A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens
Abstract
1. Introduction
1.1. Acquired Immunodeficiency Syndrome
1.2. Tuberculosis
- Group A: Highly effective drugs that unless contraindicated, are strongly recommended for inclusion in all regimens (fluoroquinolones such as levofloxacin and moxifloxacin, bedaquiline and linezolid).
- Group B: Conditionally recommended as agents of second choice (clofazimine and cycloserine or terizidone).
- Group C: Agents that can be used when a regimen cannot be composed of Group A or B agents (for example, ETB, PZD, delamanid, amikacin and ethionamide).
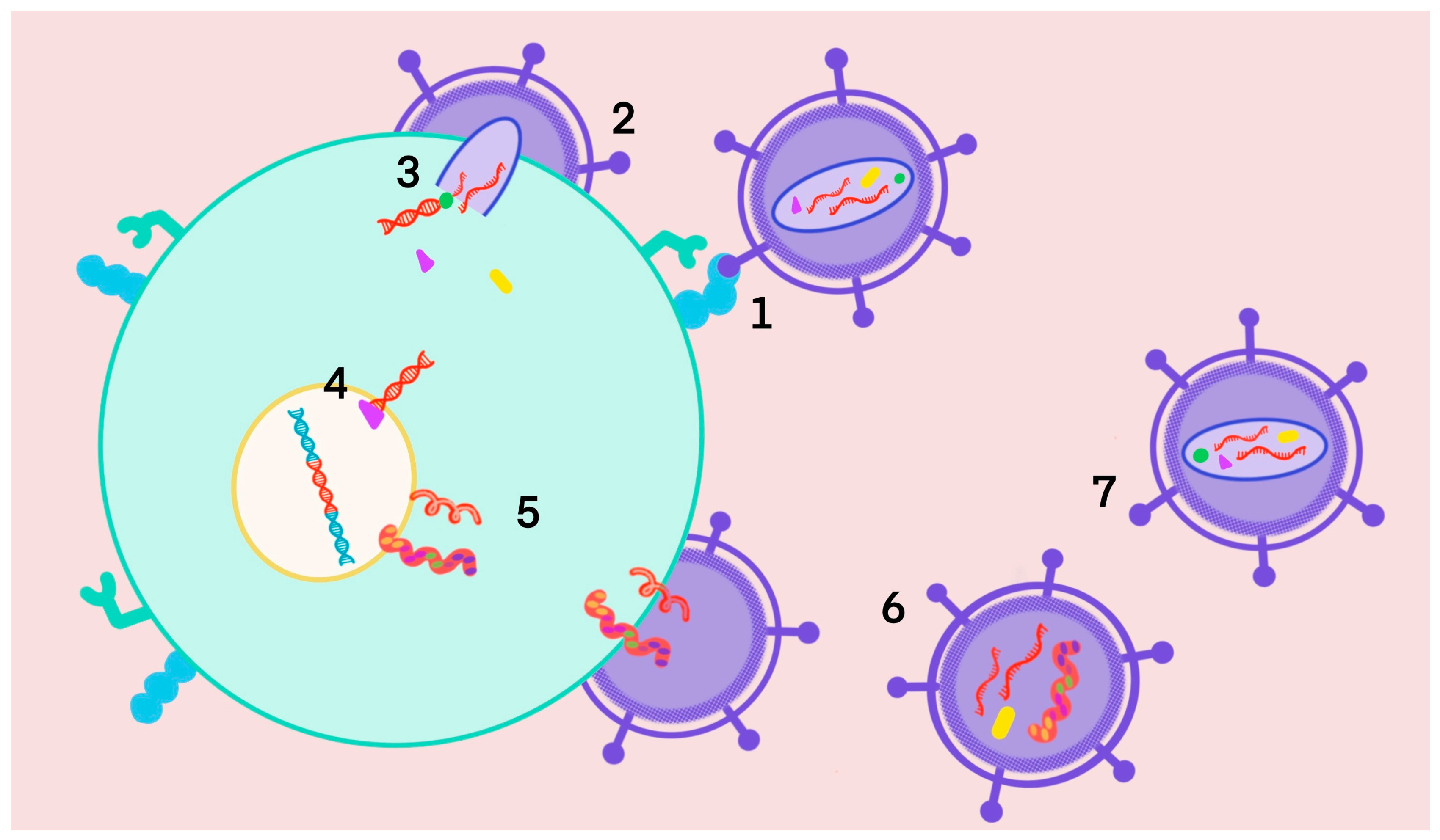
1.3. HIV-TB Coinfection
1.4. Multitargets
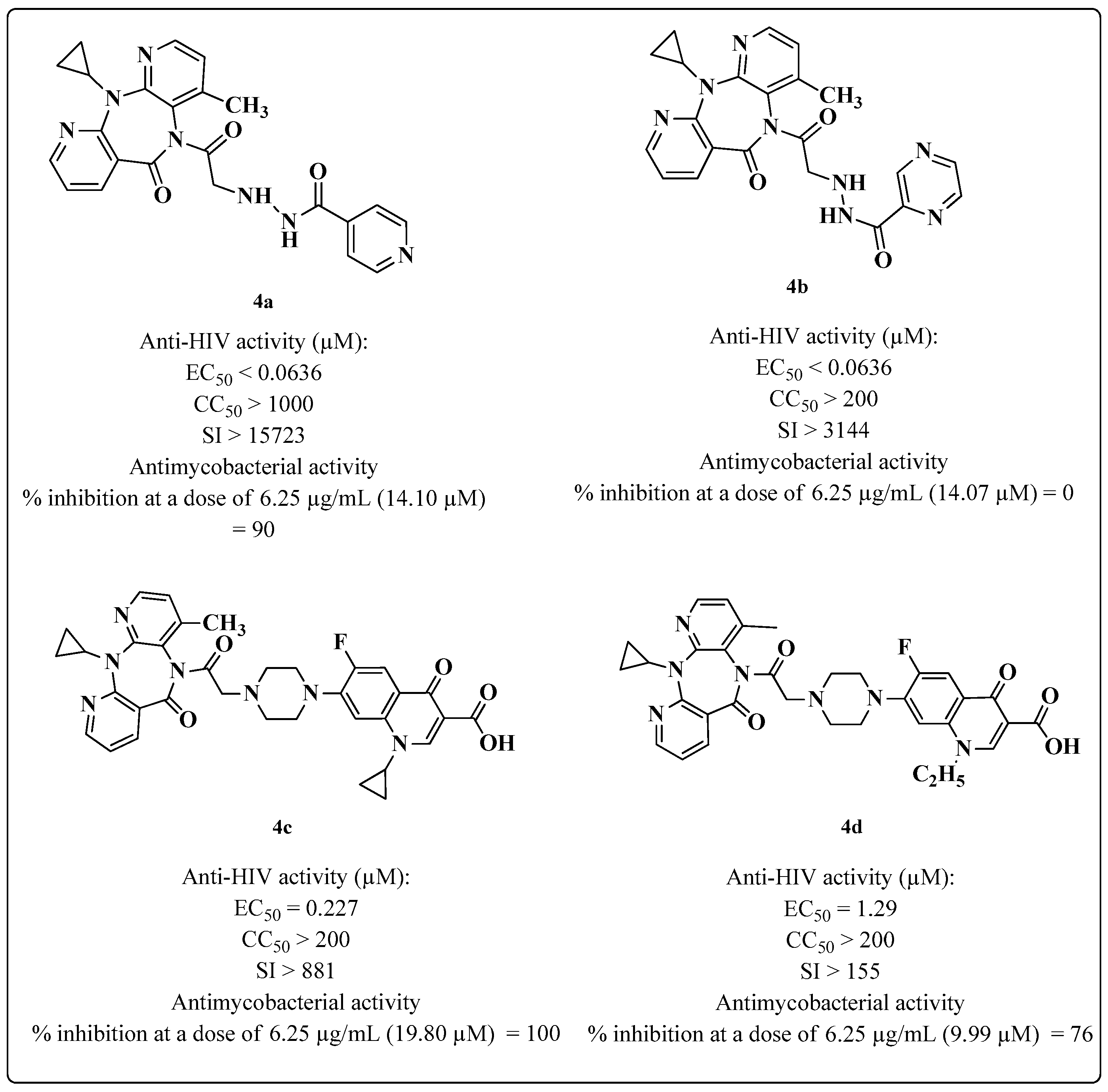
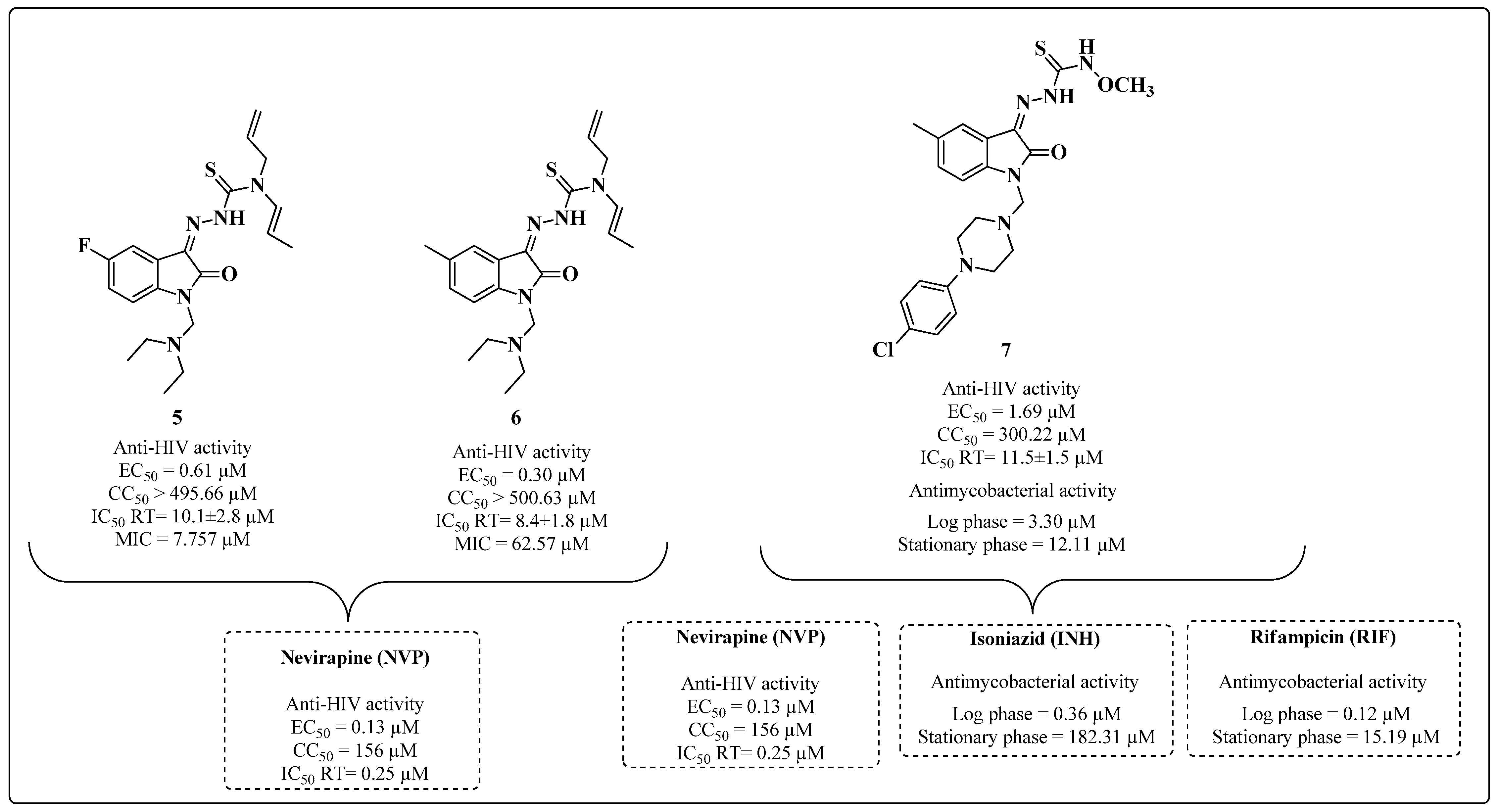
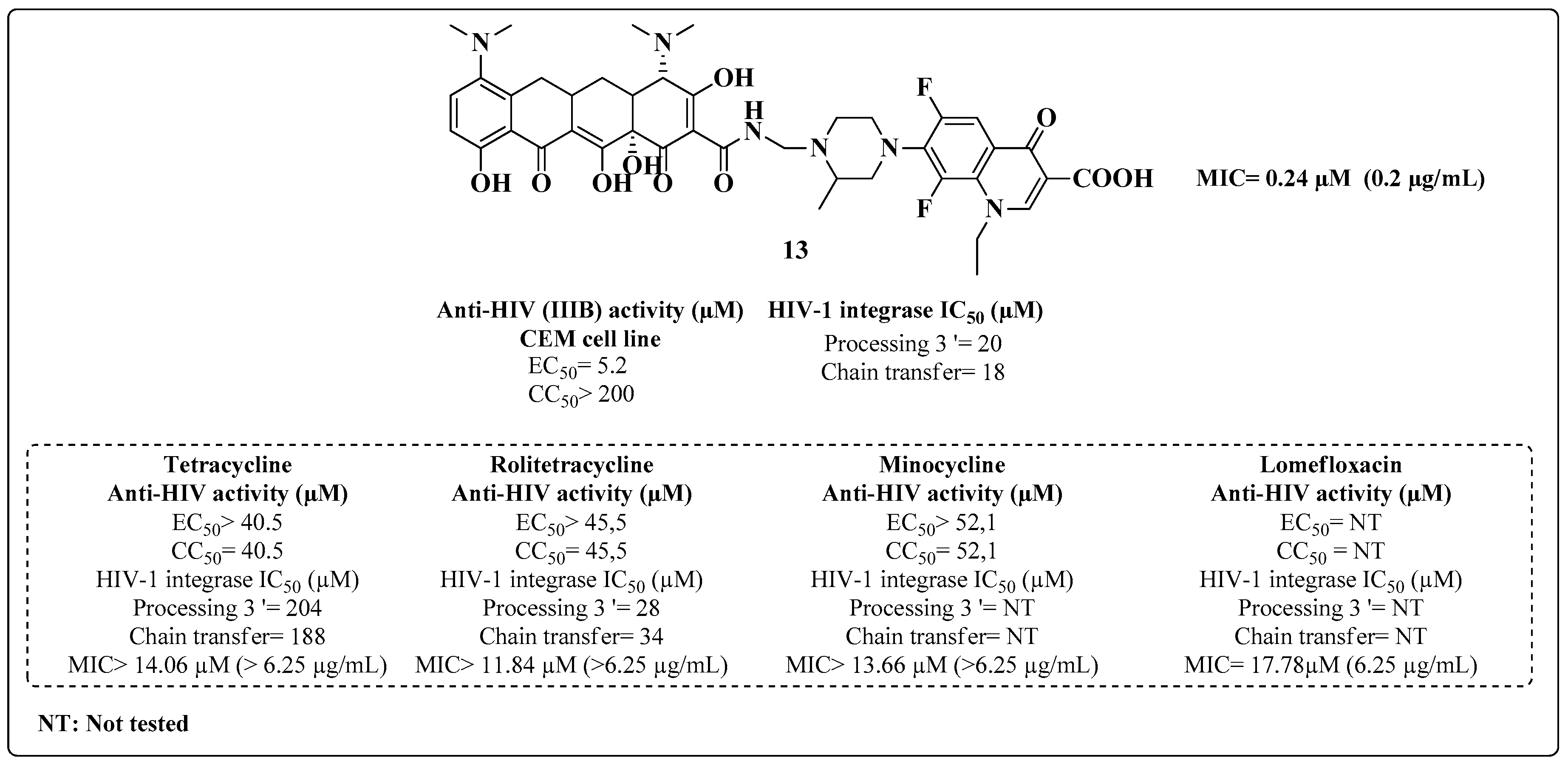
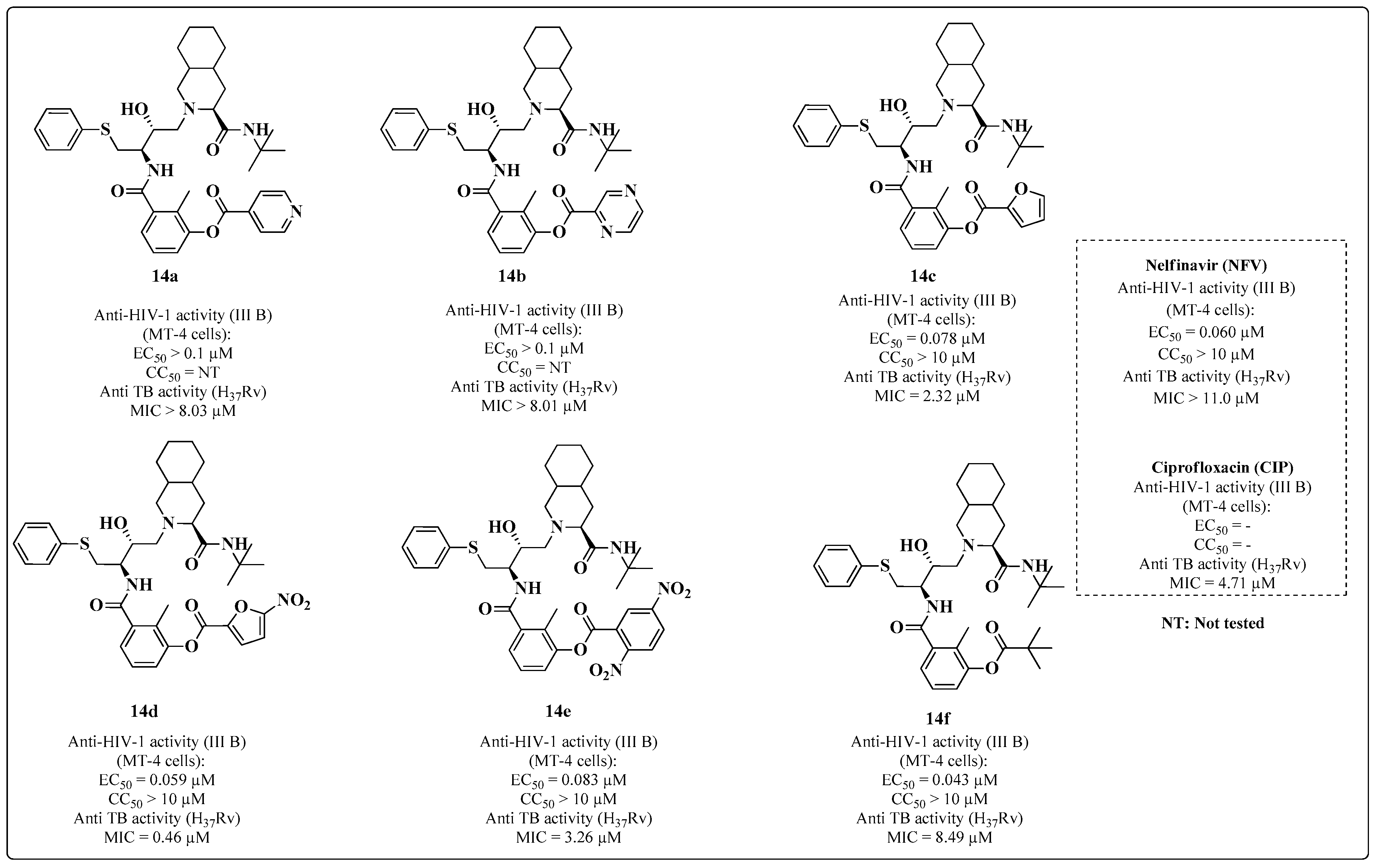
2. Multitarget HIV-TB
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaishnav, Y.N.; Wong-staal, F. The biochemistry of AIDS. Annu. Rev. Biochem. 1991, 60, 577–630. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.S.F.C.; Robinson, W.F. The comparative pathology of the lentiviruses. J. Comp. Pathol. 1998, 119, 333–395. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.W.; Morgan, W.M.; Hardy, A.M.; Jaffe, H.W.; Darrow, W.W.; Dowdle, W.R. The epidemiology of AIDS: Current status and future prospects. Science 1985, 229, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Broder, S. The development of antirretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Available online: https://unaids.org.br/estatisticas/#:~:text=As%20novas%20infec%C3%A7%C3%B5es%20por%20HIV,pessoas%20rec%C3%A9m%2Dinfectadas%20em%202021 (accessed on 23 February 2023).
- World Health Organization. HIV AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids#:~:text=There%20were%20an%20estimated%2038.4,in%20the%20WHO%20African%20Region (accessed on 1 December 2021).
- Bianco, M.C.A.D.; Leite, D.I.; Branco, F.S.C.; Boechat, N.; Uliassi, E.; Bolognesi, M.L.; Bastos, M.M. The Use of Zidovudine Pharmacophore in Multi-Target-Directed Ligands for AIDS Therapy. Molecules 2022, 27, 8502. [Google Scholar] [CrossRef]
