. Arthritis Res. Ther. 2008, 10, R4. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, F.; Engler, A.; Eckhardt, A.; Ahmed, F.; Discher, D. Cell Responses to the Mechanochemical Microenvironment—Implications for Regenerative Medicine and Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-L.; Wang, J.; Zheleznyak, A.; Brown, E.J. Ubiquitin-Related Proteins Regulate Interaction of Vimentin Intermediate Filaments with the Plasma Membrane. Mol. Cell 1999, 4, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Azcutia, V.; Routledge, M.; Williams, M.R.; Newton, G.; Frazier, W.A.; Manica, A.; Croce, K.J.; Parkos, C.A.; Schmider, A.B.; Turman, M.V.; et al. CD47 Plays a Critical Role in T-Cell Recruitment by Regulation of LFA-1 and VLA-4 Integrin Adhesive Functions. Mol. Biol. Cell 2013, 24, 3358–3368. [Google Scholar] [CrossRef]
- Ticchioni, M.; Raimondi, V.; Lamy, L.; Wijdenes, J.; Lindberg, F.P.; Brown, E.J.; Bernard, A. Integrin-associated Protein (CD47/IAP) Contributes to T Cell Arrest on Inflammatory Vascular Endothelium under Flow. FASEB J. 2001, 15, 341–350. [Google Scholar] [CrossRef]
- Park, E.; Song, K.-H.; Kim, D.; Lee, M.; Van Manh, N.; Kim, H.; Hong, K.B.; Lee, J.; Song, J.-Y.; Kang, S. 2-Amino-1,3,4-Thiadiazoles as Glutaminyl Cyclases Inhibitors Increase Phagocytosis through Modification of CD47-SIRPα Checkpoint. ACS Med. Chem. Lett. 2022, 13, 1459–1467. [Google Scholar] [CrossRef]
- Marshall, B.T.; Long, M.; Piper, J.W.; Yago, T.; McEver, R.P.; Zhu, C. Direct Observation of Catch Bonds Involving Cell-Adhesion Molecules. Nature 2003, 423, 190–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; Fang, Y.; Wu, J. Prediction of Catch-Slip Bond Transition of Kindlin2/Β3 Integrin via Steered Molecular Dynamics Simulation. J. Chem. Inf. Model. 2020, 60, 5132–5141. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, X.; Lin, J.; Ling, Y.; Fang, Y.; Wu, J. MD Simulations on a Well-Built Docking Model Reveal Fine Mechanical Stability and Force-Dependent Dissociation of Mac-1/GPIbα Complex. Front. Mol. Biosci. 2021, 8, 638396. [Google Scholar] [CrossRef]
- Pietsch, E.C.; Dong, J.; Cardoso, R.; Zhang, X.; Chin, D.; Hawkins, R.; Dinh, T.; Zhou, M.; Strake, B.; Feng, P.-H.; et al. Anti-Leukemic Activity and Tolerability of Anti-Human CD47 Monoclonal Antibodies. Blood Cancer J. 2017, 7, e536. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fang, Y.; Wu, J. A Mechanism for Localized Dynamics-Driven Affinity Regulation of the Binding of von Willebrand Factor to Platelet Glycoprotein Ibα. J. Biol. Chem. 2013, 288, 26658–26667. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable Molecular Dynamics on CPU and GPU Architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]

| Number | Residue | Occupancy | ||
|---|---|---|---|---|
| CD47 | CD172a | WT | GT | |
| 1 | GLU106 | LYS53 | 0.54 ± 0.04 | 0.48 ± 0.03 |
| 2 | GLU104 | LYS96 | 0.66 ± 0.04 | 0.52 ± 0.10 |
| 3 | GLU104 | GLN52 | 0.60 ± 0.03 | 0.22 ± 0.07 |
| 4 | GLU100 | ARG69 | 0.79 ± 0.06 | 0.69 ± 0.01 |
| 5 | GLU97 | LYS96 | 0.66 ± 0.09 | 0.30 ± 0.09 |
| 6 | GLU97 | LYS53 | 0.34 ± 0.01 | 0.28 ± 0.04 |
| 7 | ASP51 | ARG95 | 0.07 ± 0.06 | 0.77 ± 0.07 |
| 8 | ASP46 | SER98 | 0.53 ± 0.06 | 0.30 ± 0.13 |
| 9 | GLU35 | ARG69 | 0.82 ± 0.01 | 0.81 ± 0.02 |
| 10 | PCA1 or GLN1 | SER66 | 0.52 ± 0.04 | 0.21 ± 0.08 |
| 11 | PCA1 or GLN1 | GLU54 | 0 | 0.08 ± 0.03 |
| 12 | PCA1 or GLN1 | GLN52 | 0 | 0.15 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fang, L.; Guo, P.; Fang, Y.; Wu, J. A MD Simulation Prediction for Regulation of N-Terminal Modification on Binding of CD47 to CD172a in a Force-Dependent Manner. Molecules 2023, 28, 4224. https://doi.org/10.3390/molecules28104224
Zhao Y, Fang L, Guo P, Fang Y, Wu J. A MD Simulation Prediction for Regulation of N-Terminal Modification on Binding of CD47 to CD172a in a Force-Dependent Manner. Molecules. 2023; 28(10):4224. https://doi.org/10.3390/molecules28104224
Chicago/Turabian StyleZhao, Yang, Liping Fang, Pei Guo, Ying Fang, and Jianhua Wu. 2023. "A MD Simulation Prediction for Regulation of N-Terminal Modification on Binding of CD47 to CD172a in a Force-Dependent Manner" Molecules 28, no. 10: 4224. https://doi.org/10.3390/molecules28104224
APA StyleZhao, Y., Fang, L., Guo, P., Fang, Y., & Wu, J. (2023). A MD Simulation Prediction for Regulation of N-Terminal Modification on Binding of CD47 to CD172a in a Force-Dependent Manner. Molecules, 28(10), 4224. https://doi.org/10.3390/molecules28104224





