3.1. Chemistry
All chemicals were of analytical reagent grade, and all solvents were of reagent grade. The reactions were monitored by thin-layer chromatography (TLC) (Qingdao Haiyang Chemical Co., Qingdao, China). The solutions containing the products were concentrated with a rotary evaporator. Flash column chromatography was performed in a column packed with 200–300 mesh silica gel. TLC was performed on silica gel GF254-coated glass sheets, and the spots were visualized under UV light (254 nm). NMR spectra were recorded with the Agilent DD2 600 MHz NMR spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) in CDCl3 containing 0.03% v/v tetramethylsilane (TMS) as the internal standard. The HRMS was recorded on a Xevo G2-XS QTOF instrument (Waters, MA, USA).
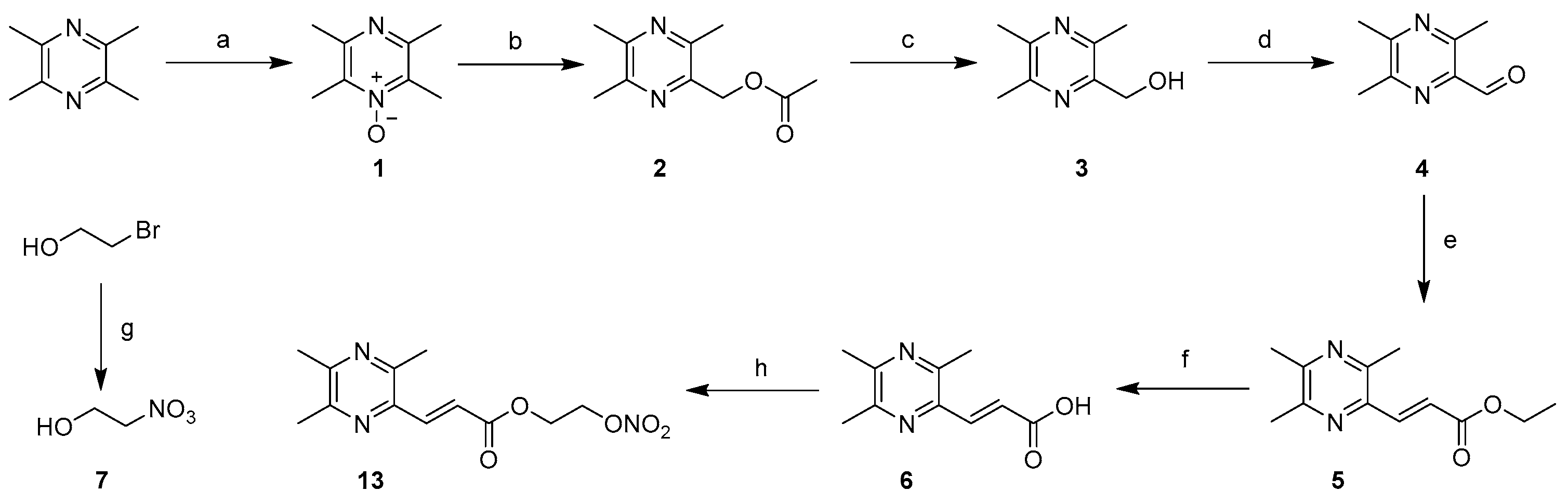
3.1.1. General Procedure for the Preparation of 1
NaOH (8.0 g, 200 mmol) and ligustrazine hydrochloride (41.8 g, 200 mmol) were dissolved in 300 mL and 100 mL of water at 0 °C to prepare solutions, respectively. To the stirred solution of ligustrazine hydrochloride was added slowly the NaOH solution. After stirring for one hour, water was removed under reduced pressure, and the product was collected by filtration and dried by a standard method to obtain ligustrazine trihydrate. Then, a solution of ligustrazine trihydrate (20.4 g, 107 mmol) in glacial acetic acid (30 mL) was added to the 30% H2O2 aqueous solution (12.1 mL, 107 mmol). The reaction mixture was stirred at 90 °C for 2 h before another portion of the 30% H2O2 aqueous solution (12.1 mL, 107 mmol) was added and stirred at 90 °C for another 2 h. The resultant mixture was cooled to room temperature, alkalized to pH = 10 with 50% sodium hydroxide and extracted with dichloromethane (150 mL, 50 mL × 3). The organic layer was combined and dried with anhydrous sodium sulfate for 8 h and evaporated in vacuo to afford ligustrazine mono-N-oxide (1).
