Synthesis, Characterization and Biological Activities of Zinc Oxide Nanoparticles Derived from Secondary Metabolites of Lentinula edodes
Abstract
1. Introduction
2. Results
2.1. HPLC Analysis
2.2. Characterization of Nanoparticles
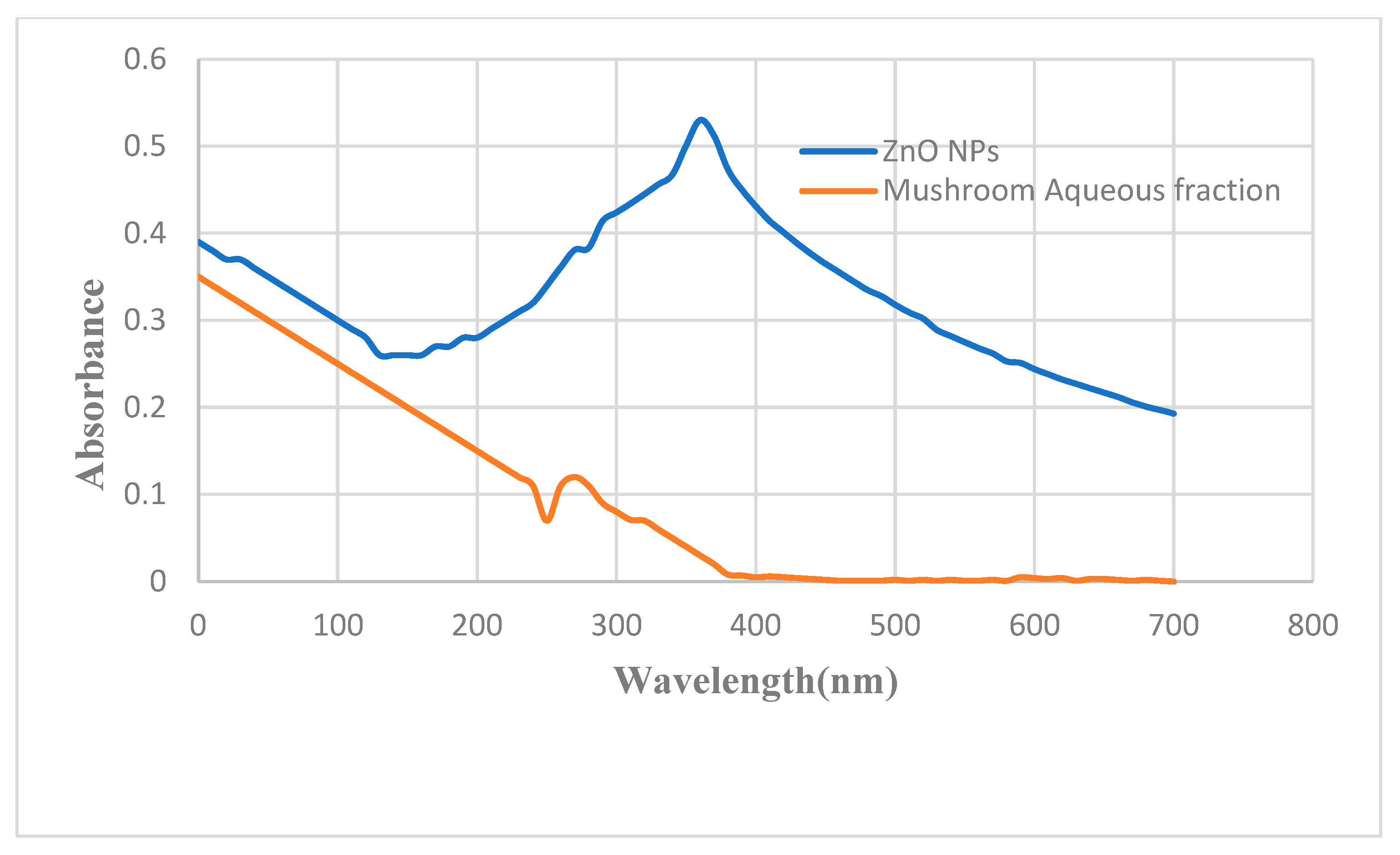
2.2.1. UV-Visible Spectroscopy
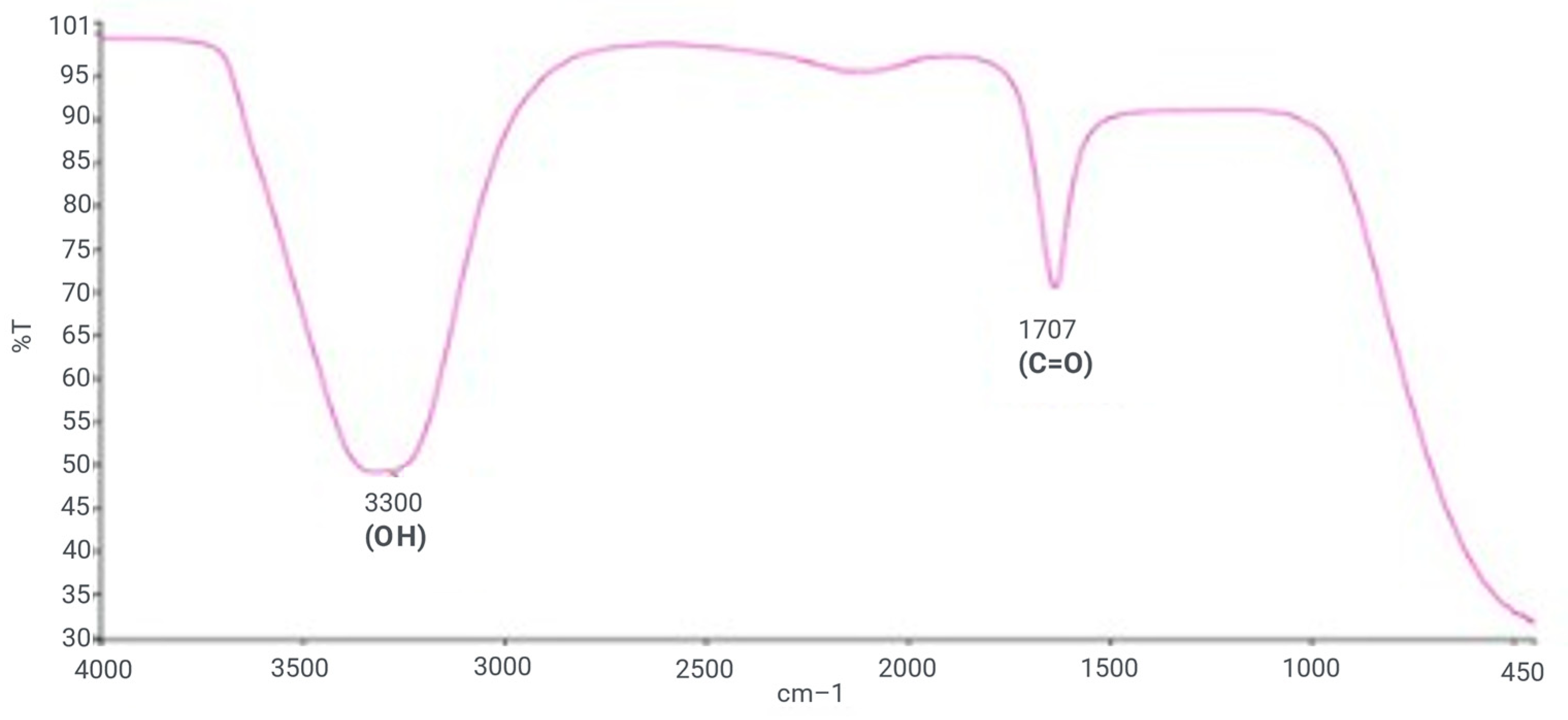
2.2.2. FTIR Analysis
2.2.3. XRD Analysis
2.2.4. SEM Analysis
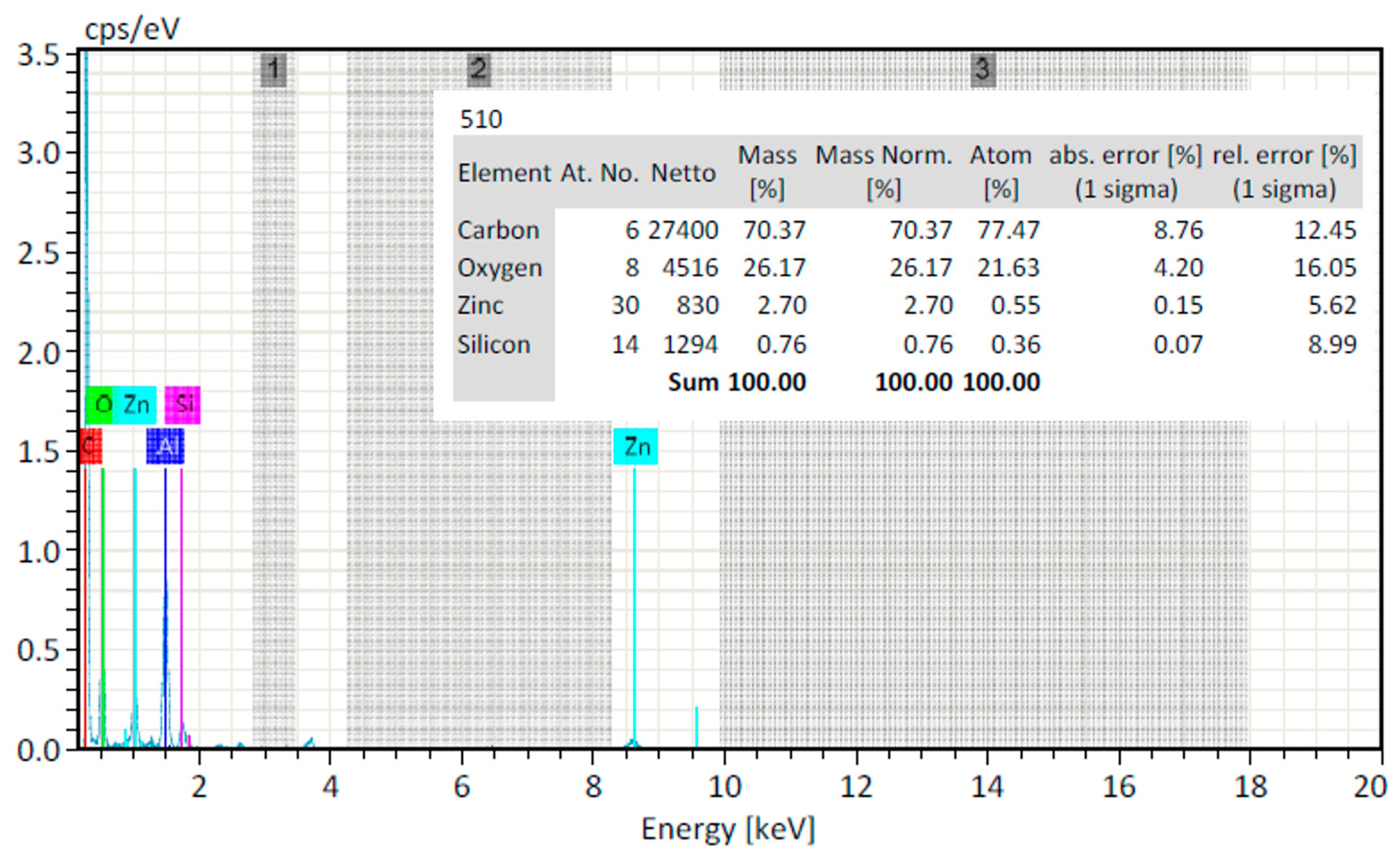
2.2.5. EDX Analysis
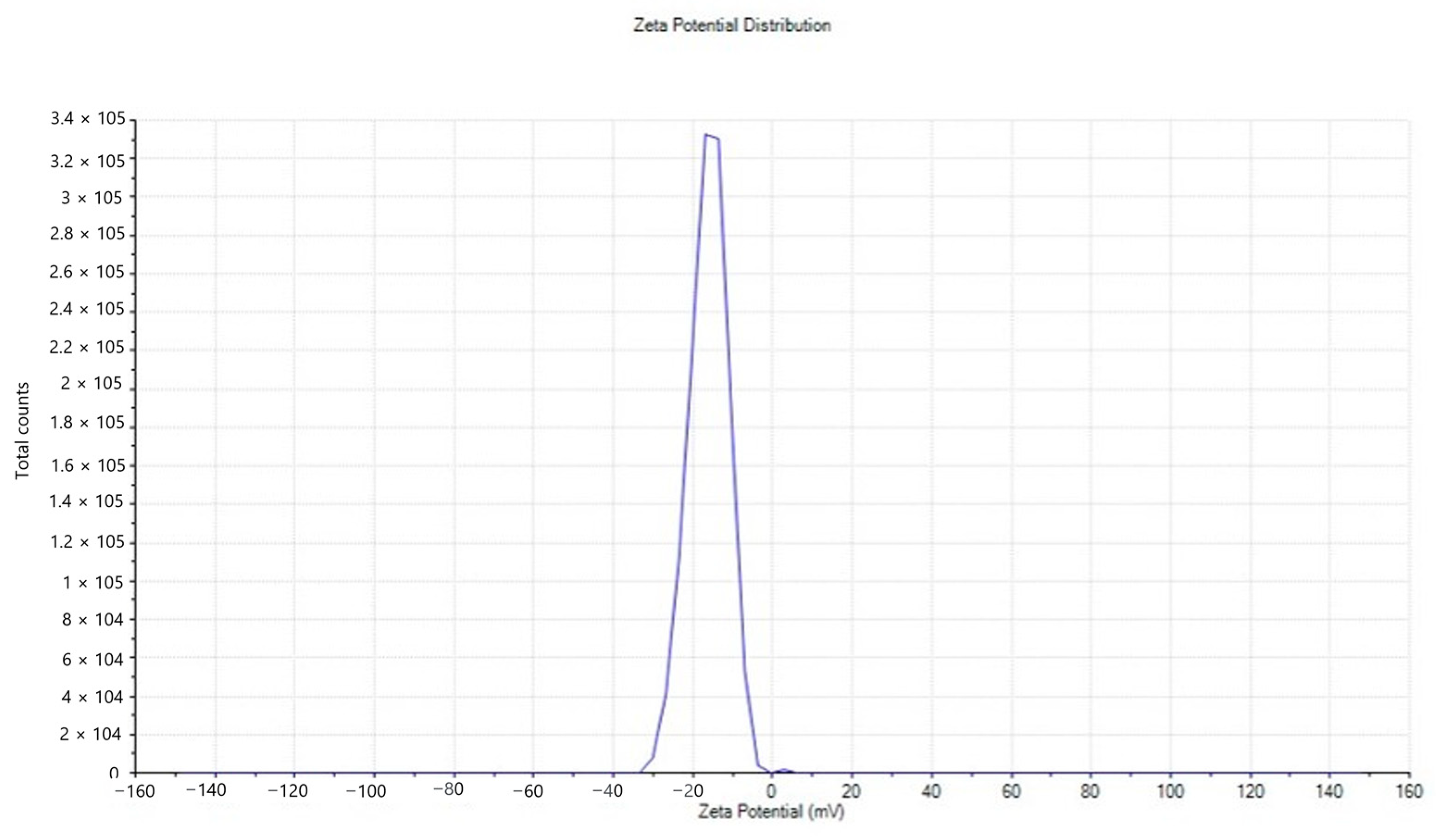
2.2.6. Zeta Potential
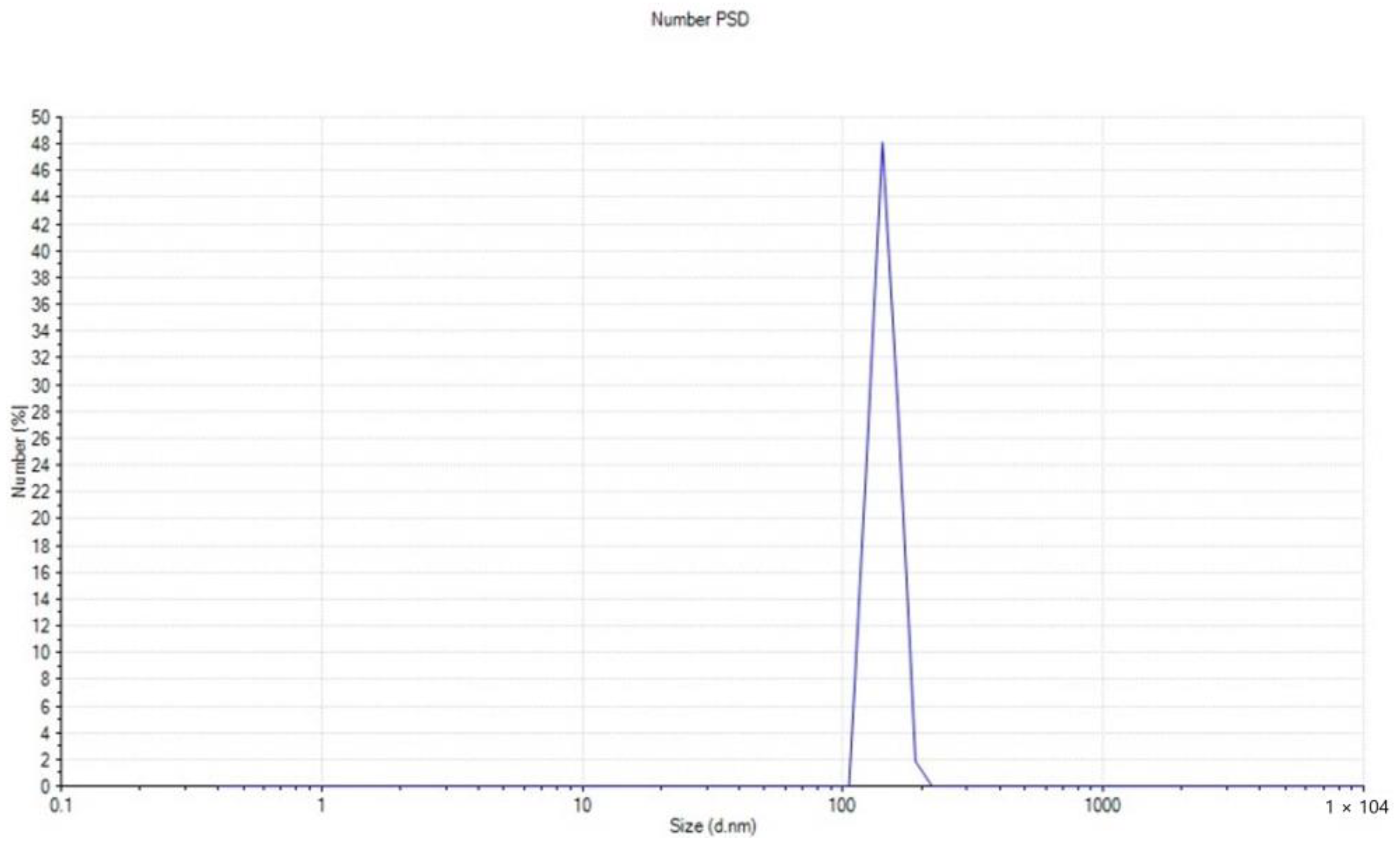
2.2.7. Size Distribution Analysis (Zeta Sizer)
2.3. Biological Activities
2.3.1. Antioxidant Potential
2.3.2. Antidiabetic Activity
2.3.3. Hemolytic Activity
2.3.4. Antibacterial Activity
2.3.5. In Vitro Anti-Inflammatory Activity
2.3.6. In Vivo Anti-Inflammatory Activity
2.3.7. In Vivo Antipyretic Activity
3. Materials and Methods
3.1. High-Performance Liquid Chromatography (HPLC)
3.2. Synthesis of ZnO NPs
3.3. Optimisation of Nanoparticles
3.4. Characterization of ZnO Nanoparticles
3.5. Biogical Activities
3.5.1. Antioxidant Potential (DPPH Assay)
3.5.2. Antidiabetic Activity
3.5.3. Hemolytic Activity
3.5.4. Anti-Bacterial Activity
3.5.5. In Vitro Anti-Inflammatory Activity
3.5.6. In Vivo Anti-Inflammatory Activity
3.5.7. Antipyretic Activity
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Chinnapaiyan, M.; Selvam, Y.; Bassyouni, F. Nanotechnology, Green Synthesis and Biological Activity Application of Zinc Oxide Nanoparticles Incorporated Argemone Mxicana Leaf Extract. Molecules 2022, 27, 1545. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green synthesis of zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 2019, 1, 117. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Petrovic, P.; Vunduk, J.; Pavlovic, V.; Van Griensven, L.J.L.D. The antimicrobial activities of silver nanoparticles synthesized from medicinal mushrooms. Int. J. Med. Mushrooms 2020, 22, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Šebesta, M.; Urík, M.; Bujdoš, M.; Kolenčík, M.; Vávra, I.; Dobročka, E.; Kim, H.; Matúš, P. Fungus Aspergillus niger processes exogenous zinc nanoparticles into a biogenic oxalate mineral. J. Fungi 2020, 6, 210. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Chen, H.-P.; Liu, J.-K. Secondary metabolites from higher fungi. Prog. Chem. Org. Nat. Prod. 2017, 106, 1–201. [Google Scholar] [PubMed]
- Stephany, M.P.; Chung, S.; Handler, M.Z.; Handler, N.S.; Handler, G.A.; Schwartz, R.A. Shiitake mushroom dermatitis: A review. Am. J. Clin. Derm. 2016, 17, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Atila, F. Cultivation and utilization of shiitake mushroom. In Medicinal Plants: Domestication, Biotechnology and Regional Importance; Springer: Berlin, Heidelberg, 2021; pp. 383–413. [Google Scholar]
- Ishikawa, N.K.; Kasuya, M.C.M.; Vanetti, M.C.D. Antibacterial activity of lentinula edodes grown in liquid medium. Braz. J. Microbiol. 2001, 32, 206–210. [Google Scholar] [CrossRef]
- Jeena, G.S.; Punatha, H.; Prakash, O.; Chandra, M.; Kushwaha, K. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian J. Nat. Prod. Radiance 2016, 5, 56–61. [Google Scholar]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Veklich, T.N. HPLC analysis of phenolic compounds in leaves and inflorescences of Sorbaria pallasii. In Proceedings of the BIO Web of Conferences, Novosibirsk, Russia, 30 September–3 October 2020; p. 00040. [Google Scholar]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Ngoepe, N.; Mbita, Z.; Mathipa, M.; Mketo, N.; Ntsendwana, B.; Hintsho-Mbita, N. Biogenic synthesis of ZnO nanoparticles using Monsonia burkeana for use in photocatalytic, antibacterial and anticancer applications. Ceram. Int. 2018, 44, 16999–17006. [Google Scholar] [CrossRef]
- Raj, L.; Jayalakshmy, E. Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiber officinale. Orient. J. Chem 2015, 31, 51–56. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Ali, T.F.S. Anti-Inflammatory Potential of Green Synthesized Silver Nanoparticles of the Soft Coral Nephthea Sp. Supported by Metabolomics Analysis and Docking Studies. Int. J. Nanomed. 2020, 15, 5345–5360. [Google Scholar] [CrossRef] [PubMed]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Synthesis, characterization and photocatalytic activity of ZnO nanoparticles prepared by biological method. Spectrochim. Acta A Mol. Biomol. 2015, 143, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Ramzan, M.; Karobari, M.I.; Heboyan, A.; Mohamed, R.N.; Mustafa, M.; Basheer, S.N.; Desai, V.; Batool, S.; Ahmed, N.; Zeshan, B. Synthesis of silver nanoparticles from extracts of wild ginger (Zingiber zerumbet) with antibacterial activity against selective multidrug resistant oral bacteria. Molecules 2022, 27, 2007. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-induced RAW 264.7 macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M. Nutritional profile of spinach and its antioxidant & antidiabetic evaluation. Int. J. Green Pharm. 2017, 11. [Google Scholar] [CrossRef]
