Abstract
In the current article, the effect of Si/Al ratio on the NOx adsorption and storage capacity over Pd/Beta with 1 wt% Pd loading was investigated. The XRD, 27Al NMR and 29Si NMR measurements were used to determine the structure of Pd/Beta zeolites. XAFS, XPS, CO-DRIFT, TEM and H2-TPR were used to identify the Pd species. The results showed that the NOx adsorption and storage capacity on Pd/Beta zeolites gradually decreased with the increase of Si/Al ratio. Pd/Beta-Si (Si-rich, Si/Al~260) rarely has NOx adsorption and storage capacity, while Pd/Beta-Al (Al-rich, Si/Al~6) and Pd/Beta-C (Common, Si/Al~25) exhibit excellent NOx adsorption and storage capacity and suitable desorption temperature. Pd/Beta-C has slightly lower desorption temperature compared to Pd/Beta-Al. The NOx adsorption and storage capacity increased for Pd/Beta-Al and Pd/Beta-C by hydrothermal aging treatment, while the NOx adsorption and storage capacity on Pd/Beta-Si had no change.
1. Introduction
NOx is an important precursor for ozone and haze pollution, which is harmful to natural environment and human health [1,2]. Most of the NOx in the atmosphere comes from mobile sources, so it is very important to control the NOx emission of diesel exhaust. At present, the most widely used and commercialized technology for removing NOx from diesel vehicle exhaust is ammonia selective catalytic reduction (NH3-SCR) technology. However, NH3-SCR technology only has excellent removal efficiency of NOx at high temperatures above 200 °C, while the NOx reduction efficiency is extremely low when the exhaust temperature is below 200 °C [3,4,5]. With the increasingly stringent emissions regulations in future, controlling NOx emissions from diesel vehicle exhaust during cold-start period is a considerable challenge due to the low temperature of the exhaust [6]. Therefore, the passive NOx adsorber (PNA) technology came into being. The PNA technology can trap NOx at low temperatures and release it at 200–500 °C, which is the temperature window of NH3-SCR catalyst working efficiently [7,8,9]. The desorbed NOx is further removed by the downstream SCR catalyst, thus achieving the purpose of efficiently removing NOx at low temperatures.
The most widely used PNA materials can be divided into two types. One is Pd dispersed on oxides (CeO2, Al2O3), but the weak hydrothermal stability and sulfur resistance limit their application [9,10,11,12,13]. In the past years, Pd/zeolites with various framework structures were paid much attention as PNA materials due to the large NOx adsorption and storage capacity, appropriate desorption temperature and better resistance to hydrothermal aging and sulfur resistance [9,14,15,16,17,18].
The pore size and structure have significant influences on the PNA performance and stability of Pd/zeolites. Chen et al. investigated the PNA performance of Pd/BEA, Pd/MFI, Pd/CHA and Pd/CeO2 materials and found that Pd/BEA zeolites showed the largest NOx storage capacity regardless of sulphation [9]. Further, Khivantsev et al. established the structure-storage property relationships of Pd/BEA zeolites as PNA materials [19]. They uncovered the inhibition effect of H2O and promotion effect of CO on PNA storage of Pd/BEA zeolites due to the formation of Pd(II)(NO)(CO) species. Pace et al. compared the distribution of Pd species in Pd/Beta and Pd/CHA zeolites under various pretreatment conditions and found that ionic Pd2+ in Pd/Beta is less stable and easy to form either Pd metal or PdO particles [20].
The Si/Al ratios of zeolite significantly influence the metal active sites and framework structure. Many researchers have proved that Pd2+ is the active site to store NOx in Pd/zeolite that is mainly riveted on the framework Al. Therefore, the Si/Al ratio of zeolite has a significant impact on dispersion of Pd2+ [21]. The Al-rich zeolite favors the presence of highly dispersed Pd ions compared to the zeolite with high Si/Al ratio [22]. However, the structure of Al-rich zeolite is less stable. Zhao et al. reported that Pd/SSZ-13 with Si/Al ratio of 6 is easier to dealuminate at high temperatures than Pd/SSZ-13 with Si/Al ratio of 13, which damages the zeolite structure and leads to lower PNA activity [21]. In addition, the Si/Al ratio can also influence the NOx desorption property of PNA materials. Mihai et al. found that Pd/Beta with a SiO2/Al2O3 ratio of 38 and 300 have two NOx desorption peaks in the absence of CO at 100 °C and 180 °C, which is relatively low for NH3-SCR catalyst [23]. Nevertheless, Pd/Beta with a low SiO2/Al2O3 ratio of 25 have an additional desorption peak, despite only a small amount of NOx release, in the absence of CO at 250 °C, where the downstream SCR catalyst can significantly decrease the NOx [23]. In the presence of CO, the Pd/BEA with a SiO2/Al2O3 ratio of 25 and 38 showed an intensified NOx desorption peak at 250–350 °C, while the Pd/BEA with a SiO2/Al2O3 ratio of 300 only showed a NOx desorption peak at 100–200 °C.
To sum up, Pd/BEA zeolite is a great potential candidate as PNA material due to its large NOx adsorption and suitable desorption temperature as well as its economy and accessibility [9,20,24]. However, the Pd2+ species on Pd/BEA zeolites were less stable, resulting in weak hydrothermal stability, especially compared to the Pd/CHA zeolite with a small pore structure. Therefore, this work mainly concentrated on the framework and Pd speciation stability of Pd/Beta zeolites. The Pd-loaded Al-rich, common, and Si-rich Beta zeolites with Si/Al ratios of 6, 25 and 260 were selected as PNA materials before and after the hydrothermal aging treatment. The adsorption and storage amounts, desorption temperature and Pd species and framework structure were systematically investigated.
2. Results and Discussion
2.1. NOx Adsorption/Storage Performance on Pd/Beta Zeolites with Various Si/Al Ratios
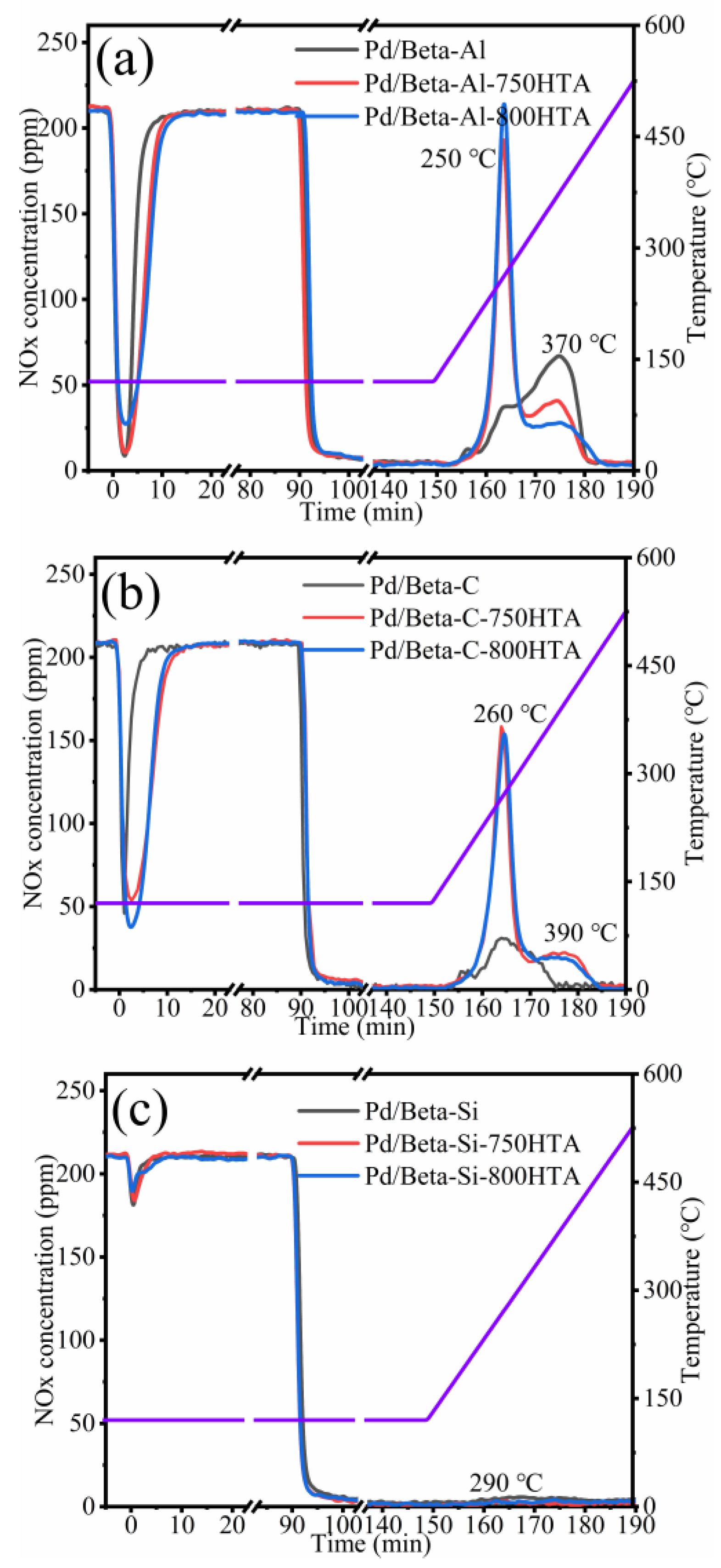
Figure 1 depicts the NOx adsorption and storage profiles of Pd/Beta zeolites with various Si/Al ratios. The NOx adsorption/storage amounts and absolute content of NOx/Pd (the ratio of the NOx storage amount and Pd loading) were listed in Table 1. The Pd/Beta-Al zeolite shows a rapid adsorption capacity in the first 3 min and levels off at 20 min. The NOx storage amount of Pd/Beta-Al zeolite is up to 65.0 umol/g. In the desorption temperature range, two peaks at 250 °C and 370 °C were observed, which located in the ideal operation window of Cu-zeolite NH3-SCR catalysts [4,25,26,27]. NO was the main desorption species, while the content of NO2 was lower (Figure S1a). Moreover, the Pd/Beta-Al showed NOx/Pd storage of 0.69. This indicated that 69% of Pd species exist as isolated Pd2+, corroborating reports that dispersed Pd2+ ion species are active sites for NOx adsorption [20]. The other two samples, however, only showed that 27% and 3% of Pd species are isolated Pd2+, indicating the severe accumulation of Pd species.
