“Green” Extraction and On-Site Rapid Detection of Aflatoxin B1, Zearalenone and Deoxynivalenol in Corn, Rice and Peanut
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Sample Preparation
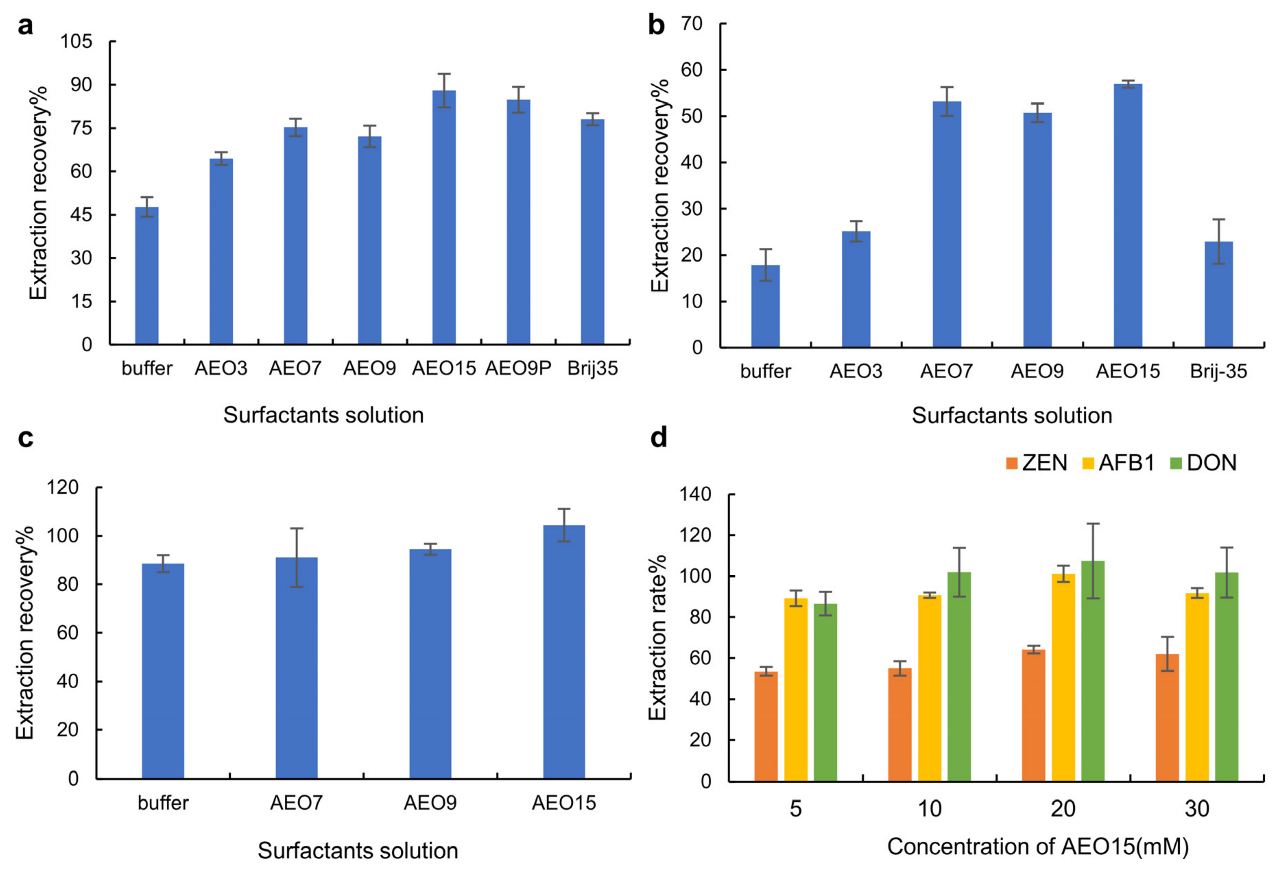
2.1.1. Optimization of the Composition of the Mycotoxin Extraction Solution
2.1.2. Optimization of the Concentration of Mycotoxin Extraction Solution
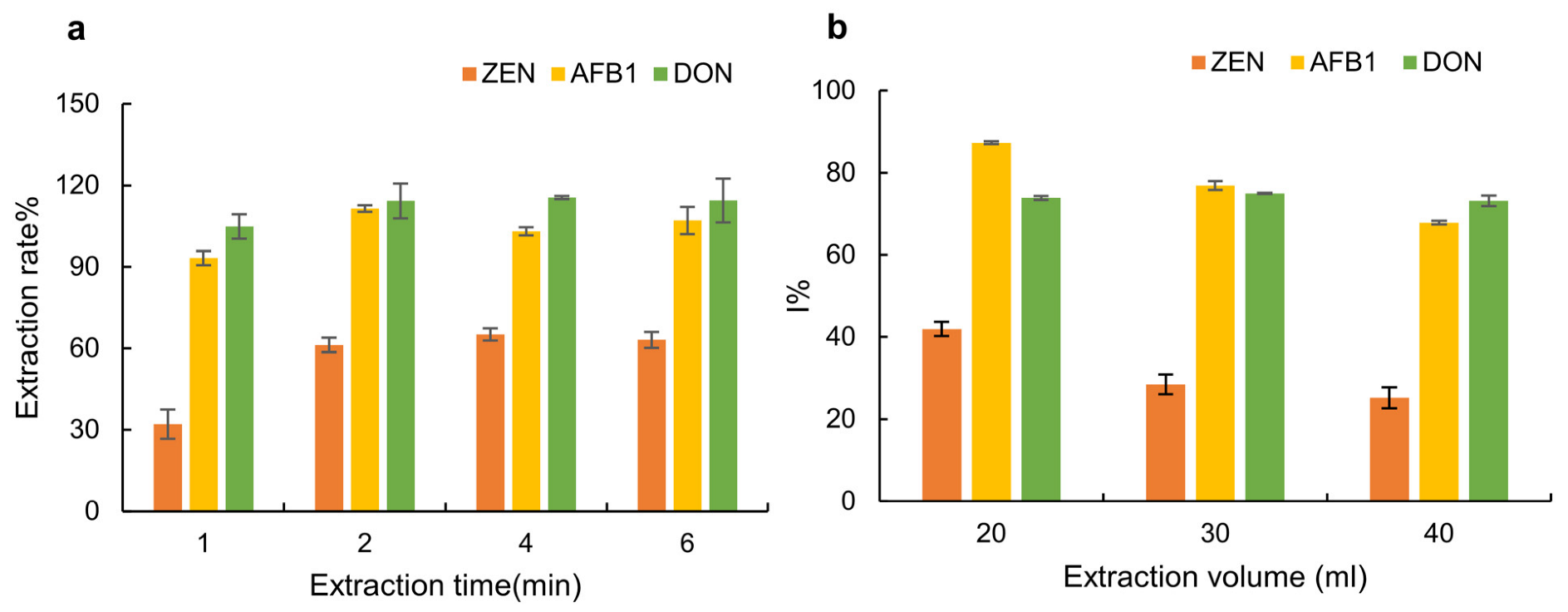
2.1.3. Optimization of Extraction Time
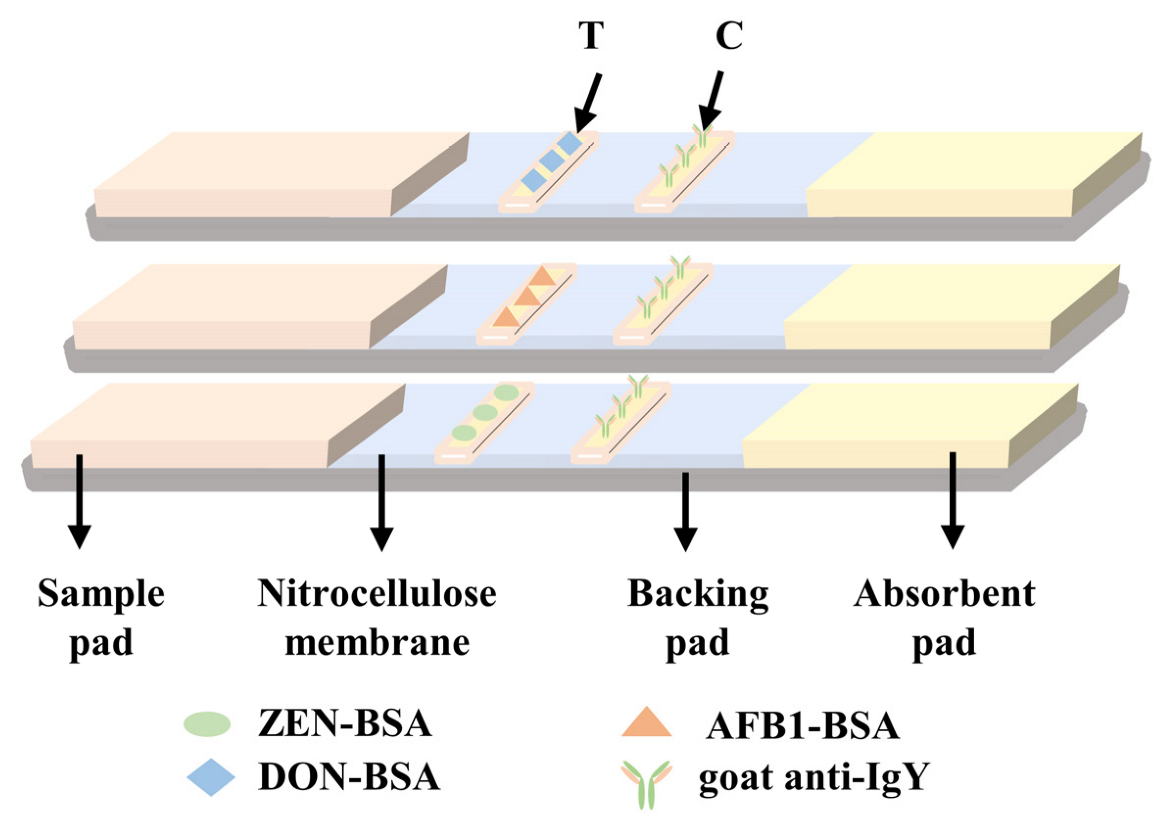
2.2. Optimization of Preparation Conditions for TRF-LFIA Test Strips
2.3. Optimization of Extraction Volume
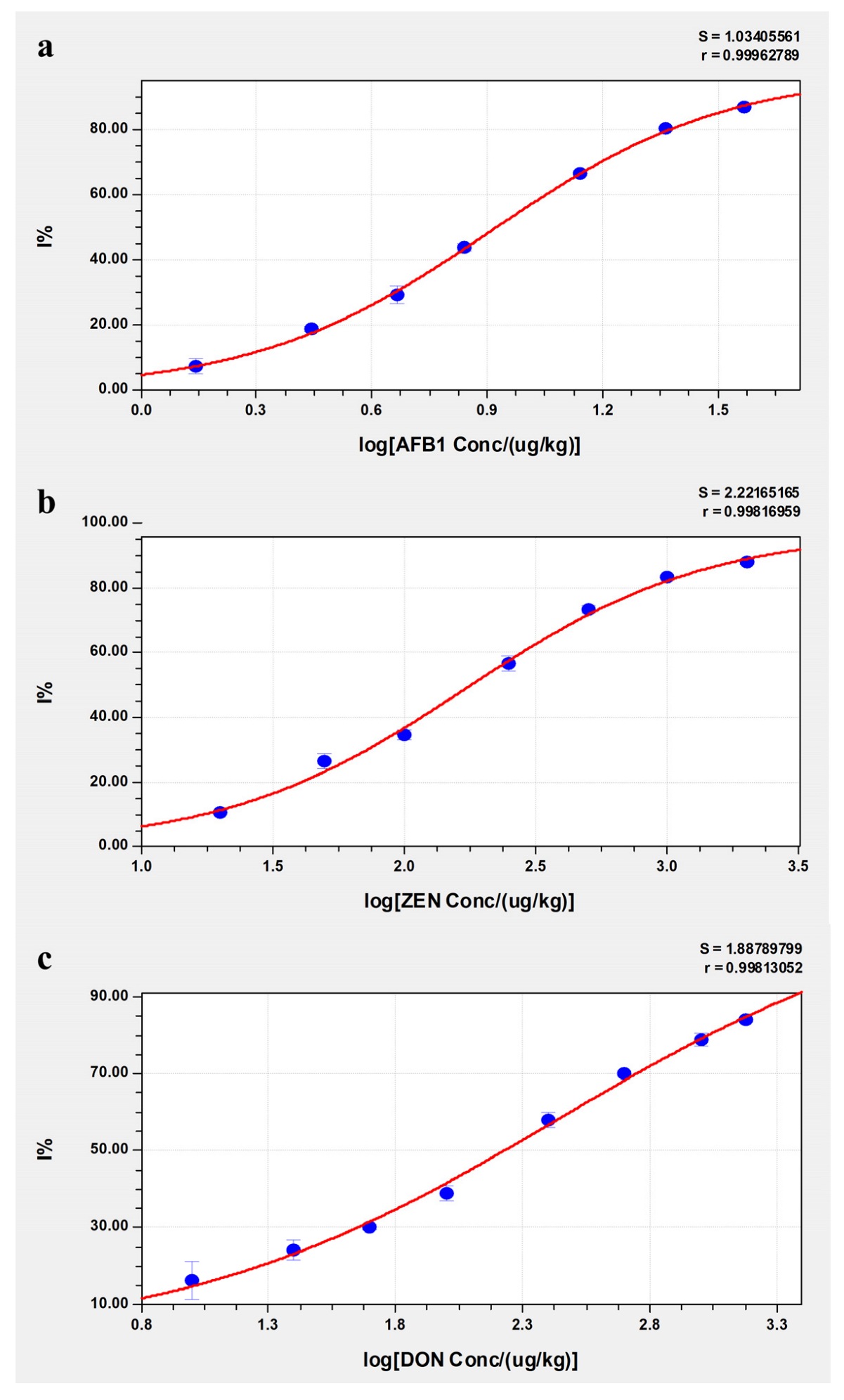
2.4. Standard Curves
2.5. Recovery of AFB1, ZEN and DON in Grains
2.6. Cross-Reactivity
2.7. Assessment of the Trueness
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Preparation of mAb-TRF Nanoparticle Conjugates