3.1.2. General Procedure for the Preparation of 3
Compound 1 (13.8 g, 90.6 mmol) was treated with acetic anhydride (17.1 mL, 181.2 mmol) at 130 °C for 2.5 h. The mixture was added in 20% NaOH (aq, 100 mL) and stirred at room temperature overnight. The resultant mixture was extracted with dichloromethane (160 mL, 40 mL × 4). The extract was dried with anhydrous sodium sulfate for 8 h and evaporated in vacuo to obtain the crude product. The crude product was recrystallized from a mixed solvent of petroleum ether (PE) and ethyl acetate (EA) (PE/EA = 10/1, v/v) to obtain 3 as a yellow solid (5.5 g), an overall yield of 33.7%.
3.1.3. General Procedure for the Preparation of 4
To a solution of 3 (6.09 g, 40 mmol) in anhydrous ethanol (20 mL), active MnO2 was added (20.8 g, 240 mmol). After heating to reflux for 6 h, the reaction mixture was filtered through a sintered glass funnel layered with a layer of diatomite, and the filtrate was evaporated in vacuo to obtain 3,5,6-trimethylpyrazine-2-carbaldehyde (4) as a yellow solid (5.67 g).
3.1.4. General Procedure for the Preparation of 5
To a solution of compound 4 (5.5 g, 36.6 mmol) in toluene (50 mL), NaH (1.76 g, 73.2 mmol) was added carefully in an ice bath, and after 0.5 h, triethyl phosphonoacetate (9.0 g, 40.3 mmol) was added dropwise, and the mixture was stirred at room temperature overnight. The reaction solution was diluted by adding EtOAc (50 mL), then washed with saturated brine (50 mL × 3), and the combined extract was dried over anhydrous sodium sulfate for 8 h and concentrated in vacuo. The crude product was purified by column chromatography (PE/EA = 8/1, v/v) to obtain 5 as a pale-yellow solid (6.45 g).
3.1.5. General Procedure for the Preparation of 6
Compound 5 (6.45 g, 29.3 mmol) was dissolved in a mixed solvent of tetrahydrofuran (THF) and water (THF/H2O = 2/1, v/v, 30 mL), and then sodium hydroxide (2.35 g, 58.6 mmol) was slowly added, and the mixture was stirred at room temperature for 4 h. The reaction mixture was adjusted with 2 mol/L of hydrochloric acid to pH = 4, followed by the addition of sodium chloride solid until it dissolved more. Next, the mixture was extracted with EtOAc (90 mL, 30 mL × 3), and the combined organic phase was dried over anhydrous sodium sulfate for 8 h and evaporated in vacuo to obtain 6 as a white solid (5.5 g).
3.1.6. General Procedure for the Preparation of 7
2-Bromoethanol (1.25 g, 10 mmol) was dissolved in acetonitrile (50 mL), and AgNO3 (2.55 g, 15 mmol) was added to the reaction solution, which was then stirred under darkness at 70 °C for 3 h. After cooling, the reaction mixture was filtered to remove the solid product, and the filtrate was concentrated in vacuo. The concentrate was stirred for 10 min after adding 30 mL EtOAc, then filtered and concentrated again to obtain 7 (1.0 g) as a yellow liquid.