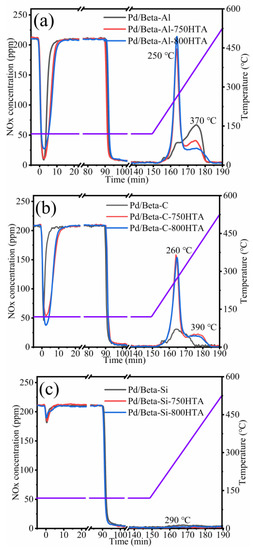
Figure 1.
The NOx adsorption and storage capacity of (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; (c) Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA. Feed: 200 ppm NO; 200 ppm CO, 5%H2O, 10%O2 and N2 balance.

Table 1.
The calculation of NOx adsorption/desorption capacity of Pd/Beta with different Si/Al ratios Feed: 200 ppm NO; 200 ppm CO, 5%H2O, 10%O2 and N2 balance.
After hydrothermal aging treatment, the NOx adsorption and desorption amount both increased clearly for Pd/Beta-Al. In addition, the adsorption time to saturation of hydrothermally aged samples is similar to the fresh ones, indicating an increasing adsorption rate of NOx. The NOx/Pd increased from 0.69 to 0.92 and 0.96 (Table 1) after hydrothermal aging treatment at 750 °C and 800 °C, respectively. This demonstrated that Pd species redispersed into ionic Pd2+ during hydrothermal aging treatment. Moreover, the desorption peak at 250 °C significantly intensified, accompanying the decrease of the desorption peak at 370 °C, again indicating the redistribution of Pd species. This is a beneficial change for diesel aftertreatment system, since 250 °C is sufficiently high for the SCR catalyst to efficiently eliminate NOx. Moreover, the Pd/Beta-Al can be easily recovered and adsorb NOx at low temperatures again.
The Si/Al ratio has a significant impact on the activity and hydrothermal stability of Pd/Beta zeolites as PNA materials. The NOx adsorption and storage capacity on Pd/Beta zeolites gradually decreased with the increase of Si/Al ratio. The desorption temperature also shifted to high temperatures with the increase of the Si/Al ratio. The Pd/Beta-C with a medium Si/Al ratio of 25 showed a lower adsorption ability and similar variation tendency after hydrothermal aging treatment compared to Pd/Beta-Al materials, with the result shown in Figure 1b. The NOx/Pd increased from 0.27 to 0.75 and 0.82 after hydrothermal aging at 750 °C and 800 °C treatment, respectively, which indicated that the redistribution of Pd species can also occur on the Pd/Beta-C zeolites. On the other hand, too high of a Si/Al ratio of Pd/Beta led to the deactivation of PNA performance regardless of hydrothermal aging, with the result shown in Figure 1c. Only a slight desorption peak of NOx was observed at 290 °C for fresh Pd/Beta-Si zeolite. Next, the zeolite framework structure and Pd active species were analyzed.
2.2. The Structure Analysis of Pd/Beta Zeolites
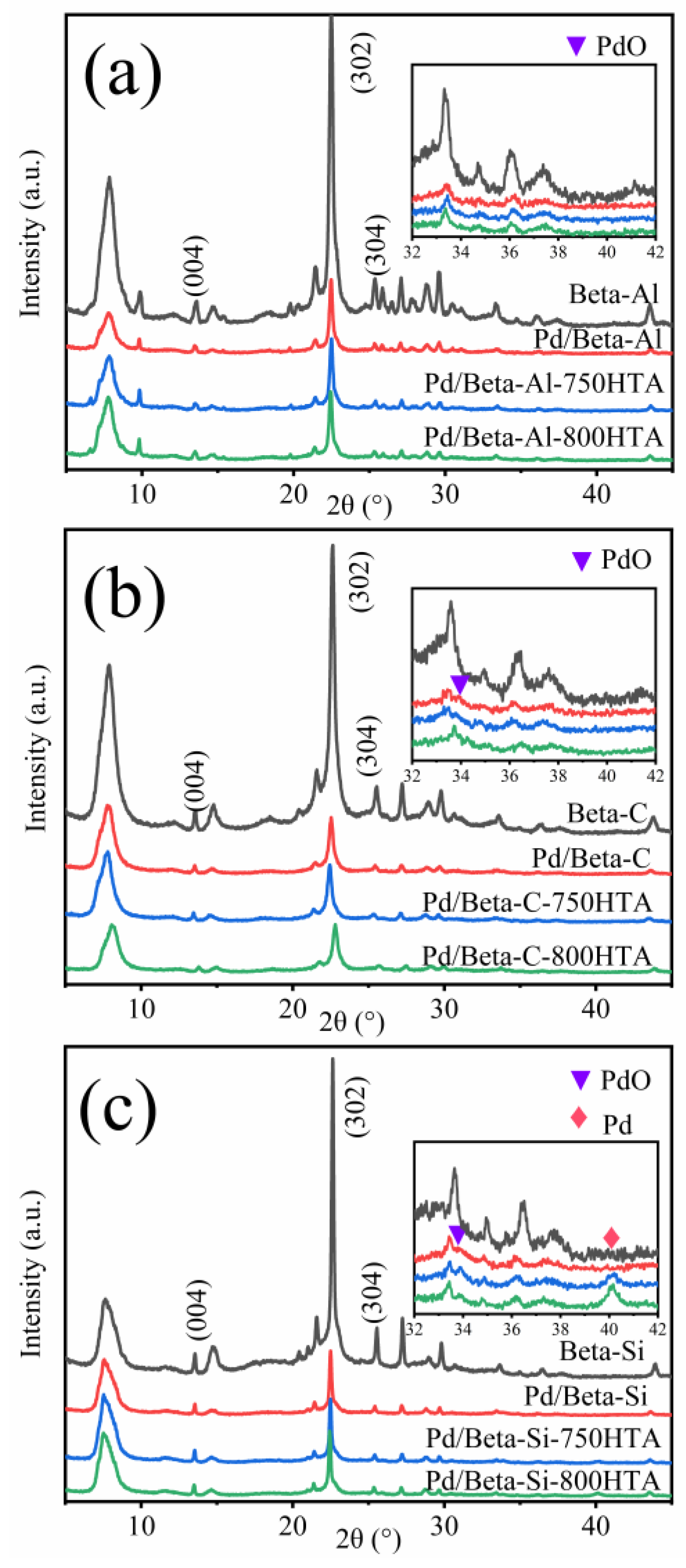
To understand the crystallinity and structure of the zeolite, XRD characterization was carried out as shown in Figure 2. For the fresh Pd/Beta-Al zeolite, the diffraction peaks at 7.7°, 13.4°, 21.5°, 22.5°, 25.4°, 26.8°, 29.6° and 43.5° were corresponding to pure Beta phase crystallization [28,29,30]. Among them, the diffraction peaks at 13.4°, 22.5° and 25.4° were ascribed to (004), (302) and (304) crystal planes, respectively [31]. The weak signal at 22.5–32.5° appeared, which may be due to amorphous phase (non-framework Al species) [9]. The Pd/Beta zeolites with different Si/Al ratio have excellent crystallinity, which shows that Si/Al ratio has little effect on the crystallinity. After hydrothermal aging at 750 °C and 800 °C treatment, the crystallinity of Beta zeolites with different Si/Al ratios scarcely changed, indicating the high stability of BEA zeolite framework structure. For the identification of Pd species, diffraction peaks at 33.8° and 40.2° were observed, corresponding to the (101) crystal plane of PdO and (111) crystal plane of metal Pd, respectively [32,33,34]. For fresh and hydrothermally aged Pd/Beta-Al zeolites, no characteristic diffraction peaks of PdO and Pd species are observed, indicating the high disperse of Pd species regardless of hydrothermal aging treatment. For the Pd/Beta-C zeolite, only a slight diffraction peak of PdO was observed, and the peak intensity decreased after hydrothermal aging treatment, indicating redisperse of Pd species. However, besides the observation of the PdO diffraction peak, the diffraction peak, attributed to metal Pd, also appeared for the hydrothermally aged Pd/Beta-Si zeolites. This indicated Pd species were more facile to accumulate on the Si-rich Beta zeolite. Moreover, with progressively hydrothermal aging, the peak of metal Pd intensified accompanying the decrease of the peak intensity that represented the PdO species. This demonstrated that the accumulated PdO species turned into metal Pd rather than Pd2+ species due to the few ion exchange sites in the Si-rich Beta zeolite.
