Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators
Abstract
1. Introduction
2. Unchecked Inflammation and CID
3. Anti-Inflammatory Effects of Canthin-6-Ones
3.1. Canthin-6-Ones Suppress the Progression of Several CIDs
3.1.1. Inflammatory Bowel Disease (IBD)
3.1.2. Alzheimer’s Disease (AD)
3.1.3. Parkinson’s Disease (PD)
3.1.4. Diabetes Mellitus (DM)
3.1.5. Rheumatoid Arthritis (RA)
3.1.6. Ulcers
3.1.7. Erectile Dysfunction (ED)
3.1.8. Tumors
| Compound No. | Compound Name | Source | Study Type | Effective Dose/IC50 | Reference |
|---|---|---|---|---|---|
| (10) | 9-Hydroxycanthin-6-one | Eurycoma longifolia | In vitro | Suppressed the proliferation of a melanoma cell line (5.4 μM) | [52] |
| (11) | 4,5-Dimethoxy-10-hydroxycanthin-6-one | Picrasma quassioides (D. Don) Benn | In vitro | Suppressed the proliferation of CNE2 (11.6 ± 2.48 μM) and Bel-7402 (118.91 ± 67.42μM) cell lines | [47] |
| (12) | 4,5-Dimethoxy- canthin-6-one | Picrasma quassioides Benn (Simarobaceae) | In vitro | Suppressed the proliferation of CNE2 (9.86 ± 1.49 μM) and Bel-7402 (32.27 ± 9.74 μM) cell lines | [47] |
| (14) | 9-Methoxycanthin-6-one | Eurycoma longifolia | In vitro | Suppressed the proliferation of A2780 (4.04 ± 0.36 μM), SKOV-3 (5.80 ± 0.40 μM), MCF-7 (15.09 ± 0.99 μM), HT-29 (3.79 ± 0.069 μM), A375 (5.71 ± 0.20 μM), and HeLa (4.30 ± 0.27 μM) cell lines | [29,93] |
| (15) | 6-Hydroxy-4-methoxy canthin-6-one | Picrasma quassioides BENN | In vitro | Suppressed the proliferation of CT26.WT, K-562, SGC-7901, Hep G2, and A-549 cell lines (>50 μM) | [47,102] |
| (16) | 9-Methoxycanthin-6-one-N-oxide | Eurycoma longifolia | In vitro | Suppressed the proliferation of a melanoma cell line (6.5 μM) | [52,93] |
| (17) | 9-Hydroxycanthin-6-one-N-oxide | Eurycoma longifolia | In vitro | Suppressed the proliferation of a melanoma cell line (7.0 μM) | [52] |
| (18) | 8-Hydroxycanthin-6-one | Picrasma quassioides (D. Don) Benn | In vitro | Suppressed the proliferation of CNE2(13.43 ± 2.29 μM) and Bel-7402(39.27 ± 9.72 μM) cell lines | [47] |
| (19) | 1-Methoxy-canthin-6-one | Ailanthus altissima Swingle | In vitro | Suppressed the proliferation of thyroid carcinoma and hepatocellular carcinoma cell lines (40 μM). | [103] |
| (20) | 1,11-Dimethoxycanthin-6-one | Brucea antidysentrica | In silico | Showed consecutive binding affinity with mainly hydrophobic interaction (−11.0 kcal/mol) | [94] |
| (21) | 2-Methoxycanthin-6-one | Brucea antidysentrica | In silico | Showed consecutive binding affinity with mainly hydrophobic interaction (−11.9 kcal/mol) | [94] |
| (22) | 10-Methoxycanthin-6-one | Chemical synthesis | In vitro | Suppressed the proliferation of Kasumi-1 (80μM) and KG-1 (36μM) | [95] |
3.2. Mechanism of Action and Pathways for Canthin-6-Ones
3.2.1. NF-κB Pathway
3.2.2. MAPK Pathway
3.2.3. JAK/STAT Pathway
3.2.4. PI3K–AKT Pathway
3.3. Major Inflammatory Mediators Regulated by Canthin-6-Ones
3.3.1. Pro-Inflammatory Cytokines
3.3.2. NO
3.3.3. PGE2
4. Structure–Activity Relationships of Canthin-6-Ones
5. Limitations and Possible Solutions
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of Macrophage Polarization in Autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The Effects of Inflammation, Aging and Oxidative Stress on the Pathogenesis of Diabetes Mellitus (Type 2 Diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent Inflammatory and Non-Inflammatory Mechanisms in Refractory Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C. Review: Systemic Inflammation and Alzheimer’s Disease: Systemic Inflammation and AD. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Resolution of Inflammation in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2017, 2, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Pei, J. Innovating Chinese Herbal Medicine: From Traditional Health Practice to Scientific Drug Discovery. Front. Pharmacol. 2017, 8, 381. [Google Scholar] [CrossRef]