- Bianco, M.C.A.D.; Marinho, D.I.L.F.; Hoelz, L.V.B.; Bastos, M.M.; Boechat, N. Pyrroles as Privileged Scaffolds in the Search for New Potential HIV Inhibitors. Pharmaceuticals 2021, 14, 893. [Google Scholar] [CrossRef]
- World Health Organization. First Case of HIV Cure in a Woman after Stem Cell Transplantation Reported at CROI-2022. Available online: https://www.who.int/news/item/24-03-2022-first-case-of-hiv-cure-in-a-woman-after-stem-cell-transplantation-reported-at-croi-2022 (accessed on 22 March 2023).
- Ambinder, R.F.; Capoferri, A.A.; Durand, C.M. Haemopoietic cell transplantation in patients living with HIV. Lancet 2020, 7, e652–e660. Available online: https://www.sciencedirect.com/science/article/abs/pii/S235230182030117X (accessed on 27 January 2023). [CrossRef]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 27 January 2023).
- Weichseldorfer, M.; Reitz, M.; Latinovic, O.S. Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients. Pharmaceutics 2021, 13, 1798. [Google Scholar] [CrossRef]
- Moranguinho, I.; Valente, S.T. Block-and-Lock: New Horizons for a Cure for HIV-1. Viruses 2020, 12, 1443. [Google Scholar] [CrossRef]
- Joseph, J.; Daley, W.; Lawrence, D.; Lorenzo, E.; Perrin, P.; Rao, V.R.; Tsai, S.-Y.; Varthakavi, V. Role of macrophages in HIV pathogenesis and cure: NIH perspectives. J. Leukoc. Biol. 2022, 112, 1233–1243. Available online: https://academic.oup.com/jleukbio/article/112/5/1233/6976233?login=true (accessed on 28 December 2022). [CrossRef] [PubMed]
- Waight, E.; Zhang, C.; Mathews, S.; Kevadiya, B.D.; Lloyd, K.C.K.; Gendelman, H.E.; Gorantla, S.; Poluektova, L.Y.; Dash, P.K. Animal models for studies of HIV-1 brain reservoirs. J. Leukoc Biol. 2022, 102, 1285–1295. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9804185/ (accessed on 28 December 2022). [CrossRef] [PubMed]
- Campaniço, A.; Moreira, R.; Lopes, F. Drug discovery in tuberculosis. New drug targets and antimycobacterial agents. Eur. J. Med. Chem. 2018, 150, 525–545. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2022. Available online: https://www.who.int/publications/i/item/9789240051157 (accessed on 17 June 2022).
