Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst
Abstract
1. Introduction
2. Results and Discussions
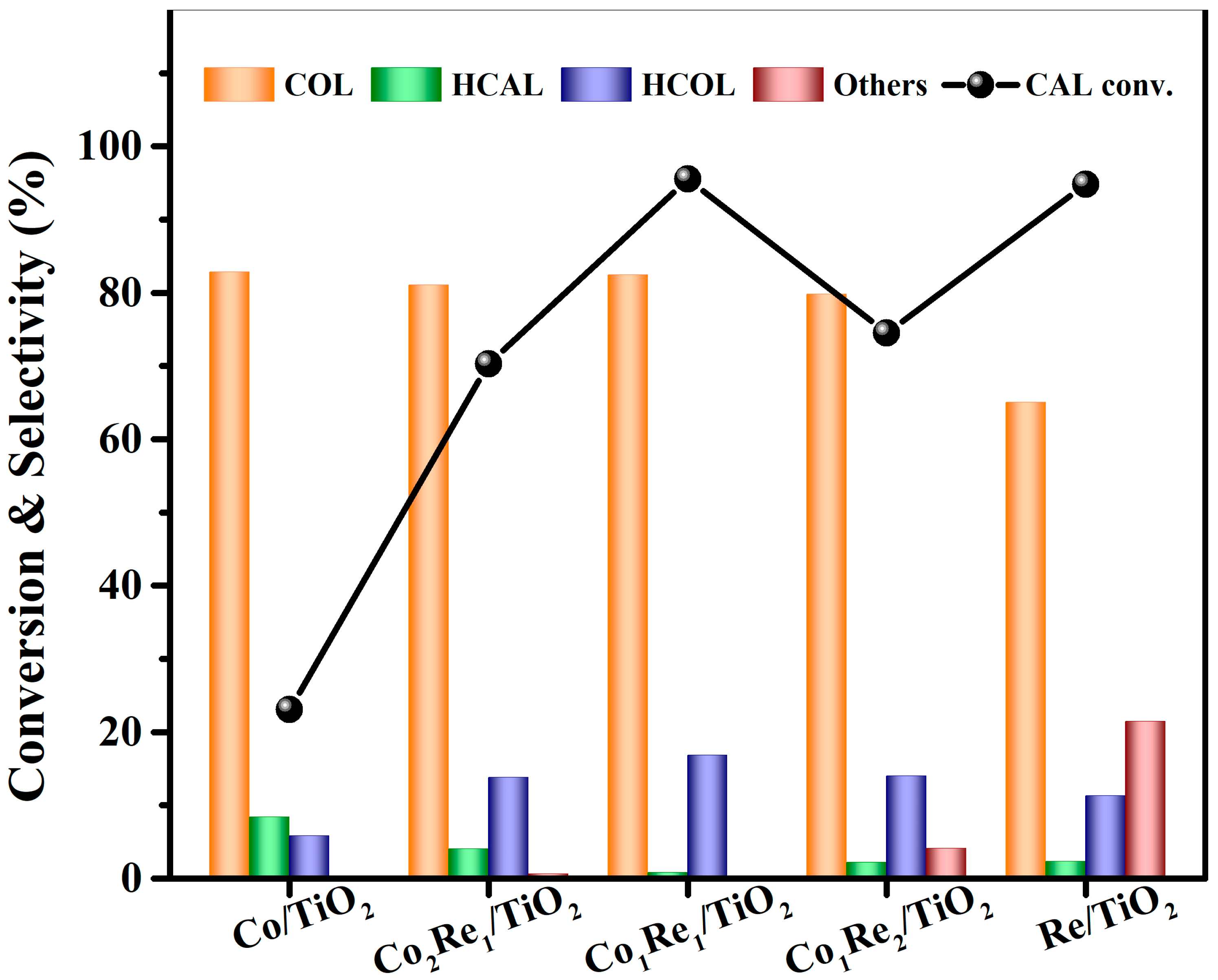
2.1. Catalytic Activity Test
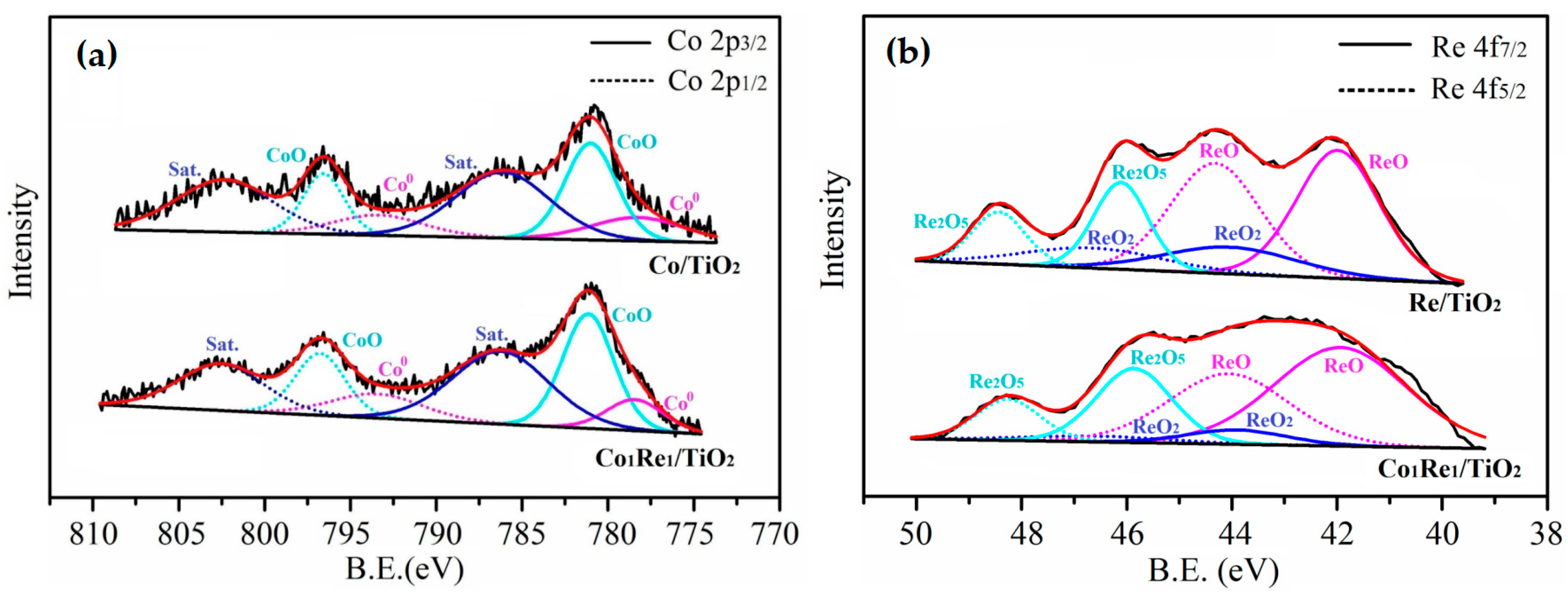
2.2. Catalyst Characterization
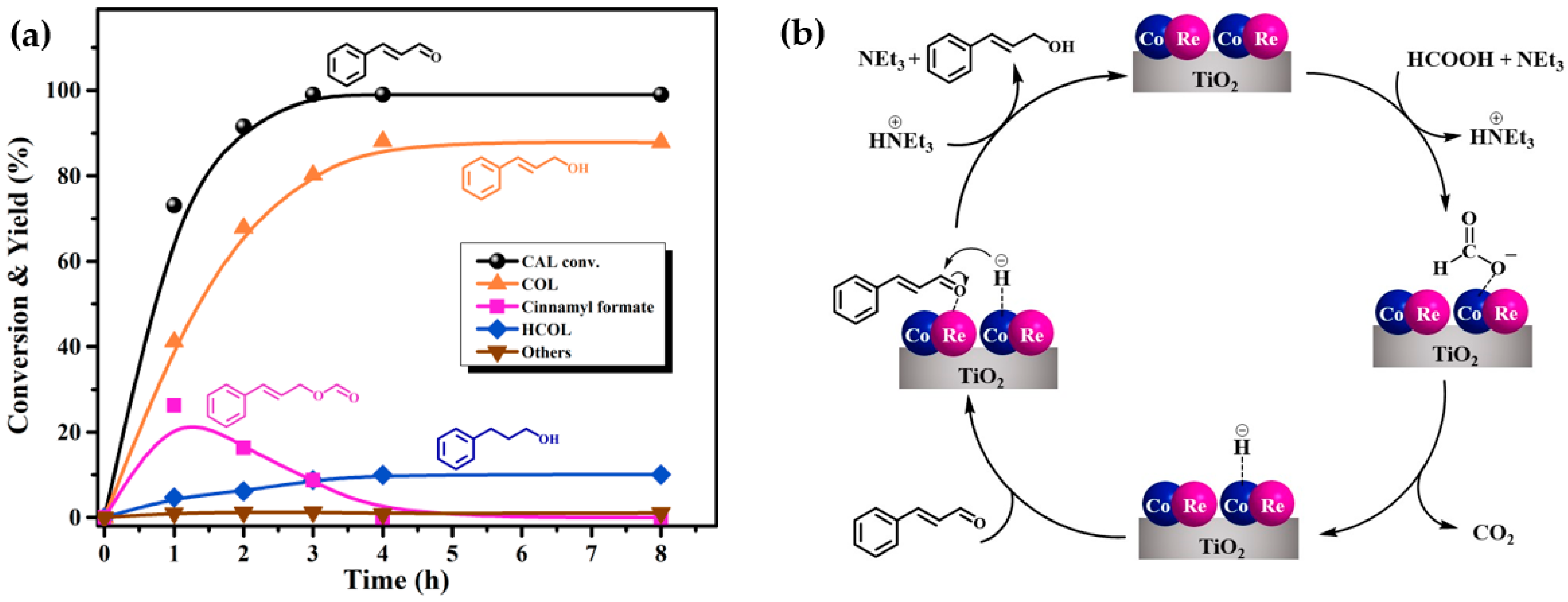
2.3. Effects of Reaction Conditions
3. Experimental Section
3.1. Materials
3.2. Preparation of Various CoRe/TiO2 Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gallezot, P.; Richard, D. Selective hydrogenation of α,β-unsaturated aldehydes. Catal. Rev. 1998, 40, 81–126. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef]
- Hao, C.H.; Guo, X.N.; Pan, Y.T.; Chen, S.; Jiao, Z.F.; Yang, H.; Guo, X.Y. Visible-light-driven selective photocatalytic hydrogenation of cinnamaldehyde over Au/SiC catalysts. J. Am. Chem. Soc. 2016, 138, 9361–9364. [Google Scholar] [CrossRef] [PubMed]
- Luneau, M.; Lim, J.S.; Patel, D.A.; Sykes EC, H.; Friend, C.M.; Sautet, P. Guidelines to achieving high selectivity for the hydrogenation of α,β-unsaturated aldehydes with bimetallic and dilute alloy catalysts: A review. Chem. Rev. 2020, 120, 12834–12872. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent advances in selective hydrogenation of cinnamaldehyde over supported metal-based catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Chen, Y.; Li, Y.; Su, H.; Liu, Q.; Li, G. Ir nanoclusters confined within hollow MIL-101(Fe) for selective hydrogenation of α,β-unsaturated aldehyde. Chin. Chem. Lett. 2022, 33, 374–377. [Google Scholar] [CrossRef]