- dos Santos Costa, R.; do Espírito-Santo, R.F.; Abreu, L.S.; de Oliveira Aguiar, L.; Leite Fontes, D.; Fechine Tavares, J.; Sobral da Silva, M.; Botelho Pereira Soares, M.; da Silva Velozo, E.; Flora Villarreal, C. Fluorescent Canthin-6-one Alkaloids from Simaba Bahiensis: Isolation, Identification, and Cell-Labeling Properties. ChemPlusChem 2019, 84, 260–267. [Google Scholar] [CrossRef]
- Arunachalam, K.; Damazo, A.S.; Macho, A.; Matchado, M.S.; Pavan, E.; de Figueiredo, F.F.; Oliveira, D.M.; Duckworth, C.A.; Thangaraj, P.; Leonti, M.; et al. Canthin-6-One Ameliorates TNBS-Induced Colitis in Rats by Modulating Inflammation and Oxidative Stress: An in Vivo and in Silico Approach. Biochem. Pharmacol. 2021, 186, 114490. [Google Scholar] [CrossRef]
- Guo, E.; Hu, Y.; Du, T.; Zhu, H.; Chen, L.; Qu, W.; Zhang, J.; Xie, N.; Liu, W.; Feng, F.; et al. Effects of Picrasma Quassioides and Its Active Constituents on Alzheimer’s Disease in Vitro and in Vivo. Bioorg. Chem. 2019, 92, 103258. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.-N.; Cai, C.-Z.; Wu, M.-Y.; Zhu, Q.; Su, H.; Li, M.; Ren, J.; Tan, J.-Q.; Lu, J.-H. Canthin-6-One Accelerates Alpha-Synuclein Degradation by Enhancing UPS Activity: Drug Target Identification by CRISPR-Cas9 Whole Genome-Wide Screening Technology. Front. Pharmacol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Sethiya, N.K.; Mishra, S.H. Antidiabetic Activity of Alkaloids of Aerva Lanata Roots on Streptozotocin-Nicotinamide Induced Type-II Diabetes in Rats. Pharm. Biol. 2013, 51, 635–642. [Google Scholar] [CrossRef]
- Fan, H.; Qi, D.; Yang, M.; Fang, H.; Liu, K.; Zhao, F. In Vitro and in Vivo Anti-Inflammatory Effects of 4-Methoxy-5- Hydroxycanthin-6-One, a Natural Alkaloid from Picrasma Quassioides. Phytomedicine 2013, 20, 319–323. [Google Scholar] [CrossRef] [PubMed]
- de Souza Almeida, E.S.; Filho, V.C.; Niero, R.; Clasen, B.K.; Balogun, S.O.; de Oliveira Martins, D.T. Pharmacological Mechanisms Underlying the Anti-Ulcer Activity of Methanol Extract and Canthin-6-One of Simaba Ferruginea A. St-Hil. in Animal Models. J. Ethnopharmacol. 2011, 134, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Choonong, R.; Chaingam, J.; Chantakul, R.; Mukda, S.; Temkitthawon, P.; Ingkaninan, K.; Juengwatanatrakul, T.; Yusakul, G.; Kanchanapoom, T.; Putalun, W. Phosphodiesterase-5 Inhibitory Activity of Canthin-6-One Alkaloids and the Roots of Eurycoma Longifolia and Eurycoma Harmandiana. Chem. Biodivers. 2022, 19, e202200121. [Google Scholar] [CrossRef]
- Stramer, B.M.; Mori, R.; Martin, P. The Inflammation–Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Roth, J.; Barczyk-Kahlert, K. More Than Suppression: Glucocorticoid Action on Monocytes and Macrophages. Front. Immunol. 2019, 10, 2028. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage Polarization and Meta-Inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Huang, C.; Sali, A.; Stevens, R.L. Regulation and Function of Mast Cell Proteases in Inflammation. J. Clin. Immunol. 1998, 18, 15. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1β Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Yue, Q.; Xu, Y.; Lin, L.; Hoi, M.P.M. Canthin-6-One (CO) from Picrasma Quassioides (D.Don) Benn. Ameliorates Lipopolysaccharide (LPS)-Induced Astrocyte Activation and Associated Brain Endothelial Disruption. Phytomedicine 2022, 101, 154108. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-ΚB Inhibitors from Eurycoma Longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef]
