Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectral Data
2.2. Evaluation of the Biological Activities of Compounds Isolated from Cyperus sexangularis Leaf
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Gradient Extraction
3.4. Isolation of Compounds
3.4.1. Column Chromatography of n-Hexane Extract of C. sexangularis (CS) Leaf
3.4.2. Column Chromatography of DCM Extract of C. sexangularis (CS) Leaf
3.5. Antioxidant Tests
3.5.1. DPPH Spectrophotometric Assay
3.5.2. Nitric Oxide (NO) Inhibition Assay
3.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. In Vitro Anti-Inflammatory Test
3.7. Anti-Elastase Assay
3.7.1. Cell Culture
3.7.2. Test Procedure
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sumit, K.; Vivek, S.; Sujata, S.; Ashish, B. Herbal cosmetics: Used for skin and hair. Inventi J. 2012, 10, 1–7. [Google Scholar]
- Melendez-Martinez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar] [CrossRef]
- Gediya, S.K.; Mistry, R.B.; Patel, U.K.; Blessy, M.; Jain, H.N. Herbal plants: Used as a cosmetics. J. Nat. Prod. Plant Resour. 2011, 1, 24–32. [Google Scholar]
- Hamdani, S.S.; Khan, B.A.; Saeed, A.; Larik, F.A.; Hameed, S.; Channar, P.A.; Ahmad, K.; Mughal, E.U.; Abbas, Q.; Amin, N.U. Densely substituted piperidines as a new class of elastase inhibitors: Synthesis and molecular modeling studies. Archiv. Der Pharm. 2019, 352, 1900061. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquatic. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Daamen, W.F.; Veekamp, J.; Van Hest, J.; Van Kuppevelt, T. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four Southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- Nar, H.; Werle, K.; Bauer, M.M.; Dollinger, H.; Jung, B. Crystal structure of human macrophage elastase (MMP-12) in complex with a hydroxamic acid inhibitor. J. Mol. Biol. 2001, 312, 743–751. [Google Scholar] [CrossRef]
- Syntia, F.; Nehme, R.; Claude, B.; Morin, P. Human neutrophil elastase inhibition studied by capillary electrophoresis with laser induced fluorescence detection and microscale thermophoresis. J. Chrom. A 2016, 1431, 215–223. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin ageing. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Jabs, H.-U. Elastase-the target of a novel anti-ageing strategy to defy skin aging, loss of skin elasticity and wrinkle formation. Ästhetische Dermatol. 2012, 6, 38–40. [Google Scholar]