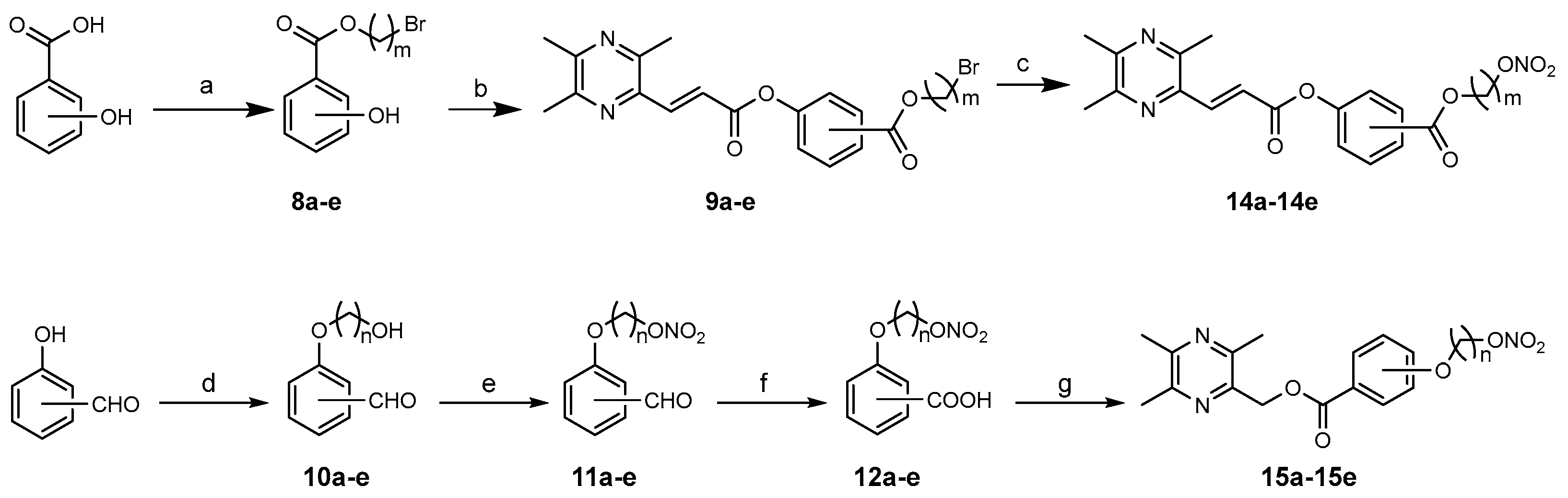
3.1.7. General Procedure for the Preparation of 8a–e
The corresponding dibromoethane (7.5 mmol,1.5 eq) and triethylamine (7.5 mmol, 1.5 eq) were added to a solution of hydroxybenzoic acid (0.69 g, 5 mmol) in acetone (20 mL). After heating to reflux for 6 h under nitrogen, the reaction mixture was cooled to room temperature and evaporated in vacuo. The residue was stirred for 5 min after adding 30 mL of EtOAc and then filtered and concentrated. The concentrate was purified by column chromatography (PE/EA = 10/1, 8/1, v/v) to obtain 8a–e: 8a, colorless oil, PE/EA = 8/1, yield 22%; 8b, colorless oil, PE/EA = 8/1, yield 27%; 8c, white solid, PE/EA = 8/1, yield 29%; 8d, colorless oil, PE/EA = 10/1, yield 61%; 8e, colorless oil, PE/EA = 10/1, yield 60%.
3.1.8. General Procedure for the Preparation of 9a–e
DCC (0.23 g, 1.1 mmol) and 6 (0.19 g, 1.0 mmol) were dissolved in anhydrous dichloromethane (20 mL), and a catalytic amount of DMAP was added to the reaction solution, which was then stirred for about 15 min. Next, 8a–e (1.1 mmol,1.1 eq) was added to the solution and stirred for 4 h at room temperature. The resultant mixture was filtered, and the filtrate was concentrated in vacuo. The concentrate was purified by column chromatography (PE/EA = 5/1, 4/1, v/v) to obtain 9a–e: 9a, white powder, PE/EA = 5/1, yield 67%; 9b, white powder, PE/EA = 5/1, yield 70%; 9c, white powder, PE/EA = 5/1, yield 70%; 9d, yellow solid, PE/EA = 5/1, yield 72%; 9e, white solid, PE/EA = 5/1, yield 93%.