- Cho, S.-K.; Jeong, M.; Jang, D.; Choi, J.-H. Anti-Inflammatory Effects of Canthin-6-One Alkaloids from Ailanthus Altissima. Planta Med. 2018, 84, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Gao, Z.; Jiao, W.; Chen, L.; Chen, L.; Yao, X. In Vitro Anti-Inflammatory Effects of Beta-Carboline Alkaloids, Isolated from Picrasma Quassioides, through Inhibition of the INOS Pathway. Planta Med. 2012, 78, 1906–1911. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.; Luan, R.; Huang, G.; Liu, Y.; Wang, M.; Chao, Q.; Wang, L.; Li, D.; Fan, H.; et al. Identification of β-Carboline and Canthinone Alkaloids as Anti-Inflammatory Agents but with Different Inhibitory Profile on the Expression of INOS and COX-2 in Lipopolysaccharide-Activated RAW 264.7 Macrophages. J. Nat. Med. 2019, 73, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J. Inflammatory Markers of Coronary Risk. N. Engl. J. Med. 2000, 343, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First?: Inflammation in Psychiatric Disorders. Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Arienti, S.; Barth, N.D.; Dorward, D.A.; Rossi, A.G.; Dransfield, I. Regulation of Apoptotic Cell Clearance During Resolution of Inflammation. Front. Pharmacol. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Gudernatsch, V.; Stefańczyk, S.A.; Mirakaj, V. Novel Resolution Mediators of Severe Systemic Inflammation. ImmunoTargets Ther. 2020, 9, 31–41. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.L.; Henson, P.M. Neutrophil Clearance: When the Party Is over, Clean-up Begins. Trends Immunol. 2011, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 327–343. ISBN 978-3-319-48380-1. [Google Scholar]
- Yao, C.; Narumiya, S. Prostaglandin-Cytokine Crosstalk in Chronic Inflammation: PGs in Chronic Inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-Ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Showalter, H.D.H. Progress in the Synthesis of Canthine Alkaloids and Ring-Truncated Congeners. J. Nat. Prod. 2013, 76, 455–467. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial Activity of Two Canthin-6-One Alkaloids FromAllium Neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Aragozzini, F.; Maconi, E.; Gualandris, R. Evidence for Involvement of Ketoglutarate in the Biosynthesis of Canthin-6-One from Cell Cultures of Ailanthus Altissima. Plant Cell Rep. 1988, 7, 213–215. [Google Scholar] [CrossRef]
- Farouil, L.; Sylvestre, M.; Fournet, A.; Cebrián-Torrejón, G. Review on Canthin-6-One Alkaloids: Distribution, Chemical Aspects and Biological Activities. Eur. J. Med. Chem. Rep. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D. A New Canthinone-Type Alkaloid Isolated from Ailanthus Altissima Swingle. Molecules 2016, 21, 642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dai, J.-K.; Liu, D.; Wang, S.-J.; Wang, J.-R. Synthesis and Evaluation of Ester Derivatives of 10-Hydroxycanthin-6-One as Potential Antimicrobial Agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-X.; Zhou, Y.-J. Canthin-6-One Alkaloids from Picrasma quassioides and Their Cytotoxic Activity. J. Asian Nat. Prod. Res. 2008, 10, 1009–1012. [Google Scholar] [CrossRef]
- Zhao, W.; He, J.; Zhang, Y.; Ito, Y.; Su, Q.; Sun, W. Preparative isolation and purification of alkaloids from Picrasma quassiodes (D. Don) Benn. by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gong, Y.-X.; Jeong, H.; Seo, H.; Xie, D.-P.; Sun, H.-N.; Kwon, T. Pharmacological Effects of Picrasma Quassioides (D. Don) Benn for Inflammation, Cancer and Neuroprotection (Review). Exp. Ther. Med. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, J.; Su, Q.; Liang, J.; Zhao, L.; Zhang, Y.; Sun, W. Antihypertensive Effects of Extract from Picrasma Quassiodes (D. Don) Benn. in Spontaneously Hypertensive Rats. J. Ethnopharmacol. 2013, 145, 187–192. [Google Scholar] [CrossRef]