- Lall, N. Natural Cosmetics from South African Wetland Plants; WRC Report No. 2020, TT 817/20; Water Research Commission: Gezina, South Africa, 2020; 83p. [Google Scholar]
- Zhang, C.; Wang, N.; Feng, Y. Oxidative stress, Chinese herbals and toxicity: A focused review with examples. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; pp. 215–224. [Google Scholar]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Rehman, V. Revisiting the fairness paradigm in India: Synthesis of literature and application of the self-concept theory. Soceity Bus. Rev. 2018, 14, 31–42. [Google Scholar] [CrossRef]
- Shivanand, P.; Nilam, M.; Viral, D. Herbs play an important role in the field of cosmetics. Int. J. Pharm. Technol. Res. 2010, 2, 632–639. [Google Scholar]
- Raimondo, D.; Staden, L.V.; Foden, W.; Victor, J.; Helme, N.; Turner, R.; Kamundi, D.; Manyama, P. Red List of South African Plants; South African National Biodiversity Institute: Pretoria, South Africa, 2009. [Google Scholar]
- Taheri, Y.; Herrera-Bravo, J.; Huala, L.; Salazar, L.A.; Sharifi-Rad, J.; Akram, M.; Shahzad, K.; Melgar-Lalanne, G.; Baghalpour, N.; Tamimi, K.; et al. Cyperus spp.: A review on phytochemical composition, biological activity, and health-promoting effects. Oxid. Med. Cell Longev. 2021, 2021, 4014867. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Loran, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagan, R.; Conchello, P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res. Int. 2012, 45, 313–319. [Google Scholar] [CrossRef]
- Gugsa, T.; Yaya, E.E. Chemical constituents of the traditional skin care and fragrance nut, Cyperus esculentus (Tigernut). Am. J. Essent. Oils Nat. Prod. 2018, 6, 4–12. [Google Scholar]
- Kakarla, L.; Katragadda, S.B.; Tiwari, A.K.; Komtamraju, K.S.; Madhusudana, K.; Kumar, D.A.; Botlagunta, M. Free radical scavenging, α-glucosidase inhibitory and anti-inflammatory constituents from Indian sedges, Cyperus scariosus R. Br and Cyperus rotundus L. Pharmacog. Mag. 2016, 12, S488. [Google Scholar]
- Shah, H.; Khan, A.A. Phytochemical characterisation of an important medicinal plant, Chenopodium ambrosioides Linn. Nat. Prod. Res. 2017, 31, 2321–2324. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharma. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Cyril-Olutayo, C.M.; Adeyemo, T.A.; Oriola, A.O.; Agbedahunsi, J.M. Bioactivity-directed isolation of antisickling Compounds from Cnidoscolus acontifolius (Mill.) I.M. Johnst leaf extract. J. Pharm. Pharmacog. Res. 2020, 8, 580–590. [Google Scholar]