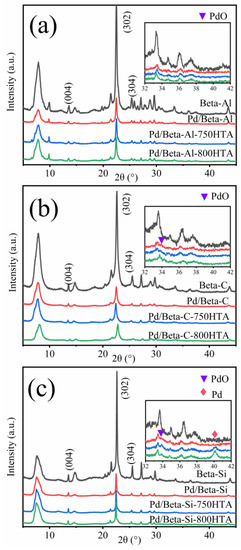
Figure 2.
XRD patterns of (a) Beta-Al, Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Beta-C, Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; and (c) Beta-Si, Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA.
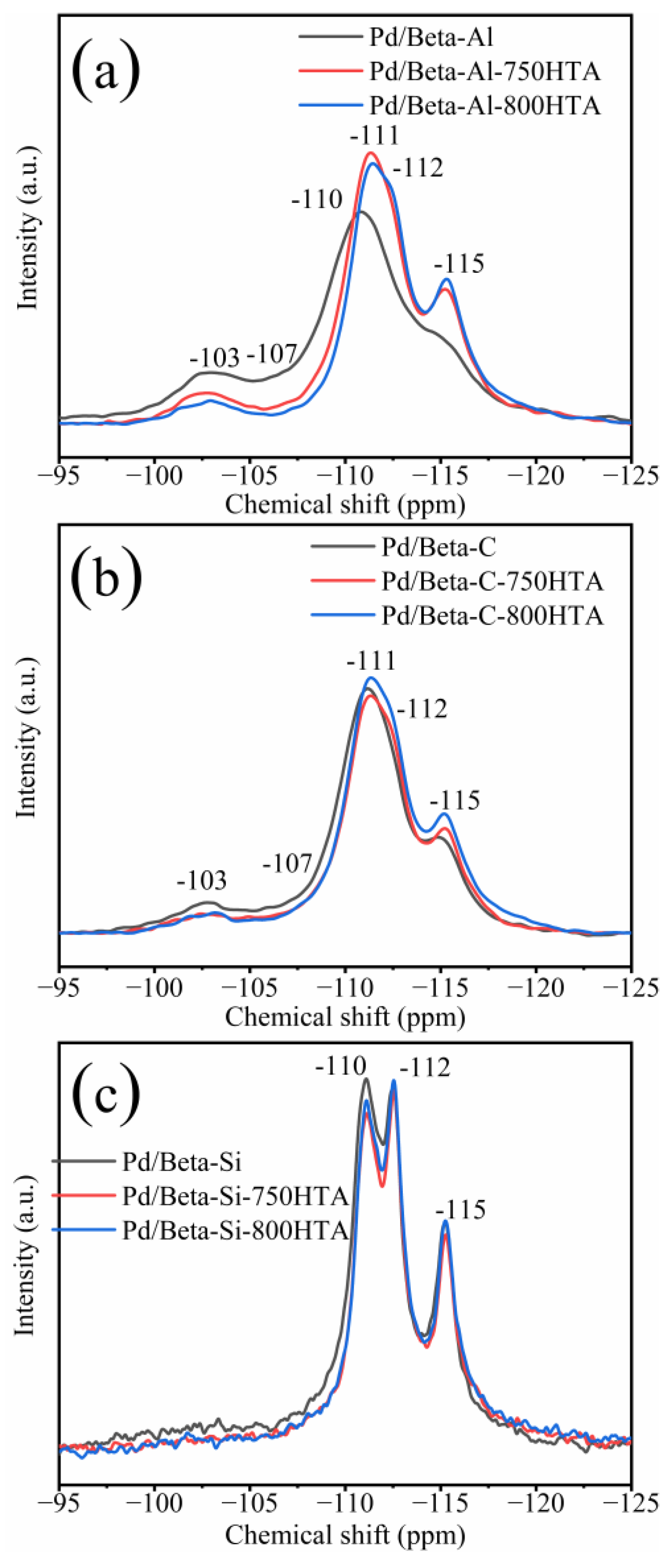
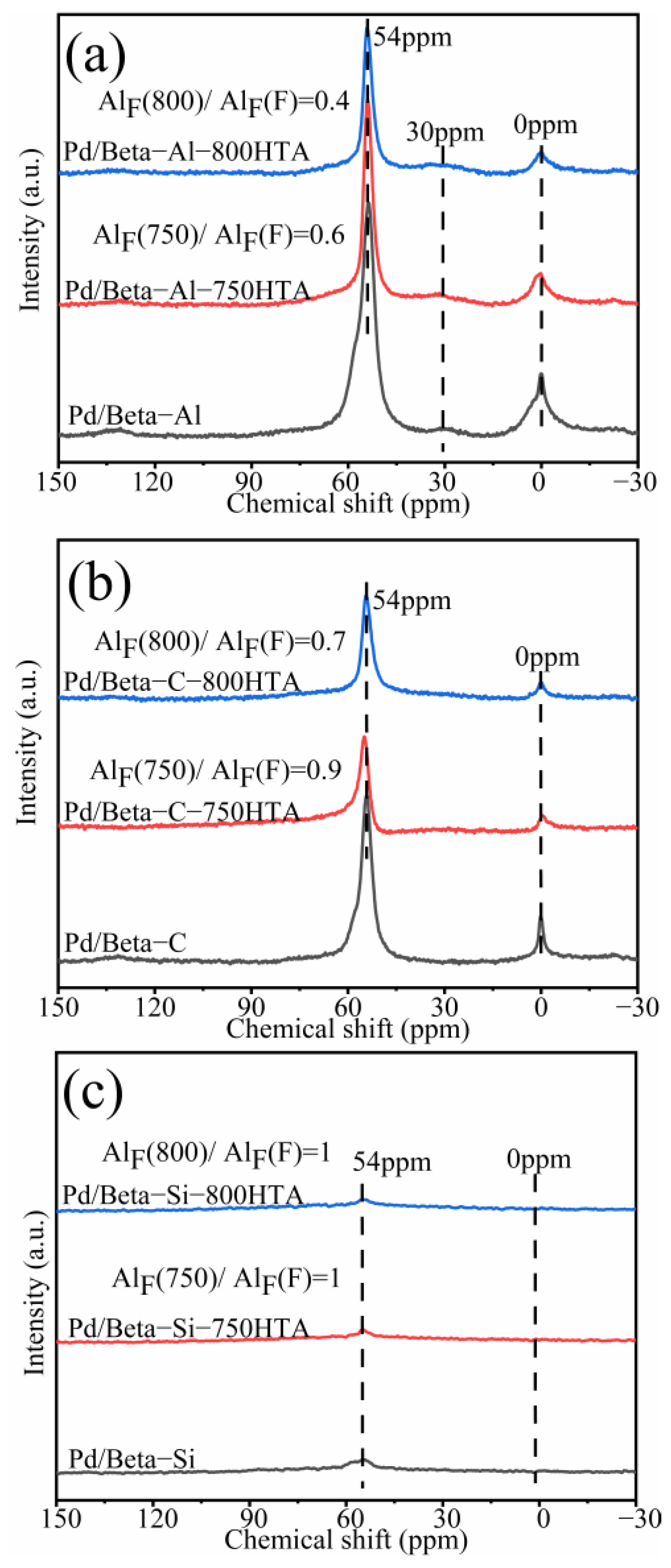
In addition to XRD characterization of the zeolite framework, NMR was also used to characterize the local framework Si and Al atoms of zeolite. Figure 3 shows the 29Si NMR profiles of Pd/Beta zeolites, and the deconvolution diagram of 29Si NMR is shown in Figure S2. The peaks with chemical shifts at −115 ppm, −112 ppm and −110 ppm were assigned to Si (4Si) species [35,36,37]. The peak with chemical shifts at −103 ppm was assigned to Si (3Si, 1Al) or Si (3Si, 1OH) species [35,36,37]. Obviously, dealumination occurred and intensified with progressively hydrothermal aging for Pd/Beta-Al as indicated by the decrease of the peak at −103 ppm. Nevertheless, it should be noted that the hydrothermal aging treatment did not lead to deterioration in PNA activity, which may be attributed to the fact that the strong interaction between the framework and Pd ions’ coordinated Al sites can survive from dealumination [7]. The similar results are also observed in the Pd/Beta-C zeolite but the dealumination is weaker. For the Pd/Beta-Si zeolite, however, there only weak dealumination occurs due to the few framework Al sites. The results of 27Al NMR measurements were consistent with 29Si NMR results as shown in Figure 4. Pd/Beta with different Si/Al ratios show resonances at chemical shifts of 0 ppm and 54 ppm, which are attributed to the octahedral extra-framework Al and tetrahedral Al in the framework, respectively [38,39]. An additional peak was also observed, which was attributed to pentahedral Al [19]. With the decrease of Si/Al ratio, dealumination occurred more easily during hydrothermal aging. For example, 40% of framework Al was reserved for Pd/Beta-Al, while 70% of framework Al was reserved for the Pd/Beta-C zeolite, and there is no obvious change of framework Al peak for the Pd/Beta-Si zeolite after hydrothermal aging at 800 °C treatment. Attentionally, some framework Al suffered from hydrothermal aging and favored the Pd distribution, which may be the reason that the Pd/Beta-Al zeolite behaved the best in PNA performance and hydrothermal stability despite severe dealumination. We further characterized the Pd species to prove this inference.
