Abstract
A sulfur doping strategy has been frequently used to improve the sodium storage specific capacity and rate capacity of hard carbon. However, some hard carbon materials have difficulty in preventing the shuttling effect of electrochemical products of sulfur molecules stored in the porous structure of hard carbon, resulting in the poor cycling stability of electrode materials. Here, a multifunctional coating is introduced to comprehensively improve the sodium storage performance of a sulfur-containing carbon-based anode. The physical barrier effect and chemical anchoring effect contributed by the abundant C-S/C-N polarized covalent bond of the N, S-codoped coating (NSC) combine to protect SGCS@NSC from the shuttling effect of soluble polysulfide intermediates. Additionally, the NSC layer can encapsulate the highly dispersed carbon spheres inside a cross-linked three-dimensional conductive network, improving the electrochemical kinetic of the SGCS@NSC electrode. Benefiting from the multifunctional coating, SGCS@NSC exhibits a high capacity of 609 mAh g−1 at 0.1 A g−1 and 249 mAh g−1 at 6.4 A g−1. Furthermore, the capacity retention of SGCS@NSC is 17.6% higher than that of the uncoated one after 200 cycles at 0.5 A g−1.
1. Introduction
Over the past decade, the energy crisis, environmental degradation, and political orientation across the world have combined to fire the explosive growth of the new energy vehicle market [1,2]. However, limited by barren lithium resources, Lithium-ion batteries (LIBs) cannot support the rapid development of electric vehicles and large-scale energy storage alone [3]. The high crustal abundance, low standard reduction potential (−2.71 V vs. SHE), and inert nature of sodium when it encounters aluminum make sodium-ion batteries (SIBs) a cheaper and more widely available alternative to LIBs [3,4,5]. More importantly, the operating principles and battery components of SIBs are similar to LIBs, which means the advanced manufacturing line and technique of LIBs can be applied to the production of SIBs without resistance [2,3,5].
Currently, the biggest obstacle to the commercial production of SIBs is the lack of suitable electrode materials. Although various anode materials have been intensively studied, such as conversion materials (oxide [6], sulfide [7]), alloy materials (Sn [8], Sb [9], and Ge [10], etc.), organic compounds (Schiff base polymer [11] and polyamide [12]), and intercalation materials (carbon-based material [13] and titanium-based material [14], etc.), a carbon-based material is still the most promising one for its low cost, chemical inertness, adjustable structure, and abundant sources (Table S1). For example, Kang et al. reported that graphite as a host for Na+-solvent complexes could provide a sodium storage specific capacity in excess of 120 mAh g−1 [15]. In 2000, glucose-derived hard carbon (HC) as the anode of SIBs was first reported by Dahn et al. and demonstrated a high reversible capacity of 300 mAh g−1 [16]. From then on, HC, as carbonaceous materials that cannot be converted into graphite even at temperatures above 3000 °C, has attracted intensive investigation [17]. HC materials with different interlayer distance, doping elements, and pore structure have been reported in succession [18]. However, the stress-induced process accompanying the insertion/desertion of sodium ions deteriorates the cycling stability of HC [19]. Additionally, the sluggish reaction kinetics during the insertion and pore-filling process make the rate capability of HC very poor [20].
An effective way to overcome the shortages of HC is to dope with heteroatoms such as B, N, P, and S [21]. Doped heteroatoms can optimize interlayer distance, electron/ion conductivity, porosity, and defect concentration of hard carbon material to increase the active sites for sodium storage [22,23,24,25]. For example, benefiting from the expanded interlayer distance (0.406 nm) and enhanced electronic conductivity of hierarchical N/S–coped carbon microspheres (NSC-SP), a NSC-SP electrode delivered a high specific capacity of 280 mAh g−1 at 30 mA g−1 and 130 mAh g−1 at 10 A g−1 [26]. Additionally, the introduction of heteroatoms can also enhance the rate capability of electrode material because of the increase in sodium storage capacity dominated by pseudocapacitance [21]. According to the report of Jin et al., the capacitive contributions of N, S co-doped carbon nanoparticles (NSC2) at 5 mV s−1 is 94%, which is higher than that of undoped carbon nanosheets (DCs, 86%). As a result, NSC2 exhibited higher discharge specific capacities at various current densities [27]. Among the common non-metallic doping elements, sulfur is preferred because sulfur atoms can exist not only in the covalent bonding state (C-S-C, C-S-S-C), but also in the molecular state of S2, S4, S6, and S8 stored in the pores of HC [28,29]. Each single sulfur atom can anchor two sodium ions during the discharge to significantly improve the sodium storage capacity of HC. However, some HC materials, such as glucose-derived carbon spheres (GCS), have difficulty in preventing the shuttling effects of soluble polysulfide intermediates when sulfur molecules are in the pores of GCS, deteriorating the cyclic stability of electrode materials. Fortunately, the research findings of Lithium-Sulfur batteries inspire us to solve this problem from a multifunctional carbon coating layer [27,30]. For example, Song [31] designed a polypyrrole coated loose mesh carbon/sulfur composite (C/S@PPy). In this structure, the conductive network of mesoporous carbon has good electrical conductivity, and the carbon coating enhances the electronic conductivity of the sulfur cathode. In addition, the coating inhibits the dissolution of polysulfide. The initial discharge capacity of the C/S@PPy electrode material was 1209.6 mAh g−1 at 0.1 C. The discharge capacity retention rate was 62.2% at 0.2 C for 300 cycles.