3.1.9. General Procedure for the Preparation of 10a–e
To a solution of hydroxybenzaldehyde (1.22 g, 10 mmol) in acetone (20 mL), the corresponding brominated alcohol (20 mmol, 2.0 eq) and anhydrous K2CO3 (20 mmol, 2.0 eq) were added. After heating to reflux for 6 h under nitrogen, the reaction mixture was cooled to room temperature and concentrated in vacuo. The concentrate was purified by column chromatography (PE/EA = 4/1, 2/1, v/v) to obtain 10a–e: 10a, yellow oil, PE/EA = 4/1–2/1, yield 38%; 10b, cream oil, PE/EA = 4/1, 2/1, yield 29%; 10c, mauve oil, PE/EA = 3/1, yield 67%; 10d, yellow oil, PE/EA = 4/1, yield 35%; 10e, yellow oil, PE/EA = 4/1, yield 58%.
3.1.10. General Procedure for the Preparation of 11a–e
Compounds 10a–e (1.0 eq) and a small amount of carbamide were dissolved in glacial acetic acid (5 mL), and mixtures of Fuming HNO3 (5.0 eq) and acetic anhydride (5 mL) were slowly added dropwise at −5 °C. The reaction solution was stirred at −5 °C for 0.5 h and then kept at room temperature for 2 h. The resultant solution was poured into ice water, then adjusted to pH = 7 with the saturated NaHCO3 solution and extracted with EtOAc (60 mL, 20 mL × 3). The combined organic layer was washed with brine, dried over anhydrous sodium sulfate for 8 h and concentrated in vacuo. The concentrate was purified by column chromatography (PE/EA = 4/1, 3/1, v/v) to obtain 11a–e: 11a, yellow oil, PE/EA = 4/1, yield 66%; 11b, yellow oil, PE/EA = 4/1, yield 63%; 11c, yellow oil, PE/EA = 3/1, yield 60%; 11d, yellow oil, PE/EA = 4/1, yield 61%; 11e, yellow oil, PE/EA = 4/1, yield 63%.
3.1.11. General Procedure for the Preparation of 12a–e
KMnO4 (1.5 eq) was added to a solution of 11a–e (1.0 eq) in acetone (20 mL) and heated to reflux for 2 h. The reaction mixture was added to the saturated NaHSO3 solution until its red faded completely. Then, the pH of the solution was adjusted to 5 with 2 mol/L of hydrochloric acid, and after being filtered through a sintered glass funnel layered with a layer of diatomite, the filtrate was extracted with EtOAc (60 mL, 20 mL × 3). The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate for 8 h and evaporated in vacuo to afford 12a–e: 12a, yellow oil, yield 82%; 12b, yellow solid, yield 88%; 12c, white solid, yield 90%; 12d, yellow oil, yield 86%; 12e, yellow oil, yield 85%.
3.1.12. 2-(Nitrooxy)ethyl (E)-3-(3,5,6-Trimethylpyrazin-2-yl)acrylate (13)
Both 6 (0.74 g, 3.8 mmol) and DCC (0.79 g, 3.8 mmol) were dissolved in anhydrous dichloromethane (30 mL), and a catalytic amount of DMAP was added to the reaction solution, which was then stirred for about 15 min. Next, compound 7 (0.38 g, 3.5 mmol) was added to the solution and stirred for 6 h at room temperature. The resultant mixture was filtered, and the filtrate was concentrated in vacuo. The concentrate was stirred for 15 min after adding 20 mL EtOAc, then filtered and concentrated again, and was purified by column chromatography (PE/EA = 5/1, v/v) to obtain target compound 13 (0.40 g), with an overall yield of 41%. White solid, 1H NMR (600 MHz, Chloroform-d): δ 7.88 (d, 1H, J = 15.3 Hz, ArCH = CH), 7.06 (d, 1H, J = 15.3 Hz, ArCH = CH), 4.79–4.72 (m, 2H, CH2CH2NO3), 4.55–4.48 (m, 2H, CH2CH2NO3), 2.62 (s, 3H, ArCH3), 2.52 (d, 6H, J = 4.6 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 166.28, 153.01, 150.02, 149.12, 142.25, 140.06, 121.87, 77.20, 76.99, 76.77, 70.44, 60.36, 22.02, 21.69, 20.66; HRMS (ESI): m/z: 281.1112 calc. for C12H15N3O5 [M + H]+, found 281.1109, ppm error −1.1.
