Validation of a Novel RP-HPLC Technique for Simultaneous Estimation of Lignocaine Hydrochloride and Tibezonium Iodide: Greenness Estimation Using AGREE Penalties
Abstract
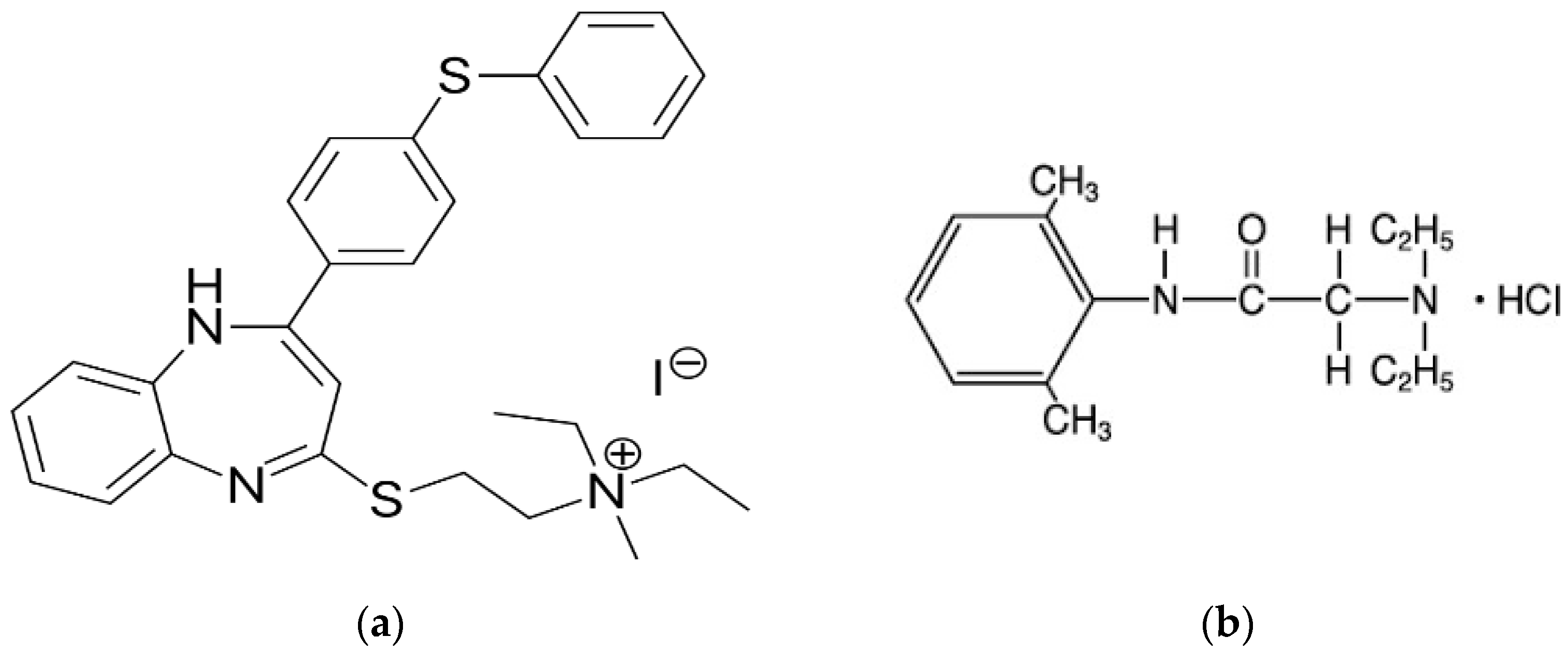
1. Introduction
2. Results and Discussion
2.1. Method Development and Optimization
2.2. System Suitability
2.2.1. Retention Time
2.2.2. Resolution
2.2.3. Tailing Factor
2.2.4. Theoretical Plate Count
2.3. Method Validation
2.3.1. Linearity
2.3.2. Accuracy
2.3.3. Precision
2.3.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.3.5. Robustness
2.3.6. Specificity
2.4. In Vitro Dissolution Analysis
2.5. Salivary Drug Concentrations in Volunteers
2.6. Greenness of HPLC Method
3. Materials and Methods
3.1. Materials/Chemicals/Reagents
3.2. Instrumental Conditions
3.3. Mobile Phase Preparation
3.4. Standard Solution Preparation
3.5. System Suitability Parameters
3.5.1. Retention Time
3.5.2. Resolution
3.5.3. Tailing Factor
3.5.4. Theoretical Plate Count
3.6. Validation Parameters for the Proposed Method
3.6.1. Linearity
3.6.2. Accuracy
3.6.3. Precision
3.6.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.6.5. Robustness
3.6.6. Specificity
3.7. In Vitro Dissolution Studies
3.8. Salivary Drug Concentration
3.9. Greenness of HPLC Method
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hanif, S.; Irfan, N.; Danish, Z.; Hussain, N.; Ali, M.; Nasir, B.; Iqbal, J.; Saeed, H.; Ali, R.; Saleem, Z. Computer Aided Formulation and Characterization of Propranolol Hcl Buccal Tablet Using Polymeric Blend. Open Conf. Proc. J. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Glick, M.; Williams, D.M.; Kleinman, D.V.; Vujicic, M.; Watt, R.G.; Weyant, R.J. A new definition for oral health developed by the FDI World Dental Federation opens the door to a universal definition of oral health. Br. Dent. J. 2016, 221, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and Evaluation of Biodegradable Chitosan Films of Metronidazole and Levofloxacin for the Management of Periodontitis. AAPS PharmSciTech 2016, 17, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Syed, M.A.; Abbas, N.; Hanif, S.; Arshad, M.S.; Bukhari, N.I.; Hussain, K.; Akhlaq, M.; Ahmad, Z. Development of an ANN optimized mucoadhesive buccal tablet containing flurbiprofen and lidocaine for dental pain. Acta Pharm. 2016, 66, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Maslamani, M.; Sedeqi, F. Antibiotic and Analgesic Prescription Patterns among Dentists or Management of Dental Pain and Infection during Endodontic Treatment. Med. Princ. Pract. 2018, 27, 66–72. [Google Scholar] [CrossRef]