- Kahsar, K.R.; Schwartz, D.K.; Medlin, J.W. Control of metal catalyst selectivity through specific noncovalent molecular interactions. J. Am. Chem. Soc. 2014, 136, 520–526. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Ren, J.; Hu, C.; Lv, B. Effect of boron nitride overlayers on Co@BNNSs/BN-catalyzed aqueous phase selective hydrogenation of cinnamaldehyde. J. Colloid Interface Sci. 2023, 630, 549–558. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Li, Q.; Liu, Y.; Wei, T.; Liu, Y.; Zeng, Z.; Bradshaw, D.; Zhang, B.; Huo, J. Precise control of selective hydrogenation of α,β-unsaturated aldehydes in water mediated by ammonia borane. Appl. Catal. B Environ. 2022, 311, 121348. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Wang, J.; Liu, H.; Zhang, X. Geometrically embedding dispersive Pt nanoparticles within silicalite-1 framework for highly selective α,β-unsaturated aldehydes hydrogenation via oriented C=O adsorption configuration. Chem. Eng. J. 2022, 446, 137064. [Google Scholar] [CrossRef]
- Chen, H.; Peng, T.; Liang, B.; Zhang, D.; Lian, G.; Yang, C.; Zhang, Y.; Zhao, W. Efficient electrocatalytic hydrogenation of cinnamaldehyde to value-added chemicals. Green Chem. 2022, 24, 3655–3661. [Google Scholar] [CrossRef]
- Xue, Y.; Yao, R.; Li, J.; Wang, G.; Wu, P.; Li, X. Efficient Pt–FeOx/TiO2@SBA-15 catalysts for selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. Catal. Sci. Technol. 2017, 7, 6112–6123. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zhang, C.; Xu, Q.; Dang, L.; Zhao, X.; Tan, H.; Li, Y.; Zhao, F. Defect engineering and spilt-over hydrogen in Pt/(WO3–TH2) for selective hydrogenation of C=O bonds. New J. Chem. 2022, 46, 15950–15958. [Google Scholar] [CrossRef]