3.4. Preparation of the TRF LIFA Test Strip
3.5. Detection Procedure
3.6. Selection of the Mycotoxin Extraction Solutions
3.7. Standard Curves
3.8. LOD and Working Range
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Pereg, L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019, 70, 882–897. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Advances in Mycotoxin Research: Public Health Perspectives. J. Food Sci. 2015, 80, T2970–T2983. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Wall-Martínez, H.A.; Ramírez-Martínez, A.; Wesolek, N.; Brabet, C.; Durand, N.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; Roudot, A.C. Risk assessment of exposure to mycotoxins (aflatoxins and fumonisins) through corn tortilla intake in Veracruz City (Mexico). Food Addit. Contam. Part A 2019, 36, 929–939. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Hell, K.; Adjovi, Y.; Fandohan, P.; Koudande, D.O.; Mensah, G.A.; Sanni, A.; Brimer, L. A Review on Aflatoxin Contamination and Its Implications in the Developing World: A Sub-Saharan African Perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 349–365. [Google Scholar] [CrossRef]
- Wu, Q.; Jezkova, A.; Yuan, Z.; Pavlikova, L.; Dohnal, V.; Kuca, K. Biological degradation of aflatoxins. Drug Metab. Rev. 2009, 41, 1–7. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yan, Z.; Yang, M.; Waalwijk, C.; van der Lee, T.A.J.; van Diepeningen, A.; Brankovics, B.; Chen, W.; Feng, J.; Zhang, H. Contamination and translocation of deoxynivalenol and its derivatives associated with Fusarium crown rot of wheat in Northern China. Plant Dis. 2021, 105, 3397–3406. [Google Scholar] [CrossRef]
- Kebede, H.; Abbas, H.K.; Fisher, D.K.; Bellaloui, N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins 2012, 4, 1385–1403. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Amagwula, I.O. Nutritional Diseases and Nutrient Toxicities: A Systematic Review of the Diets and Nutrition for Prevention and Treatment. Int. J. Adv. Acad. Res. (Sci. Technol. Eng.) 2020, 6, 1–46. [Google Scholar]
- Wegulo, S.N. Factors Influencing Deoxynivalenol Accumulation in Small Grain Cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Annunziata, L.; Schirone, M.; Visciano, P.; Campana, G.; De Massis, M.R.; Migliorati, G. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in organic wheat flour under different storage conditions. Int. J. Food Sci. Technol. 2021, 56, 4139–4148. [Google Scholar] [CrossRef]
- FAO. Mycotoxins. In Food safety and Quality. Retrieved 01.22.13. Available online: http://www.fao.org/food/food-safety-quality/a-zindex/mycotoxins/en/ (accessed on 15 May 2022).