- Razzaq, S.; Hanif, S.; Syed, M.A.; Iqbal, J.; Hassan, S.S.; Raza, S.A.; Riaz, H.; Abid, F. Development and evaluation of mucoadhesive buccal tablet containing metronidazole for the treatment of periodontitis and gingivitis. Pak. J. Pharm. Sci. 2018, 31, 1903–1910. [Google Scholar]
- Razzaq, S.; Syed, M.A.; Irfan, M.; Khan, I.; Sarfraz, R.M.; Shakir, R.; Ali, S.; Iqbal, Z.; Niaz, Y.; Mujtaba, S.H.; et al. Optimization of metronidazole sr buccal tablet for gingivitis using genetic algorithm. Pak. J. Pharm. Sci. 2021, 34, 2149–2158. [Google Scholar]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Minhas, M.U.; Irfan, M. Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: A pharmacokinetic investigation among healthy humans. Drug Dev. Ind. Pharm. 2022, 47, 1209–1222. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Ali, S.; Iqbal, Z.; Shakir, R.; Iqbal, J. Formulation and Evaluation of Chitosan-Based Polymeric Biodegradable Mucoadhesive Buccal Delivery for Locally Acting Drugs: In Vitro, Ex Vivo and In Vivo Volunteers Characterization. Lat. Am. J. Pharm. 2021, 40, 670–681. [Google Scholar]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Hussain, Z. Smart mucoadhesive buccal chitosan/HPMC scaffold for sore throat: In vitro, ex vivo and pharmacokinetic profiling in humans. J. Drug Deliv. Sci. Technol. 2022, 71, 103271. [Google Scholar] [CrossRef]
- Syed, M.A.; Aziz, G.; Jehangir, M.B.; Tabish, T.A.; Zahoor, A.F.; Khalid, S.H.; Khan, I.U.; Hosny, K.M.; Rizg, W.Y.; Hanif, S.; et al. Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.A.; Khan, I.U.; Iqbal, M.S.; Syed, H.K.; Irfan, M. Development of a Novel, Fast, Simple, Non-derived RP-HPLC Method for simultaneous Estimation of Benzocaine and Tibezonium Iodide from Mucoadhesive Dosage Form as well as Human Saliva and its Validation. Lat. Am. J. Pharm. 2021, 40, 1281–1287. [Google Scholar]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Orseth, M.; Srivastava, D. Local and Regional Infiltrated Anesthesia (Excluding Topical Anesthesia). In Evidence-Based Procedural Dermatology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 555–569. [Google Scholar] [CrossRef]
- Hermeto, L.C.; Rossi, R.d.; Bicudo, N.d.A.; Assis, K.T.; Escobar, L.L.; Camargo, P.S.d. The effect of epidurally administered dexamethasone with lignocaine for post-operative analgesia in dogs undergoing ovariohysterectomy. A dose-response study. Acta Cir. Bras. 2017, 32, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Gudin, J.; Nalamachu, S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad. Med. 2020, 132, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.; Cooper, B.; Jermakoff, A.; Knott, J.C. Antacid Monotherapy Is More Effective in Relieving Epigastric Pain Than in Combination With Lidocaine: A Randomized Double-blind Clinical Trial. Acad. Emerg. Med. 2020, 28, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, G. Recent Developments in Medicated Lozenges: A Promising Dosage Form for the Effective Delivery of Therapeutic Agents. Drug Deliv. Lett. 2021, 11, 97–109. [Google Scholar] [CrossRef]