- Mateen, M.; Akhtar, M.N.; Gao, L.; Cheong, W.-C.M.; Lv, S.; Zhou, Y.; Chen, Z. Engineering electrophilic atomic Ir sites on CeO2 colloidal spheres for selectivity control in hydrogenation of α,β-unsaturated carbonyl compounds. Nano Res. 2022, 15, 7107–7115. [Google Scholar] [CrossRef]
- Johar, P.; McElroy, C.R.; Rylott, E.L.; Matharu, A.S.; Clark, J.H. Biologically bound nickel as a sustainable catalyst for the selective hydrogenation of cinnamaldehyde. Appl. Catal. B 2022, 306, 121105. [Google Scholar] [CrossRef]
- Ning, L.; Zhang, M.; Liao, S.; Zhang, Y.; Jia, D.; Yan, Y.; Gu, W.; Liu, X. Differentiation of Pt−Fe and Pt−Ni3 surface catalytic mechanisms towards contrasting products in chemoselective hydrogenation of α,β-unsaturated aldehydes. ChemCatChem 2020, 13, 704–711. [Google Scholar] [CrossRef]
- Egeberg, A.; Dietrich, C.; Kind, C.; Popescu, R.; Gerthsen, D.; Behrens, S.; Feldmann, C. Bimetallic Nickel-Iridium and Nickel-Osmium alloy nanoparticles and their catalytic performance in hydrogenation reactions. ChemCatChem 2017, 9, 3534–3543. [Google Scholar] [CrossRef]
- Padmanaban, S.; Gunasekar, G.H.; Yoon, S. Direct heterogenization of the Ru-Macho catalyst for the chemoselective hydrogenation of α,β-unsaturated carbonyl compounds. Inorg. Chem. 2021, 60, 6881–6888. [Google Scholar] [CrossRef]
- Tao, R.; Shan, B.Q.; Sun, H.D.; Ding, M.; Xue, Q.S.; Jiang, J.G.; Wu, P.; Zhang, K. Surface molecule manipulated Pt/TiO2 catalysts for selective hydrogenation of cinnamaldehyde. J. Phys. Chem. C 2021, 125, 13304–13312. [Google Scholar] [CrossRef]
- Xin, H.; Li, M.; Chen, L.; Zhao, C.; Wu, P.; Li, X. Lanthanide oxide supported Ni nanoparticles for the selective hydrogenation of cinnamaldehyde. Catal. Sci. Technol. 2023, 13, 1048–1055. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Zhang, L.; Liu, F.; Wang, A.; Zhang, T. Producing of cinnamyl alcohol from cinnamaldehyde over supported gold nanocatalyst. Chin. J. Catal. 2021, 42, 470–481. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, Y.J.; Chen, F.; Xiang, Y.Z.; Zhang, B.; Xu, L.Y.; Zhang, T.R. Efficient selective hydrogenation of cinnamaldehyde over zeolite supported cobalt catalysts in water. Reac. Kinet. Mech. Cat. 2015, 115, 283–292. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.B.; Xu, L.Y.; Zhang, Y.J.; Qin, Y.H.; Liang, C.F. Selective hydrogenation of cinnamaldehyde over ZSM-5 supported Co catalysts. Reac. Kinet. Mech. Cat. 2013, 110, 207–214. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, X.; Liu, X.; Xu, H.; Li, X.; Hou, Y.; Liu, Y. Pt-Re/rGO bimetallic catalyst for highly selective hydrogenation of cinnamaldehyde to cinnamylalcohol. Chin. J. Chem. Eng. 2019, 27, 369–378. [Google Scholar] [CrossRef]
- Stucchi, M.; Vasile, F.; Cattaneo, S.; Vomeri, A.; Hungria, A.B.; Prati, L. Pt-WOx/C catalysts for α,β-unsaturated aldehydes hydrogenation: An NMR study of the effect of the reactant adsorption on activity and selectivity. Eur. J. Org. Chem. 2022, 2022, e202200735. [Google Scholar] [CrossRef]
- Yang, Y.; Rao, D.; Chen, Y.; Dong, S.; Wang, B.; Zhang, X.; Wei, M. Selective hydrogenation of cinnamaldehyde over Co-based intermetallic compounds derived from layered double hydroxides. ACS Catal. 2018, 8, 11749–11760. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, D.; Pan, Y.; Pu, X.; Xia, Y.; Wang, J.; Xiong, W. Magnetic anchored CoPt bimetallic nanoparticles as selective hydrogenation catalyst for cinnamaldehyde. Catal. Lett. 2018, 149, 851–859. [Google Scholar] [CrossRef]