- Ouyang, Y.; Koike, K.; Ohmoto, T. Canthin-6-One Alkaloids from Brucea Mollis Var. Tonkinensis. Phytochemistry 1994, 36, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Kardono, L.B.S.; Angerhofer, C.K.; Tsauri, S.; Padmawinata, K.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic and Antimalarial Constituents of the Roots of Eurycoma Longifolia. J. Nat. Prod. 1991, 54, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the Pathogenesis of Inflammatory Bowel Disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ilias, A.; Gonczi, L.; Kurti, Z.; Lakatos, P.L. Biosimilars in Ulcerative Colitis: When and for Who? Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Szeto, M.C.H.; Yalçın, M.D.; Khan, A.; Piotrowicz, A. Successful Use of Tocilizumab in a Patient with Coexisting Rheumatoid Arthritis and Ulcerative Colitis. Case Rep. Immunol. 2016, 2016, 7562123. [Google Scholar] [CrossRef]
- Holmstroem, R.B.; Nielsen, O.H.; Jacobsen, S.; Riis, L.B.; Theile, S.; Bjerrum, J.T.; Vilmann, P.; Johansen, J.S.; Boisen, M.K.; Eefsen, R.H.L.; et al. COLAR: Open-Label Clinical Study of IL-6 Blockade with Tocilizumab for the Treatment of Immune Checkpoint Inhibitor-Induced Colitis and Arthritis. J. Immunother. Cancer 2022, 10, e005111. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, Y.; Shimizu, K.; Yamagami, K.; Yao, S.; Nakamura, R.; Nakamura, T.; Goto, H. Exacerbation of Ulcerative Colitis with Tocilizumab: A Report of Two Cases, One with Takayasu Arteritis and the Other with Relapsing Polychondritis. Intern. Med. 2021, 60, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate Promotes Macrophage HMGB1 Lactylation, Acetylation, and Exosomal Release in Polymicrobial Sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-Induced Colitis Animal Model: An Overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Pinto, R.; Mateus, V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. J. Clin. Med. 2019, 8, 1574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, Z.; Wang, Q.; Liu, F.; Wang, M.; Lin, C.; Zhu, C. Pharmacokinetic Study of Four Major Bioactive Components of Liandan Xiaoyan Formula in Ulcerative Colitis and Control Rats Using UPLC-MS/MS. Front. Pharmacol. 2022, 13, 936846. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 684486. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Malinski, T. Nitric Oxide and Nitroxidative Stress in Alzheimer’s Disease. J. Alzheimer’s Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef]
- Tropea, M.R.; Gulisano, W.; Vacanti, V.; Arancio, O.; Puzzo, D.; Palmeri, A. Nitric Oxide/CGMP/CREB Pathway and Amyloid-Beta Crosstalk: From Physiology to Alzheimer’s Disease. Free Radic. Biol. Med. 2022, 193, 657–668. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural Product for the Treatment of Alzheimer’s Disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Tolar, M. Aducanumab, Gantenerumab, BAN2401, and ALZ-801—The First Wave of Amyloid-Targeting Drugs for Alzheimer’s Disease with Potential for near Term Approval. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Chung, K.K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin Ubiquitinates the α-Synuclein–Interacting Protein, Synphilin-1: Implications for Lewy-Body Formation in Parkinson Disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.I.; Park, H.; Chou, S.C.; Van Vranken, J.G.; Mittenbühler, M.J.; Kim, H.; Choi, Y.R.; Biswas, D.; Wang, J.; Shin, Y.; et al. Amelioration of Pathologic α-Synuclein-Induced Parkinson’s Disease by Irisin. Proc. Natl. Acad. Sci. USA 2022, 119, e2204835119. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Chesselet, M.-F. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef]