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.A.; Shahid, M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Bairwa, K.; Jachak, S.M. Nanoparticle formulation of 11-keto-β-boswellic acid (KBA): Anti-inflammatory activity and in vivo pharmacokinetics. Pharm. Biol. 2016, 54, 2909–2916. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A. In vivo anti-inflammatory, analgesic and antipyretic activities of a medicinal plant, Caesalpinia bonducella F. Pak. J. Pharm. Sci. 2015, 28, 1517–1521. [Google Scholar] [PubMed]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Sushma, N.J.; Mahitha, B.; Mallikarjuna, K.; Raju, B. Bio-inspired ZnO nanoparticles from Ocimum tenuiflorum and their in vitro antioxidant activity. Appl. Phys. A 2016, 122, 544. [Google Scholar] [CrossRef]
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green synthesis of zinc oxide nanoparticles using pomegranate fruit peel and solid coffee grounds vs. chemical method of synthesis, with their biocompatibility and antibacterial properties investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Shameer, P.M.; Nishath, P.M. Exploration and enhancement on fuel stability of biodiesel: A step forward in the track of global commercialization. In Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–213. [Google Scholar]
- Krpetic, Z.; Scari, G.; Caneva, E.; Speranza, G.; Porta, F. Gold nanoparticles prepared using cape aloe active components. Langmuir 2009, 25, 7217–7221. [Google Scholar] [CrossRef]