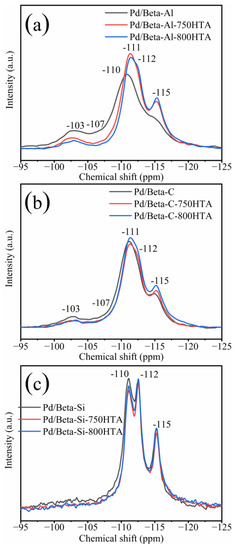
Figure 3.
29Si NMR spectra for the (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; and (c) Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA.
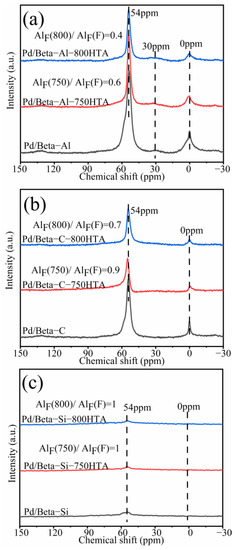
Figure 4.
27Al NMR spectra for the (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; (c) Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA.
2.3. The Analysis of Pd Species in Pd/Beta Zeolites
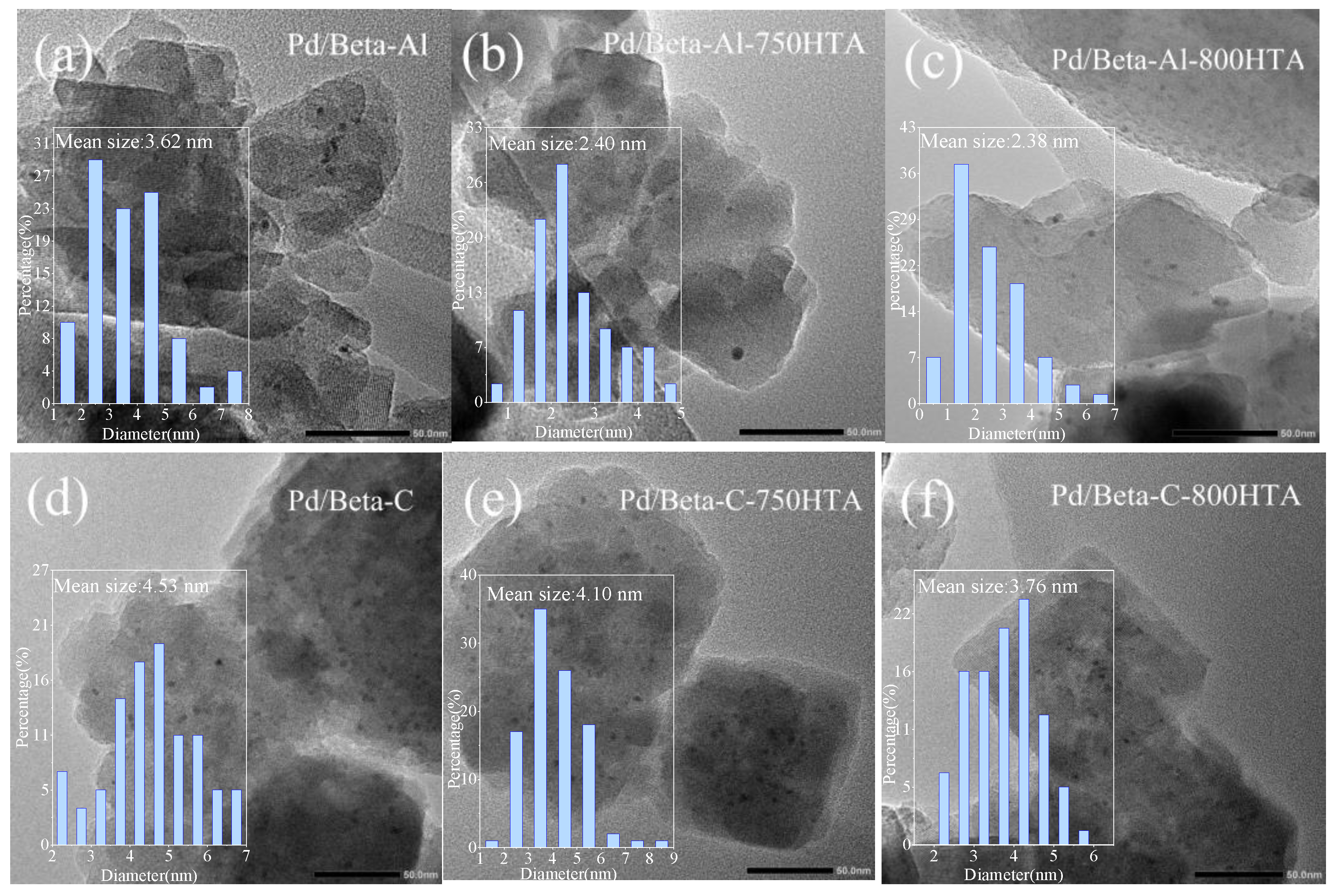
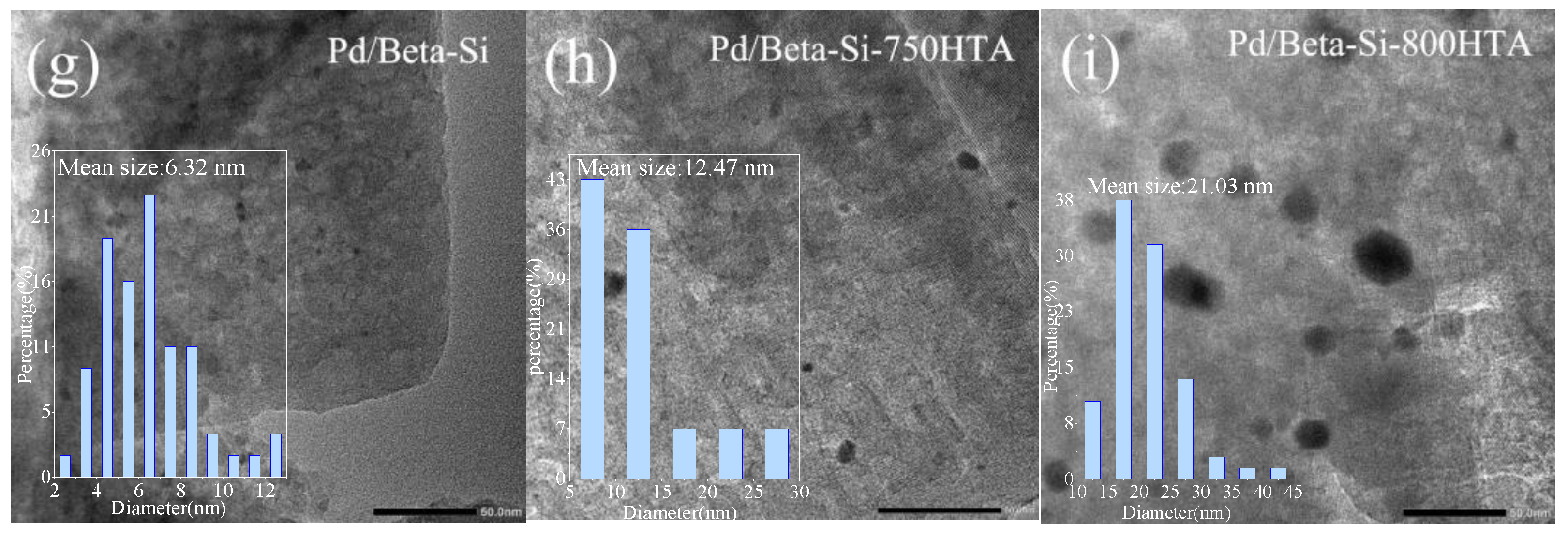
TEM measurement was first used to observe the particle size and distribution of Pd species in Pd/Beta zeolites with the result shown in Figure 5. For Pd/Beta-Al, the Pd species were homogeneously and highly distributed due to the observation of only sporadic particles. The average size of the observed particle was only about 3.6 nm and minified with progressively hydrothermal aging, indicating the high dispersion of Pd species regardless of hydrothermal aging. Compared to Pd/Beta-Al zeolite, more particles were observed on the Pd/Beta-C and Pd/Beta-Si zeolites with the average size of about 4.5 nm and 6.3 nm, respectively. This suggest that increasing the Si/Al ratio led to the accumulation of Pd species. Differently, the Pd/Beta zeolites with a medium Si/Al ratio (Pd/Beta-C) showed a decrease in particle size after the hydrothermal aging treatment, indicating the dispersion of Pd species. However, obvious agglomeration of Pd species occurred on Pd/Beta-Si zeolites after hydrothermal aging treatment. The particle size even grew to about 12 nm and 21 nm for Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA samples, respectively, indicating the significant accumulation of Pd species. This can explain why the NOx storage amounts of Pd/Beta-Al and Pd/Beta-C increased, while that of Pd/Beta-Si decreased after hydrothermal aging treatment.