- Sanguanphun, T.; Sornkaew, N.; Malaiwong, N.; Charlorak, P.; Jattujan, P.; Niamnoth, N. Neuroprotective effects of a medium chain fatty acid, decanoic acid, isolated from H. leucospilota against Parkinsonism in C. elegans PD model. Front. Pharmacol. 2022, 13, 4568. [Google Scholar] [CrossRef]
- Abe, M.; Ito, Y.; Suzuki, A.; Onoue, S.; Nogushi, H.; Yamada, S. Isolation and pharmacological characterization of fatty acids from Saw Palmetto extract. Anal. Sci. 2009, 25, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-C.; Han, J.-M.; Nam, S.-K.; Ko, O.-H.; Choi, C.-H.; Kee, K.-H.; Sohng, J.-K.; Jo, J.-S.; Seong, C.-N. Characterization and cytotoxic activities of nonadecanoic acid produced by Streptomyces scabei subsp. Chosunensis M0137 (KCTC 9927). J. Microbiol. 2002, 40, 331–334. [Google Scholar]
- Jauah, N.M.; Nafiah, M.A.; Hasnan, M.H.H.; Tan, S.-P. Anti-diabetic activity and nuclear magnetic resonance (NMR) characterization of natural esters from hexane and dichloromethane crude extracts from Calyces of Hibiscus sabdariffa Linn. Int. J. Rec. Tech. Eng. 2019, 8, 102–105. [Google Scholar]
- Vivancos, M.; Moreno, J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Rad. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vit. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Pinto, M.E.A.; Araujo, S.G.; Morais, M.I.; Sa, N.P.; Lima, C.M.; Rosa, C.A.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef]
- Holanda-Pinto, S.A.; Pinto, L.M.; Cunha, G.M.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of alpha, beta-amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.-C.; Hornebeck, W.; Sauvain, M.; Zèches-Hanrot, M. Triterpenes and phytosterols as human leucocyte elastase inhibitors. Planta Med. 2002, 68, 930–932. [Google Scholar] [CrossRef]
- Ashhraf, R.; Bhatti, H.N. Chapter 10-Stigmasterol. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 213–232. ISBN 9780128229231. [Google Scholar] [CrossRef]