3.1.13. General Procedure for the Preparation of 14a–14e
9a–e (1.0 eq) was dissolved in acetonitrile (20 mL), and AgNO3 (1.5 eq) was added to the reaction solution, which was then stirred under darkness at 70 °C for 3 h. After cooling, the reaction mixture was filtered to remove the solid product, and the filtrate was concentrated in vacuo. The concentrate was stirred for 10 min after adding 30 mL of EtOAc, then filtered and concentrated again, and purified by column chromatography (PE/EA = 4/1, 2/1, v/v) or TLC (DCM/MeOH = 50/1, v/v) to obtain target compounds 14a–14e.
2-(nitrooxy)ethyl (E)-2-((3-(3,5,6-trimethylpyrazin-2-yl)acryloyl)oxy)benzoate (14a). Cream solid, PE/EA = 2/1, yield 37%; 1H NMR (600 MHz, Chloroform-d): δ 8.08 (d, 1H, J = 6.4 Hz, 6-ArH), 8.05 (d, 1H, J = 15.6 Hz, ArCH = CH), 7.60 (t, 1H, J = 7.8 Hz, 4-ArH), 7.36 (t,1H, J = 7.6 Hz, 5-ArH), 7.31 (d, 1H, J = 15.3 Hz, ArCH = CH), 7.19 (d, 1H, J = 8.0 Hz, 3-ArH), 4.38 (t, 2H, J = 4.5 Hz, CH2CH2NO3), 3.84 (t, 2H, J = 4.5 Hz, CH2CH2NO3), 2.66 (s, 3H, ArCH3), 2.56 (d, 6H, J = 5.9 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 165.28, 164.98, 152.75, 150.56, 150.22, 148.86, 142.79, 140.50, 133.99, 132.03, 126.19, 123.78, 123.43, 122.71, 77.22, 77.01, 76.80, 66.93, 60.71, 21.63, 21.61, 20.29; HRMS (ESI): m/z: 402.1301 calc. for C19H20N3O7 [M + H]+, found 402.1291, ppm error −2.5.
2-(nitrooxy)ethyl (E)-3-((3-(3,5,6-trimethylpyrazin-2-yl)acryloyl)oxy)benzoate (14b). Yellow solid, PE/EA = 2/1, yield 64%; 1H NMR (600 MHz, Chloroform-d) δ 8.03 (d, 1H, J = 15.2 Hz, ArCH = CH), 7.96 (d, 1H, J = 7.8 Hz, 6-ArH), 7.86 (s, 1H, 2-ArH), 7.49 (m, 1H, J = 7.9 Hz, 5-ArH), 7.39 (d, 1H, J = 8.1 Hz, 4- ArH), 7.24 (d, 1H, J = 15.3 Hz, ArCH = CH), 4.47 (t, 2H, J = 4.6 Hz, CH2CH2NO3), 3.96 (t, 2H, J = 4.6 Hz, CH2CH2NO3), 2.64 (s, 3H, ArCH3), 2.55 (s, 6H, ArCH3); 13C NMR (151 MHz, CDCl3) δ 165.93, 164.95, 153.15, 150.68, 150.26, 149.17, 142.28, 140.95, 131.41, 129.49, 127.12, 126.51, 122.89, 121.88, 77.22, 77.01, 76.80, 66.90, 61.19, 21.95, 21.71, 20.58; HRMS (ESI): m/z: 402.1301 calc. for C19H20N3O7 [M + H]+, found 402.1300, ppm error −0.2.