In this paper, a polyacrylonitrile derived N, S-codoped carbon layer (NSC) is coated on the glucose-derived and sulfur-loaded carbon spheres (SGCS@NSC) through a solvothermal reaction and subsequent thermal treatment. As a physical barrier layer, the NSC layer can confine polysulfide in the GCS to prevent its dissolution in the electrolyte. More importantly, the abundant polarized C-S and C-N covalent bonds in NSC have a strong anchoring ability to further eliminate the shuttling effect of polysulfide. As a result, SGCS@NSC exhibits a significantly improved cyclic stability, with a 17.6% higher capacity retention than SGCS after 200 cycles at 0.5 A g−1. Besides, SGCS@NSC inherits the cross-linked three-dimensional network structure of NSC, which can serve as a continuous conductive network to achieve excellent electrochemical performance. SGCS@NSC exhibits a considerable sodium storage capacity of 609.8 mAh g−1 at 0.1 A g−1 and excellent rate capability of 249 mAh g−1 at 6.4 A g−1. The synergistic effect of physical confinement and chemical adsorption can be used to improve the electrochemical performance of other sulfur-rich electrode materials.
2. Results and Discussion
Glucose-derived carbon spheres (GCS) of about 150 nm were firstly prepared according to the reports elsewhere (Figure 1a). After p-GCS were fully dispersed in the polyacrylonitrile (PAN) solution, the homogeneous suspension was titrated into the high-speed rotating mixed alcohol solution to encapsulate p-GCS into a cross-linked 3D PAN network. SGCS@NSC, NSC, and SGCS samples were finally obtained by annealing a mixture of the corresponding precursor and sublimated sulfur. As shown in Figure 1b,c and Figure S1a1–a3, both SGCS@NSC and NSC maintain the cross-linked network structure that facilitates fast electron transport. The difference is that there are more sphere-like particles in SGCS@NSC due to the encapsulation of SGCS into the network. The TEM image in Figure 1d further reveals the detailed structure of SGCS@NSC and validates the conclusion drawn from SEM images. The HRTEM (Figure 1e) shows that SGCS particles are tightly encapsulated with NSC of uneven thickness. The NSC layer acts as a physical barrier to prevent the diffusion of soluble polysulfides from the SGCS particles to a certain extent. Energy dispersive X-ray spectroscopy (EDS) demonstrates the coexistence and uniform distribution of C, N, and S elements in SGCS@NSC. The sulfur element in the NSC layer in the form of C-S covalent bonding also plays an important role in eliminating the shuttling effects of polysulfides, which will be discussed later.
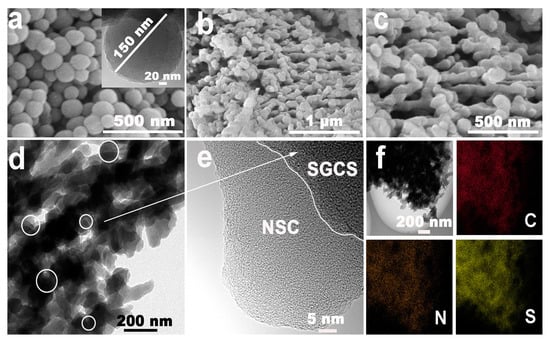
Figure 1.
(a) The SEM and TEM images of GCS. (b,c) The SEM images of SGCS@NSC. (d,e) The TEM images of SGCS@NSC. (f) Element mapping images (C, N, and S) of SGCS@NSC.
X-ray diffraction (XRD) patterns, nitrogen adsorption/desorption curves, Raman spectra, and XPS spectra were obtained to understand the structural characteristics and chemical properties of obtained samples. The XRD patterns of prepared samples all show broad (002) peaks around 25° corresponding to characteristics of amorphous carbon (Figure S2a) [32,33]. The interlayer distance (d002) of SGCS@NSC, NSC, SGCS, and GCS samples are 0.361 nm, 0.352 nm, 0.367 nm, and 0.389 nm, respectively. The interlayer distance of SGCS is lower than that of GCS due to the low thermal treatment temperature and the promoting effect of molten sulfur on the uniformity of the thermal field. This also implicitly suggests that the sulfur species in SGCS are not primarily presented in the form of chemical bonds, a view that will be discussed in detail in conjunction with subsequent XPS characterization. On the other hand, it is completely reasonable that the interlayer distance of SGCS@NSC composite is between SGCS and NSC itself. Nitrogen adsorption/desorption tests indicate that GCS owns the highest Brunauer–Emmett–Teller (BET) surface area (56.1 m2 g−1, Table S4). After the introduction of sulfur, the BET surface area of SGCS decreases to 18.7 m2 g−1, corresponding to a severe loss of pores between 10 and 50 nm (Figure 2a, obtained from the adsorption curve). Considering the BET surface of NSC is 31.2 m2 g−1, its reasonable that the BET surface area and pore volume exhibit the sequence of GCS > SGCS@NSC > SGCS.
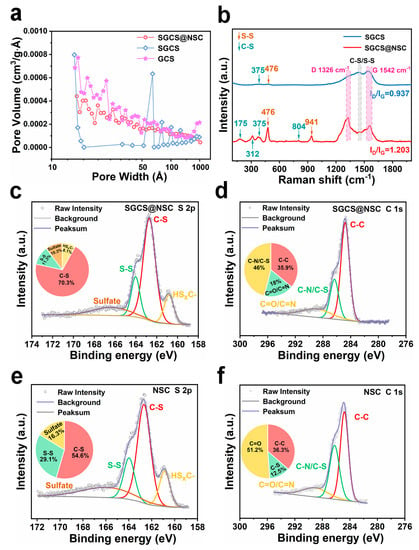
Figure 2.
(a) Pore distribution curves of SGCS@NSC, SGCS, and GCS. (b) Raman scattering spectra of SGCS@NSC and SGCS. (c–f) The high-resolution XPS of S 2p and C 1s of SGCS@NSC and SGCS.