- Esperon-Rojas, A.A.; Torres-Palacios, C.; Santos-Luna, D.; Baeza-Jimenez, R.; Cano-Sarmiento, C.; Garcia, H.S. A specific thin-layer chromatography method for the identification and separation of medium chain acylglycerols. J. Oleo Sci. 2018, 67, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, R. Emollient: Fatty acid esters. In Cosmetic Science and Technology: Theoretical Principle and Applications; Elsevier Inc.: London, UK, 2017. [Google Scholar]
- Antwi, O.; Obiri, D.D.; Osafo, N.; Essel, L.B.; Forkuo, A.D.; Atobiga, C. Stigmasterol alleviates cutaneous allergic responses in rodents. Biomed. Res. Int. 2018, 2018, 39840. [Google Scholar] [CrossRef] [PubMed]
- Han, N.-R.; Kim, H.-M.; Jeong, H.-J. The β-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLP. Exp. Biol. Med. 2014, 239, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An experimental and theoretical study on essential oil of Aethionema sancakense: Characterization, molecular properties and RDG analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef]
- Dederen, J.C.; Chavan, B.; Rawlings, A.V. Emollients are more than sensory ingredients: The case of isostearyl isostearate. Int. J. Cosmet. Sci. 2012, 34, 502–510. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.G.; Snyder, P.W.; et al. Safety assessment of stearyl heptanoate and related stearyl alkanoates as used in cosmetics. Int. J. Toxicol. 2012, 31, 141S–146S. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 2nd ed.; Springer: Amsterdam, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Oriola, A.O.; Aladesanmi, A.J.; Idowu, T.O.; Akinwumi, F.O.; Obuotor, E.M.; Idowu, T.; Oyedeji, A.O. Ursane-type triterpenes, phenolics and phenolic derivatives from Globimetula braunii leaf. Molecules 2021, 26, 6528. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef]
- Lall, N.; Van Staden, A.B.; Rademan, S.; Lambrechts, I.; De Canha, M.N.; Mahore, J.; Winterboer, S.; Twilley, D. Antityrosinase and anti-acne potential of plants traditionally used in the Jongilanga community in Mpumalanga. S. Afr. J. Bot. 2019, 126, 241–249. [Google Scholar] [CrossRef]
- Bieth, J.G.; Dirrig, S.; Jung, M.-L.; Boudier, C.; Papamichael, E.; Sakarellos, C.; Dimicoli, J.-L. Investigation of the active center of rat pancreatic elastase. Biochim. Biophys. Acta (BBA)-Protein Str. Mol. Enzymol. 1989, 994, 64–74. [Google Scholar] [CrossRef]
- Fibrich, B.; Gao, X.; Puri, A.; Banga, A.; Lall, N. In vitro antioxidant, anti-inflammatory and skin permeation of Myrsine africana and its isolated compound myrsinoside B. Front. Pharmacol. 2020, 10, 1410. [Google Scholar] [CrossRef]