- Maug, A.K.J.; Hossain, M.A.; Gumusboga, M.; Decroo, T.; Mulders, W.; Braet, S.; Buyze, J.; Arango, D.; Schurmans, C.; Herssens, N.; et al. First-line tuberculosis treatment with double-dose rifampicin is well tolerated. Int. J. Tuberc. Lung Dis. 2020, 24, 499–505. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Susceptible Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240048126 (accessed on 17 December 2022).
- Gruzdev, D.A.; Musiyak, V.V.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Purine derivatives with antituberculosis activity. Russ. Hem. Rev. 2018, 87, 604–618. [Google Scholar] [CrossRef]
- Rusu, A.; Munteanu, A.-C.; Arbanasi, E.-M.; Uivarosi, V. Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics 2023, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.; Asif, M. Pyrazinamide Analogs Designed for Rational Drug Designing Strategies against Resistant Tuberculosis. Russ. J. Bioorg. Chem. 2022, 48, 491–512. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N. Development of new antituberculosis drugs among of 1,3- and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. 2019, 68, 2172–2189. [Google Scholar] [CrossRef]
- Egelund, E.F.; Dupree, L.; Huesgen, E.; Peloquin, C.A. The pharmacological challenges of treating tuberculosis and HIV coinfections. Expert Rev. Clin. Pharmacol. 2017, 10, 213–223. [Google Scholar] [CrossRef]
- Murphy, D.J.; Brown, J.R. Novel drug target strategies against Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2008, 11, 422–427. [Google Scholar] [CrossRef]
- Ghebrevesus, T.A.; Kazatchkine, M.; Sidibé, M.; Nakatani, H. Tuberculosis and HIV: Time for an intensified response. Lancet 2010, 375, 1757–1758. [Google Scholar] [CrossRef] [PubMed]
- Fitzgeral, J.M.; Houston, S. Tuberculosis: The disease in association with HIV infection. CMAJ 1999, 161, 47–51. Available online: https://www.cmaj.ca/content/cmaj/161/1/47.full.pdf (accessed on 1 December 2022).
- Rosas-Taraco, A.G.; Arce-Mendoza, A.Y.; Caballero-Olín, G.; Salinas-Carmona, M.C. Mycobacterium tuberculosis upregulates coreceptors CCR5 and CXCR4 while HIV modulates CD14 favoring concurrent infection. AIDS Res. Hum. Retrovir. 2006, 22, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Harrington, M.; O’Brien, R.; Nunn, P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes. Lancet 2007, 369, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Noursadeghi, M.; Katz, D.R.; Miller, R.F. HIV-1 infection of mononuclear phagocytic cells: The case of bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006, 6, 794–804. [Google Scholar] [CrossRef]
- Pawlowski, A.; Jansson, M.; Skold, M.; Rottenberg, M.E.; Kallenius, G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012, 8, e1002464. [Google Scholar] [CrossRef]
- Deffur, A.; Mulder, N.J.; Wilkinson, R.J. Co-infection with Mycobacterium tuberculosis and human immunodeficiency virus: An overview and motivation for systems approaches. Pathog. Dis. 2013, 69, 101–113. Available online: https://academic.oup.com/femspd/article/69/2/101/2398867 (accessed on 10 January 2023). [CrossRef]
- Geldmacher, C.; Schuetz, A.; Nqwenyama, N.; Casazza, J.P.; Sanga, E.; Saathoff, E.; Boehme, C.; Geis, S.; Maboko, L.; Singh, M.; et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1-cell responses after HIV-1 infection. J. Infect. Dis. 2008, 198, 1590–1598. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The role of granuloma in expansion and dissemination of early tuberculous infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef]
- Roca, F.J.; Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef]
- Cambier, C.J.; Takaki, K.K.; Larson, R.P.; Hernandez, R.E.; Tobin, D.M.; Urdhal, K.B.; Cosma, C.L.; Ramakrishnan, L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 2014, 505, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, J.; Herit, F.; Bouchet, J.; Benmerah, A.; Benichou, S.; Niedergang, F. Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood 2010, 115, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Wentzel-Larsen, T.; Asjo, B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect. Immun. 2010, 78, 4022–4032. [Google Scholar] [CrossRef] [PubMed]
- Tornheim, J.A.; Dooley, K.E. Tuberculosis associated with HIV infection. Microbiol. Spectr. 2017, 5, 577–594. [Google Scholar] [CrossRef]
- Mcllleron, H.; Meintjes, G.; Burman, W.J.; Maartens, G. Complications of antiretroviral therapy in pacients with tuberculosis: Drug interactions, toxicity and immune reconstitution inflammatory syndrome. J. Infect. Dis. 2007, 196, 63–75. [Google Scholar] [CrossRef]
- Shu, Y.; Deng, Z.; Wang, H.; Chen, Y.; Yuan, L.; Deng, Y.; Tu, X.; Zhao, X.; Shi, Z.; Huang, M.; et al. Integrase inhibitors versus efavirenz combination antiretroviral therapies for TB/HIV coinfection: A meta-analysis of randomized controlled trials. AIDS Res. Ther. 2021, 18, 25. [Google Scholar] [CrossRef]
- Ma, Z.; Lienhardt, C. Toward an optimized therapy for tuberculosis? Drugs in clinical trials and in preclinical development. Clin. Chest Med. 2009, 30, 755–768. [Google Scholar] [CrossRef]
- Peters, J.U. Polypharmacology–foe or friend? J. Med. Chem. 2013, 56, 8955–8971. [Google Scholar] [CrossRef]
- AIDSInfo. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/tuberculosishiv-coinfection (accessed on 2 December 2022).
- World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. Available online: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/ (accessed on 4 December 2022).
- De Souza MV, N. Rifampicina, um importante fármaco no combate à tuberculose. Rev. Bras. Farm. 2005, 86, 92–94. Available online: http://www.rbfarma.org.br/files/pag_92a94_RIFAMPICINA.pdf (accessed on 12 January 2023).
- Naiker, S.; Connolly, C.; Wiesner, L.; Kellerman, T.; Reddy, T.; Harries, A.; Mcllleron, H.; Lienhardt, C.; Pym, A. Randomized pharmacokinetic evaluation of different rifabutin doses in African HIV-infected tuberculosis patients on lopinavir/ritonavir-based antiretroviral therapy. BMC Pharmacol. Toxicol. 2014, 15, 61. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4277828/pdf/40360_2014_Article_353.pdf (accessed on 12 January 2023). [CrossRef]
- Burman, W.J.; Galllicano, K.; Peloquin, C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharm. 2001, 40, 327–341. Available online: https://link.springer.com/article/10.2165/00003088-200140050-00002 (accessed on 15 January 2023). [CrossRef] [PubMed]