- Heidari, Z.; Salehzadeh, A.; Sadat Shandiz, S.A.; Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 2018, 8, 177. [Google Scholar] [CrossRef]
- Thimmaraju, A.; Govindan, S. Novel studies of characterization, antioxidant, anticoagulant and anticancer activity of purified polysaccharide from Hypsizygus ulmarius mushroom. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100308. [Google Scholar] [CrossRef]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta A Mol. Biomol. 2015, 144, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S. Antimicrobial activity of ZnO nanoparticles against pathogenic bacteria and fungi. Sci. Med. Cent. 2015, 3, 1033. [Google Scholar]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Bayrami, A.; Parvinroo, S.; Habibi-Yangjeh, A.; Rahim Pouran, S. Bio-extract-mediated ZnO nanoparticles: Microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 730–739. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Sulaiman, S.; Ahmad, S.; Naz, S.S.; Qaisar, S.; Muhammad, S.; Ullah, R.; Al-Sadoon, M.K.; Gulnaz, A. Synthesis of zinc oxide based etoricoxib and montelukast nanoformulations and their evaluation through analgesic, anti-inflammatory, anti-pyretic and acute toxicity activities. J. King Saud Univ. Sci. 2022, 34, 101938. [Google Scholar] [CrossRef]


| Sr. No | Name of Compounds | Retention Time (min) | Area (%) | Concentration (ppm) |
|---|---|---|---|---|
| 1 | Quercetin | 3.521 | 2.53 | 45 |
| 2 | Benzoic acid | 14.847 | 3.3 | 13.239 |
| 3 | m-coumaric acid | 20.453 | 3.8 | 1.712 |
| 4 | Sinapic acid | 26.840 | 18.7 | 9.183 |