- Srivastava, R.; Jyoti, B.; Parashar, P.; Chokotiya, H.; Pradhan, D.; Kundu, A. A Comparative Study to Evaluate the Efficacy of Curcumin Lozenges (TurmNova®) and Intralesional Corticosteroids with Hyaluronidase in Management of Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 2021, 22, 751–755. [Google Scholar] [CrossRef]
- Darshan, D.D.; Kumar, C.N.V.; Kumar, A.D.M.; Manikantan, N.S.; Balakrishnan, D.; Uthkal, M.P. Clinical study to know the efficacy of Amlexanox 5% with other topical Antiseptic, Analgesic and Anesthetic agents in treating minor RAS. J. Int. Oral Health. 2014, 6, 5–11. [Google Scholar]
- Syed, M.A.; Hanif, S.; Ain, N.U.; Syed, H.K.; Zahoor, A.F.; Khan, I.U.; Abualsunun, W.A.; Jali, A.M.; Qahl, S.H.; Sultan, M.H.; et al. Assessment of Binary Agarose–Carbopol Buccal Gels for Mucoadhesive Drug Delivery: Ex Vivo and In Vivo Characterization. Molecules 2022, 27, 7004. [Google Scholar] [CrossRef]
- Kapoor, B.; Gupta, R.; Gulati, M.; Singh, S.K.; Khatik, G.L.; Chawla, M.; Nagappan, K.V.; Khursheed, R.; Kumar, R. High-Performance Liquid Chromatography and Liquid Chromatography/Mass Spectrometry Studies on Stress Degradation Behavior of Sulfapyridine and Development of a Validated, Specific, Stability-Indicating HPLC Assay Method. ASSAY Drug Dev. Technol. 2020, 18, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Raza, A.; Murtaza, S.H.; Hanif, S.; Iqbal, J.; Ali, I.; Aftab, T.; Shakir, R.; Bedar, R.; Syed, M.A. Validation of a rapid and economical RP-HPLC method for simultaneous determination of metformin hydrochloride and sitagliptin phosphate monohydrate: Greenness evaluation using AGREE score. Pak. J. Pharm. Sci. 2022, 35, 15–21. [Google Scholar] [PubMed]
- Weinstein, R.D.; Muske, K.R.; Moriarty, J.; Schmidt, E.K. The Solubility of Benzocaine, Lidocaine, and Procaine in Liquid and Supercritical Carbon Dioxide. J. Chem. Eng. Data 2004, 49, 547–552. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Shehnaz, H.; Haider, A. RP-HPLC method for the quantitative determination of fexofenadine hydrochloride in coated tablets and human serum. Med. Chem. Res. 2011, 20, 55–61. [Google Scholar] [CrossRef]
- Dolan, J.W. Peak tailing and resolution. LC GC N. Am. 2002, 20, 430–437. [Google Scholar]