- Decloedt, E.H.; Maartens, G.; Smith, P.; Merry, C.; Bango, F.; Mcllleron, H. The safety, effectiveness and concentrations of adjusted lopinavir/ritonavir in HIV-infected adults on rifampicin-based antitubercular therapy. PLoS ONE 2012, 7, e32173. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3296695/ (accessed on 15 January 2023). [CrossRef] [PubMed]
- Coelho, L.E.; Escada, R.O.S.; Barbosa, H.P.P.; Santos, V.G.V.; Grinsztejn, B.G.J. O Tratamento da Coinfecção HIV-TB. Braz. J. Infect. Dis. 2016, 2, 134–148. Available online: https://www.elsevier.es/pt-revista-the-brazilian-journal-infectious-diseases-269-articulo-o-tratamento-da-coinfeccao-hiv-tb-X2177511716600168 (accessed on 15 January 2023). (In Portuguese).
- Haas, D.W.; Koletar, S.L.; Laughlin, L.; Kendall, M.A.; Suckow, C.; Gerber, J.G.; Zolopa, A.R.; Bertz, R.; Child, M.J.; Hosey, L.; et al. Hepatotoxicity and Gastrointestinal Intolerance when Healthy Volunteers Taking Rifampin Add Twice-Daily Atazanavir and Ritonavir. J. Acquir. Immune Defic. Syndr. 2009, 50, 290–293. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2653210/ (accessed on 17 January 2023). [CrossRef] [PubMed]
- Nijland, H.M.; L’homme, R.F.; Rongen, G.A.; Van-Crevel, R.; Boeree, M.J.; Aarnoutse, R.E.; Koopmans, P.P.; Burger, D.M. High Incidence of Adverse Events in Healthy Volunteers Rifampicin and Adjusted Doses of Lopinavir/Ritonavir Tablets. AIDS 2008, 22, 931–935. Available online: https://journals.lww.com/aidsonline/Fulltext/2008/05110/High_incidence_of_adverse_events_in_healthy.3.aspx (accessed on 17 January 2023). [CrossRef] [PubMed]
- Gautam, C.S.; Lekha, S. Fixed dose drug combinations (FDCs): Rational or irrational: A view point. Br. J. Clin. Pharmacol. 2007, 65, 795–796. [Google Scholar] [CrossRef]
- Sherman, E.M.; Worley, M.V.; Unger, N.R.; Gauthier, T.P.; Schafer, J.J. Cobicistat: Review of a pharmacokinetic enhancer for HIV infection. Clin. Ther. 2015, 37, 1876–1893. [Google Scholar] [CrossRef]
- Nøhr-Nielsen, A.; Lange, T.; Forman, J.L.; Papathanasiou, T.; Foster, D.J.; Upton, R.N.; Bjerrun, O.J.; Lund, T.M. Demonstrating Contribution of Components of Fixed-Dose Drug Combinations Through Longitudinal Exposure-Response Analysis. AAPS J. 2020, 22, 32. [Google Scholar] [CrossRef]
- Wensing, A.M.; Van Maarseveen, N.M.; Nijhuis, M. Fifteen years of HIV protease inhibitors: Raising the barrier to resistence. Antivir. Res. 2010, 85, 59–74. [Google Scholar] [CrossRef]
- Hull, M.W.; Montaner, J.S. Ritonavir-boosted protease inhibitors in HIV therapy. Ann. Med. 2011, 43, 375–388. [Google Scholar] [CrossRef]
- De Castro, S.; Camarasa, M.J. Polypharmacology in HIV inhibition: Can a drug with simultaneous action against two relevant targets be an alternative to combination therapy? Eur. J. Med. Chem. 2018, 150, 206–227. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two Diseases, One Approach: Multitarget Drug Discovery in Alzheimer’s and Neglected Tropical Diseases. Med. Chem. Commun. 2014, 5, 853–861. Available online: https://pubs.rsc.org/en/content/articlelanding/2014/md/c4md00069b (accessed on 20 January 2023). [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; Da Silva, B.V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Bottegoni, G.; Flavia, A.D.; Recanatini, M.; Cavalli, A. The role of fragment-based and computational methods in polypharmacology. Drug Discov. Today 2012, 17, 23–34. [Google Scholar] [CrossRef]
- Scotti, L.; Filho, F.J.; De Moura, R.O.; Ribeiro, F.F.; Ishiki, H.; Da Silva, M.S.; Filho, J.M.; Scotti, M.T. Multi-target drugs for neglected diseases. Curr. Pharm. Des. 2016, 22, 3135–3163. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Bolognesi, M.L. Polypharmacology in a single drug: Multitarget drugs. Curr. Med. Chem. 2013, 20, 1639–1645. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. Available online: https://pubs.acs.org/doi/10.1021/jm7009364 (accessed on 25 January 2023). [CrossRef]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From Combinations to Multitarget-Directed Ligands: A Continuum in Alzheimer’s Disease Polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. Available online: https://www.researchgate.net/publication/342318801_From_combinations_to_multitarget-directed_ligands_A_continuum_in_Alzheimer’s_disease_polypharmacology (accessed on 1 February 2023). [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.A.M. Drug hybridization strategies: Before or after lead identification? Expert Opin. Drug Discov. 2009, 4, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Walles, M.; Connor, A.; Hainzl, D. ADME and Safety Aspects of Non-cleavable Linkers in Drug Discovery and Development. Curr. Top. Med. Chem. 2017, 17, 3463–3475. Available online: https://www.eurekaselect.com/article/88035 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Vijayakumar, S.; John, S.F.; Nusbaum, R.J.; Ferguson, M.R.; Cirillo, J.D.; Olaleye, O.; Endsley, J.J. In vitro model of mycobacteria and HIV-1 co-infection for drug discovery. Tuberculosis 2013, 93, S66–S70. [Google Scholar] [CrossRef]
- Fan, X.; Xu, J.; Files, M.; Cirillo, J.D.; Endsley, J.J.; Zhou, J.; Endsley, M.A. Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing). Tuberculosis 2019, 116, S28–S33. [Google Scholar] [CrossRef]
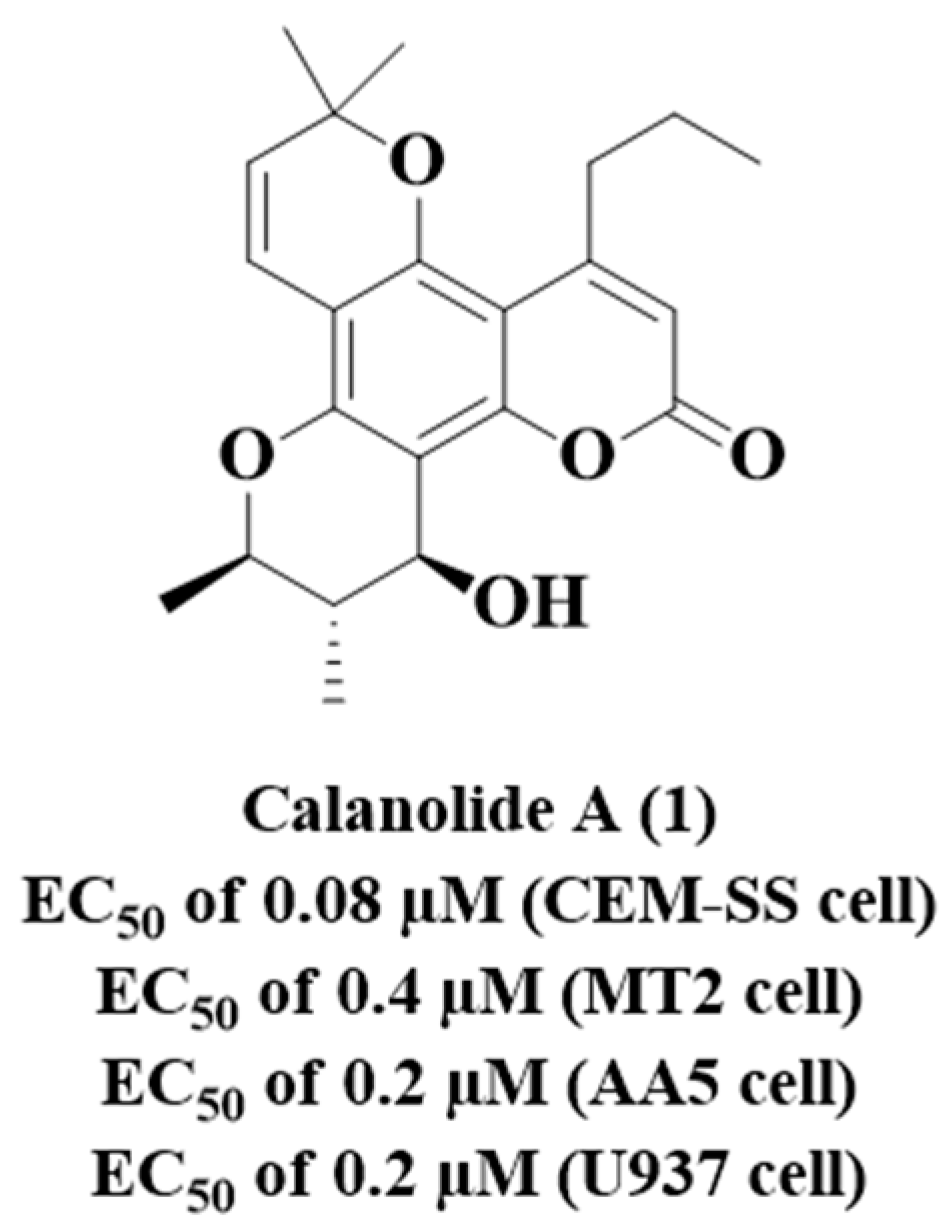
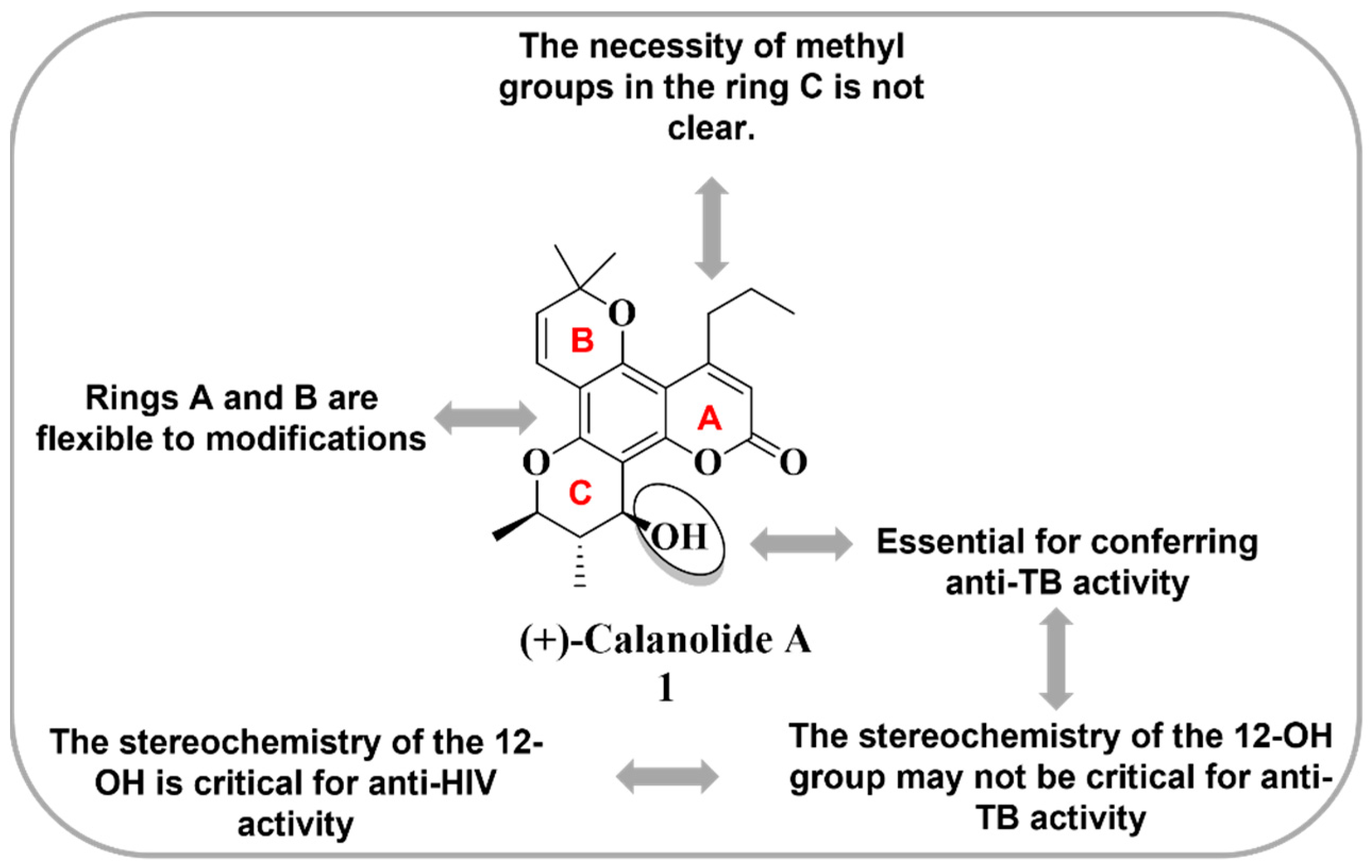
- Xu, Z.Q.; Flavin, M.T.; Jenta, T.R. Calanolides, the Naturally Occurring Anti-HIV Agents. Curr. Opin. Drug Discov. Devel. 2000, 3, 155–166. Available online: https://pubmed.ncbi.nlm.nih.gov/19649847/ (accessed on 5 February 2023).
- Buckheit, R.W., Jr.; White, E.L.; Fliakas-Boltz, V.; Russell, J.; Stup, T.L.; Kinjerski, T.L.; Osterling, M.C.; Weigand, A.; Bader, J.P. Unique anti-human immunodeficiency virus activities of the nonnucleoside reverse transcriptase inhibitors calanolide A, costatolide, and dihydrocostatolide. Antimicrob. Agents Chemother. 1999, 43, 1827–1834. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Barrow, W.W.; Suling, W.J.; Westbrook, L.; Barrow, E.; Lin, Y.M.; Flavin, M.T. Anti-HIV natural product (+)-calanolide A is active against both drug-susceptible and drug-resistant strains of Mycobacterium tuberculosis. Bioorg. Med. Chem. 2004, 12, 1199–1207. [Google Scholar] [CrossRef]
- Currens, M.J.; Gulakowski, R.J.; Mariner, J.M.; Moran, R.A.; Buckheit, R.W.; Gustafson, K.R.; McMahon, J.B.; Boyd, M.R. Antiviral Activity and Mechanism of Action of Canolide a Against the Human Immunodeficiency Virus Type 1. J. Pharmacol. Exp. Ther. 1996, 279, 645–651. Available online: https://jpet.aspetjournals.org/content/279/2/645.long (accessed on 5 February 2023).