The Raman spectra of SGCS@NSC and NSC show peaks at 175, 312, 375, and 804 cm−1 in the 100–1000 cm−1 wavenumber region, indicating the stretching, bending, and deformation of the C-S bond (Figure 2b and Figure S2b) [33,34]. The stretching of the S-S bond contributes to the two peaks at 476 and 941 cm−1 [35]. In addition, the two peaks near 1480 cm−1 also correspond to the stretching of C-S/S-S bonds [33,36]. The characteristic peaks of C-S/S-S bonds in SGCS are hardly observed. Sulfur in SGCS is mainly stored in the pores as an independent molecular state, making C-S/S-S bonds difficult to detect on surface, and the few sites in GCS where sulfur can form covalent bonds cause the absence of C-S/S-S bonds. In the high wavenumber region, all the prepared samples show two main peaks: one centered at 1326 cm−1 corresponds to the D band caused by defects or disordered structures, and the other around 1580 cm−1 represents the G band generated by the in-plane stretching vibration of sp2 hybridized carbon [37,38]. The exact wavenumber of the D band of SGCS and SGCS@NSC are close to each other, while the G band of SGCS (1542 cm−1) displays a red shift compared to SGCS@NSC (1556 cm−1), which may be due to more edge defects induced by a larger interlayer distance of SGCS. The Raman characteristic peaks of SGCS@NSC are consistent with NSC, suggesting that SGCS particles are well wrapped by the NSC layer. The D-band to G-band intensity ratio of SGCS@NSC (1.203) is much higher than SGCS (0.937) and GCS (0.168), indicating N/S co-doping can introduce more structural defects, which is conducive to improving the sodium storage performance [39].
The high content of the C-S bond in SGCS@NSC is also reflected in XPS spectra. Figure S3a and Table S5 confirm the coexistence of C, O, and S elements in SGCS, NSC, and SGCS@NSC, and the latter two also contain significant amounts of the N element. Figure 2c shows the fitted S 2p high-resolution XPS spectra of SGCS@NSC. The two main peaks at 162.7 and 164.0 eV are contributed by C-S and S-S bonds, respectively [27,36], and another peak at 160.8 eV can be assigned to HSXC- by-products produced by PAN during the vulcanization process [40]. According to the quantitative analysis, the ratio of the C-S bond to S-S bond in SGCS@GCS is 6.2, much higher than 1.9 in SGCS (Table S2). As for C 1s spectra, the area percentage of C-N/C-S bonds in SGCS@GCS (18.0%) far exceeds the percentage of C-S in SGCS (12.5%, Table S3). These two comparisons demonstrate that SGCS@NSC possesses much more C-S bonds, which can serve as anchoring sites of polysulfide. In addition, the abundant pyridine nitrogen (389.9 eV), pyrrole nitrogen (400.7 eV), and oxidized nitrogen (402.7 eV) in SGCS@NSC exhibit strong dipole–dipole electrostatic interactions with polysulfides, preventing polysulfides from shuttling (Figure S3c) [41,42]. Therefore, the chemical adsorption of the NSC layer is helpful to enhance the cyclic stability of SGCS@NSC.
The effectiveness of the NSC coating layer is verified by the electrochemical performance in sodium ion half-cells. Figure 3a shows the initial five cycles cyclic voltammetry (CV) curves of SGCS@NSC in the range of 0.01–3.0 (V vs. Na/Na+) with a sweep speed of 0.1 mV s−1 [43]. After the first cycle, two pairs of peaks at 2.239/1.855 and 1.903/1.137 V in the subsequent curves correspond to the step-by-step redox reaction of sulfur [44,45], and the curves overlap well, indicating the excellent cycling stability of the electrode. However, the cathodic peaks current of SGCS decreases rapidly from the second cycle, and the redox peaks’ intensities are weaker than SGCS@NSC despite the higher sulfur content of SGCS (Figure S4c).
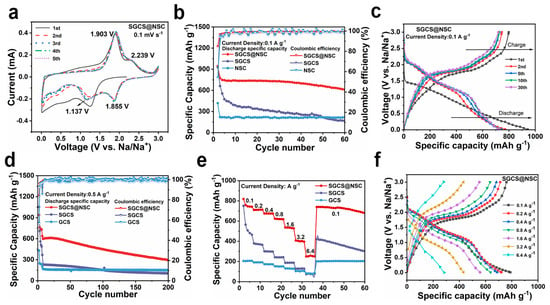
Figure 3.
The electrochemical characterization of SGCS, SGCS@NSC, and NSC in the voltage range of 0.01–3.0 V versus Na/Na+. (a) The first five CV curves of SGCS@NSC at 0.1 mV s−1. (b) Cycling test at 0.1 A g−1. (c) The 1st, 2nd, 5th, 10th, and 30th cycles discharge–charge profiles of SGCS@NSC at 100 mA g−1. (d) Cycling test at 0.5 A g−1. (e) Rate capability electrodes at increasing current densities from 0.1 to 6.4 A g−1. (f) Galvanostatic discharge–charge profiles of SGCS@NSC at increasing current densities from 0.1 to 6.4 A g−1.