- Pasupuleti, R.R.; Wang, Z.-F.; Ya, W.-J.; Kuo, C.-A.; Chao, Y.-Y.; Huang, Y.-L. Extraction and detection of chlorophenols in water samples using deep eutectic solvent-based dispersive liquid–liquid microextraction coupled with HPLC-UV. Microchem. J. 2022, 182, 107843. [Google Scholar] [CrossRef]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Waraszkiewicz, S.M.; Milano, E.; Dirubio, R. Stability-indicating High-Performance Liquid Chromatographic Analysis of Lidocaine Hydrochloride and Lidocaine Hydrochloride with Epinephrine Injectable Solutions. J. Pharm. Sci. 1981, 70, 1215–1218. [Google Scholar] [CrossRef]
- Plenis, A.; Konieczna, L.; Miękus, N.; Bączek, T. Development of the HPLC Method for Simultaneous Determination of Lidocaine Hydrochloride and Tribenoside Along with Their Impurities Supported by the QSRR Approach. Chromatographia 2013, 76, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, P.; Harrison, J.; Procter, G.; Andrews, G.; Svirskis, D.; Sharma, M.; Jones, D.S.; Hill, A.G. Development, Validation and Application of a Stability Indicating HPLC Method to Quantify Lidocaine from Polyethylene-co-Vinyl Acetate (EVA) Matrices and Biological Fluids. J. Chromatogr. Sci. 2017, 55, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Hanif, S.; Azhar, F.; Ali, I.; Alafnan, A.; Hussain, T.; Moin, A.; Alamri, M.A.; Syed, M.A. HPLC Method Validation for the Estimation of Lignocaine HCl, Ketoprofen and Hydrocortisone: Greenness Analysis Using AGREE Score. Int. J. Mol. Sci. 2023, 24, 440. [Google Scholar] [CrossRef] [PubMed]
- Bose, A. HPLC calibration process parameters in terms of system suitability test. Austin Chromatogr. 2014, 1, 4. [Google Scholar]
- Pasupuleti, R.R.; Hsieh, J.-R.; Pasupuleti, V.R.; Huang, Y.-L. Eco-friendly magnetic Solid-Phase extraction and deep eutectic solvent for the separation and detection of parabens from the environmental water and urine samples. Microchem. J. 2022, 178, 107330. [Google Scholar] [CrossRef]
- Louw, C.J.; Hamnca, S.; Baker, P.G.L. Voltammetric and impedimetric detection of norfloxacin at co nanoparticle modified polymer composite electrodes. Electroanalysis 2020, 32, 3170–3179. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Tibezonium Iodide | Lignocaine Hydrochloride |
|---|---|---|
| System Suitability Parameters | ||
| Retention time (min) | 4.20 ± 0.28 | 2.33 ± 0.18 |
| Tailing factor | 1.86 | 1.32 |
| Peak area | 70.32 ± 0.74 | 98.97 ± 0.84 |
| Theoretical plates (USP) | 5598 | 4339 |
| Resolution | 2.591 | --- |
| Linearity Parameters | ||
| Linear function | y = 26.321x + 0.7419 | y = 1.9737x − 0.5297 |
| Regression coefficient (r2) | 0.9995 | 0.9992 |
| Linearity range (µg/mL) | 0.14–10.08 | 0.28–20.18 |
| LOD (µg/mL) | 0.011 | 0.115 |
| LOQ (µg/mL) | 0.037 | 0.384 |
| Percent Level | Tibezonium Iodide | Lignocaine Hydrochloride | ||||||
|---|---|---|---|---|---|---|---|---|
| Theoretical Content (µg/mL) | Amount Recovered (µg/mL) | Recovery (%) | RSD (%) | Theoretical Content (µg/mL) | Amount Recovered (µg/mL) | Recovery (%) | RSD (%) | |