- Eizirik, D.L. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Bouchmaa, N.; Ainani, H.; El Fatimy, R.; Malka, G.; Mazini, L. Anti-Rheumatoid Drugs Advancements: New Insights into the Molecular Treatment of Rheumatoid Arthritis. Biomed. Pharmacother. 2022, 151, 113126. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, R.; Peng, L.; Liu, M.; Sun, Z. Efficacy and Safety of Adalimumab Biosimilars: Current Critical Clinical Data in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 638444. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.B.; Chen, L.Z.; Wang, B.S.; Huang, X.; Jiao, M.M.; Liu, M.M.; Tang, W.J.; Liu, X.H. Novel Pyrazolo [4, 3-d]Pyrimidine as Potent and Orally Active Inducible Nitric Oxide Synthase (INOS) Dimerization Inhibitor with Efficacy in Rheumatoid Arthritis Mouse Model. J. Med. Chem. 2019, 62, 4013–4031. [Google Scholar] [CrossRef] [PubMed]
- Fazalda, A.; Quraisiah, A.; Nur Azlina, M.F. Antiulcer Effect of Honey in Nonsteroidal Anti-Inflammatory Drugs Induced Gastric Ulcer Model in Rats: A Systematic Review. Evid.-Based Complement. Altern. Med. 2018, 2018, 7515692. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Ye, K.; Xu, L.; Zhang, Y. Smoking, Alcohol Consumption, Diabetes, Body Mass Index, and Peptic Ulcer Risk: A Two-Sample Mendelian Randomization Study. Front. Genet. 2022, 13, 992080. [Google Scholar] [CrossRef] [PubMed]
- Kavitt, R.T.; Lipowska, A.M.; Anyane-Yeboa, A.; Gralnek, I.M. Diagnosis and Treatment of Peptic Ulcer Disease. Am. J. Med. 2019, 132, 447–456. [Google Scholar] [CrossRef]
- de Vries, R.R.P.; Huizinga, T.W.J.; Toes, R.E.M. Redefining the HLA and RA Association: To Be or Not to Be Anti-CCP Positive. J. Autoimmun. 2005, 25, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.O.; Fonseca, A.P.R.; de Aguiar, M.T.; Dias, C.C.; Avelar, R.L.; Sousa, F.B.; Alves, A.P.N.N.; de Barros Silva, P.G. Tumor Necrosis Factor Alpha (TNF-α) Blockage Reduces Acute Inflammation and Delayed Wound Healing in Oral Ulcer of Rats. Inflammopharmacology 2022, 30, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Blans, M.C.A.; Visseren, F.L.J.; Banga, J.D.; Hoekstra, J.B.L.; van der Graaf, Y.; Diepersloot, R.J.A.; Bouter, K.P. Infection Induced Inflammation Is Associated with Erectile Dysfunction in Men with Diabetes. Eur. J. Clin. Investig. 2006, 36, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Pyrgidis, N.; Mykoniatis, I.; Haidich, A.-B.; Tirta, M.; Talimtzi, P.; Kalyvianakis, D.; Ouranidis, A.; Hatzichristou, D. The Effect of Phosphodiesterase-Type 5 Inhibitors on Erectile Function: An Overview of Systematic Reviews. Front. Pharmacol. 2021, 12, 735708. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Speina, E.; Trzybiński, D.; Winiewska-Szajewska, M.; Wińska, P.; Borsuk, E.M.; Podsiadła-Białoskórska, M.; Przygodzki, T.; Drabikowski, K.; Stanczyk, L.; et al. Diketopiperazine-Based, Flexible Tadalafil Analogues: Synthesis, Crystal Structures and Biological Activity Profile. Molecules 2021, 26, 794. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Ioakeimidis, N.; Rokkas, K.; Angelis, A.; Terentes-Printzios, D.; Stefanadis, C.; Tousoulis, D. Acute Effect of Sildenafil on Inflammatory Markers/Mediators in Patients with Vasculogenic Erectile Dysfunction. Int. J. Cardiol. 2015, 182, 98–101. [Google Scholar] [CrossRef]