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 39. [Google Scholar] [CrossRef]
- De Girolamo, A.; Ciasca, B.; Pascale, M.; Lattanzio, V.M.T. Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins 2020, 12, 786. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC–MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Huang, Y.H.; Xu, Y.; He, Q.H.; Chu, J.S.; Du, B.B.; Liu, J. Determination of zearalenone in corn based on a biotin-avidin amplified enzyme-linked immunosorbent assay. Food Agric. Immunol. 2014, 25, 186–199. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q. A simple fluorescent aptamer based assay coupled with fluorescence scanning capillary array for aflatoxin B1. Analyst 2018, 143, 4600–4605. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. One-step rapid detection of fumonisin B1, dexyonivalenol and zearalenone in grains. Food Control 2020, 117, 107107. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a Rainbow Lateral Flow Immunoassay for the Simultaneous Detection of Four Mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 749. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Mokoena, M.P.; Zhao, H.; Olaniran, A.O.; Shi, J. Development of an immunochromatographic strip test for the rapid detection of zearalenone in wheat from Jiangsu province, China. Toxicon 2019, 158, S67. [Google Scholar] [CrossRef]
- McNamee, S.E.; Bravin, F.; Rosar, G.; Elliott, C.T.; Campbell, K. Development of a nanoarray capable of the rapid and simultaneous detection of zearalenone, T2-toxin and fumonisin. Talanta 2017, 164, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, Y.; Chen, H.; Wang, Y.; Yu, B.; Shi, G. A mix-and-detect method based on colloidal gold immunochromatographic assay for on-site detection of zearalenone in edible oils. Anal. Methods 2020, 12, 5628–5634. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Huang, Q.; Yang, J. Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals. Foods 2022, 11, 1319. [Google Scholar] [CrossRef]
- Xing, C.; Dong, X.; Xu, T.; Yuan, J.; Yan, W.; Sui, X.; Zhao, X. Analysis of multiple mycotoxins-contaminated wheat by a smart analysis platform. Anal. Biochem. 2020, 610, 113928. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.Z.; Wu, X.L.; Li, Y.; Liu, L.Q.; Song, S.S.; Zheng, Q.K.; Kuang, H.; Xu, C.L. Ultrasensitive and eco-friendly immunoassays based monoclonal antibody for detection of deoxynivalenol in cereal and feed samples. Food Chem. 2019, 270, 130–137. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Yang, L.; Liu, A.; Wang, F.; He, P. Dual quantum dot nanobeads-based fluorescence-linked immunosorbent assay for simultaneous detection of aflatoxin B1 and zearalenone in feedstuffs. Food Chem. 2022, 366, 130527. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Duan, H.; Guo, L.; Leng, Y.; Lai, W.; Xiong, Y. Quantum dot nanobead-based multiplexed immunochromatographic assay for simultaneous detection of aflatoxin B1 and zearalenone. Anal. Chim. Acta 2018, 1025, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, Q.; Luo, S.; He, L.; Fan, R.; Zhang, S.; Yang, C.; Chen, Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021, 336, 127718. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics; Eurachem: Gembloux, Belgium, 2014. [Google Scholar]