| Concentrations µg/mL | DPPH Inhibition (%) (Mean ± SD) | IC50 | Ascorbic Acid (Mean ± SD) | IC50 |
|---|---|---|---|---|
| 50 | 28.78 ± 0.54 | 29.84 | 51.15 ± 1.73 | 5.477 |
| 100 | 35.60 ± 0.64 | 61.52 ± 0.23 | ||
| 150 | 41.7 ± 0.68 | 69.03 ± 0.67 | ||
| 200 | 48.6 ± 0.80 | 75.37 ± 0.08 | ||
| 250 | 56.5 ± 0.61 | 85.11 ± 0.63 | ||
| 300 | 65.7 ± 1.09 | 91.02 ± 0.54 |
| Concentrations µg/mL | α-Amylase Inhibition (%) (Mean ± SD) | IC50 | Metformin Inhibition (%) (Mean ± SD) | IC50 |
|---|---|---|---|---|
| 50 | 44.99 ± 0.99 | 15.102 | 46.35 ± 0.96 | 12.48 |
| 100 | 50.72 ± 0.98 | 54.33 ± 0.43 | ||
| 150 | 58.57 ± 0.90 | 61.4 ± 1.09 | ||
| 200 | 67.76 ± 1.14 | 68.53 ± 0.59 | ||
| 250 | 78.07 ± 0.90 | 77.92 ± 0.39 | ||
| 300 | 85.18 ± 0.48 | 87.69 ± 1.57 |
| Sr. # | Concentrations µg/mL | Percentage Hemolysis (%) (Mean ± SD) |
|---|---|---|
| 1 | 50 | 2.19 ± 0.34 |
| 2 | 100 | 2.64 ± 0.98 |
| 3 | 150 | 3.52 ± 0.60 |
| 4 | 200 | 4.21 ± 1.14 |
| 5 | 250 | 4.74 ± 0.70 |
| 6 | 300 | 6.90 ± 0.06 |
| 7 | 0.1% Titron X | 96.60 ± 0.10 |
| 8 | PBS | 2.31 ± 0.002 |
| Sr. # | Microorganism | Zone of Inhibition in mm Concentration µg | |||
|---|---|---|---|---|---|
| A 500 | B 750 | C 1000 | D 1500 | ||
| 1 | Staphylococcus aureus | 9 | 11 | 12 | 14 |
| 2 | Klebsiella pneumoniae | 8 | 10 | 13 | 15 |
| 3 | Escherichia coli | 8 | 9 | 9 | 10 |
| Sr. # | Concentrations µg/mL | % Denaturation Proteins (Mean ± SD) | IC50 | Dicloran Inhibition (%) (Mean ± SD) | IC50 |
|---|---|---|---|---|---|
| 1 | 50 | 50.04 ± 0.44 | 6.74 | 54.76 ± 0.57 | 4.97 |
| 2 | 100 | 58.17 ± 0.37 | 57.76 ± 2.01 | ||
| 3 | 150 | 65.22 ± 0.81 | 65.76 ± 1.18 | ||
| 4 | 200 | 72.69 ± 0.43 | 73.76 ± 0.94 | ||