- Demirtaş Şahin, T.; Yazir, Y.; Utkan, T.; Gacar, G.; Furat Rençber, S.; Gocmez, S.S. TNF-α Antagonism with Etanercept Enhances Penile NOS Expression, Cavernosal Reactivity, and Testosterone Levels in Aged Rats. Can. J. Physiol. Pharmacol. 2018, 96, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, D.; Gao, X.; Wang, J.; Li, R.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front. Pharmacol. 2020, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Dejos, C.; Voisin, P.; Bernard, M.; Régnacq, M.; Bergès, T. Canthin-6-One Displays Antiproliferative Activity and Causes Accumulation of Cancer Cells in the G2/M Phase. J. Nat. Prod. 2014, 77, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Amin, N.D.M.; Jauri, M.H.; Ling, S.K.; Hassan, N.H.; Sallehudin, N.J. The In Vitro Anti-Cancer Activities and Mechanisms of Action of 9-Methoxycanthin-6-One from Eurycoma Longifolia in Selected Cancer Cell Lines. Molecules 2022, 27, 585. [Google Scholar] [CrossRef]
- Bultum, L.E.; Tolossa, G.B.; Lee, D. Combining Empirical Knowledge, in Silico Molecular Docking and ADMET Profiling to Identify Therapeutic Phytochemicals from Brucea Antidysentrica for Acute Myeloid Leukemia. PLoS ONE 2022, 17, e0270050. [Google Scholar] [CrossRef]
- Torquato, H.F.V.; Junior, M.T.R.; Lima, C.S.; de Júnior, R.T.A.; Talhati, F.; Dias, D.A.; Justo, G.Z.; Ferreira, A.T.; Pilli, R.A.; Paredes-Gamero, E.J. A Canthin-6-One Derivative Induces Cell Death by Apoptosis/Necroptosis-like with DNA Damage in Acute Myeloid Cells. Biomed. Pharmacother. 2022, 145, 112439. [Google Scholar] [CrossRef]
- Ciordia, R.; Supko, J.; Gatineau, M.; Batchelor, T. Cytotoxic Chemotherapy: Advances in Delivery, Pharmacology, and Testing. Curr. Oncol. Rep. 2000, 2, 445–453. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Tolcher, A.W.; Rowinsky, E.K. The Future of Cytotoxic Therapy: Selective Cytotoxicity Based on Biology Is the Key. Breast Cancer Res. 2003, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Vieira Torquato, H.F.; Ribeiro-Filho, A.C.; Buri, M.V.; Araújo Júnior, R.T.; Pimenta, R.; de Oliveira, J.S.R.; Filho, V.C.; Macho, A.; Paredes-Gamero, E.J.; de Oliveira Martins, D.T. Canthin-6-One Induces Cell Death, Cell Cycle Arrest and Differentiation in Human Myeloid Leukemia Cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 958–967. [Google Scholar] [CrossRef]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 Promotes NLRP1/NLRP3 Inflammasome Activation and IL-1β Production in Acute Glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Chien, C.-Y.; Yang, Y.-H.; Hour, T.-C.; Yang, S.-F.; Chen, H.-R.; Tsai, K.-L.; Ko, J.-Y.; Chen, J.Y.-F. Autophagy Is Deficient and Inversely Correlated with COX-2 Expression in Nasal Polyps: A Novel Insight into the Inflammation Mechanism. Rhin 2015, 53, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and Autophagy-Related Proteins in Cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Lai, Z.-Q.; Liu, W.-H.; Ip, S.-P.; Liao, H.-J.; Yi, Y.-Y.; Qin, Z.; Lai, X.-P.; Su, Z.-R.; Lin, Z.-X. Seven Alkaloids from Picrasma Quassioides and Their Cytotoxic Activities. Chem. Nat. Compd. 2014, 50, 884–888. [Google Scholar] [CrossRef]
- Ammirante, M.; Di Giacomo, R.; De Martino, L.; Rosati, A.; Festa, M.; Gentilella, A.; Pascale, M.C.; Belisario, M.A.; Leone, A.; Caterina Turco, M.; et al. 1-Methoxy-Canthin-6-One Induces c-Jun NH2-Terminal Kinase–Dependent Apoptosis and Synergizes with Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Activity in Human Neoplastic Cells of Hematopoietic or Endodermal Origin. Cancer Res. 2006, 66, 4385–4393. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.; Zhai, Y.; Li, W.; Lukiw, W. Lipopolysaccharide-Stimulated, NF-KB-, MiRNA-146a- and MiRNA-155-Mediated Molecular-Genetic Communication between the Human Gastrointestinal Tract Microbiome and the Brain. Folia Neuropathol. 