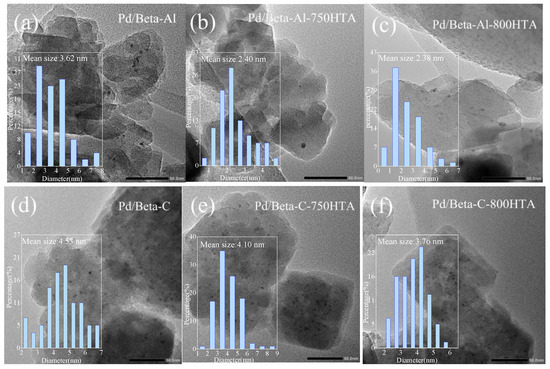
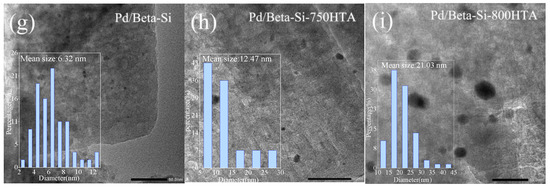
Figure 5.
TEM images of (a) Pd/Beta-Al, (b) Pd/Beta-Al-750HTA, (c) Pd/Beta-Al-800 HTA, (d) Pd/Beta-C, (e) Pd/Beta-C-750HTA, (f) Pd/Beta-C-800 HTA, (g) Pd/Beta-Si, (h) Pd/Beta-Si-750HTA and (i) Pd/Beta-Si-800 HTA.
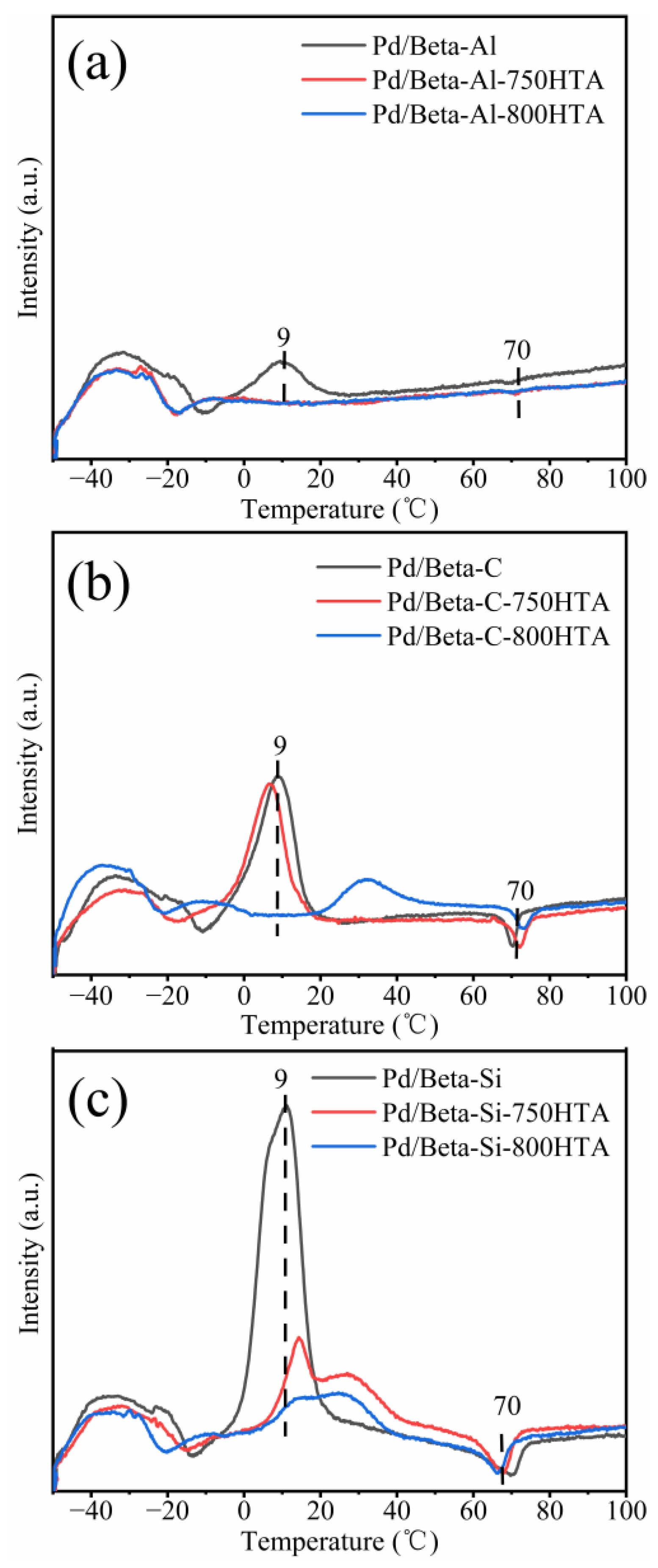
Furthermore, the Pd species on Pd/Beta zeolites with different Si/Al ratios were identified by H2-TPR measurement (Figure 6). In the temperature range of 0–50 °C, a H2 consumption peak appeared, which was attributed to the reduction of PdO species [15,40,41]. A negative peak at about 70 °C was attributed to decomposition of Pd hydride species [31,42]. For the fresh Pd/Beta-Al, the peak of PdO at 9 °C was observed and disappeared after the hydrothermal aging treatment, which again indicated that PdO was highly dispersed after the hydrothermal aging treatment. For the Pd/Beta-C zeolite, the peak at 9 °C was stronger than that of Pd/Beta-Al, suggesting the presence of more PdO species. Hydrothermal aging treatment at 750 °C also decreased the amounts of PdO species, which was similar to Pd/Beta-Al zeolites. Moreover, after the hydrothermal aging treatment at 800 °C, the temperature of PdO reduction shifted to higher temperature, also indicating the redispersion of Pd species as well as increased stability of PdO species. For the fresh Pd/Beta-Si zeolites, on the other hand, a large amount of PdO species were observed. After hydrothermal aging treatments at 750 °C and 800 °C, although the peak representing PdO species also decreased, this rarely meant high dispersion of Pd species due to the observation of a large-size particle in Figure 5i. Instead, the PdO species were reduced during hydrothermal aging due to the weak nature of ion exchange sites, and some Pd0 species were formed as indicated by the observation of a Pd0 diffraction peak in Figure 2c.
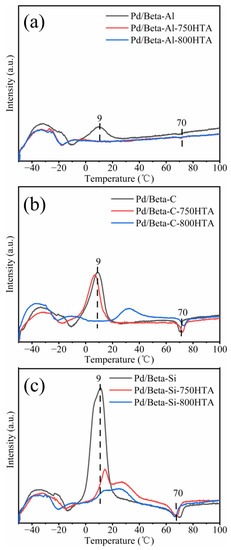
Figure 6.
H2-TPR profiles of (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; (c) Pd/Beta-Si, Pd/Beta-Si-750HTA andPd/Beta-Si-800HTA HTA.