As shown in Figure 3b, the initial discharge/charge specific capacity of SGCS@NSC, SGCS, and GCS at 0.1 A g−1 is 942.9/798.2, 928.1/661.3, and 417.1/226 mAh g−1, respectively. SGCS@NSC has the highest initial coulombic efficiency (ICE, 84.7%) due to the moderate BET surface area and the suppressed polysulfides dissolution. Although GCS has the largest interlayer distance, the high BET surface area and the lack of highly reversible active sites introduced by N, S co-doping resulted in an ICE as low as 54.2% for GCS. The 5th, 10th, and 30th galvanostatic charge and discharge curves of SGCS@NSC overlap each other (Figure 3c), exhibiting the same electrochemical phenomena as the CV curves. After 60 cycles at 0.1 A g−1, the discharge specific capacity of SGCS@NSC is 609.8 mAh g−1, far exceeding that of SGCS (164.9 mAh g−1) and GCS (215.4 mAh g−1). The significant improvement in sodium storage performance can be attributed to N, S co-doped active sites, which can not only act as sodium storage sites themselves, but also enhance the reversibility of polysulfide intermediates in SGCS. After 200 cycles at 0.5 A g−1 (Figure 3d), the discharge specific capacity of SGCS@NSC, SGCS, and GCS are 294.1, 105.1, and 148.3 mAh g−1, corresponding to the capacity retentions of 46.1%, 28.5%, and 74.1%, respectively (all the electrodes were tested at 100 mA g−1 for 5 cycles to fully activate the electrode materials, and the capacity retention was calculated based on the 6th discharge specific capacity). GCS has a better cycling stability but lacks sufficient active sites for sodium storage, so the specific capacity is lower than SGCS@NSC by up to 146 mAh g−1. The inability of SGCS to maintain the reversibility of the sulfur-containing active sites resulted in the capacity retention and specific capacity to be 17.6% and 189 mAh g−1 lower than SGCS@NSC, respectively. Benefiting from the synergistic effect of the physical barrier and chemical adsorption of NSC layer, SGCS@NSC displays high sodium storage specific capacity and enhanced cycling stability.
Meanwhile, Figure 3e presents the rate performance of SGCS@NSC, NSC, and SGCS measured at various current densities. At the current densities of 0.1, 0.2, 0.4, 0.8, 1.6, and 3.2 A g−1, the discharge specific capacity of SGCS@NSC are 818.6, 710.2, 674.8, 623.6, 536.4, and 407.9 mAh g−1. At 3.2 A g−1, the discharge profiles of SGCS@NSC show an obvious step-by-step sodiation phenomenon, implying the excellent electrochemical kinetics (Figure 3f). Even when the current density is increased to 6.4 A g −1, a high specific capacity of 248.9 mAh g−1 can still be provided, much higher than the 39.1 mAh g−1 of SGCS and 72.9 mAh g−1 of GCS. In addition, when the current density is reset to 0.1 A g−1, the SGCS@NSCC electrode still provides a reversible specific capacity of 609.8 mAh g−1, exhibiting excellent high-rate cycling performance. These results indicate that the successful incorporation of S and N elements into the carbon framework can effectively improve the reactivity and electronic conductivity of GCS by producing external defects, and the resulting defects can enhance the transport rates of sodium ions. More contact sites are provided between the active material and the electrolyte, which is beneficial to the high-rate performance.
To explore the reaction kinetics of the prepared electrodes, CV curves at different sweep speeds of 0.1–1.0 mV s−1 were obtained (Figure 4a and Figure S4a–c). The area of the closed CV curves represents the total charge storage for Faraday and non-Faraday processes [46], which are usually divided into capacitive-control and diffusive-control charge storage [33]. The contribution of the two charge storage modes can be calculated according to the following equations [47]:
where a and b are variable parameters, means the sweep speed, and is the response current. When the b value is close to 0.5 or 1, the diffusion-controlled process or the surface capacitance-controlled process dominates the electrochemical reaction [48]. The value of b is determined by the slope of the log () vs. log () plot of the redox peaks. As can be seen from Figure 4b, the b value of peak A of SGCS@NSC is between NSC and SGCS, which are both below 0.7, indicating a diffusion-controlled process. However, the b value of peak C of SGCS@NSC is around 0.8, higher than that of NSC (0.61) and SGCS (0.57), manifesting a surface capacitance-controlled discharge process. To quantify the contribution of the diffusion and surface capacitance-controlled processes at various sweep speeds, Equation (1) can be rewritten as:
where k1 and k2 are constants [49]. Figure 4c shows that the contribution of pseudocapacitance for SGCS@NSC are 44, 49, 57, 65, 77, and 90% from 0.1 to 1 mV s−1, exceeding SGCS and NSC at various sweep speeds. This result is consistence with the optimal rate capability of SGCS@NSC, demonstrating the resistance to high current pulses.
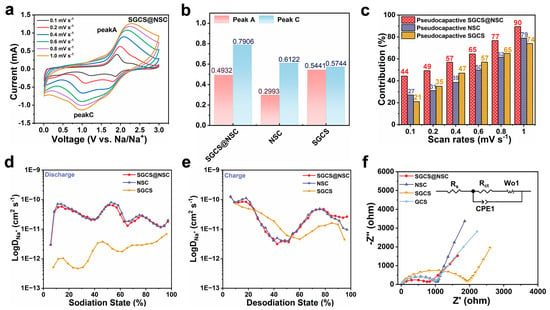
Figure 4.
(a) CV curves of SGCS@NSC with scan rates from 0.1 to 1 mV s−1. (b) Log () versus log () plots of SGCS@NSC, NSC, and SGCS at peak A and C. (c) Normalized pseudocapacitive contribution ratios of SGCS@NSC, NSC, and GCS at different scan rates (0.1–1 mV s−1). (d,e) The Na+ diffusion coefficient of SGCS@NSC, SGCS, and NSC (discharge and charge) obtained by GITT tests. (f) EIS spectra of SGCS@NSC, NSC, SGCS, and GCS electrodes and equivalent circuit.