2-(nitrooxy)ethyl (E)-4-((3-(3,5,6-trimethylpyrazin-2-yl)acryloyl)oxy)benzoate (14c). Yellow solid, PE/EA = 2/1, yield 50%; 1H NMR (600 MHz, Chloroform-d): δ 8.12 (d, 2H, J = 8.5 Hz, 2,6-ArH), 8.03 (d, 1H, J = 15.2 Hz, ArCH = CH), 7.31–7.24 (m, 3H, 3,5-ArH and ArCH = CH), 4.48 (t, 2H, J = 4.7 Hz, CH2CH2NO3), 3.97 (t, 2H, J = 4.6 Hz, CH2CH2NO3), 2.67 (s, 3H, ArCH3), 2.58 (d, 6H, J = 7.2 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 166.11, 164.47, 154.54, 152.61, 150.83, 148.59, 142.77, 140.60, 131.29, 127.43, 122.34, 121.63, 77.21, 77.00, 76.79, 66.77, 61.33, 21.71, 21.52, 20.12; HRMS (ESI): m/z: 402.1301 calc. for C19H20N3O7 [M + H]+, found 402.1299, ppm error −0.5.
4-(nitrooxy)butyl (E)-2-((3-(3,5,6-trimethylpyrazin-2-yl)acryloyl)oxy)benzoate (14d). Cream solid, PE/EA = 4/1, yield 39%; 1H NMR (600 MHz, Chloroform-d) δ 8.06 (d, 1H, J = 14.7 Hz, ArCH = CH), 8.04 (d, 1H, J = 7.9 Hz, 6-ArH), 7.60 (t, 1H, J = 7.8 Hz, 4-ArH), 7.36 (t, 1H, J = 7.7 Hz, 5-ArH), 7.32 (d, 1H, J = 15.2 Hz, ArCH = CH), 7.19 (d, 1H, J = 8.1 Hz, 3-ArH), 4.43 (t, 2H, J = 5.9 Hz, CH2NO3), 4.30 (t, 2H, J = 5.6 Hz, COOCH2), 2.65 (s, 3H, ArCH3), 2.55 (d, 6H, J = 5.6 Hz, ArCH3), 1.87–1.77 (m, 4H, CH2CH2CH2CH2); 13C NMR (151 MHz, CDCl3) δ 165.17, 164.62, 153.10, 150.39, 150.23, 149.14, 142.28, 140.79, 133.91, 131.78, 126.11, 123.84, 123.52, 122.00, 77.22, 77.01, 76.79, 72.53, 64.18, 24.99, 23.62, 21.97, 21.70, 20.58; HRMS (ESI): m/z: 430.1614 calc. for C21H24N3O7 [M + H]+, found 430.1617, ppm error 0.7.
6-(nitrooxy)hexyl (E)-2-((3-(3,5,6-trimethylpyrazin-2-yl)acryloyl)oxy)benzoate (14e). Yellow oil, DCM/MeOH = 50/1, yield 59%; 1H NMR (600 MHz, Chloroform-d) δ 8.01 (d, 1H, J = 15.3 Hz, ArCH = CH), 8.00 (d, 1H, J = 8.5 Hz, 6-ArH), 7.55 (t, 1H, J = 7.7 Hz, 4-ArH), 7.31 (t, 1H, J = 6.8 Hz, 5-ArH), 7.29 (d, 1H, J = 15.4 Hz, ArCH = CH), 7.15 (d, 1H, J = 8.1 Hz, 3-ArH), 4.34 (t, 2H, J = 6.6 Hz, CH2NO3), 4.22 (t, 2H, J = 6.6 Hz, COOCH2), 2.61 (s, 3H, ArCH3), 2.51 (d, 6H, J = 4.1 Hz, ArCH3), 1.65 (td, 4H, J = 14.5, 7.6 Hz, CH2CH2CH2CH2CH2CH2), 1.37 (td, 4H, J = 11.7, 10.4, 6.8 Hz, CH2CH2CH2CH2CH2CH2); 13C NMR (151 MHz, CDCl3) δ 165.15, 164.69, 153.08, 150.38, 150.08, 149.15, 142.27, 140.63, 133.71, 133.68, 131.78, 131.74, 126.04, 126.02, 123.81, 123.79, 123.77, 122.12, 122.09, 77.27, 77.06, 76.85, 73.06, 65.07, 64.92, 28.44, 28.39, 26.55, 25.62, 25.54, 25.29, 25.17, 22.03, 22.01, 21.73, 21.69, 20.67, 20.65; HRMS (ESI): m/z: 458.1927 calc. for C23H28N3O7 [M + H]+, found 458.1927, ppm error 0.0.