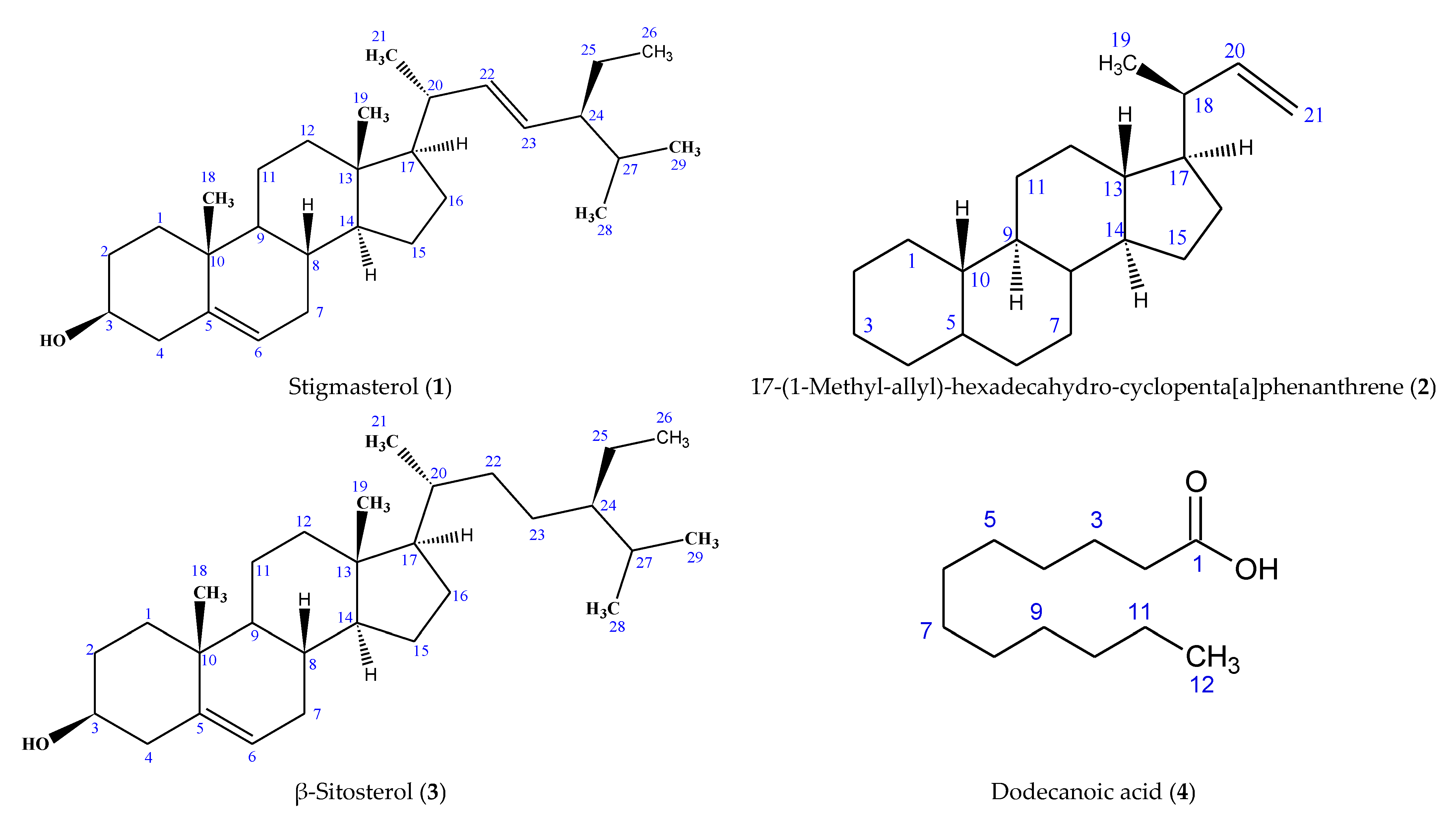

| Position | Type of Carbon | Compound 1 | Compound 3 | Stigmasterol [25] | ||
|---|---|---|---|---|---|---|
| 1H NMR (Multiplicity, J) | 13C NMR | 1H NMR (Multiplicity, J) | 13C NMR | 13C NMR | ||
| 1 | CH2 | 37.40 | 37.48 | 37.6 | ||
| 2 | CH2 | 34.08 | 31.86 | 32.1 | ||
| 3 | CH | 3.53 (1H, dt, J = 6.0 Hz) | 71.95 | 3.52 (1H, tdd, J = 9.6, 6.4, 6.0 Hz) | 71.78 | 72.1 |
| 4 | CH2 | 2.25 (2H, d, J = 10.9 Hz) | 42.42 | 2.25 (2H, d, J = 6.8 Hz) | 42.28 | 42.4 |
| 5 | C=CQ | 140.89 | 140.66 | 141.1 | ||
| 6 | HC=C | 5.35 (1H, bd, J = 5.1 Hz) | 121.87 | 5.32 (1H, bd, J = 5.2 Hz) | 121.39 | 121.8 |
| 7 | CH2 | 31.78 | 30.36 | 31.8 | ||
| 8 | CH | 32.04 | 29.73 | 31.8 | ||
| 9 | CH | 50.27 | 50.02 | 50.2 | ||
| 10 | CQ | 36.29 | 36.86 | 36.6 | ||
| 11 | CH2 | 21.23 | 22.93 | 21.5 | ||
| 12 | CH2 | 39.92 | 39.52 | 39.9 | ||
| 13 | CQ | 42.45 | 42.28 | 42.4 | ||
| 14 | CH | 56.91 | 56.60 | 56.8 | ||
| 15 | CH2 | 24.45 | 28.09 | 24.4 | ||
| 16 | CH2 | 29.29 | 29.73 | 29.3 | ||
| 17 | CH | 56.20 | 55.82 | 56.2 | ||
| 18 | CH3 | 1.00 (3H, s) | 12.12 | 0.99 (3H, s) | 11.84 | 12.2 |
| 19 | CH3 | 0.68 (3H, s) | 18.93 | 0.66 (3H, s) | 17.98 | 18.9 |
| 20 | CH | 2.02 (1H, m) | 40.63 | 2.02 (1H, m) | 36.24 | 40.6 |
| 21 | CH3 | 0.92 (3H, d, J = 6.7 Hz) | 19.97 | 0.91 (3H, d, J = 6.4 Hz) | 19.23 | 21.7 |
| 22 | HC=C | 5.15 (1H, dd) | 138.46 | 35.61 | 138.7 | |
| 23 | C=CH | 5.01 (1H, dd) | 129.41 | 25.74 | 129.6 | |
| 24 | CH | 45.97 | 45.71 | 46.1 | ||
| 25 | CH2 | 26.21 | 24.33 | 25.4 | ||
| 26 | CH3 | 0.84 (3H, t, J = 8.1 Hz) | 12.00 | 0.82 (3H, t, J = 4.5 Hz) | 11.96 | 12.1 |
| 27 | CH | 29.85 | 29.69 | 29.6 | ||
| 28 | CH3 | 0.82 (3H, d, J = 6.9 Hz) | 19.54 | 0.80 (3H, d, J = 4.0 Hz) | 20.88 | 20.2 |
| 29 | CH3 | 0.80 (3H, d, J = 6.9 Hz) | 19.18 | 0.78 (3H, d, J = 4.0 Hz) | 19.28 | 19.8 |
| Position | Type of Carbon | Chemical Shift δ (ppm) | 5β-Pregnane [27] | ||
|---|---|---|---|---|---|
| 1H NMR (Multiplicity, J) | 13C NMR | 1H NMR | 13C NMR | ||
| 1 | CH2 | 1.21–1.27 (m) | 28.74 | 1.20–1.30 (m) | 37.29 |
| 2 | CH2 | 1.27–1.37 (m) | 26.45 | 1.25–1.35 (m) | 24.47 |
| 3 | CH2 | 1.27–1.37 (m) | 24.18 | 1.20–1.30 (m) | 29.25 |
| 4 | CH2 | 1.21–1.27 (m) | 29.40 | 1.20–1.30 (m) | 29.45 |
| 5 | CH | 31.70 | 25.20 | ||
| 6 | CH2 | 1.27–1.37 (m) | 29.07 | 1.20–1.30 (m) | 29.70 |