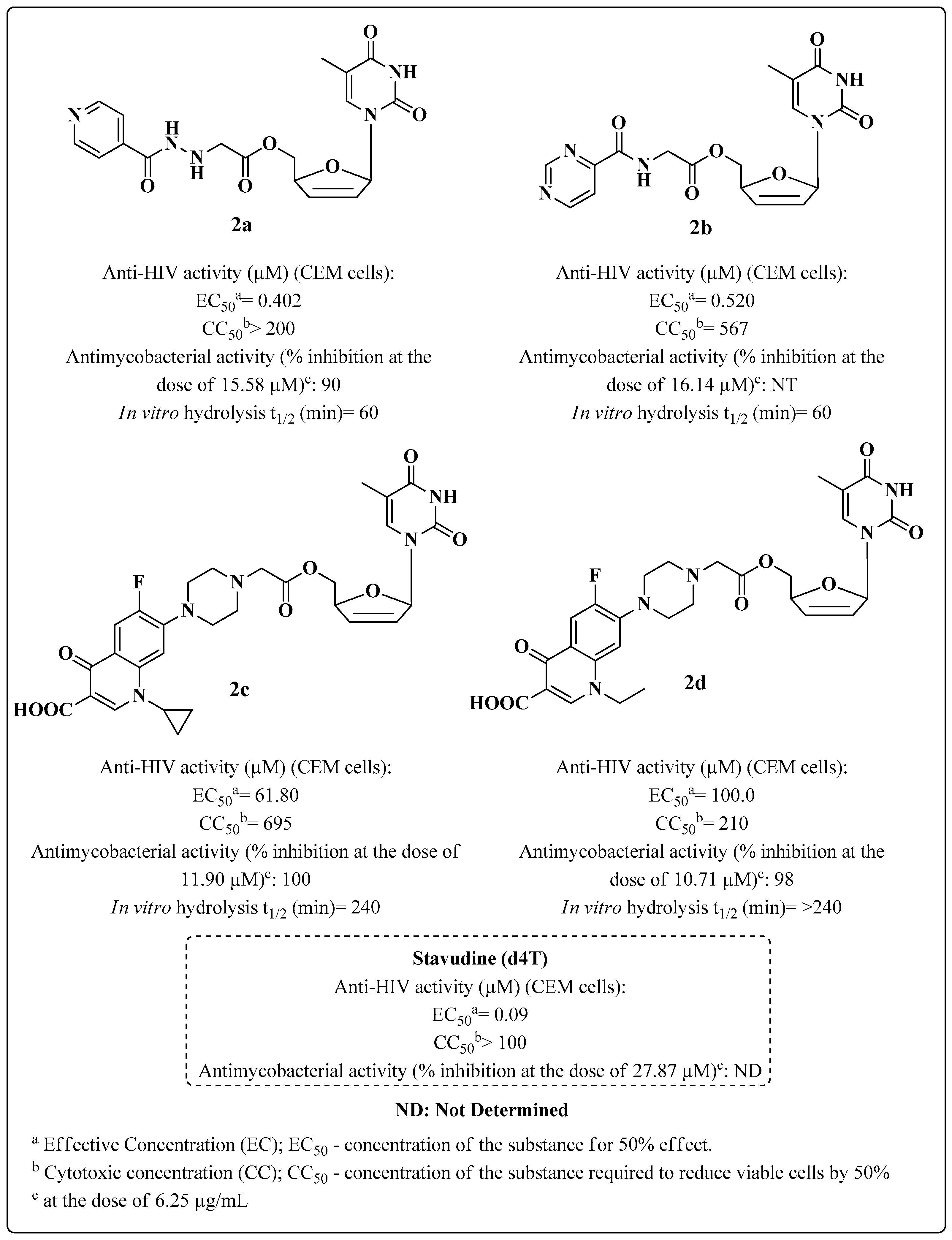
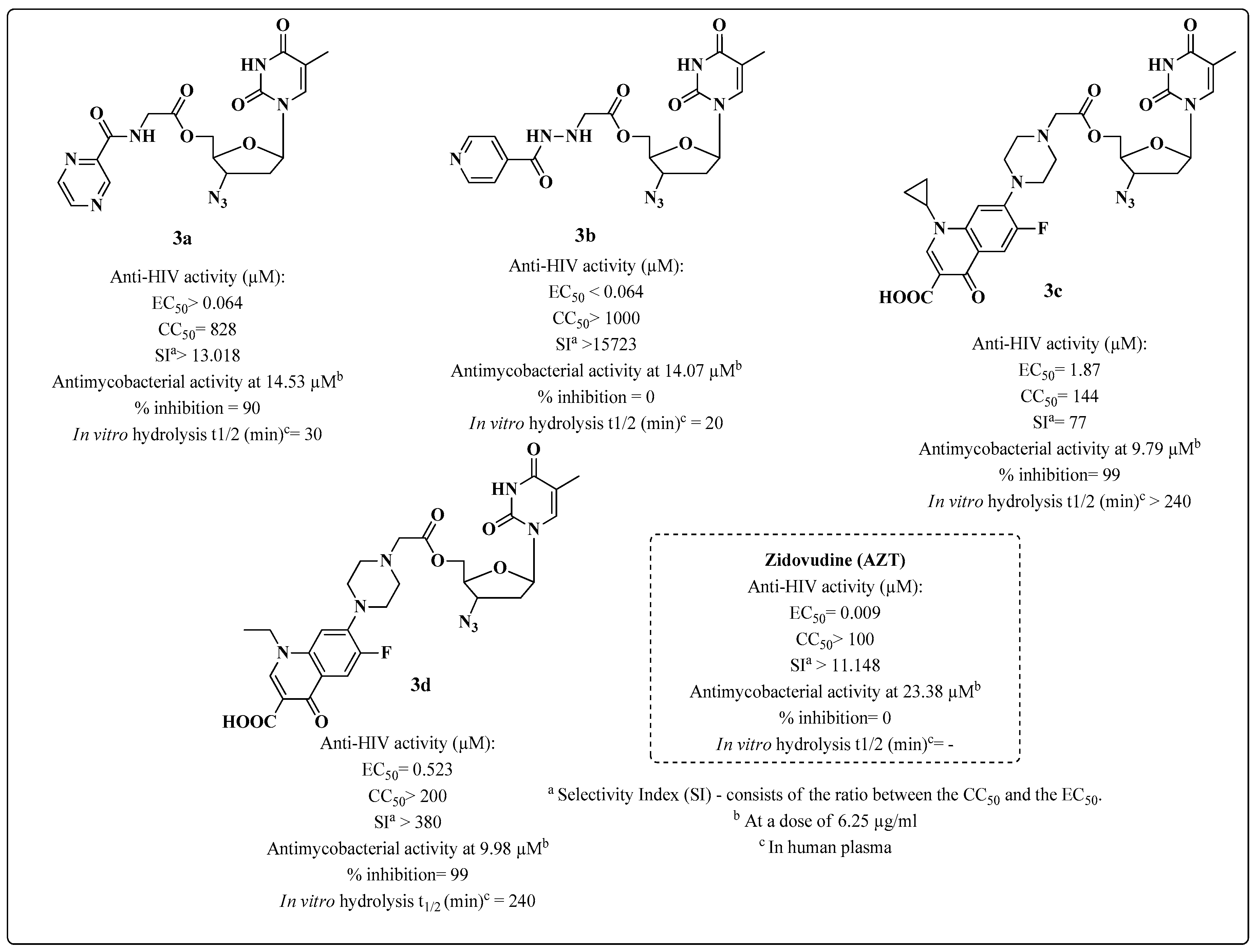
- Sriram, D.; Yogeeswari, P.; Srichakravarthy, N.; Bal, T.R. Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorg. Med. Chem. Lett. 2004, 14, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Srichakravarthy, N.; Bal, T.R.; Yogeeswari, P. Synthesis of zidovudine prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Biomed. Pharmacother. 2005, 59, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Srichakravarthy, N.; Bal, T.R.; Yogeeswari, P. Nevirapine derivatives with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Biomed. Pharmacother. 2005, 59, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Teitz, Y.; Ronen, D.; Vansover, A.; Stematsky, T.; Riggs, J.L. Inhibition of human immunodeficiency virus by N-methylisatin-beta 4’:4’-diethylthiosemicarbazone and N-allylisatin-beta-4’:4’-diallythiosemicarbazone. Antiviral Res. 1994, 24, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Bal, T.R.; Yogeeswari, P. Design, synthesis and biological evaluation of novel non-nucleoside HIV-1 reverse transcriptase inhibitors with broad-spectrum chemotherapeutic properties. Bioorg. Med. Chem. 2004, 12, 5865–5873. [Google Scholar] [CrossRef]
- Sriram, D.; Bal, T.R.; Yogeeswari, P. Newer aminopyrimidinimino isatin analogues as non-nucleoside HIV-1 reverse transcriptase inhibitors for HIV and other opportunistic infections of AIDS: Design, synthesis and biological evaluation. II Farm. 2005, 60, 377–384. [Google Scholar] [CrossRef]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Meena, K. Synthesis, anti-HIV and antitubercular activities of isatin derivatives. Pharmazie 2006, 61, 274–277. [Google Scholar] [CrossRef]
- Banerjee, D.; Yogeeswari, P.; Bhat, P.; Thomas, A.; Sriram, D. Synthesis, in vitro evaluation and computational studies of novel isatynil derivatives for their activity against HIV-TB co-infection. Int. J. Drug Des. Discov. 2010, 1, 65–80. [Google Scholar]
- Banerjee, D.; Yogeeswari, P.; Bhat, P.; Thomas, A.; Srividya, M.; Sriram, D. Novel isatinyl thiosemicarbazones derivatives as potential molecule to combat HIV-TB co-infection. Eur. J. Med. Chem. 2011, 46, 106–121. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Gopal, G. Synthesis, anti-HIV and antitubercular activities of lamivudine prodrugs. Eur. J. Med. Chem. 2005, 40, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Neamati, N.; Hong, H.; Sunder, S.; Milne, G.W.A.; Pommier, Y. Potent inhibitors of human immunodeficiency virus type 1 integrase: Identification of a novel four-point pharmacophore and tetracyclines as novel inhibitors. Mol. Pharmacol. 1997, 52, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Senchani, G.; Banerjee, D. Newer tetracycline derivatives: Synthesis, anti-HIV, antimycobacterial activities and inhibition of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2007, 17, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Dinakaran, M.; Sowmya, M. Synthesis, anti-HIV and antitubercular activities of nelfinavir diester derivatives. Biomed. Pharmacother. 2008, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Banerjee, D.; Yogeeswari, P. Efavirenz mannich bases: Synthesis, anti-HIV and antitubercular activities. J. Enzyme Inhib. Med. Chem. 2009, 24, 1–5. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Long, J.; Swetha, R.; Shruthi, V.; Wang, R.R.; Preethi, S.; Yogeeswari, P.; Zheng, Y.T.; Sriram, D. Synthesis of Zidovudine Derivatives with Anti-HIV-1 and Antibacterial Activities. Nucleosides Nucleotides Nucleic Acids 2009, 28, 89–102. Available online: https://www.tandfonline.com/doi/full/10.1080/15257770902736442 (accessed on 7 February 2023). [CrossRef]
- Bhikshapathi, D.V.R.N.; Krishna, D.R.; Kishan, V. Anti-HIV, Anti-Tubercular and Mutagenic Activities of Borrelidin. Indian J. Biotechnol. 2010, 9, 265–270. Available online: https://nopr.niscpr.res.in/bitstream/123456789/9883/1/IJBT%209(3)%20265-270.pdf (accessed on 8 February 2023).