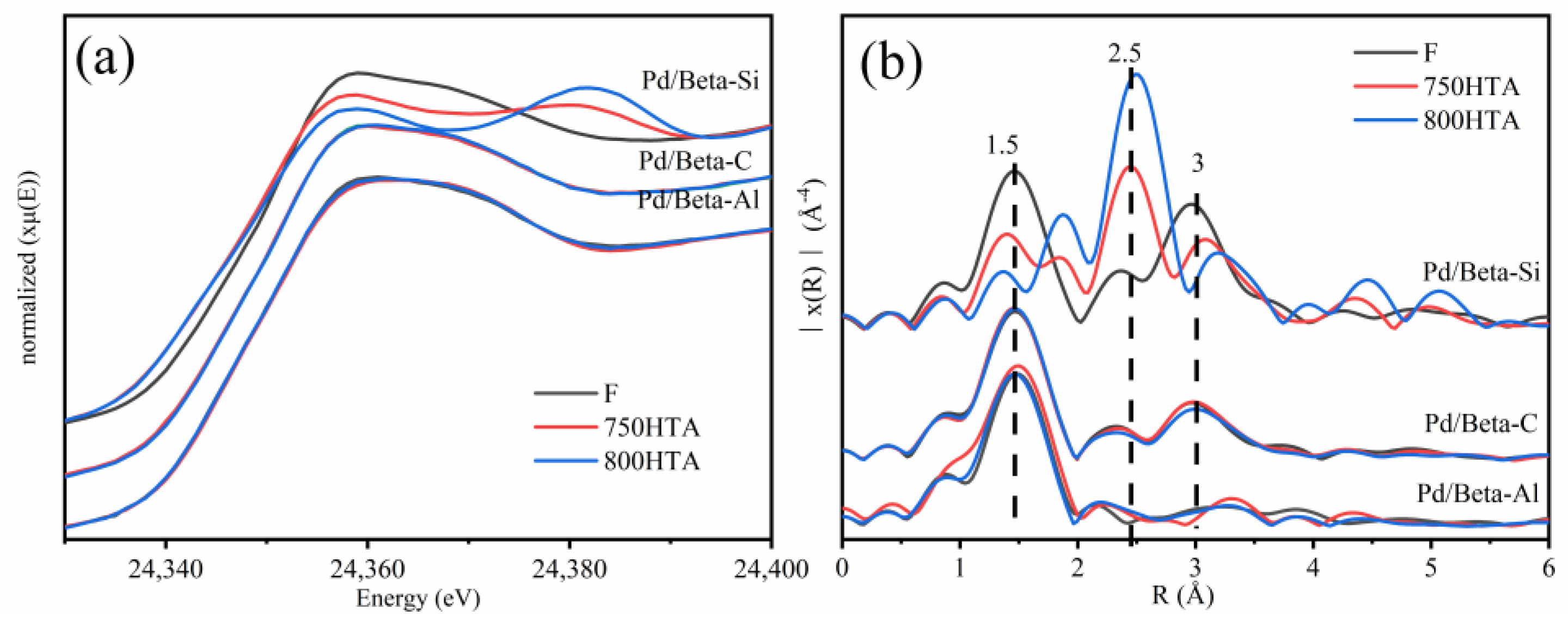
The effect of the Si/Al ratio on the local structure and state of Pd species for Pd/Beta zeolites was further investigated by X-ray adsorption fine structure (XAFS) (Figure 7). Pd K-edge X-ray absorption near-edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS) spectra of PdO and Pd foil are listed in Figure S3. For XANES, the profiles were almost coincident before and after hydrothermal aging for the Pd/Beta-Al and Pd/Beta-C zeolites and resembled the profiles of reference PdO, indicating that Pd species existed in the form of PdO clusters or Pd2+ [9]. The results of EXAFS (Figure 7b) further indicated that the primary Pd species were the Pd2+ species coordinated with the zeolite framework for Pd/Beta-Al zeolites due to the absence of second scattering peak of Pd-Pd at ~3.0 Å [29,43]. However, for the Pd/Beta-C, PdO species existed as seen by the appearance of the second scattering peak at ~3.0 Å. The XANES and EXAFS profiles of Pd/Beta-Si zeolite were strikingly distinctive from the other two zeolites. With progressively hydrothermal aging, the XANES profile was closer to that of metallic Pd reference. The dramatical increase of the peak at ~2.5 Å (metal Pd-Pd) and decrease of the peak at ~1.5 Å (Pd-O) in EXAFS profiles also indicated the transformation from PdO into metallic Pd species during hydrothermal aging for Pd/Beta-Si [44,45,46].
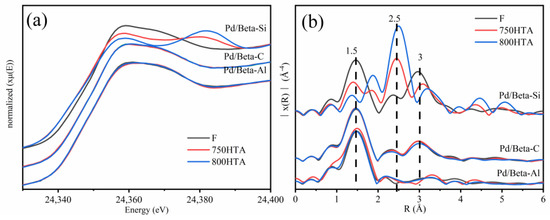
Figure 7.
(a) Pd K-edge XANES spectra and (b) Fourier transforms of the k3-weighted Pd K-edge EXAFS of Pd1/Beta.
The ionic Pd2+ were also verified by the DFIFTS measurement using CO as a probe molecule, with the result shown in Figure S4. All adsorbents showed distinct peaks in the range of 2215–2097 cm−1, ascribed to CO adsorbed on the Pdn+ (n = 0–3) ions, which indicated that isolated Pd exits at the exchange sites of the zeolite [9,47]. The peaks in the range of 2215–2097 cm−1 decreased with the increase of the Si/Al ratio, which indicated that the exchanged Pd ions were decreasing with the increase of the Si/Al ratio. Moreover, the hydrothermally aged Pd/Beta-Al and Pd/Beta-C also showed more intensified peaks than the fresh ones. This demonstrated the exchanged Pd ions species raised after hydrothermal aging.
Additionally, the in situ DRIFTS measurement was conducted after NO + O2 adsorption as shown in Figure S5. The peaks at 1570–1700 cm−1 were attributed to polydentate nitrate species on the zeolite framework [38]. The peaks at 1869 cm−1 and 1827 cm−1 were related to Pd(II)-NO and Pd(II)(NO)(CO) species, respectively [38]. The Pd/Beta-Al zeolites showed the largest NOx adsorption regardless of hydrothermal aging. However, the hydrothermally aged Pd/Beta-Si showed scarce NOx adsorption despite only a little of NOx adsorption on Pd(II) ions on the fresh one. This indicated that Pd ions easily accumulated and were reduced to metallic Pd on Beta-Si zeolite during hydrothermal aging, which is consistent with TEM and XANES results.
3. Materials and Methods
3.1. Preparation of PNA Materials
Commercial H-Beta with specific Si/Al ratios of 6, 25 and 260, which were determined by inductively coupled plasma-optical emission spectroscopy (OPTIMA 8300), were written as Beta-Al, Beta-C and Beta-Si, respectively.
Pd/Beta zeolites were obtained by common impregnation method using Palladium (II) nitrate dihydrate (Sigma) as the Pd precursor. The Pd loading of all the adsorbent was 1 wt.%. The definite preparation method is as follows. First, 0.25 g Palladium (II) nitrate dihydrate was put into 600 mL deionized water and stirred for 10 min at room temperature. Second, 10.00 g of Beta was added, and the solution continued to be stirred for 2 h at room temperature. Third, the solution was transferred to a rotary evaporation flask with temperature of 80 °C for drying. The obtained sample was placed in an oven and dried for 12 h at 70 °C. Finally, the sample was calcined in a muffle furnace at 600 °C for 5 h with a heating rate of 5 °C/min. A synthetic path description diagram is shown in Figure S6.
In order to explore the hydrothermal stability of the adsorbent, the prepared adsorbent was hydrothermally aged at 750 °C and 800 °C for 16 h in 10% H2O/air, denoted as Pd/Beta-750HTA and Pd/Beta-800HTA, respectively.
3.2. Characterization of PNA Materials
The test of NOx adsorption and storage performance on Pd/Beta zeolites was carried out on a self-built fixed reaction bed under a simulate exhaust condition. Mass flow controllers were used to control the flow rate of gas and the temperature is controlled by a K-type thermocouple. The Pd/Beta zeolite particle of 150 mg with 40–60 mesh was placed in a quartz tube (inner diameter of the quartz tube is 4 mm), and both ends of the Pd/Beta were blocked by quartz wool, in which case the sample was not blown into the detector. Pd/Beta were pretreated at 500 °C for 60 min with the feed atmosphere of 5% H2O, 10% O2/N2, following by dropping the temperature to 120 °C to collect background before testing. After deducting the background, 210 ppm NOx (200 ppm NO, ~10 ppm NO2) and 200 ppm CO were introduced for isothermal adsorption at 120 °C. In order to understand more clearly the desorption ability of the adsorbent, we turned off NOx and purged for 1 h at 120 °C before desorption. Finally, the temperature was raised to 520 °C with a heating rate of 10 °C/min for desorption process and the feed atmosphere was 5% H2O, 10% O2/N2. The Antaris™ IGS analyzer (Thermofisher Scientific, Waltham, MA, USA) was used to measure the concentration of the gas during the reaction.
X-ray diffraction (XRD) measurement was used to understand the crystallinity and structure of the zeolite. It was carried out in a Bruker D8 advance diffractometer (Bruker Corporation, Karlsruhe, Germany) with Cu kα as the light source. The scanning range was 4–45° and the step length was 0.02°.
A field emission transmission electron microscope (FETEM) (JEOL, Tokyo, Japan) was used to understand the morphology and the dispersion of Pd of the adsorbent. For analysis, the catalyst was dispersed over the channel and photographed with a camera.
Temperature-programmed reduction of hydrogen (H2-TPR) was used to understand the Pd species of the adsorbent. The experiment was carried out on a Micromeritics AutoChem 2920 chemisorption instrument (Micromeritics, GA, USA). The 50 mg adsorbent was placed into a U-tube and pretreated in O2/Ar atmosphere for 60 min at 500 °C. The temperature was reduced to −50 °C by liquid nitrogen injection followed by sweeping with Ar for 30 min. The temperature was controlled by programmed heating. Then, 10% H2/Ar was introduced into the atmosphere after the baseline was stable. The Pd species of the adsorbent were determined by recording the TCD signal.
Diffuse reflection infrared Fourier transform spectroscopy (DRIFTS) with CO as a probe was used to understand the Pd species, which was carried out on a Nicolet IS50 Fourier infrared spectrometer (Thermofisher Scientific, Waltham, MA, USA) equipped with an MCT detector. The adsorbent, ground into fine powder, was put into the sample pool and heated to 500 °C through the heating module at a heating rate of 10 °C/min. After Pd/Beta zeolite was pretreated at 500 °C for 60 min in the air inlet atmosphere of 10% O2 and N2, the adsorbent was cooled to room temperature. It stayed at room temperature for 50 min to collect background data. After deducting the corresponding temperature background, 200 ppm CO was injected, and the surface species were recorded at this time.