| 7 | CH2 | 1.27–1.37 (m) | 25.83 | 1.20–1.30 (m) | 22.73 |
| 8 | CH | 31.39 | 32.68 | ||
| 9 | CH | 29.74 | 27.98 | ||
| 10 | CH | 32.33 | 36.50 | ||
| 11 | CH2 | 1.27–1.37 (m) | 28.42 | 1.20–1.30 (m) | 22.63 |
| 12 | CH2 | 1.27–1.37 (m) | 26.79 | 1.20–1.30 (m) | 39.37 |
| 13 | CH | 32.96 | 42.40 | ||
| 14 | CH | 33.36 | 33.66 | ||
| 15 | CH2 | 22.24 | 25.20 | ||
| 16 | CH2 | 19.26 | 29.93 | ||
| 17 | CH | 1.55 (m, 1H) | 36.88 | 1.6 (m, 1H) | 31.93 |
| 18 | CH | 2.33 (m, 1H) | 32.02 | 1.28 (m, 2H) | 21.40 |
| 19 | CH3 | 0.87 (d, 3H, J = 4.0 Hz) | 13.81 | 0.85 (t, 3H) | 19.75 |
| 20 | CH | 5.82 (m, 1H) | 138.72 | 0.98 (s, 3H) | 24.87 |
| 21 | CH2 | 4.96 (d, 2H, J = 16.0 Hz) | 113.65 | 1.10 (s, 3H) | 14.12 |
| Position | Type of Carbon | Compound 4 | Lauric Acid [29] | ||
|---|---|---|---|---|---|
| 1H NMR (Multiplicity, J) | 13C NMR | 1H NMR (Multiplicity, J) | 13C NMR | ||
| 1 | CQ | 179.84 | 180.0 | ||
| 2 | CH2 | 2.31 (t, J = 10.0 Hz) | 31.92 | 2.35 (t, J = 7.5 Hz) | 34.0 |
| 3 | CH2 | 1.56 (m) | 29.36 | 1.63 (quintet, J = 7.5 Hz) | 31.9 |
| 4 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 | 29.6 |
| 5 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 | 29.4 |
| 6 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 | 29.3 |
| 7 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 | 29.2 |
| 8 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 (m) | 29.1 |
| 9 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 (m) | 24.7 |
| 10 | CH2 | 1.23–1.28 (m) | 29.85 | 1.20–1.40 (m) | 22.1 |
| 11 | CH2 | 1.26–1.32 (m) | 22.89 | 0.88 (t, J = 6.9 Hz) | 14.1 |
| 12 | CH3 | 0.86 (t, J = 8.0, 8.0 Hz) | 14.12 | ||
| Position | Type of Carbon | Compound 5 | Compound 6 | Ethyl Stearate [31] 13C NMR | ||
|---|---|---|---|---|---|---|
| 1H NMR (Multiplicity, J) | 13C NMR | 1H NMR (Multiplicity, J) | 13C NMR | |||
| 1 | C=O, ester | 173.01 | 173.01 | 174.1 | ||
| O-CH2 | 4.07 (q, J = 6.7 Hz) | 64.31 | 4.08 (q, J = 6.7, 6.7 Hz) | 64.31 | 60.3 | |
| CH3 | 1.26 | 14.30 | 1.26 (t, J = 21.6 Hz) | 14.29 | 14.2 | |
| 2 | CH2 | 2.21 (t, J = 7.4 Hz) | 34.59 | 2.21 (t, J = 7.4 Hz) | 34.59 | 34.5 |
| 3 | CH2 | 1.61 (m) | 25.49 | 1.56–1.62 (m) | 25.49 | 25.1 |
| 4 | CH2 | 1.24 (m) | 26.41 | 1.24–1.30 (m) | 26.41 | 29.4 |
| 5 | CH2 | 1.24–1.32 (m) | 29.27 | 1.46–1.52 (m) | 29.27 | 29.5 |
| 6 | CH2 | 1.24–1.32 (m) | 29.60 | 1.46–1.52 (m) | 29.59 | 29.7 |