| 5 | 250 | 79.99 ± 0.43 | 81.76 ± 1.56 | ||
| 6 | 300 | 86.45 ± 0.60 | 89.76 ± 0.86 |
| Sample Fraction | Dose mg/kg Body Weight | Change in Paw Thickness (mm) 0 h | 1 h | 2 h | 3 h | 4 h |
|---|---|---|---|---|---|---|
| Nanoparticles | 6 | 1.31 ± 0.05 18.75% | 1.41 ± 0.04 25.39% | 1.51 ± 0.03 31.36% | 1.62 ± 0.03 42.14% | 1.31 ± 0.03 53.73% |
| 8 | 1.21 ± 0.03 28.9% | 1.3 ± 0.06 35% | 1.34 ± 0.02 36.19% | 1.51 ± 0.06 46.07% | 1.1 ± 0.03 60.99% | |
| 10 | 1.12 ± 0.04 34.91% | 1.31 ± 0.02 37.61% | 1.42 ± 0.05 40.83% | 1.46 ± 0.04 47.85% | 1.1 ± 0.06 63.21% | |
| Neg Control | 6 | 1.38 ± 0.04 30% | 1.47 ± 0.03 1% | 1.57 ± 0.04 2% | 1.65 ± 0.04 15% | 1.64 ± 0.03 0% |
| 8 | 1.41 ± 0.02 40% | 1.52 ± 0.06 2% | 1.63 ± 0.03 3% | 1.72 ± 0.04 1% | 1.73 ± 0.03 0% | |
| 10 | 1.25 ± 0.05 3% | 1.35 ± 0.03 1% | 1.45 ± 0.04 3% | 1.52 ± 0.03 1% | 1.53 ± 0.06 0% | |
| Diclofenac Sodium Standard | 6 | 1.12 ± 0.03 31 | 1.31 ± 0.04 45% | 1.41 ± 0.02 58% | 1.53 ± 0.02 61% | 1.2 ± 0.02 70% |
| 8 | 1.14 ± 0.02 35% | 1.21 ± 0.06 47% | 1.32 ± 0.03 56% | 1.41 ± 0.04 67% | 1.13 ± 0.03 75% | |
| 10 | 1.25 ± 0.05 38% | 1.35 ± 0.03 45% | 1.45 ± 0.04 57% | 1.56 ± 0.03 66% | 1.21 ± 0.06 76% |
| Drug | Dose mg/kg Body Weight | Temperature Before Yeast Injection (F°) | 0 h | 1 h | 2 h | 3 h | 4 h |
|---|---|---|---|---|---|---|---|
| Nanoparticles | 6 | 97.5 | 101.2 ± 0.37 | 99.7 ± 0.34 | 98.6 ± 0.02 | 97.6 ± 0.37 | 97.3 ± 0.5 |
| 8 | 97.4 | 101 ± 0.31 | 99.5 ± 0.41 | 98.4 ± 0.7 | 97.3 ± 0.22 | 97.3 ± 0.4 | |
| 10 | 98.1 | 100.3 ± 0.41 | 99.3 ± 0.05 | 98.1 ± 0.3 | 97.3 ± 0.4 | 97.4 ± 0.51 | |