Pd K-edge X-ray absorption fine structure (XAFS) was carried out on beamline BL14W1 at Shanghai synchrotron radiation facility using XAFS beams Si (111) monochromatic light mirrors. Pd foil was used as the energy calibration, and PdO was used as the standard sample. The X-ray absorption near-edge structure (XANES) data were background-corrected and normalized using the Athena module implemented in the IFFEFIT software package [48].
27Al-nuclear magnetic resonance (27Al NMR) and 29Si-nuclear magnetic resonance (29Si NMR) values were collected on a nuclear magnetic resonance spectrometer (JEOL, Tokyo, Japan) equipped with a 3.2 mm MAS probe at a mass frequency of 12 KHz, which aimed to determine the framework Si and Al in the zeolite. The sampling times were 128 and 700 scans, respectively, and the relaxation delay was 5 s.
X-ray photoelectron spectroscopy (XPS) spectra were collected from the XPS spectrometer (Thermofisher Scientific, Waltham, MA, USA) to determine the valence states of Pd and the proportion of each valence state.
Diffuse reflection infrared Fourier transform spectroscopy (DRIFTS) was used to understand the NOx adsorption and storage mechanism of adsorbent, which was carried out on a Fourier infrared spectrometer (Thermofisher Scientific, Waltham, MA, USA) equipped with an MCT detector. Pd/Beta zeolite was pretreated at 500 °C for 40 min in the atmosphere of 10% O2 and N2 followed by reducing the temperature to 150 °C. After cooling down, it stayed at 150 °C for 50 min to collect background data. After deducting the background, the isothermal adsorption was performed at 150 °C with the feed atmosphere of 500 ppm NO, 200 ppm CO and 10% O2 and N2.
4. Conclusions
Pd/Beta-Al has excellent NOx adsorption and desorption capacity, suitable desorption temperature and perfect hydrothermal stability, which shows that it has great potential as a PNA candidate. Increasing the Si/Al ratio contributed to the formation of PdO, which led to the decrease of NOx adsorption and storage capacity of fresh Pd/Beta zeolites. The desorption temperature also increases with the increase of the Si/Al ratio regardless of hydrothermal aging. After hydrothermal aging treatment, however, the NOx adsorption–desorption capacity of Pd/Beta-Al and Pd/Beta-C significantly increased, which resulted from the redispersion of Pd species. The strong interaction with Pd ions and coordinated Al sites can survive from dealumination of Al-rich Beta zeolite. Hydrothermal aging treatment led to the formation of metal Pd0 species for Pd/Beta-Si zeolite, which made it an inappropriate candidate as a PNA material. The results inspired us to use Pd/Beta with a low Si/Al ratio as a PNA material candidate.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28083501/s1. Figure S1. The NO and NO2 storage capacity of (a) Pd/Beta-Al, (b) Pd/Beta-Al-750HTA, (c) Pd/Beta-Al-800 HTA, (d) Pd/Beta-C, (e) Pd/Beta-C-750HTA, (f) Pd/Beta-C-800 HTA, (g) Pd/Beta-Si, (h) Pd/Beta-Si-750HTA and (i) Pd/Beta-Si-800 HTA. Figure S2. 29Si NMR for the (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta-C, Pd/Beta-C-750HTA and Pd/Beta-C-800HTA; and (c) Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA HTA. Figure S3. Pd K-edge (a) XANES spectra and (b) EXAFS spectra of PdO and Pd foil. Figure S4. CO-FTIR patterns of (a) Pd/Beta-Al, Pd/Beta-Al-750HTA and Pd/Beta-Al-800HTA; (b) Pd/Beta, Pd/Beta-750HTA and Pd/Beta-800HTA; and (c) Pd/Beta-Si, Pd/Beta-Si-750HTA and Pd/Beta-Si-800HTA. Figure S5. DRIFT spectra of (a) Pd/Beta-Al, Pd/Beta-C and Pd/Beta-Si; (b) Pd/Beta-Al-750HTA, Pd/Beta-C-750HTA and Pd/Beta-Si-750-HTA; and (c) Pd/Beta-Al-800HTA, Pd/Beta-C-800HTA and Pd/Beta-Si-800-HTA. Figure S6. The synthetic path description diagram of Pd/Beta.
Author Contributions
Conceptualization, Q.W. and Y.S.; Software, Y.S.; Data curation, S.H. and Z.L.; Writing—original draft, S.H.; Writing—review and editing, S.H. and Y.S.; Supervision, Q.W., Y.S., X.S., Z.L. and H.H.; Funding acquisition, Y.S. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (U1810209, 52270112, 52225003, 22076206).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the funding agencies, and the authors would like to acknowledge the beamline BL14W1 (SSRF) for performing XAFS experiments.
Conflicts of Interest
The authors have no conflict of interest.
Sample Availability
Samples are available from the authors.
References
- Jin, Q.; Shen, Y.S.; Zhu, S.M. Effect of fluorine additive on CeO2(ZrO2)/TiO2 for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2017, 487, 401–409. [Google Scholar] [CrossRef]
- Li, D.; Meng, Y.; Hao, D.P.; Ding, Q.Z.; Pang, L.; Yang, G.J.; Guo, Y.B.; Yu, J.H.; Li, T. Deactivation of Pd/SSZ-13 passive NOx adsorber from the perspectives of phosphorus poisoning and hydrothermal aging. Chem. Eng. J. 2022, 446, 136779. [Google Scholar] [CrossRef]
- Yao, D.; Ilmasani, R.F.; Wurzenberger, J.C.; Thomas Glatz, T.; Han, J.; Ho, P.H.; Creaser, D.; Olsson, L. Insight into CO induced degradation mode of Pd/SSZ-13 in NOx adsorption and release: Experiment and modeling. Chem. Eng. J. 2022, 439, 135714. [Google Scholar] [CrossRef]
- Shan, Y.L.; Du, J.P.; Zhang, Y.; Shan, W.P.; Shi, X.Y.; Yu, Y.B.; Zhang, R.D.; Meng, X.J.; Xiao, F.S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Cheng, S.Y.; Kang, T.F.; Dai, H.X. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Theis, J.R.; Lambert, C.K. An assessment of low temperature NOx adsorbers for cold-start NOx control on diesel engines. Catal. Today 2015, 258, 367–377. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, Y.; Hwang, S.; Lee, H.; Kim, C.H.; Kim, D.H. Improving NOx storage and CO oxidation abilities of Pd/SSZ-13 by increasing its hydrophobicity. Appl. Catal. B Environ. 2020, 277, 119190. [Google Scholar] [CrossRef]
- Chen, H.Y.; Mulla, S.; Weigert, E.; Camm, K.; Ballinger, T.; Cox, J.; Blakeman, P. Cold start concept (CSC™): A novel catalyst for cold start emission control. SAE Int. J. Fuels Lubr. 2013, 6, 372–381. [Google Scholar] [CrossRef]
- Chen, H.Y.; Collier, J.E.; Liu, D.X.; Mantarosie, L.; Duran-Martin, D.; Novak, V.; Rajaram, R.R.; Thompsett, D. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Lee, H.; Kim, C.H.; Kim, D.H. Low temperature NO adsorption over hydrothermally aged Pd/CeO2 for cold start application. Catal. Today 2018, 307, 93–101. [Google Scholar] [CrossRef]
- Ji, Y.Y.; Bai, S.L.; Crocker, M. Al2O3-based passive NOx adsorbers for low temperature applications. Appl. Catal. B Environ. 2015, 170, 283–292. [Google Scholar] [CrossRef]
- Jones, S.; Ji, Y.Y.; Crocker, M. Ceria-based catalysts for low temperature NOx storage and release. Catal. Lett. 2016, 146, 909–917. [Google Scholar] [CrossRef]
- Cao, Y.D.; Ran, R.; Wu, X.D.; Zhao, B.H.; Wan, J.; Weng, D. Comparative study of ageing condition effects on Pd/Ce0.5Zr0.5O2 and Pd/Al2O3 catalysts: Catalytic activity, palladium nanoparticle structure and Pd-support interaction. Appl. Catal. A Gen. 2013, 457, 52–61. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Engelhard, M.H.; Wang, Y.; Wang, Y.; Gao, F.; Szanyi, J. Low-temperature Pd/zeolite passive NOx adsorbers: Structure, performance, and adsorption chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Investigation of the active sites and optimum Pd/Al of Pd/ZSM–5 passive NO adsorbers for the cold-start application: Evidence of isolated-Pd species obtained after a high-temperature thermal treatment. Appl. Catal. B Environ. 2018, 226, 71–82. [Google Scholar] [CrossRef]