The galvanostatic intermittent titration technique (GITT) was performed to investigate the Na+ diffusion coefficient in prepared electrodes during the discharge and charge process. The calculation based on Equation (S1) shows that SGCS@NSC inherits the electrochemical kinetics process of NSC with similar Na+ diffusion coefficient and variation trends. The average Na+ diffusion coefficient of SGCS@NSC and NSC is higher than SGCS in the whole process, and the difference in the discharge process reaches an order of magnitude (Figure 4d,e). As shown in Figure 4f, the Nyquist plots of all samples were composed of two parts: one is a depressed half circle, which is composed of two half-circles in the high and middle frequency areas; and the other is an oblique linear in the low frequency area. Furthermore, the corresponding equivalent circuit model in Figure 4f is used to simulate the experimental data, where RS stands for the resistance of electrolyte solution obtained from the high frequency region data and Rct represents the charge transfer resistance fitted from the intermediate frequency region data [50]. The electrochemical impedance spectroscopy (EIS) results illustrate decreased semicircle diameters at high-frequency regions and increased slopes of the inclined lines at low-frequency regions for SGCS@NSC. As we can see from the fitted EIS parameters in Table S6, the Rs (4.9 Ω) and Rct (637.2 Ω) values of SGCS@NSC are significantly reduced compared to other samples. Profiting from the cross-linked conductive network constructed by NSC coating, the charge transfer between the SGCS@NSC electrode and the electrolyte is easier. Thus, it is demonstrated that the electron and sodium ion transfer rates in SGCS@NSC are both improved [50]. The fast Na+ diffusion coefficient and electron transport in the 3D cross-linked NSC coating layer account for the high sodium storage specific capacity and excellent rate capability of the SGCS@NSC electrode.
3. Materials and Methods
Synthesis of glucose-derived carbon spheres (GCS): typically, 8.0 g glucose (D-(+)-glucose, 99.5%, Aladdin) was dissolved in 80 mL deionized water. Then, the obtained transparent solution was placed in a sealed 100 mL Teflon-lined stainless-steel autoclave and heated to 160 °C for 8 h. After cooling naturally to room temperature, the precipitate was separated from the supernatant by centrifugation at 10,000 r/min for 10 min. The resulting solid was purified three times with deionized water and, finally, once with absolute ethanol. The obtained product was vacuum dried at 80 °C overnight. Finally, the dry powder was calcined at 200 °C for 12 h in a muffle furnace to obtain the glucose-derived carbon spheres precursor (p-GCS), which was then carbonized at 500 °C in an argon atmosphere for 2 h to obtain glucose-derived carbon spheres (GCS).
Synthesis of p-GCS@H-PAN and H-PAN: generally, 0.1 g of polyacrylonitrile (PAN, Mw = 150,000) was dissolved in 3 mL of N, N-dimethyl formamide (DMF), followed by the addition of 0.05 g of p-GCS. The suspension endured ultrasonic treatment for 2 h to fully disperse p-GCS. A total of 3 mL glycerin and 30 mL isopropyl alcohol were stirred at 500 rpm for 1 h in a 50 mL Teflon polytetrafluoroethylene (PTFE) beaker. Next, the speed was increased to 700 rpm and the as-prepared DMF suspension was added dropwise into the mixed alcohol system. After stirring for another 10 min, the reactor was transferred into 180 °C electrical oven for 6 h. The resulting brown precipitate was thoroughly cleaned with deionized water and dried by freeze-drying. The hydrothermally treated PAN coated p-GCS was abbreviated as p-GCS@H-PAN. The H-PAN was prepared by the same procedures as p-GCS@H-PAN but without the addition of p-GCS.
Synthesis of SGCS@NSC, NSC, and SGCS: the above-prepared p-GCS@H-PAN, H-PAN, and p-GCS were mixed with sublimed sulfur in a ratio of 1:5 and heated to 500 °C for 2 h at 3 °C min−1 in an argon atmosphere. The obtained black products were labeled as SGCS@NSC, NSC, and SGCS.
Materials Characterization: S4800 Field emission scanning electron microscopy (SEM Hitachi, Tokyo, Japan) and JEM2100 Transmission electron microscope (TEM) tests were used to characterize the microstructure of the samples. The X-ray diffraction (XRD) powder diffraction pattern of the samples was recorded on a D8 ADVANCE DAVINCI X-ray diffractometer (Cu kα radiation, = 0.154 nm BRUKER AXS, Bremen, Germany). Axis Ultra DLD X-ray photoelectron spectroscopy (XPS, Kratoms Britain) were used to analyze the chemical composition of the samples. The structure of carbon component of the prepared samples was characterized by Renishaw India Reflex confocal Raman microscopy (Renishaw, Kingswood, UK). ASAP 2020 surface area and a porosity analyzer were used to analyze the pore structure of the sample.
Electrochemical measurements: The synthesized products were mixed with a conductive carbon (Super P) and sodium carboxymethyl cellulose (CMC) binder with a mass ratio of 8:1:1 in deionized water. The electrodes were fabricated by spreading the slurry on Cu foil and then dried at 80 °C in a vacuum overnight. Whatman glass fiber was used as the separator, sodium metal foil as the counter electrode, and 1 M NaPF6 dissolved in ethylene (EC) and dimethyl carbonate (DMC) (1:1, vol/vol) as the electrolyte. The Cyclic Voltammetry (CV) curves were obtained by an electrochemical workstation (CHI 660E) at different sweep speeds between 0.01 and 3.0 V (vs. Na+/Na). On the LAND instrument, the constant current charge and discharge experiments with different current densities were performed in the same voltage range. Electrochemical Impedance Spectroscopy (EIS) tests were conducted on an electrochemical station (Model 1470E multi-channel electrochemical workstation, Solartron Metrology) in the frequency range of 100 kHz to 10 mHz at room temperature.