3.1.14. General Procedure for the Preparation of 15a–15e
Compound 12a–e (1.2 mmol, 1.2 eq) and DCC (1.2 mmol,1.2 eq) were dissolved in anhydrous dichloromethane (20 mL), and a catalytic amount of DMAP was added to the reaction solution, which was then stirred for about 15 min. Next, 3 (1.0 mmol,1.0 eq) was added to the solution and stirred for 6 h at room temperature. The resultant mixture was filtered, and the filtrate was concentrated in vacuo. The concentrate was purified by TLC (PE/EA/EtOH = 30/10/1, v/v/v) to obtain the target compounds 15a–15e.
(3,5,6-trimethylpyrazin-2-yl)methyl 2-(2-(nitrooxy)ethoxy)benzoate (15a). Yellow solid, 43% yield; 1H NMR (600 MHz, Chloroform-d): δ 7.85 (d, 1H, J = 7.8 Hz, 6-ArH), 7.47 (t, J = 7.8 Hz, 1H, 4-ArH), 7.04 (t, J = 7.6 Hz, 1H, 5-ArH), 6.94 (d, 1H, J = 8.3 Hz, 3-ArH), 5.42 (s, 2H, ArCH2), 4.72 (t, 2H, J = 4.7 Hz, CH2CH2NO3), 4.28 (t, 2H, J = 4.7 Hz, CH2CH2NO3), 2.59 (s, 3H, ArCH3), 2.52 (d, 6H, J = 6.9 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 165.54, 157.63, 151.38, 149.24, 148.97, 144.81, 133.69, 132.09, 121.60, 120.81, 114.28, 77.24, 77.02, 76.81, 70.75, 65.70, 65.57, 21.61, 21.36, 20.52; HRMS (ESI): m/z: 362.1352 calc. for C17H20N3O6 [M + H]+, found 362.1361, ppm error 2.5.
(3,5,6-trimethylpyrazin-2-yl)methyl 3-(2-(nitrooxy)ethoxy)benzoate (15b). Cream solid, 54% yield; 1H NMR (600 MHz, Chloroform-d): δ 7.69 (dd, 1H, J = 7.7, 1.4 Hz, 6-ArH), 7.55 (s, 1H, 2-ArH), 7.35 (t, 1H, J = 7.9 Hz, 5-ArH), 7.11 (dd, 1H, J = 8.3, 2.9 Hz, 4-ArH), 5.44 (s, 2H, ArCH2), 4.82 (t, 2H, J = 4.6 Hz, CH2CH2NO3), 4.28 (t, 2H, J = 4.5 Hz, CH2CH2NO3), 2.59 (s, 3H, ArCH3), 2.52 (d, 6H, J = 9.8 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 165.81, 157.90, 151.47, 149.26, 149.02, 144.68, 131.19, 129.65, 123.06, 120.22, 114.69, 77.24, 77.03, 76.82, 70.73, 65.93, 64.22, 21.63, 21.37, 20.54; HRMS (ESI): m/z: 362.1352 calc. for C17H20N3O6 [M + H]+, found 362.1353, ppm error 0.3.
(3,5,6-trimethylpyrazin-2-yl)methyl 4-(2-(nitrooxy)ethoxy)benzoate (15c). White solid, 45% yield; 1H NMR (600 MHz, Chloroform-d): δ 8.01 (d, 2H, J = 8.5 Hz, 2,6-ArH), 6.90 (d, 2H, J = 8.3 Hz, 3,5-ArH), 5.42 (s, 2H, ArCH2), 4.83 (t, 2H, J = 4.5 Hz, CH2CH2NO3), 4.30 (t, 2H, J = 4.5 Hz, CH2CH2NO3), 2.58 (s, 3H, ArCH3), 2.52 (d, 6H, J = 8.3 Hz, ArCH3); 13C NMR (151 MHz, CDCl3) δ 165.69, 161.72, 151.33, 149.28, 148.93, 144.93, 131.86, 131.80, 123.06, 114.15, 114.10, 77.26, 77.05, 76.84, 70.57, 65.65, 64.07, 21.63, 21.38, 20.55; HRMS (ESI): m/z: 362.1352 calc. for C17H20N3O6 [M + H]+, found 362.1351, ppm error −0.3.