2019, 57, 211–219. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Compromised MAPK Signaling in Human Diseases: An Update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Paris, F.; Huot, J. The P38 Pathway, a Major Pleiotropic Cascade That Transduces Stress and Metastatic Signals in Endothelial Cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Schabbauer, G.; Tencati, M.; Pedersen, B.; Pawlinski, R.; Mackman, N. PI3K-Akt Pathway Suppresses Coagulation and Inflammation in Endotoxemic Mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt Signalling Pathway Plays a Crucial Role in the Anti-Inflammatory Effects of Curcumin in LPS-Activated Microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, S.J.; Kim, H.-Y.; Ryu, B.; Kwak, H.; Hur, J.; Choi, J.-H.; Jang, D.S. Constituents of the Stem Barks of Ailanthus Altissima and Their Potential to Inhibit LPS-Induced Nitric Oxide Production. Bioorg. Med. Chem. Lett. 2015, 25, 1017–1020. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Ruan, J.; Wichai, N.; Li, Z.; Han, L.; Zhang, Y.; Wang, T. Anti-Inflammatory Canthin-6-One Alkaloids from the Roots of Thailand Eurycoma Longifolia Jack. J. Nat. Med. 2020, 74, 804–810. [Google Scholar] [CrossRef]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular Mechanisms Underlying Prostaglandin E2-Exacerbated Inflammation and Immune Diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 Inhibitors as a Therapeutic Target in Inflammatory Diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Guha, R. On Exploring Structure–Activity Relationships. In In Silico Models for Drug Discovery; Kortagere, S., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 993, pp. 81–94. ISBN 978-1-62703-341-1. [Google Scholar]
- Ferreira, M.E.; Cebrián-Torrejón, G.; Corrales, A.S.; Vera de Bilbao, N.; Rolón, M.; Gomez, C.V.; Leblanc, K.; Yaluf, G.; Schinini, A.; Torres, S.; et al. Zanthoxylum Chiloperone Leaves Extract: First Sustainable Chagas Disease Treatment. J. Ethnopharmacol. 2011, 133, 986–993. [Google Scholar] [CrossRef]
- Tian, J.; Shen, Y.; Li, H.; Liu, R.; Shan, L.; Gao, J.; Zhang, W. Carboline Alkaloids from Psammosilene Tunicoides and Their Cytotoxic Activities. Planta Med. 2012, 78, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Mas, E. Drug Resistance in Inflammatory Bowel Diseases. Curr. Opin. Pharmacol. 2015, 25, 56–61. [Google Scholar] [CrossRef]
- Shivaji, U.N.; Sharratt, C.L.; Thomas, T.; Smith, S.C.L.; Iacucci, M.; Moran, G.W.; Ghosh, S.; Bhala, N. Review Article: Managing the Adverse Events Caused by Anti-TNF Therapy in Inflammatory Bowel Disease. Aliment. Pharm. Ther. 2019, 49, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Li, N.; Wang, X.; He, S.; Li, W.; Fan, W.; Li, R.; Liu, J.; Hou, S. Icariin Alleviates Uveitis by Targeting Peroxiredoxin 3 to Modulate Retinal Microglia M1/M2 Phenotypic Polarization. Redox Biol. 2022, 52, 102297. [Google Scholar] [CrossRef] [PubMed]
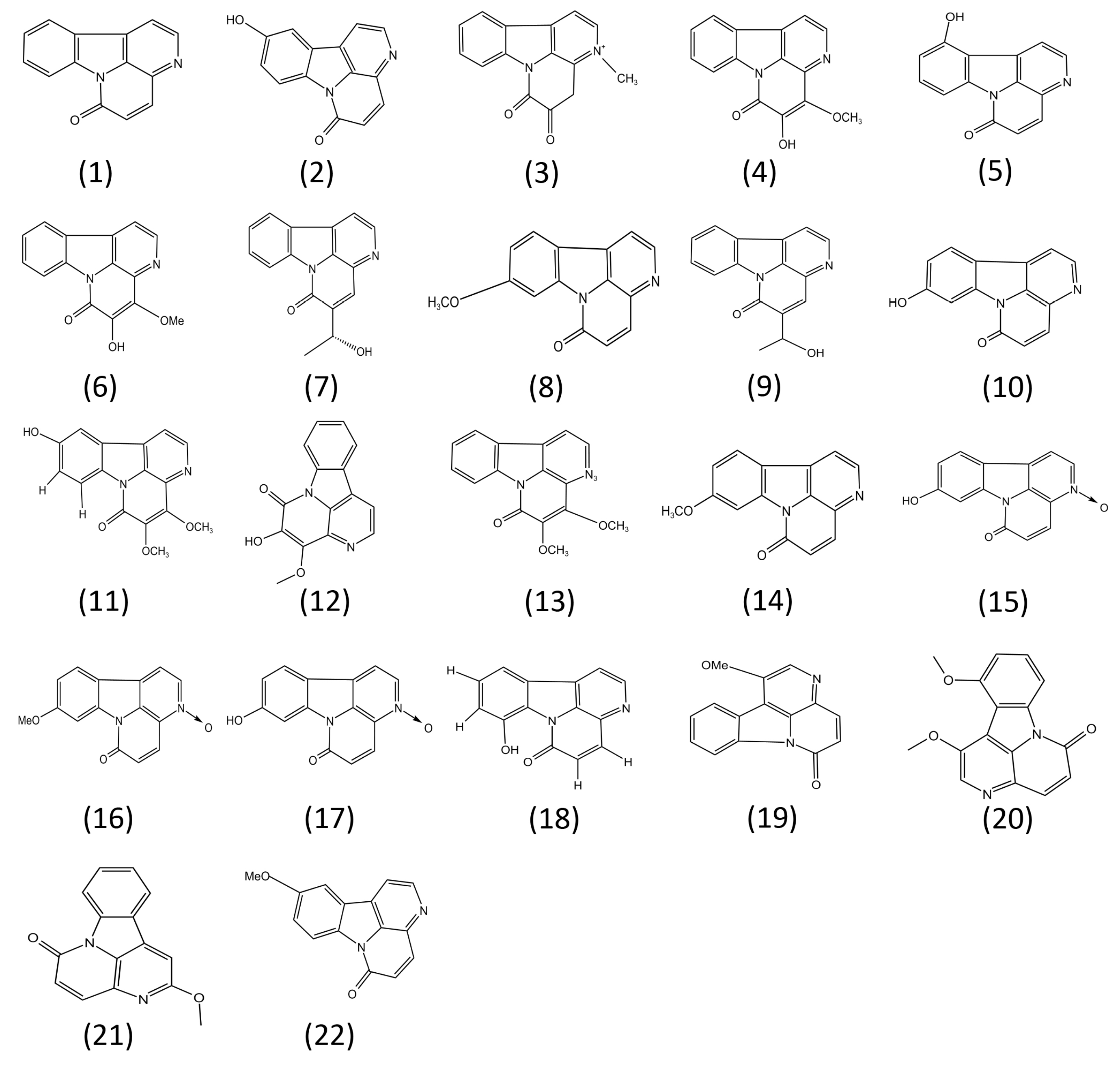
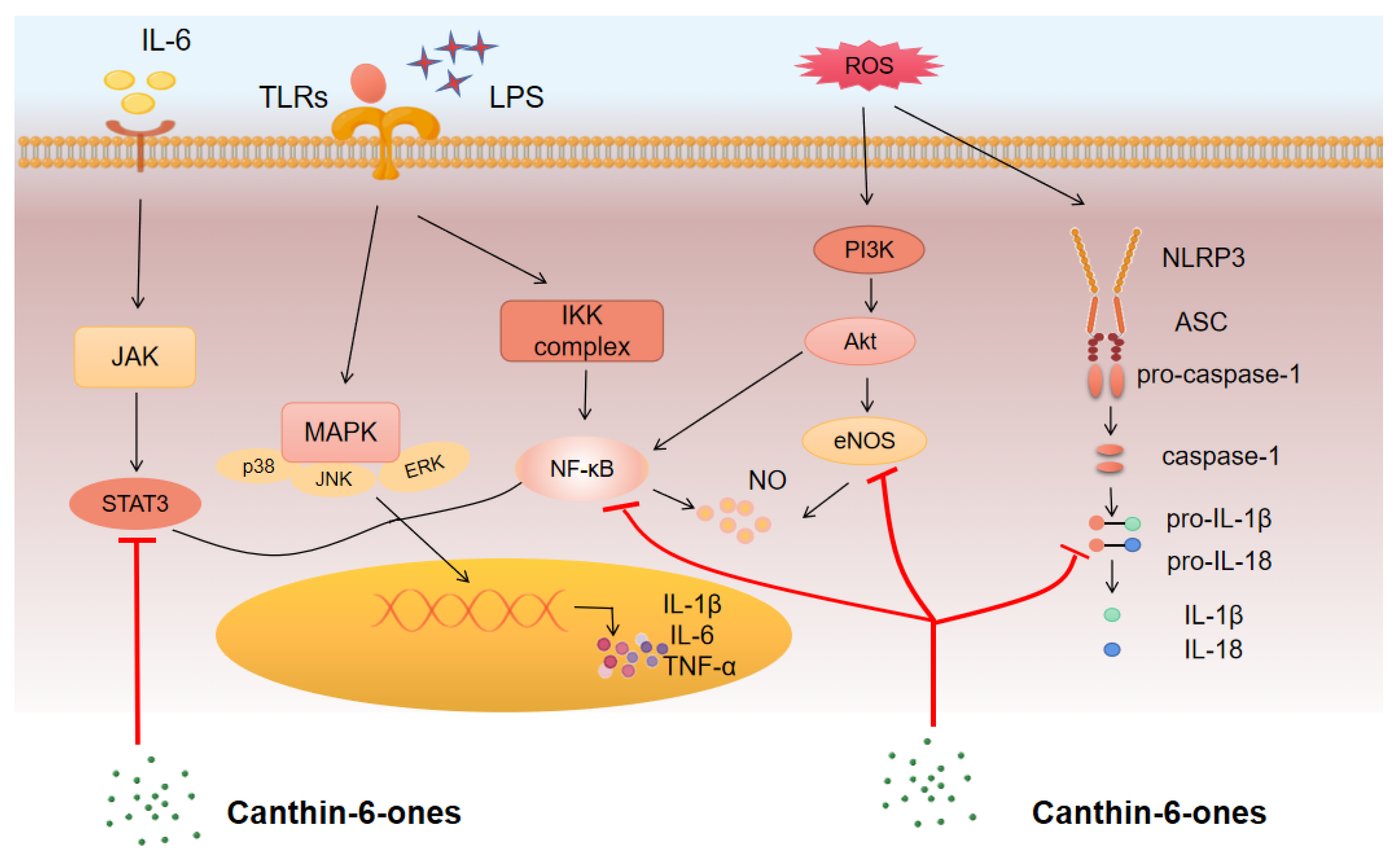
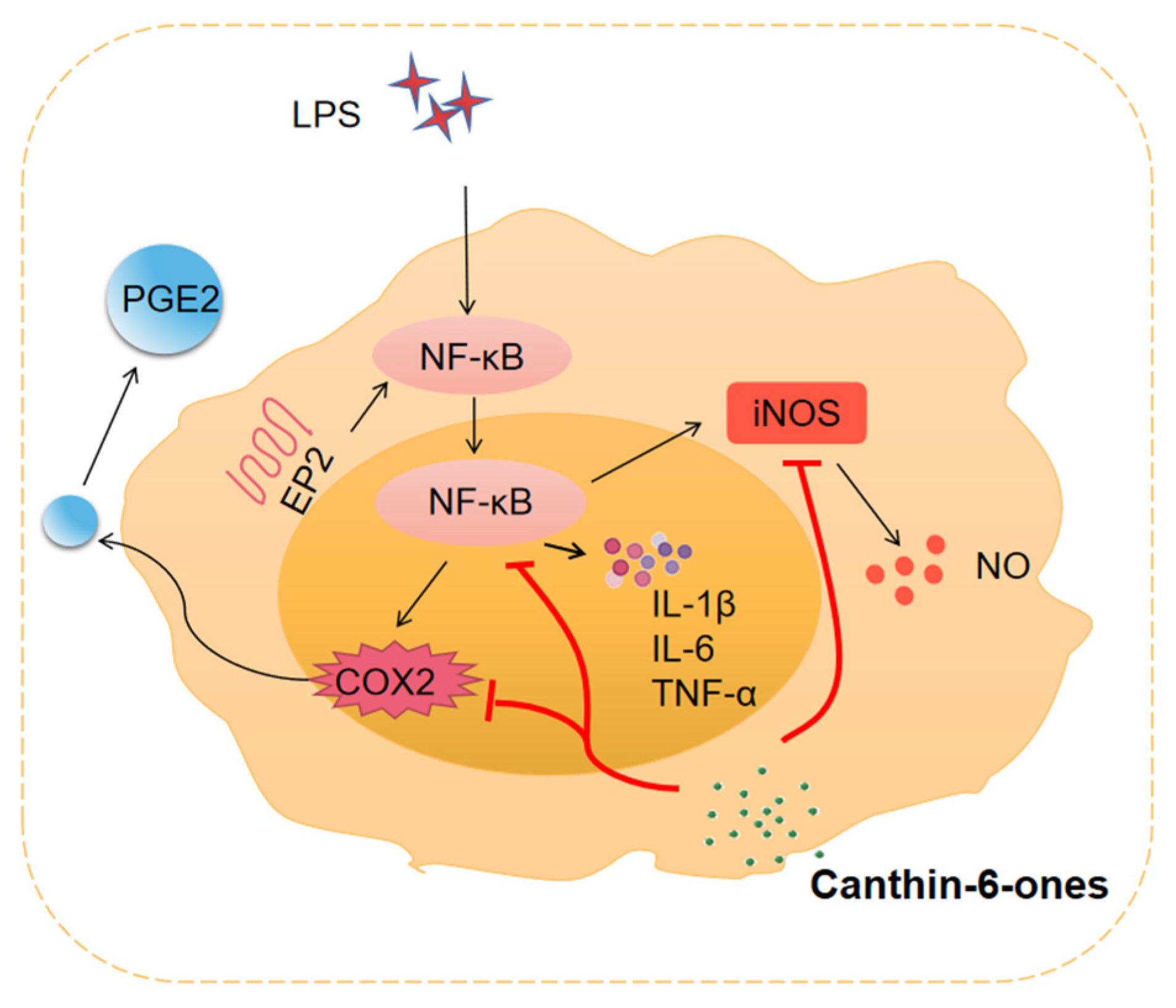
| Compound No. | Compound Name | Source | Study Type | Effects on Disease Models | Effects on Inflammatory Pathways | Reference |
|---|---|---|---|---|---|---|
| (1) | Canthin-6-one | Several plants, including the initial source, Pentaceras australis | In vivo /In vitro | Suppresses colitis model in Wistar rats by oral gavage (1, 5, and 25 mg/kg) | Suppresses the expression of NO and COX2 (12.5, 25, and 50 μM), PDE-5 (4.31 ± 0.52 μM), NF-κB, MAPK, STAT3, PI3K/Akt, and the NLRP3 inflammasome (6.5, 12.5, and 25 μM) | [15,17,25,27,29,40,45] |