4. Conclusions
In summary, glucose-derived carbon spheres were encapsulated into a N, S co-doped 3D cross-linked network for the construction of high-performance sulfur-containing anode for sodium-ion batteries. The tight coating relationship of the NSC layer on SGCS acting as a physical barrier and abundance of C-S/C-N bonds serving as chemical adsorption sites together protect SGCS@NSC from the shuttling effect of polysulfides. In addition, the doped sites can provide additional sodium storage sites and improve the sodium diffusion coefficient of the composite to ensure the comprehensive electrochemical performance of SGCS@NSC. Thus SGCS@NSC still delivers 293.7 mAh g−1 sodium storage specific capacity after 200 cycles at 0.5 A g−1, higher than 107.4 mAh g−1 of SGCS, demonstrating improved cycling stability. With the assistance of a 3D high-speed electron and ion transport network of the NSC layer, SGCS@NSC illustrates enhanced pseudocapacitive charge storage and rate performance. SGCS@NSC provides a high specific capacity of 248.9 mAh g−1 at 6.4 A g−1 and the pseudocapacitive contribution, of which is as high as 90% at 1.0 mV s−1. This multifunctional coating greatly improves the electrochemical performance of SGCS and is also applicable to other sulfur-containing electrodes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083335/s1, Table S1: Summary of some different carbon-based materials for SIBs. Figure S1: The SEM images of (a1,a2,a3) NSC, (b1,b2,b3) SGCS and (c1,c2,c3) GCS at different magnifications. TEM and HRTEM images of (a4,a5) NSC, (b4,b5) SGCS and (c4,c5) GCS; Figure S2: (a) X-ray diffraction patterns of SGCS@NSC, NSC, SGCS, and GCS. (b,c) Raman scattering spectra of NSC and GCS. (d) N2 adsorption-desorption isotherms of SGCS@NSC, NSC, SGCS, and GCS. € Pore distribution curves of NSC; Table S2: Analysis of sulfur bonding area ratio of SGCS@NSC and SGCS; Table S3: Analysis of carbon bonding area ratio of SGCS@NSC and SGCS; Table S4: Specific surface areas and pore volume analysis of SGCS@NSC, SGCS, GCS, and NSC; Figure S3: (a) Typical XPS survey spectra. (b,d,f) The high-resolution XPS of S 2p, N 1s, and C 1s for NSC, respectively. (c) The high-resolution XPS of N 1s for SGCS@NS€ (e) The high-resolution XPS of C 1s for GCS; Table S5: Element composition and ratio of the surface of four groups of samples calculated from XPS data (wt.%); Figure S4: The first five successive CV curves of (a) NSC, (c) SGCS€nd (e) GCS at the scan rate of 0.1 mV s−1. Galvanostatic discharge-charge profiles (100 mA g−1) at the 1st, 2nd, 5th, 10th, and 30th cycles of (b) NSC, (d) SGCS, and (f) GCS; Figure S5: CV curves at different scan rates of (a) NSC, (b) SGCS, and (c) GCS from 0.1 to 1.0 mV s−1. (d) Normalized pseudocapacitive contribution rations of GCS at scan rates from 0.1 to 1.0 mV s−1. (e) Log () versus log () plots of GCS at selected peak currents. (f) Rate capability of NSC electrodes at increasing current densities from 0.1 to 6.4 A g−1; Table S6: The EIS fitting parameters of SGCS@NSC, NSC, SGCS, and GCS. References [37,42,51,52,53,54,55,56,57] are cited in the supplementary materials.
Author Contributions
Conceptualization, B.Y., M.S. and H.H.; methodology, L.Z. and B.Y.; software, B.Y. and Y.Z.; validation, B.Y., M.S. and H.H.; formal analysis, L.Z. and B.Y.; investigation, L.Z. and H.D.; data curation, L.Z., Q.W. and H.X.; writing—original draft preparation, L.Z.; writing—review and editing, B.Y., M.S. and H.H.; visualization, H.X. and Q.W.; supervision, Y.Z. and Q.W.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the High-quality Development Project of Ministry of Industry and Information Technology of People’s Republic of China (TC210H041) (to H.H.), the Hundred Talents Program, the National Natural Science Foundation of China (Grant No. 51872304) (to H.H.), and Ningbo S&T Innovation 2025 Major Special Program (2018B10024; 2019B10044; 2020Z101; 2022Z022) (to H.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nayak, P.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Larramendi, I.R.; Guo, X.; Wang, G.; Rojo, T. Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Hariharan, S.; Saravanan, K.; Ramar, V.; Balaya, P. A rationally designed dual role anode material for lithium-ion and sodium-ion batteries: Case study of eco-friendly Fe3O4. Phys. Chem. Chem. Phys. 2013, 15, 2945–2953. [Google Scholar] [CrossRef]
- Dai, H.; Tang, M.; Huang, J.; Wang, Z. A Series of Molecule-Intercalated MoS2 as Anode Materials for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 10870–10877. [Google Scholar] [CrossRef]
- Sha, M.; Zhang, H.; Nie, Y.; Nie, K.; Lv, X.; Sun, N.; Xie, X.; Ma, Y.; Sun, X. Sn nanoparticles@nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef]
- Abel, P.R.; Lin, Y.-M.; de Souza, T.; Chou, C.-Y.; Gupta, A.; Goodenough, J.B.; Hwang, G.S.; Heller, A.; Mullins, C.B. Nanocolumnar Germanium Thin Films as a High-Rate Sodium-Ion Battery Anode Material. J. Phys. Chem. C 2013, 117, 18885–18890. [Google Scholar] [CrossRef]