- Narayanasamy, P.; Switzer, B.L.; Britigan, B.E. Prolonged-acting, multi-targeting gallium nanoparticles potently inhibit growth of both HIV and mycobacteria in co-infected human macrophages. Sci. Rep. 2015, 5, 8824. [Google Scholar] [CrossRef]
- Olakanmi, O.; Kesavalu, B.; Pasula, R.; Abdalla, M.Y.; Schlessinger, L.S.; Britigan, B.E. Gallium nitrate is efficacious in murine models of tuberculosis and inhibits key bacterial Fe-dependent enzymes. Antimicrob. Agents Chemother. 2013, 57, 6074–6080. [Google Scholar] [CrossRef]
- Olakanmi, O.; Britigan, B.E.; Schlessinger, L.S. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun. 2000, 68, 5619–5627. [Google Scholar] [CrossRef]
- Krakoff, I.H.; Newman, R.A.; Goldberg, R.S. Clinical Toxicologic and pharmacologic studies of gallium nitrate. Cancer 1979, 44, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Nair, V. HIV integrase as a target for antiviral chemotherapy. Rev. Med. Virol. 2002, 12, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Okello, M.O.; Mangu, N.K.; Seo, B.I.; Gund, M.G. A novel molecule with notable activity against multi-drug resistant tuberculosis. Bioorg. Med. Chem. Lett. 2015, 25, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, L.; Zicari, S.; Matyugina, E.; Khandazhinskaya, A.; Smirnova, T.; Andreevskaya, S.; Chernousova, L.; Vanpouille, C.; Kochetkov, S.; Margolis, L. Dual-targeted anti-TB/anti-HIV heterodimers. Antivir. Res. 2017, 145, 175–183. [Google Scholar] [CrossRef]
- Shmalenyuk, E.R.; Chernousova, L.N.; Karpenko, I.L.; Kochetkov, S.N.; Smirnova, T.G.; Andreevskaya, S.N.; Chizhov, A.O.; Efremenkova, O.V.; Alexandrova, L.A. Inhibition of Mycobacterium tuberculosis strains H37Rv and MDR MS-115 by a new set of C5 modified pyrimidine nucleosides. Bioorg. Med. Chem. 2013, 21, 4874–4884. [Google Scholar] [CrossRef]
- Matyugina, E.; Novikov, M.; Babkov, D.; Ozerov, A.; Chernousova, L.; Andreevskaya, S.; Smirnova, T.; Karpenko, T.; Chizhov, A.; Murthu, P.; et al. 5-arylaminouracil derivatives: New inhibitors of Mycobacterium tuberculosis. Chem. Biol. Drug Des. 2015, 86, 1387–1396. [Google Scholar] [CrossRef]
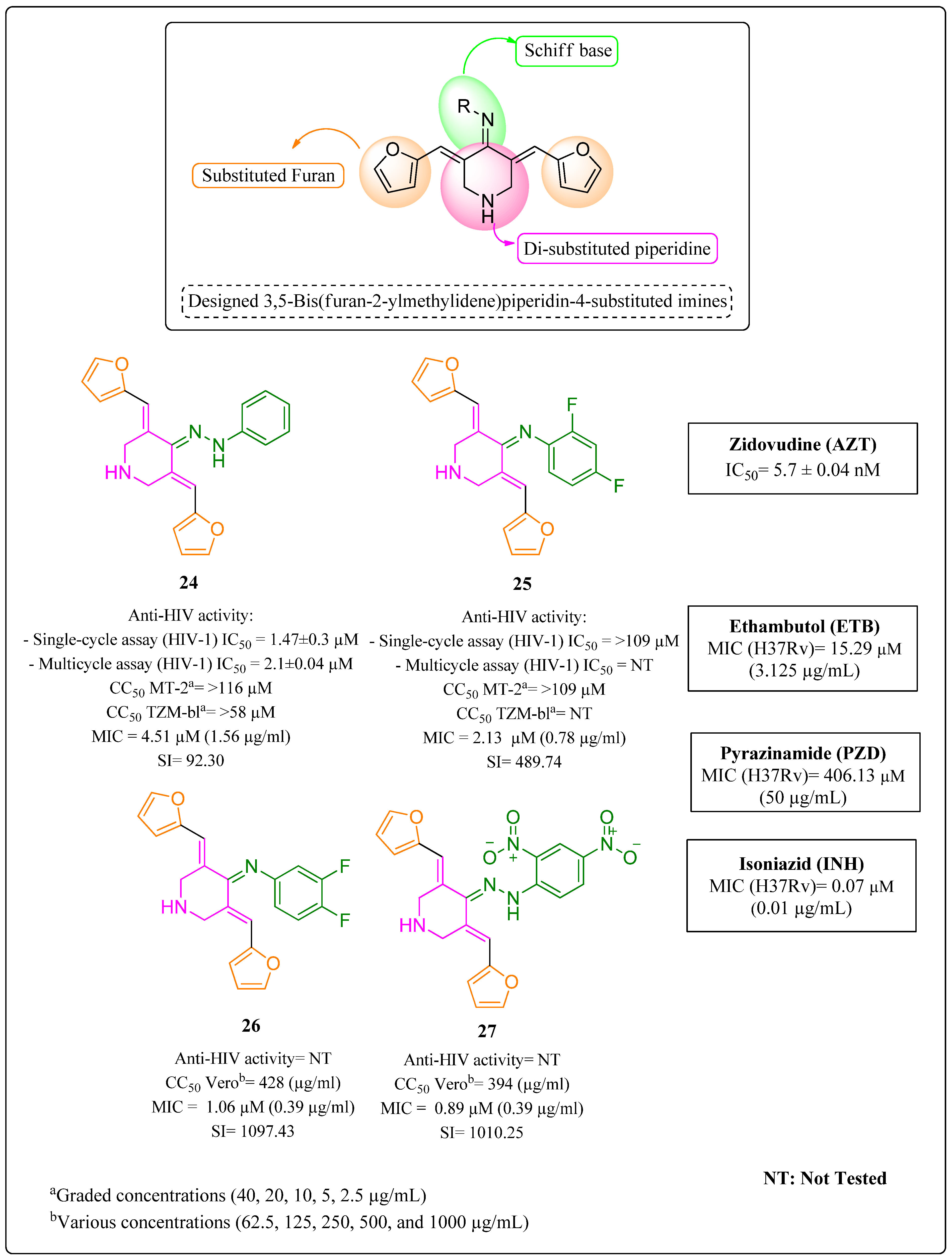
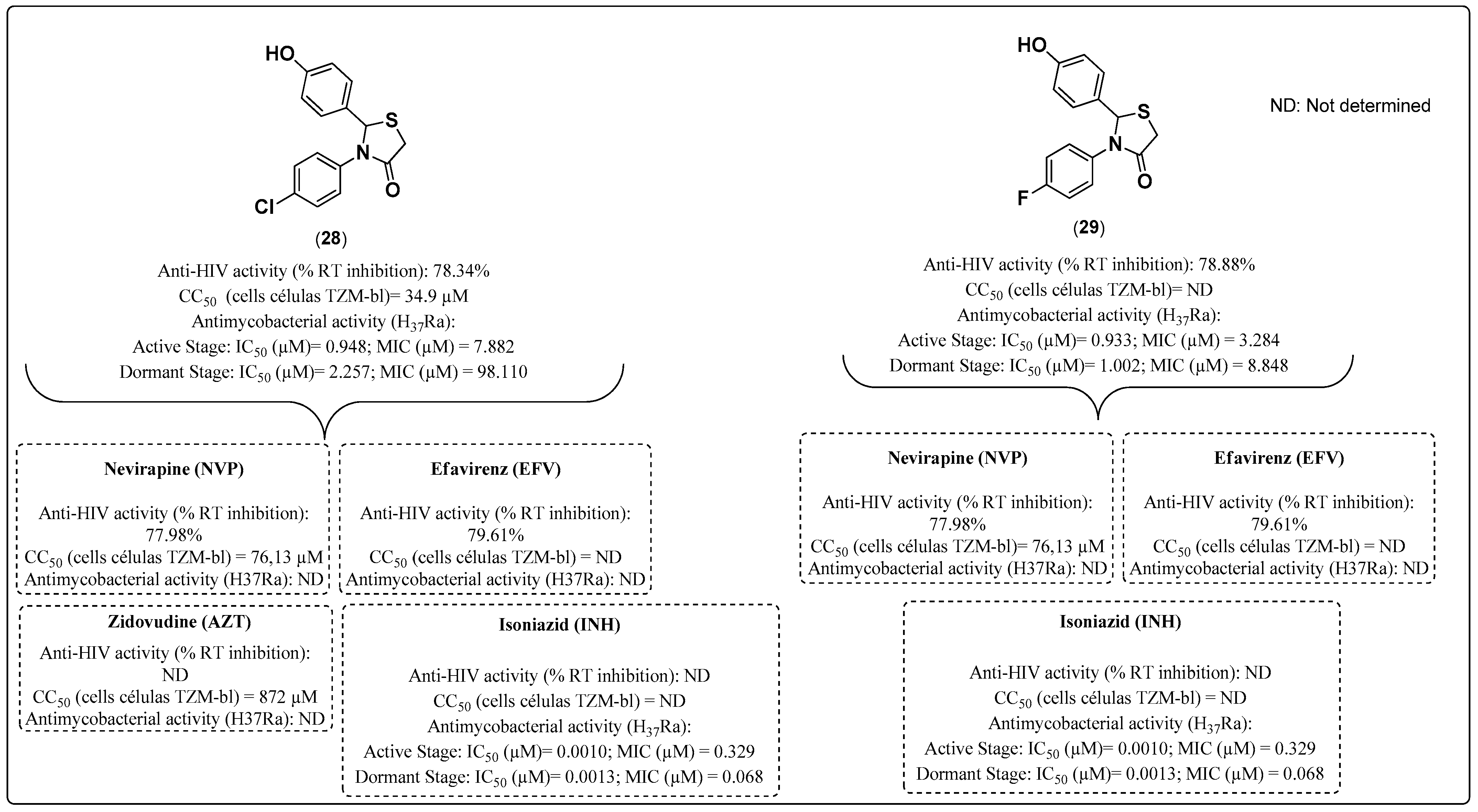
- Kumar, A.; Revathi, R.; Sriram, D.; Curreli, F.; Debnath, A.K.; Pai, K.S.; Kini, S.G. Targeting HIV-TB coinfection by developing novel piperidin-4-substituted imines: Design, synthesis, in vitro and in silico studies. Arch. Der. Pharm. 2019, 352, 1800358. [Google Scholar] [CrossRef] [PubMed]