| Negative Control | 97.6 | 100.1±0.51 | 100.2 ± 0.5 | 100.2 ± 0.51 | 100.2 ± 0.02 | 101.1 ± 0.4 | |
| Paracetamol Standard | 6 | 97.6 | 100.1 ± 0.32 | 98.4 ± 0.4 | 97.9 ± 0.21 | 97.8 ± 0.03 | 97.7 ± 0.3 |
| 8 | 98.6 | 100 ± 0.31 | 98.3 ± 0.3 | 97.5 ± 0.5 | 97.7 ± 0.04 | 97.6 ± 0.4 | |
| 10 | 98.4 | 100.1 ± 0.41 | 98.1 ± 0.3 | 97.5 ± 0.46 | 97.6 ± 0.02 | 97.6 ± 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, Z.S.; Afzal, M.; Ahmad, J.; Ahmed, N.; Zeshan, B.; Hashim, N.H.H.N.; Yean, C.Y. Synthesis, Characterization and Biological Activities of Zinc Oxide Nanoparticles Derived from Secondary Metabolites of Lentinula edodes. Molecules 2023, 28, 3532. https://doi.org/10.3390/molecules28083532
Amin ZS, Afzal M, Ahmad J, Ahmed N, Zeshan B, Hashim NHHN, Yean CY. Synthesis, Characterization and Biological Activities of Zinc Oxide Nanoparticles Derived from Secondary Metabolites of Lentinula edodes. Molecules. 2023; 28(8):3532. https://doi.org/10.3390/molecules28083532
Chicago/Turabian StyleAmin, Zeemal Seemab, Muhammad Afzal, Jamshaid Ahmad, Naveed Ahmed, Basit Zeshan, Nik Haszroel Hysham Nik Hashim, and Chan Yean Yean. 2023. "Synthesis, Characterization and Biological Activities of Zinc Oxide Nanoparticles Derived from Secondary Metabolites of Lentinula edodes" Molecules 28, no. 8: 3532. https://doi.org/10.3390/molecules28083532
APA StyleAmin, Z. S., Afzal, M., Ahmad, J., Ahmed, N., Zeshan, B., Hashim, N. H. H. N., & Yean, C. Y. (2023). Synthesis, Characterization and Biological Activities of Zinc Oxide Nanoparticles Derived from Secondary Metabolites of Lentinula edodes. Molecules, 28(8), 3532. https://doi.org/10.3390/molecules28083532