- Castillo-Martinez, E.; González, J.C.; Armand, M. Polymeric Schiff Bases as Low-Voltage Redox Centers for Sodium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2014, 53, 5341–5345. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Wang, Y.; Wang, C.; Xia, Y. Polyimide as anode electrode material for rechargeable sodium batteries. RSC Adv. 2014, 4, 25369–25373. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Liang, X.; Xiang, H. Recent developments in carbon-based materials as high-rate anode for sodium ion batteries. Mater. Chem. Front. 2021, 5, 4089–4106. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, X.; Lu, X.; Chao, C.; Liang, Y.; Gao, P.; Sun, Y.; Liu, A.; Huang, Y. Coal-Based Hierarchically Porous Carbon Nanofibers as High-Performance Anode for Sodium-Ion Batteries. Chemelectrochem 2022, 9, e202200496. [Google Scholar] [CrossRef]
- Xiao, B.; Rojo, T.; Li, X. Hard Carbon as Sodium-Ion Battery Anodes: Progress and Challenges. Chemsuschem 2019, 12, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Shao, W.; Shi, H.; Jian, X.; Wu, Z.-S.; Hu, F. Hard-Carbon Anodes for Sodium-Ion Batteries: Recent Status and Challenging Perspectives. Adv. Energy Sustain. Res. 2022, 3, 2200009. [Google Scholar] [CrossRef]
- Wang, P.; Fan, L.; Yan, L.; Shi, Z. Low-cost water caltrop shell-derived hard carbons with high initial coulombic efficiency for sodium-ion battery anodes. J. Alloys Compd. 2019, 775, 1028–1035. [Google Scholar] [CrossRef]
- Yang, L.; Hu, M.; Zhang, H.; Yang, W.; Lv, R. Pore structure regulation of hard carbon: Towards fast and high-capacity sodium-ion storage. J. Colloid Interface Sci. 2020, 566, 257–264. [Google Scholar] [CrossRef]
- Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-Doped Carbon Materials: Synthesis, Mechanism, and Application for Sodium-Ion Batteries. Small Methods 2018, 3, 1800323. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, D.-M.; Zhang, C.; Zhang, Y.; Liang, Q.; Chen, S.; Weng, Q.; Zhou, M.; Xue, Y.; Liu, J.; et al. “Protrusions” or “holes” in graphene: Which is the better choice for sodium ion storage? Energy Environ. Sci. 2017, 10, 979–986. [Google Scholar] [CrossRef]
- Lee, W.J.; Lim, J.; Kim, S.O. Nitrogen Dopants in Carbon Nanomaterials: Defects or a New Opportunity? Small Methods 2017, 1, 1600014. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Schmidt, J. Microporous sulfur-doped carbon from thienyl-based polymer network precursors. Chem. Commun. 2011, 47, 8283–8285. [Google Scholar] [CrossRef]
- Li, Z.; Bommier, C.; Chong, Z.S.; Jian, Z.; Surta, T.W.; Wang, X.; Xing, Z.; Neuefeind, J.C.; Stickle, W.F.; Dolgos, M.; et al. Mechanism of Na-Ion Storage in Hard Carbon Anodes Revealed by Heteroatom Doping. Adv. Energy Mater. 2017, 7, 1602894. [Google Scholar] [CrossRef]
- Xu, D.; Chen, C.; Xie, J.; Zhang, B.; Miao, L.; Cai, J.; Huang, Y.; Zhang, L. A Hierarchical N/S-Codoped Carbon Anode Fabricated Facilely from Cellulose/Polyaniline Microspheres for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501929. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, K.; Feng, P.; Zhang, Z.; Cheng, S.; Jiang, K. Surface-dominated storage of heteroatoms-doping hard carbon for sodium-ion batteries. Energy Storage Mater. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cheng, H.; Ni, Z.; Wang, Y.; Xia, G.; Li, X.; Zeng, X. Research Progress toward Room Temperature Sodium Sulfur Batteries: A Review. Molecules 2021, 26, 1535. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.; Chou, S.; Liu, H.; Dou, S. Remedies for Polysulfide Dissolution in Room-Temperature Sodium–Sulfur Batteries. Adv. Mater. 2020, 32, 1903952. [Google Scholar] [CrossRef]
- Ye, J.; He, F.; Nie, J.; Cao, Y.; Yang, H.; Ai, X. Sulfur/carbon nanocomposite-filled polyacrylonitrile nanofibers as a long life and high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 7406–7412. [Google Scholar] [CrossRef]
- Song, H.; Pan, Y.; Tang, A.; Xu, G.; Liu, L.; Chen, H. Polypyrrole-coated loose network mesoporous carbon/sulfur composite for high-performance lithium-sulfur batteries. Ionics 2019, 25, 3121–3127. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Liang, X.; Gong, S.; Liu, J.; Wang, Q.; Guo, S.; Yang, H. Sulfur/Oxygen Codoped Porous Hard Carbon Microspheres for High-Performance Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800171. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Wang, L.; Yang, H.; Li, H.; Zhao, Y.; Liu, T. Sulfurized Polyacrylonitrile Cathodes with High Compatibility in Both Ether and Carbonate Electrolytes for Ultrastable Lithium–Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1902929. [Google Scholar] [CrossRef]
- Yu, X.-G.; Xie, J.-Y.; Yang, J.; Huang, H.-J.; Wang, K.; Wen, Z.-S. Lithium storage in conductive sulfur-containing polymers. J. Electroanal. Chem. 2004, 573, 121–128. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, J.-Y.; Aurbach, D.; Sun, Y.-K. Electrochemical Properties of Sulfurized-Polyacrylonitrile Cathode for Lithium–Sulfur Batteries: Effect of Polyacrylic Acid Binder and Fluoroethylene Carbonate Additive. J. Phys. Chem. Lett. 2017, 8, 5331–5337. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Kim, H.M.; Sun, Y.-K. High performance potassium–sulfur batteries based on a sulfurized polyacrylonitrile cathode and polyacrylic acid binder. J. Mater. Chem. A 2018, 6, 14587–14593. [Google Scholar] [CrossRef]