- Gahtori, P.; Ghosh, S.K.; Singh, B.; Singh, U.P.; Bhat, H.B.; Uppal, A. Synthesis, SAR and antibacterial activity of hybrid chloro, dichloro-phenylthiazolyl-s-triazines. Saudi Pharm. J. 2012, 20, 35–43. [Google Scholar] [CrossRef]
- Weis, R.; Schweiger, K.; Faist, J.; Rajkovic, E.; Kungl, A.J.; Fabian, W.M.F.; Schunack, W.; Seebacher, W. Antimycobacterial and H1-antihistaminic activity of 2-substituted piperidine derivatives. Bioorg. Med. Chem. 2008, 16, 10326–10331. [Google Scholar] [CrossRef]
- Sun, D.; Scherman, M.S.; Jones, V.; Hurdle, J.G.; Woolhiser, L.K.; Knudson, S.E.; Lee, R.E. Discovery, synthesis, and biological evaluation of piperidinol analogs with anti-tuberculosis activity. Bioorg. Med. Chem. 2009, 17, 3588–3594. [Google Scholar] [CrossRef]
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Al-Obaid, A.M. Synthesis and biological evaluation of certain α, β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000, 43, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Kang, H.J.O.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D.; et al. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 44, 4169–4178. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, P.; Liu, X.; Cheng, Z.; Meng, C.; Shao, S.; Pannecouque, C.; De Clercq, E.; Liu, X. Design, synthesis, anti-HIV evaluation and molecular modeling of piperidine-linked amino-triazine derivatives as potent non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2012, 20, 3856–3864. [Google Scholar] [CrossRef]
- Chitre, T.S.; Patil, S.M.; Sujalegaonkar, A.G.; Asgaonkar, K.D.; Khedkar, V.M.; Garud, D.R.; Jah, P.C.; Gaikwad, S.Y.; Kulkarni, S.S.; Choudhari, A.; et al. Non Nucleoside Reverse Transcriptase Inhibitors, Molecular Docking Studies and Antitubercular Activity of Thiazolidin-4-one Derivatives. Curr. Comput. Aided Drug Des. 2019, 15, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stammers, D.K. HIV reverse transcriptase structures: Designing new inhibitors and understanding mechanisms of drug resistance. Trends Pharmacol. Sci. 2005, 26, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Oruç, E.E.; Rollas, S.; Sahin, F.; Özbek, A. Synthesis, characterisation and biological activity of novel 4- thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar] [CrossRef]
- Zhang, S.Z. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber Lung. Dis. 1999, 79, 319–320. [Google Scholar] [CrossRef]
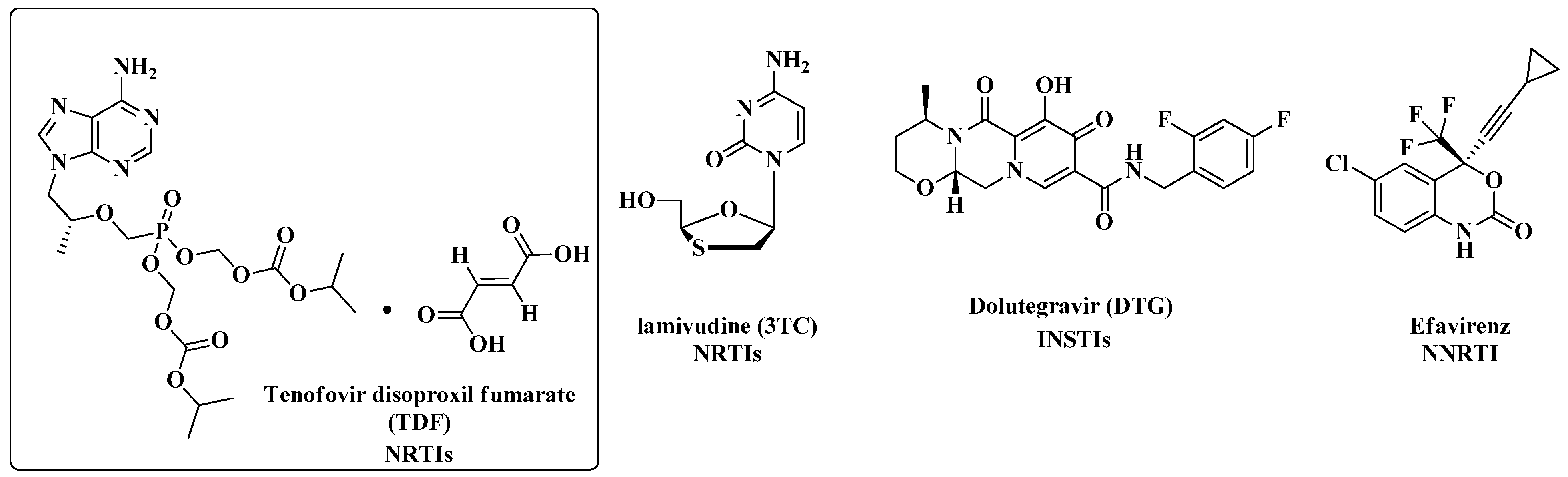
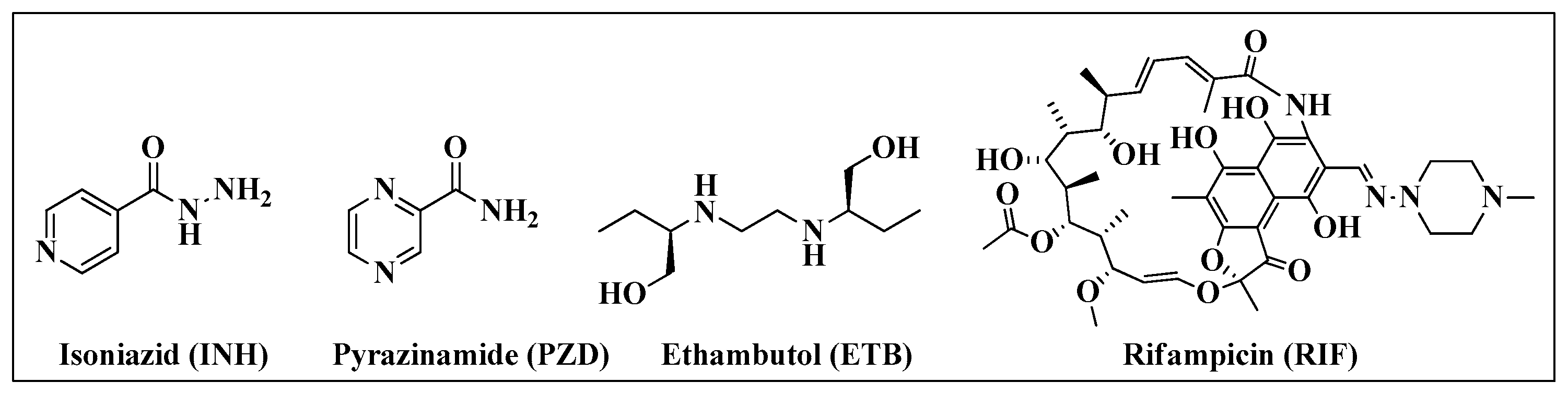

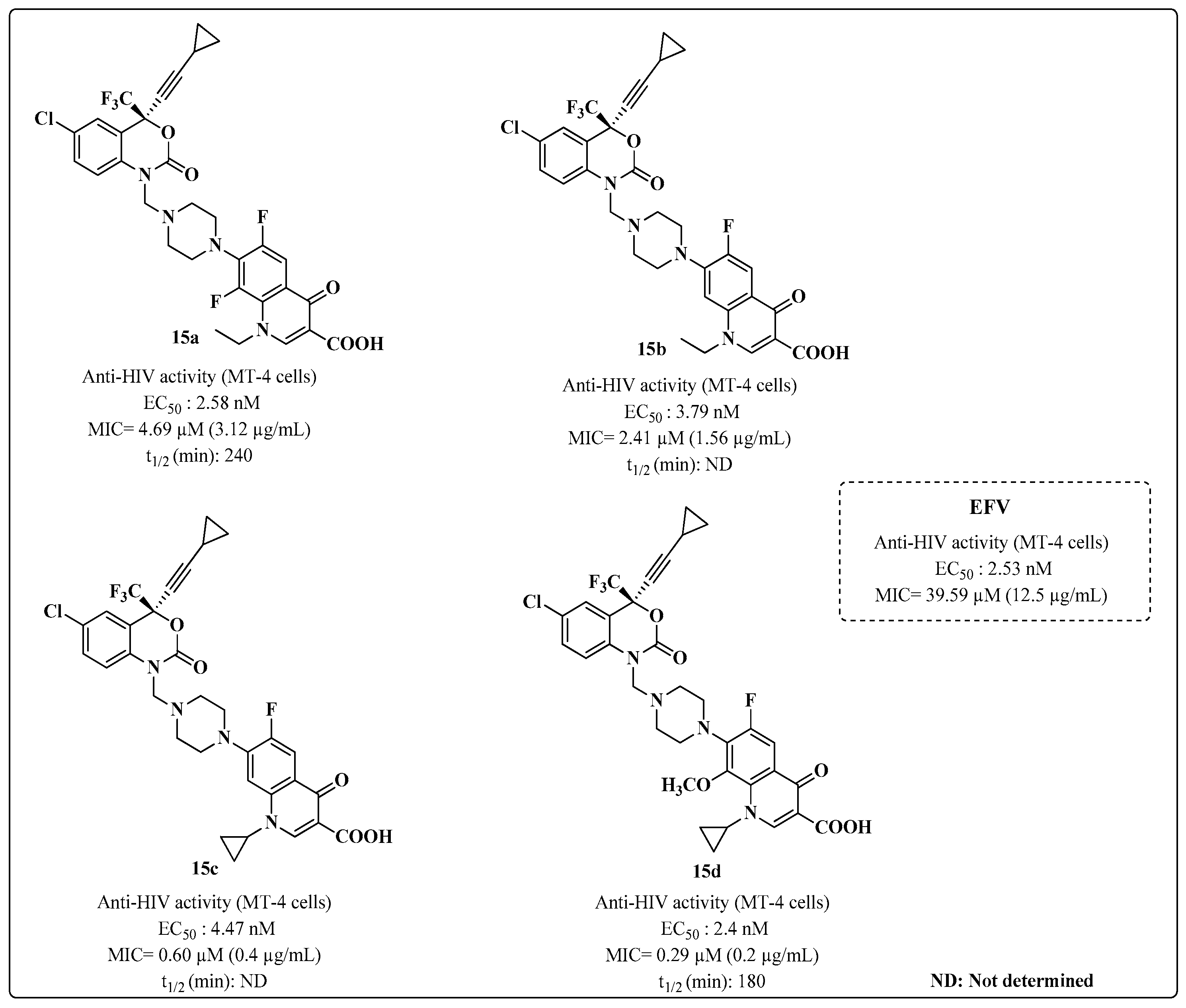