| 7 | CH2 | 1.24–1.32 (m) | 29.69 | 1.46–1.52 (m) | 29.69 | 29.7 |
| 8 | CH2 | 1.24–1.32 (m) | 29.74 | 1.46–1.52 (m) | 29.73 | 29.7 |
| 9 | CH2 | 1.24–1.32 (m) | 29.95 | 1.22–1.32 (m) | 29.95 | 29.7 |
| 10 | CH2 | 1.24–1.32 (m) | 29.82 | 1.46–1.52 (m) | 29.81 | 29.7 |
| 11 | CH2 | 1.24–1.32 (m) | 30.05 | 1.22–1.32 (m) | 30.05 | 29.7 |
| 12 | CH2 | 1.24–1.32 (m) | 30.00 | 1.22–1.32 (m) | 29.99 | 29.7 |
| 13 | CH2 | 1.24–1.32 (m) | 30.13 | 1.22–1.32 (m) | 30.14 | 29.7 |
| 14 | CH2 | 1.24–1.32 (m) | 30.09 | 1.22–1.32 (m) | 30.08 | 29.6 |
| 15 | CH2 | 1.24–1.32 (m) | 30.15 | 1.22–1.32 (m) | 30.20 | 29.2 |
| 16 | CH2 | 1.24–1.32 (m) | 30.21 | 1.26–1.30 (m) | 32.35 | 32.0 |
| 17 | CH2 | 1.30–1.36 (m) | 32.36 | 1.24–1.28 (m) | 23.10 | 22.8 |
| 18 | CH2 | 1.26–1.30 (m) | 23.10 | 0.91 (t, J = 6.8 Hz) | 14.29 | 14.2 |
| 19 | CH3 | 0.91 (t, J = 6.9 Hz) | 14.30 | |||
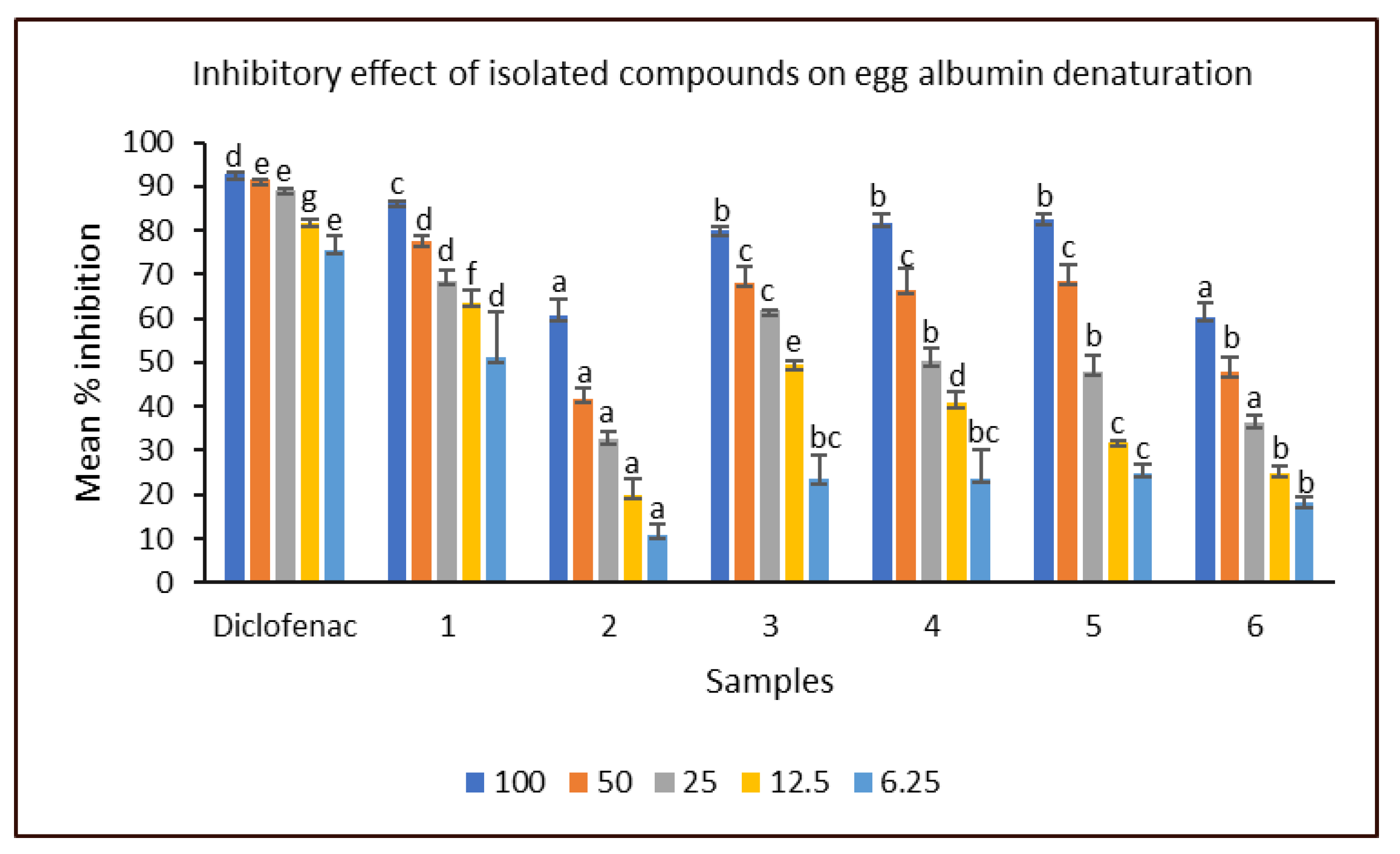
| Isolated Compound | DPPH (IC50 ± SEM) (µg/mL) | NO (IC50 ± SEM) (µg/mL) | FRAP (µgAAE/mg) |
|---|---|---|---|
| 1 | 38.18 ± 2.30 bc | 68.56 ± 4.03 bc | 303.58 ± 10.33 c |
| 2 | 57.63 ± 1.62 e | 105.73 ± 7.48 d | 196.21 ± 13.79 a |
| 3 | 40.60 ± 1.33 c | 72.48 ± 3.05 c | 257.88 ± 26.13 b |
| 4 | 35.99 ± 2.13 b | 65.04 ± 3.20 b | 245.94 ± 22.81 b |
| 5 | 43.82 ± 1.44 c | 69.74 ± 3.06 bc | 243.40 ± 19.72 b |
| 6 | 49.86 ± 2.29 d | 95.99 ± 4.93 d | 190.92 ± 11.68 a |
| ASC | 8.92 ± 1.26 a | 21.89 ± 1.32 a | N/A |
| Isolated Compound | Anti-Elastase Activity (IC50 ± SD) (µg/mL) |
|---|---|
| 1 | ≥50 b |
| 2 | ≥100 c |
| 3 | ≥50 b |
| 4 | ≥50 b |
| 5 | ≥50 b |
| 6 | ≥50 b |
| Ursolic acid | 24.80 ± 2.60 a |
| E | ≥400 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434. https://doi.org/10.3390/molecules28083434
Miya GM, Oriola AO, Payne B, Cuyler M, Lall N, Oyedeji AO. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules. 2023; 28(8):3434. https://doi.org/10.3390/molecules28083434
Chicago/Turabian StyleMiya, Gugulethu Mathews, Ayodeji Oluwabunmi Oriola, Bianca Payne, Marizé Cuyler, Namrita Lall, and Adebola Omowunmi Oyedeji. 2023. "Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties" Molecules 28, no. 8: 3434. https://doi.org/10.3390/molecules28083434
APA StyleMiya, G. M., Oriola, A. O., Payne, B., Cuyler, M., Lall, N., & Oyedeji, A. O. (2023). Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules, 28(8), 3434. https://doi.org/10.3390/molecules28083434