| Target Analyte | Grain Sample | Spiked (μg/kg) | Detected (μg/kg) | Recovery (%) |
|---|---|---|---|---|
| AFB1 | Corn | 0 | ND 1 | ND |
| 7.5 | 6.71 | 89 ± 3 | ||
| 20 | 21.3 | 106 ± 3 | ||
| Rice | 1.5 | 1.42 | 94 ± 8 | |
| 15 | 13.9 | 93 ± 3 | ||
| 40 | 41.2 | 103 ± 9 | ||
| Peanut | 1.5 | 1.39 | 93 ± 10 | |
| 7.5 | 7.27 | 97 ± 4 | ||
| 40 | 36.5 | 91 ± 2 | ||
| DON | Corn | 20 | 20.6 | 103 ± 8 |
| 200 | 200 | 100 ± 8 | ||
| 1000 | 902 | 90 ± 2 | ||
| Rice | 50 | 51.7 | 103 ± 6 | |
| 200 | 201 | 101 ± 2 | ||
| 1000 | 913 | 91 ± 6 | ||
| Peanut | 50 | 53.1 | 106 ± 10 | |
| 200 | 174 | 87 ± 4 | ||
| 1000 | 956 | 96 ± 1 | ||
| ZEN | Corn | 25 | 24.2 | 97 ± 11 |
| 200 | 196 | 98 ± 2 | ||
| 750 | 688 | 92 ± 5 | ||
| Rice | 25 | 26.3 | 105 ± 3 | |
| 200 | 174 | 86 ± 2 | ||
| 750 | 742 | 98 ± 7 | ||
| Peanut | 25 | 27 | 108 ± 7 | |
| 200 | 191 | 96 ± 5 | ||
| 750 | 786 | 105 ± 4 |
| Target Analyte | IC50 (μg/kg) | Analogue | IC50 (μg/kg) | CRs (%) |
|---|---|---|---|---|
| AFB1 | 6.54 | AFB2 | 105 | 6.22 |
| AFG1 | 76.9 | 8.50 | ||
| AFG2 | 358 | 1.83 | ||
| ZEN | >2000 | <0.33 | ||
| DON | >2000 | <0.33 | ||
| ZEN | 183 | α-ZEL | >2000 | <9.15 |
| β-ZEL | 857 | 21.4 | ||
| α-ZOL | >2000 | <9.15 | ||
| β-ZOL | 1349 | 13.6 | ||
| AFB1 | >2000 | <9.15 | ||
| DON | >2000 | <9.15 | ||
| DON | 170 | AFB1 | >2000 | <8.50 |
| ZEN | >2000 | <8.50 |
| Target Analyte | Grain Sample | Certified Value (μg/kg) | Detected (μg/kg) | Bias% |
|---|---|---|---|---|
| AFB1 | Corn | 28 | 23.1 ± 0.83 | −17 |
| 7.4 | 6.92 ± 0.60 | −6 | ||
| 2.2 | 2.26 ± 0.80 | 2 | ||
| Rice | 14.3 | 15.2 ± 0.56 | 3 | |
| 8.7 | 8.96 ± 0.33 | 8 | ||
| 2.6 | 2.27 ± 0.20 | −12 | ||
| ZEN | Corn | 85 | 84.0 ± 3.71 | −2 |
| 750 | 803 ± 19.2 | 7 | ||
| Rice | 32 | 29.1 ± 3.47 | −9 | |
| 55 | 52.3 ± 10.1 | −5 | ||
| 380 | 335 ± 12.9 | −11 | ||
| DON | Corn | 1310 | 1510 ± 120 | 15 |
| 400 | 419 ± 7.82 | 5 | ||
| 125 | 119 ± 12 | −6 | ||
| Rice | 536 | 571 ± 18.8 | 7 | |
| 720 | 737 ± 17.4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Z.; Li, X.; Fan, Q.; Chen, Y.; Shi, G. “Green” Extraction and On-Site Rapid Detection of Aflatoxin B1, Zearalenone and Deoxynivalenol in Corn, Rice and Peanut. Molecules 2023, 28, 3260. https://doi.org/10.3390/molecules28073260
Li Z, Li Z, Li X, Fan Q, Chen Y, Shi G. “Green” Extraction and On-Site Rapid Detection of Aflatoxin B1, Zearalenone and Deoxynivalenol in Corn, Rice and Peanut. Molecules. 2023; 28(7):3260. https://doi.org/10.3390/molecules28073260
Chicago/Turabian StyleLi, Zijing, Zepeng Li, Xintong Li, Qi Fan, Yinuo Chen, and Guoqing Shi. 2023. "“Green” Extraction and On-Site Rapid Detection of Aflatoxin B1, Zearalenone and Deoxynivalenol in Corn, Rice and Peanut" Molecules 28, no. 7: 3260. https://doi.org/10.3390/molecules28073260
APA StyleLi, Z., Li, Z., Li, X., Fan, Q., Chen, Y., & Shi, G. (2023). “Green” Extraction and On-Site Rapid Detection of Aflatoxin B1, Zearalenone and Deoxynivalenol in Corn, Rice and Peanut. Molecules, 28(7), 3260. https://doi.org/10.3390/molecules28073260