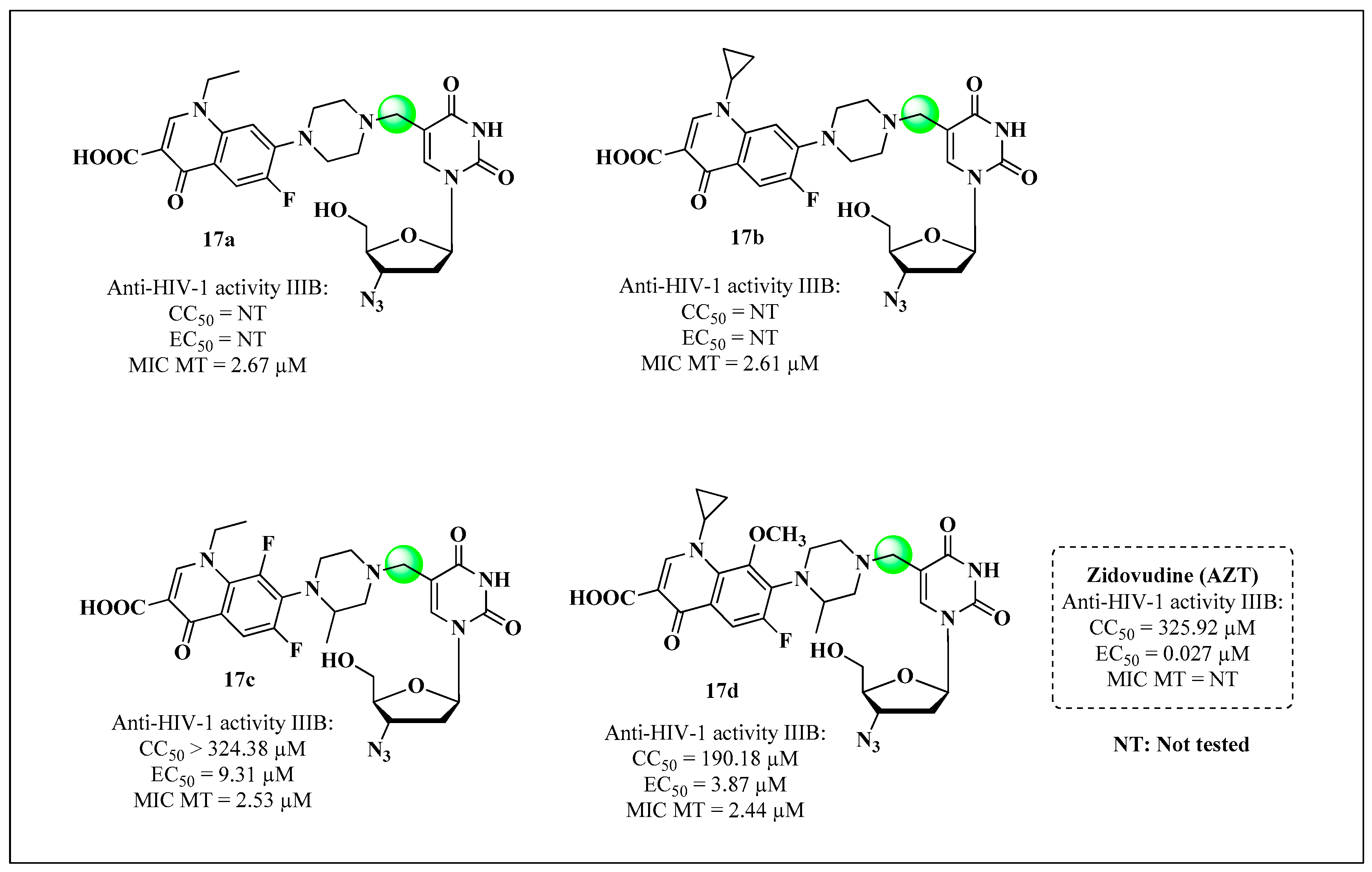

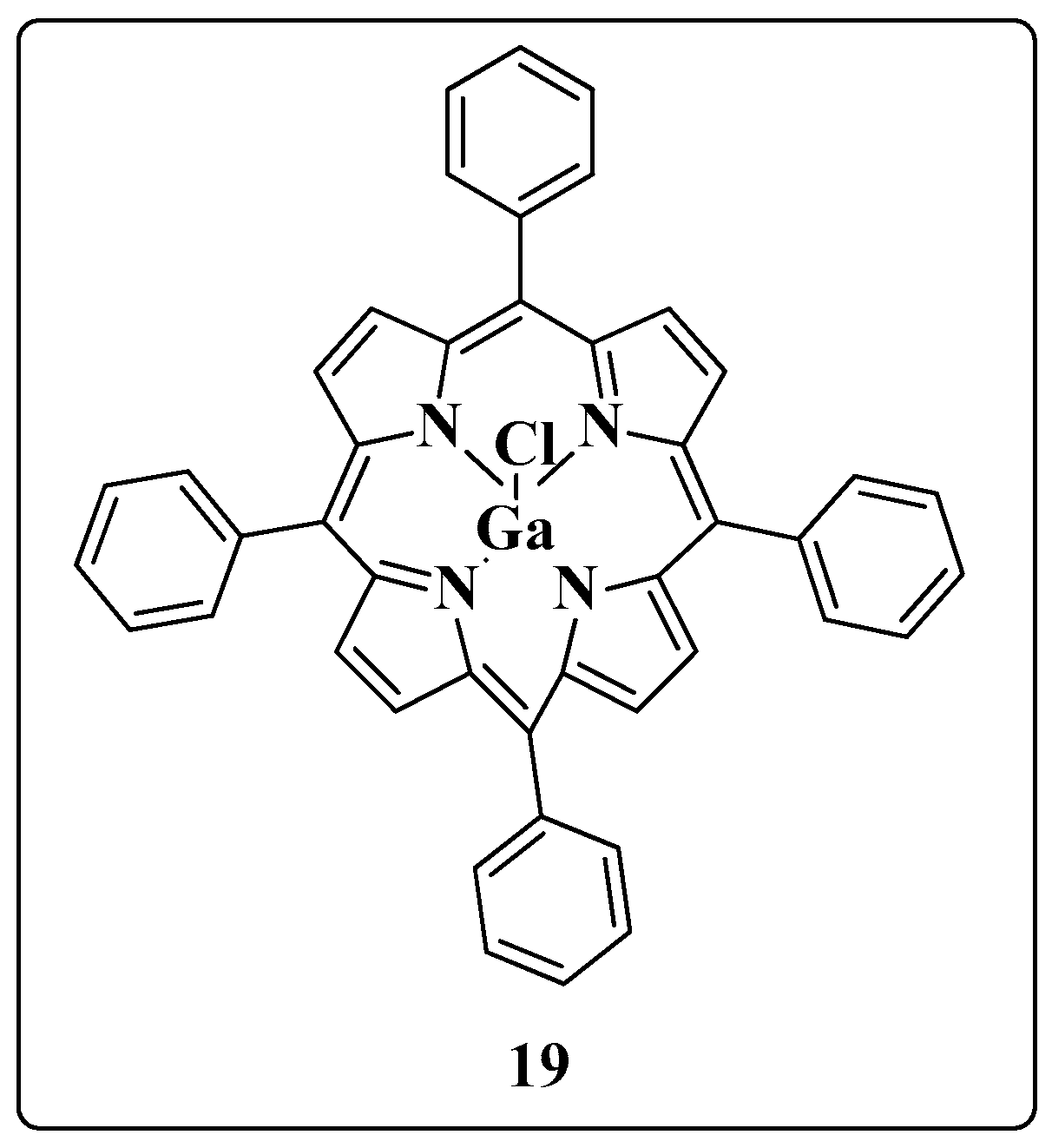
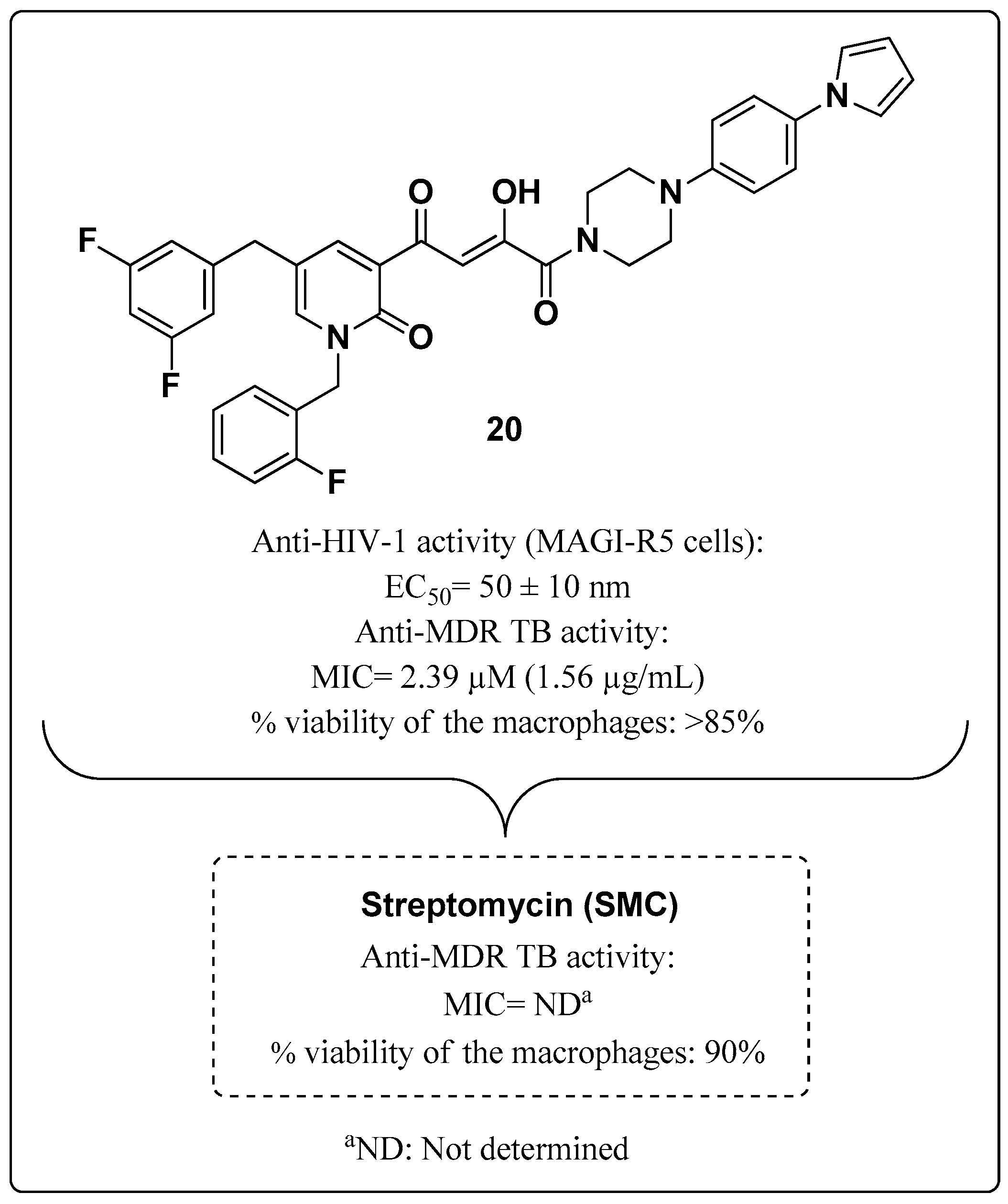


| ART and Tuberculostatic Combinations | Indication | Dosage |
|---|---|---|
| TDF/a 3TC/DTG + tuberculostatic with RIF | First choice | TDF/3TC: 300 mg tablet/day DTG: 50 mg (2 × day) |
| TDF/3TC/EFV + tuberculostatic with RIF | Alternative | DFC: 300 mg + 300 mg + 400 mg/day |
| TDF/3TC/b PI + tuberculostatic with rifabutin (RFB) | Alternative | RFB: 150 mg/day |
| TDF/3TC/c LPV-r folded dose + tuberculostatic with RIF | Alternative | - |
| MIC (μM) (Resistance Multiplication) a | |||||
|---|---|---|---|---|---|
| MTB Strain | 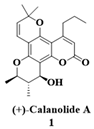 | INH | RIF | SMC | ETB |
| H37Ra | 43 | 0.45 | ND | ND | 19.57 |
| H37Rv | 21 | 0.22 | 0.019 | 0.42 | 9.78 |
| CSU b 19 | 43 | 0.22 | 0.019 | 0.42 | 9.78 |
| CSU 33 | 43 | 0.22 | 0.009 | 0.42 | 19.57 |
| H37Rv-INH-R | 21 (1) | >933 (>4100) | 0.037 (2) | 0.42 (1) | 39.15 (4) |
| CSU 36 | 21 (1) | 0.22 (1) | 77 (4000) | 0.42 (1) | 9.78 (1) |
| CSU 38 | 21 (1) | 0.22(1) | 0.019 (1) | >220 (>512) | 9.78 (1) |
| H37Rv-EMB-R | 21 (1) | 1.82 (8) | 0.037 (2) | 0.42 (1) | 313 (32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, D.I.; de Castro Bazan Moura, S.; da Conceição Avelino Dias, M.; Costa, C.C.P.; Machado, G.P.; Pimentel, L.C.F.; Branco, F.S.C.; Moreira, R.; Bastos, M.M.; Boechat, N. A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens. Molecules 2023, 28, 3342. https://doi.org/10.3390/molecules28083342
Leite DI, de Castro Bazan Moura S, da Conceição Avelino Dias M, Costa CCP, Machado GP, Pimentel LCF, Branco FSC, Moreira R, Bastos MM, Boechat N. A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens. Molecules. 2023; 28(8):3342. https://doi.org/10.3390/molecules28083342
Chicago/Turabian StyleLeite, Debora Inacio, Stefany de Castro Bazan Moura, Maria da Conceição Avelino Dias, Carolina Catta Preta Costa, Gustavo Peixoto Machado, Luiz Claudio Ferreira Pimentel, Frederico Silva Castelo Branco, Rui Moreira, Monica Macedo Bastos, and Nubia Boechat. 2023. "A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens" Molecules 28, no. 8: 3342. https://doi.org/10.3390/molecules28083342
APA StyleLeite, D. I., de Castro Bazan Moura, S., da Conceição Avelino Dias, M., Costa, C. C. P., Machado, G. P., Pimentel, L. C. F., Branco, F. S. C., Moreira, R., Bastos, M. M., & Boechat, N. (2023). A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens. Molecules, 28(8), 3342. https://doi.org/10.3390/molecules28083342