| 80% | 8.96 | 8.91 | 99.34 | 1.46 | 4.48 | 1.83 | 100.12 | 0.75 |
| 100% | 11.2 | 11.15 | 100.01 | 1.72 | 5.6 | 2.56 | 99.05 | 0.65 |
| 120% | 13.44 | 14.44 | 100.50 | 1.69 | 6.72 | 3.42 | 99.74 | 0.90 |
| Percent Level | Tibezonium Iodide | Lignocaine Hydrochloride | ||||||
|---|---|---|---|---|---|---|---|---|
| Percent Precision | Intraday RSD (%) | Percent Precision | Interday RSD (%) | Percent Precision | Intraday RSD (%) | Percent Precision | Interday RSD (%) | |
| 80% | 99.34 | 1.83 | 98.63 | 1.75 | 100.42 | 0.96 | 99.89 | 1.46 |
| 100% | 100.03 | 1.61 | 99.14 | 0.65 | 99.05 | 0.48 | 99.19 | 1.72 |
| 120% | 100.10 | 1.65 | 99.01 | 0.90 | 99.84 | 0.77 | 98.84 | 1.69 |
| Parameters | Tibezonium Iodide Content % (RSD %) | Lignocaine HCl Content % (RSD %) |
|---|---|---|
| Optimal condition | ||
| 1.00 mL/min, 35 °C, pH 4.5 | 99.83 (1.52) | 98.46 (0.65) |
| Flow rate variation | ||
| 0.85 mL/min | 97.79 (1.24) | 99.57 (0.91) |
| 1.15 mL/min | 98.11(1.86) | 98.36 (0.62) |
| Temperature variation | ||
| 30 °C | 98.60 (0.22) | 99.93 (0.31) |
| 40 °C | 98.37 (0.29) | 99.50 (0.24) |
| pH variation | ||
| pH 4.0 | 99.19 (1.91) | 100.42 (1.78) |
| pH 5.0 | 100.02 (1.81) | 99.26 (1.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, S.; Syed, M.A.; Rashid, A.J.; Alharby, T.N.; Algahtani, M.M.; Alanazi, M.; Alanazi, J.; Sarfraz, R.M. Validation of a Novel RP-HPLC Technique for Simultaneous Estimation of Lignocaine Hydrochloride and Tibezonium Iodide: Greenness Estimation Using AGREE Penalties. Molecules 2023, 28, 3418. https://doi.org/10.3390/molecules28083418
Hanif S, Syed MA, Rashid AJ, Alharby TN, Algahtani MM, Alanazi M, Alanazi J, Sarfraz RM. Validation of a Novel RP-HPLC Technique for Simultaneous Estimation of Lignocaine Hydrochloride and Tibezonium Iodide: Greenness Estimation Using AGREE Penalties. Molecules. 2023; 28(8):3418. https://doi.org/10.3390/molecules28083418
Chicago/Turabian StyleHanif, Sana, Muhammad Ali Syed, Ahmad Junaid Rashid, Tareq Nafea Alharby, Mohammad M. Algahtani, Muteb Alanazi, Jowaher Alanazi, and Rai Muhammad Sarfraz. 2023. "Validation of a Novel RP-HPLC Technique for Simultaneous Estimation of Lignocaine Hydrochloride and Tibezonium Iodide: Greenness Estimation Using AGREE Penalties" Molecules 28, no. 8: 3418. https://doi.org/10.3390/molecules28083418
APA StyleHanif, S., Syed, M. A., Rashid, A. J., Alharby, T. N., Algahtani, M. M., Alanazi, M., Alanazi, J., & Sarfraz, R. M. (2023). Validation of a Novel RP-HPLC Technique for Simultaneous Estimation of Lignocaine Hydrochloride and Tibezonium Iodide: Greenness Estimation Using AGREE Penalties. Molecules, 28(8), 3418. https://doi.org/10.3390/molecules28083418