- Hou, H.; Shao, L.; Zhang, Y.; Zou, G.; Chen, J.; Ji, X. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600243. [Google Scholar] [CrossRef]
- Shao, W.; Hu, F.; Song, C.; Wang, J.; Liu, C.; Weng, Z.; Jian, X. Hierarchical N/S co-doped carbon anodes fabricated through a facile ionothermal polymerization for high-performance sodium ion batteries. J. Mater. Chem. A 2019, 7, 6363–6373. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-Doped N-Rich Carbon Nanosheets with Expanded Interlayer Distance as Anode Materials for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–Sulfur Battery Cathodes Based on PAN–Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Liu, X.; Li, Y.; Wei, M. General Synthesis of Sulfonate-Based Metal–Organic Framework Derived Composite of MxSy@N/S-Doped Carbon for High-Performance Lithium/Sodium Ion Batteries. Chem. A Eur. J. 2021, 27, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Q.; Sun, B.; Gu, J.; Yu, B.; Zhang, W.; Zhang, D. N-doped catalytic graphitized hard carbon for high-performance lithium/sodium-ion batteries. Sci. Rep. 2018, 8, 9934. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, S.; Cheng, D.; Miao, L.; Zhong, W.; Yang, X.; Li, Z. Nitrogen, Sulfur, and Phosphorus Codoped Hollow Carbon Microtubes Derived from Silver Willow Blossoms as a High-Performance Anode for Sodium-Ion Batteries. Energy Fuels 2021, 35, 2795–2804. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Capacity Enhancement and Discharge Mechanisms of Room-Temperature Sodium-Sulfur Batteries. Chemelectrochem 2014, 1, 1275–1280. [Google Scholar] [CrossRef]
- Hu, L.; Lu, Y.; Zhang, T.; Huang, T.; Zhu, Y.; Qian, Y. Ultramicroporous Carbon through an Activation-Free Approach for Li–S and Na–S Batteries in Carbonate-Based Electrolyte. ACS Appl. Mater. Interfaces 2017, 9, 13813–13818. [Google Scholar] [CrossRef]
- Li, S.; Qiu, J.; Lai, C.; Ling, M.; Zhao, H.; Zhang, S. Surface capacitive contributions: Towards high rate anode materials for sodium ion batteries. Nano Energy 2015, 12, 224–230. [Google Scholar] [CrossRef]
- Jia, M.; Qi, T.; Yuan, Q.; Zhao, P.; Jia, M. Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries. Nanomaterials 2022, 12, 2006. [Google Scholar] [CrossRef]
- Wei, Q.; Li, Q.; Jiang, Y.; Zhao, Y.; Tan, S.; Dong, J.; Mai, L.; Peng, D.-L. High-Energy and High-Power Pseudocapacitor–Battery Hybrid Sodium-Ion Capacitor with Na+ Intercalation Pseudocapacitance Anode. Nano-Micro Lett. 2021, 13, 55. [Google Scholar] [CrossRef]
- Jena, S.; Mitra, A.; Das, S.; Das, D.; Agrawal, S.; Das, K.; Majumder, S.B.; Das, S. A strategy for designing low-cost, environment-friendly, high energy and power density sodium-ion full cells: Effect of extrinsic pseudocapacitance. J. Alloys Compd. 2021, 854, 157238. [Google Scholar] [CrossRef]
- Zhao, C.-D.; Guo, J.-Z.; Gu, Z.-Y.; Zhao, X.-X.; Li, W.-H.; Yang, X.; Liang, H.-J.; Wu, X.-L. Robust three-dimensional carbon conductive network in a NaVPO4F cathode used for superior high-rate and ultralong-lifespan sodium-ion full batteries. J. Mater. Chem. A 2020, 8, 17454–17462. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Chen, H.; Wang, J.; Ding, L.-X.; Wang, S.; Ashman, P.J.; Wang, H. Graphene-based nitrogen-doped carbon sandwich nanosheets: A new capacitive process controlled anode material for high-performance sodium-ion batteries. J. Mate. Chem. A 2016, 4, 8630–8635. [Google Scholar] [CrossRef]
- Kim, B.G.; Hong, C.-H.; Park, I.; Kim, B.G.; Hwang, G.S.; Kang, F. Sodium Storage Behavior in Natural Graphite using Ether-based Electrolyte Systems. Adv. Func. Mater. 2015, 25, 534–541. [Google Scholar] [CrossRef]
- Lee, B.; Kim, B.G.; Kim, B.G.; Nanda, J.; Kwon, S.J.; Jang, H.D.; Mitlin, D.; Lee, B. High Capacity Adsorption—Dominated Potassium and Sodium Ion Storage in Activated Crumpled Graphene. Adv. Energy Mater. 2020, 10, 1903280. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Qiu, X.; Cao, Y.; Wang, C.; Wang, Y.; Xia, Y. Extended low-voltage plateau capacity of hard carbon spheres anode for sodium ion batteries. J. Power Sources 2020, 476, 228550. [Google Scholar] [CrossRef]
- Ni, D.; Sun, W.; Wang, Z.; Bai, Y.; Lei, H.; Lai, X.; Sun, K. Heteroatom-Doped Mesoporous Hollow Carbon Spheres for Fast Sodium Storage with an Ultralong Cycle Life. Adv. Energy Mater. 2019, 9, 1900036. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, B.; Lu, B.; Xiang, H. Carbon Anode Materials: A Detailed Comparison between Na-ion and K-ion Batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Tong, H.; Wang, B.; Lu, B.; Chen, C.; Yang, C.; Huang, C.; Yuan, F.; Chen, C. Energetic Metal-Organic Frameworks Derived Highly Nitrogen-Doped Porous Carbon for Superior Potassium Storage. Small 2020, 16, e2002771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).