- Khivantsev, K.; Wei, X.; Kovarik, L.; Jaegers, N.R.; Walter, E.D.; Tran, P.; Wang, Y.; Szanyi, J. Palladium/Ferrierite versus Palladium/SSZ-13 passive NOx adsorbers: Adsorbate-controlled location of atomically dispersed Palladium(II) in Ferrierite determines high activity and stability. Angew. Chem. Int. Ed. Engl. 2022, 61, 07554. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Gu, Y.; Majumdar, S.S.; Pihl, J.A.; Epling, W.S. Investigation of NO adsorption and desorption phenomena on a Pd/ZSM-5 passive NOx adsorber. Appl. Catal. B Environ. 2021, 298, 120561. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Prodinger, S.; Derewisnki, M.; Wang, Y.; Gao, F.; Szanyi, J. Palladium/Beta zeolite passive NOx adsorbers (PNA): Clarification of PNA chemistry and the effects of CO and zeolite crystallite size on PNA performance. Appl. Catal. A Gen. 2019, 569, 141–148. [Google Scholar] [CrossRef]
- Pace, R.B.; Lardinois, T.M.; Ji, Y.Y.; Gounder, R.; Heintz, O.; Crocker, M. Effects of treatment conditions on Pd speciation in CHA and Beta zeolites for passive NOx adsorption. ACS Omega 2021, 6, 29471–29482. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Bhat, A.; Li, Y.; Schwank, J.W. Insight into hydrothermal aging effect on deactivation of Pd/SSZ-13 as low-temperature NO adsorption catalyst: Effect of dealumination and Pd mobility. Appl. Catal. B Environ. 2021, 286, 119874. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hanson, J.C.; Tao, F.F.; Tang, Y.; Zhang, X.; Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N. Achieving atomic dispersion of highly loaded transition metals in small-pore zeolite SSZ-13: High-capacity and high-efficiency low-temperature CO and passive NOx adsorbers. Angew. Chem. Int. Ed. 2018, 130, 16672–16677. [Google Scholar] [CrossRef] [PubMed]
- Oana, M.; Lidija, T.; Travis, W.; Fluxa, T.F.; Louise, O. The effect of Si/Al ratio for Pd/BEA and Pd/SSZ-13 used as passive NOx adsorbers. Top. Catal. 2018, 61, 2007–2020. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Chen, Z.X.; Wang, J.Q.; Shen, M.Q. Improvement of NOx uptake/release over Pd/Beta by propylene: Shielding effect of intermediates on adsorbed NOx species. Phys. Chem. Chem. Phys. 2021, 23, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.M.; Lezcano-Gonzalez, I.; Slawinksic, W.A.; Wragg, D.S. Correlation between Cu ion migration behaviour and deNOx activity in Cu-SSZ-13 for the standard NH3-SCR reaction. Chem. Commun. 2016, 52, 6170–6173. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Pedenc, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Khivantsev, K.; Gao, F.; Kovarik, L.; Wang, Y.; Szanyi, J. Molecular level understanding of how oxygen and carbon monoxide improve NOx storage in Palladium/ SSZ-13 passive NOx adsorbers: The role of NO+ and Pd(II)(CO)(NO) species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Gu, Y.; Zelinsky, R.P.; Chen, Y.R.; Epling, W.S. Investigation of an irreversible NOx storage degradation model on a Pd/BEA passive NOx adsorber. Appl. Catal. B Environ. 2019, 258, 118032. [Google Scholar] [CrossRef]
- Zhang, J.W.; Maximov, A.L.; Bai, X.F.; Wang, W.; Xiao, L.F.; Lin, H.L.; Wu, W. Shape selectivity in hydroisomerization of n-Hexadecane over Pd supported on zeolites: ZSM-22, ZSM-12 and Beta. Russ. J. Appl. Chem. 2020, 93, 1427–1437. [Google Scholar] [CrossRef]
- Bello, E.; Margarit, V.J.; Gallego, E.M.; Schuetze, F.; Hengst, C.; Corma, A.; Moliner, M. Deactivation and regeneration studies on Pd-containing medium pore zeolites as passive NOx adsorbers (PNAs) in cold-start applications. Microporous Mesoporous Mater. 2020, 302, 110222. [Google Scholar] [CrossRef]
- Shan, Y.L.; Sun, Y.; Li, Y.B.; Shi, X.Y.; Shan, W.P.; Yu, Y.B.; He, H. Passive NO adsorption on hydrothermally aged Pd-based small-pore zeolites. Top. Catal. 2020, 63, 944–953. [Google Scholar] [CrossRef]
- Ryou, Y.S.; Lee, J.; Kim, Y.; Hwang, S.; Lee, H.; Kim, C.H.; Kim, D.H. Effect of reduction treatments (H2 vs. CO) on the NO adsorption ability and the physicochemical properties of Pd/SSZ-13 passive NOx adsorber for cold start application. Appl. Catal. A Gen. 2019, 569, 28–34. [Google Scholar] [CrossRef]
- Yao, D.W.; Ho, P.H.; Ilmasani, R.F.; Wurzenberger, J.C.; Glatz, T.; Creaser, D.; Olsson, L. Enhanced CO resistance of Pd/SSZ-13 for passive NOx adsorption. Chem. Eng. J. 2023, 460, 141681. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Sanz, J.; Fornés, V.; Corma, A. 29Si and 27Al MAS NMR study of zeolite β with different Si/Al ratios. J. Catal. 1990, 124, 217–223. [Google Scholar] [CrossRef]
- Wu, Q.M.; Wang, X.; Qi, G.D.; Guo, Q.; Pan, S.X.; Meng, X.J.; Xu, J.; Deng, F.; Fan, F.T.; Feng, Z.C.; et al. Sustainable synthesis of zeolites without addition of both organotemplates and solvents. J. Am. Chem. Soc. 2014, 136, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Valtchev, V.; Onfroy, T.; Marichal, C.; Kno¨zinger, H.; Bein, T. Variation of the Si/Al ratio in nanosized zeolite Beta crystals. Microporous Mesoporous Mater. 2006, 90, 237–245. [Google Scholar] [CrossRef]
- Wang, A.; Lindgren, K.; Di, M.; Bernin, D.; Carlsson, P.A.; Thuvander, M.; Olsson, L. Insight into hydrothermal aging effect on Pd sites over Pd/LTA and Pd/SSZ-13 as PNA and CO oxidation monolith catalysts. Appl. Catal. B Environ. 2020, 278, 119315. [Google Scholar] [CrossRef]
- Miguel, M.A.; Camblor, A.; Valencia, S. Synthesis in fluoride media and characterisation of aluminosilicate zeolite beta. J. Mater. Chem. 1998, 9, 2137–2145. [Google Scholar] [CrossRef]
- Kim, Y.; Sung, J.; Kang, S.; Lee, J.; Kang, M.H.; Hwang, S.; Park, H.; Kim, J.; Kim, Y.; Lee, E.; et al. Uniform synthesis of palladium species confined in a small-pore zeolite via full ion-exchange investigated by cryogenic electron microscopy. J. Mater. Chem. A 2021, 9, 19796–19806. [Google Scholar] [CrossRef]
- Chen, Z.X.; Wang, M.D.; Wang, J.; Wang, C.; Wang, J.Q.; Li, W.; Shen, M.Q. Investigation of crystal size effect on the NOx storage performance of Pd/SSZ-13 passive NOx adsorbers. Appl. Catal. B Environ. 2021, 291, 120026. [Google Scholar] [CrossRef]
- Chen, D.D.; Lei, H.R.; Xiong, W.W.; Li, Y.; Ji, X.; Yang, J.Y.; Peng, B.X.; Fu, M.L.; Chen, P.R.; Ye, D.Q. Unravelling phosphorus-induced deactivation of Pd-SSZ-13 for passive NOx adsorption and CO oxidation. ACS Catal. 2021, 11, 13891–13901. [Google Scholar] [CrossRef]
- Ishida, T.; Honma, T.; Nakada, K.; Murayama, H.; Mamba, T.; Kume, K.; Izawa, Y.; Utsunomiya, M.; Tokunaga, M. Pd-catalyzed decarbonylation of furfural: Elucidation of support effect on Pd size and catalytic activity using in-situ XAFS. J. Catal. 2019, 374, 320–327. [Google Scholar] [CrossRef]
- Vu, A.; Luo, J.; Li, J.; Epling, W.S. Effects of CO on Pd/BEA passive NOx adsorbers. Catal. Lett. 2017, 147, 745–750. [Google Scholar] [CrossRef]
- Motokura, K.; Fukuda, T.; Uemura, Y.; Matsumura, D.; Ikeda, M.; Nambo, M.; Chun, W.J. Effects of mesopore internal surfaces on the structure of immobilized Pd-bisphosphine complexes analyzed by variable-temperature XAFS and their catalytic performances. Catalysts 2018, 8, 106. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Hwang, S.; Lee, E.; Lee, H.; Kim, C.H.; Kim, D.H. Deactivation of Pd/zeolites passive NOx adsorber induced by NO and H2O: Comparative study of Pd/ZSM-5 and Pd/SSZ-13. Catal. Today 2021, 360, 350–355. [Google Scholar] [CrossRef]
- Wang, A.Y.; Xie, K.P.; Kumar, A.; Kamasamudram, K.; Olsson, L. Layered Pd/SSZ-13 with Cu/SSZ-13 as PNA-SCR dual-layer monolith catalyst for NOx abatement. Catal. Today 2021, 360, 356–366. [Google Scholar] [CrossRef]
- Shan, Y.L.; He, G.Z.; Du, J.P.; Sun, Y.; Liu, Z.Q.; Fu, Y.; Liu, F.D.; Shi, X.Y.; Yu, Y.B.; He, H. Strikingly distinctive NH3-SCR behavior over Cu-SSZ-13 in the presence of NO2. Nat. Commun. 2022, 13, 4606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).