(3,5,6-trimethylpyrazin-2-yl)methyl 3-(4-(nitrooxy)butoxy)benzoate (15d). Colorless oil, 54% yield; 1H NMR (600 MHz, Chloroform-d): δ 7.59 (dd, 1H, J = 7.7, 1.3 Hz, 6-ArH), 7.49 (s, 1H, 2-ArH), 7.28 (t, J = 8.0 Hz, 1H, 5-ArH), 7.04 (dd, 1H, J = 8.3, 2.6 Hz, 4-ArH), 5.39 (s, 2H, ArCH2), 4.49 (t, 2H, J = 6.1 Hz, CH2NO3), 3.98 (t, 2H, J = 5.6 Hz, OCH2), 2.54 (s, 3H, ArCH3), 2.48 (d, 6H, J = 9.2 Hz, ArCH3), 1.88 (ddt, 4H, J = 10.7, 5.8, 2.2 Hz, CH2CH2CH2CH2); 13C NMR (151 MHz, CDCl3) δ 166.03, 158.69, 151.42, 149.31, 148.99, 144.79, 131.06, 129.48, 122.33, 119.98, 114.77, 77.22, 77.01, 76.79, 72.79, 67.13, 65.92, 25.46, 23.80, 21.65, 21.40, 20.57; HRMS (ESI): m/z: 390.1665 calc. for C19H24N3O6 [M + H]+, found 390.1668, ppm error 0.8.
(3,5,6-trimethylpyrazin-2-yl)methyl 3-((6-(nitrooxy)hexyl)oxy)benzoate (15e). Colorless oil, 49% yield; 1H NMR (600 MHz, Chloroform-d) δ 7.58 (dd, 1H, J = 7.7, 1.4 Hz, 6-ArH), 7.50 (s, 1H, 2-ArH), 7.27 (t, 1H, J = 8.0 Hz, 5-ArH), 7.04 (dd, 1H, J = 8.3, 2.6 Hz, 4-ArH), 5.39 (s, 2H, ArCH2), 4.41 (t, 2H, J = 6.7 Hz, CH2NO3), 3.94 (t, 2H, J = 6.3 Hz, OCH2), 2.54 (s, 3H, ArCH3), 2.47 (d, 6H, J = 8.9 Hz, ArCH3), 1.79–1.67 (m, 4H, CH2CH2CH2CH2CH2CH2), 1.51–1.39 (m, 4H, CH2CH2CH2CH2CH2CH2); 13C NMR (151 MHz, CDCl3) δ 166.11, 158.96, 151.40, 149.33, 148.97, 144.83, 130.99, 129.40, 122.07, 120.00, 114.84, 77.21, 77.00, 76.79, 73.16, 67.81, 65.91, 28.92, 26.69, 25.62, 25.42, 21.65, 21.40, 20.58; HRMS (ESI): m/z: 418.1978 calc. for C21H28N3O6 [M + H]+, found 418.1978, ppm error 0.0.
3.1.15. Data Analysis of NMR Spectra
NMR spectra were analyzed using the software MestReNova 14.0 after confirming that the measured molecular mass in the HRMS of individual compounds matched their calculated value. The 1H NMR spectral peak reports of the compounds were exported after processing for peak picking, integration and multiplets analysis using the software MestReNova 14.0. Individual peaks’ data were analyzed, and the corresponding hydrogen atom was found in the compound structure. The peaks in 13C NMR spectra were also picked and reported using this software, and the data of the characteristic carbon peaks (C=O, – CH3) were analyzed.