| (2) | 10-Hydroxycanthin-6-one | Chemical synthesis | In vitro | Unknown | Suppresses the expression of NO (5.92 ± 0.9~15.09 ± 1.8 μM) | [45,46] |
| (3) | 3-Methylcanthin-5,6-dione | Picrasma quassioides (D. Don) Benn | In vivo /In vitro | Suppresses Alzheimer’s disease model in ICR mice by oral administration (25, 50, 100 mg/kg) | Suppresses the expression of NO (3~30 μM) | [12,47,48,49] |
| (4) | 1:4-Methoxy-5-hyroxycanthin-6-one | Picrasma quassiodes (D. Don) Benn. | In vivo | Suppresses artery hypertension disease model in spontaneously hypertensive rats by oral gavage. Suppresses the expression of NO (50, 100, 200 mg/kg) | Unknown | [50] |
| (5) | 11-Hydroxycanthin-6-one | Brucea mollis var. tonkinensis | In vitro | Unknown | Suppresses the expression of NO (5.92 ± 0.9~15.09 ± 1.8 μM) | [45,51] |
| (6) | 4-Methoxy-5hydroxy-canthin-6-one | Picrasma quassioides | In vivo/In vitro | Suppresses chronic arthritis model in male Sprague–Dawley (SD) rats by oral administration (3, 9, 27 mg/kg) | Suppresses the expression of NO (10, 30, and 100 μM) | [15] |
| (7) | (R)-5-(1-hydroxyethyl)-canthin-6-one | Ailanthus altissima Swingle | In vitro | Unknown | Suppresses the expression of NO (5.92 ± 0.9~15.09 ± 1.8 μM) | [45] |
| (8) | 9-Methoxy-canthin-6-one | Simaroubaceae plants | In vitro | Unknown | Suppresses the expression of NO and COX2 (12.5, 25, and 50 μM) or the activity of PDE-5 (3.30 ± 1.03 μM) | [15,17,26,29] |
| (9) | 5-(1-Hydroxyethyl)- 6-canthin-6-one | Ailanthus altissima | In vitro | Unknown | Suppresses NF-κB pathway (15 μM) | [27] |
| (10) | 9-Hydroxycanthin-6-one | Eurycoma longifolia | In vivo /In vitro | Suppresses Alzheimer disease model in mice by oral administration (25, 50, 100 mg/kg) | Suppresses NF-κB pathway (3.8 μM) and the activity of PDE-5 (4.66 ± 1.13 μM) | [12,17,26,45,52] |
| (11) | 4,5-Dimethoxy-10-hydroxycanthin-6-one | Picrasma quassioides (D. Don) Benn | In vivo/In vitro | Suppresses Alzheimer disease model in mice by oral administration (25, 50, 100 mg/kg) | Suppresses the expression of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (25 μM, 50 μM, and 100 μM) | [12] |
| (12) | 4,5-Dimethoxy- canthin-6-one | Picrasma quassioides BENNET | In vivo/In vitro | Suppresses Alzheimer disease model in mice by oral administration (25, 50, 100 mg/kg) | Suppresses the expression of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (25 μM, 50 μM, and 100 μM) | [12] |
| (13) | 4-Methoxy-5-hydroxyl- 5-canthin-6-one | Picrasma quassioides BENNET | In vivo/In vitro | Suppresses Alzheimer disease model in mice by oral administration (25, 50, 100 mg/kg) | Suppresses the expression of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (25 μM, 50 μM, and 100 μM) | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, A.; Wang, Y.; Sun, W.; Zhou, X.; Xu, Q.; Mao, L.; Zhang, J. Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators. Molecules 2023, 28, 3381. https://doi.org/10.3390/molecules28083381
Zhang Z, Wang A, Wang Y, Sun W, Zhou X, Xu Q, Mao L, Zhang J. Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators. Molecules. 2023; 28(8):3381. https://doi.org/10.3390/molecules28083381
Chicago/Turabian StyleZhang, Zongying, Anqi Wang, Yunhan Wang, Weichen Sun, Xiaorong Zhou, Qiuyun Xu, Liming Mao, and Jie Zhang. 2023. "Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators" Molecules 28, no. 8: 3381. https://doi.org/10.3390/molecules28083381
APA StyleZhang, Z., Wang, A., Wang, Y., Sun, W., Zhou, X., Xu, Q., Mao, L., & Zhang, J. (2023). Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators. Molecules, 28(8), 3381. https://doi.org/10.3390/molecules28083381