- Mabate, T.P.; Meijboom, R.; Bingwa, N. The inorganic perovskite-catalyzed transfer hydrogenation of cinnamaldehyde using glycerol as a hydrogen donor. Catalysts 2022, 12, 241. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, J.; Zhao, D.; Zhao, Z.; Zaera, F.; Zhu, Y. Porous LaFeO3 prepared by an in situ carbon templating method for catalytic transfer hydrogenation reactions. ACS Appl. Mater. Interfaces 2019, 11, 15517–15527. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Long, J.; Zhang, W.; Liu, X.; Wei, D. MOFs-derived Co@CN bi-functional catalysts for selective transfer hydrogenation of α,β-unsaturated aldehydes without use of base additives. Mater. Chem. Front. 2017, 1, 2005–2012. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Liu, F.; Wang, Y.; Lan, X.; Wang, S.; Ali, B.; Wang, T. Transfer hydrogenation of cinnamaldehyde catalyzed by Al2O3 using ethanol as a solvent and hydrogen donor. ACS Sustain. Chem. Eng. 2020, 8, 8195–8205. [Google Scholar] [CrossRef]
- Siddqui, N.; Sarkar, B.; Pendem, C.; Khatun, R.; Sivakumar Konthala, L.N.; Sasaki, T.; Bordoloi, A.; Bal, R. Highly selective transfer hydrogenation of α,β-unsaturated carbonyl compounds using Cu-based nanocatalysts. Catal. Sci. Technol. 2017, 7, 2828–2837. [Google Scholar] [CrossRef]
- Hareesh, H.N.; Minchitha, K.U.; Venkatesh, K.; Nagaraju, N.; Kathyayini, N. Environmentally benign selective hydrogenation of α,β-unsaturated aldehydes and reduction of aromatic nitro compounds using Cu based bimetallic nanoparticles supported on multiwalled carbon nanotubes and mesoporous carbon. RSC Adv. 2016, 6, 82359–82369. [Google Scholar] [CrossRef]
- Butt, M.; Feng, X.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Bao, M. Unsupported nanoporous Gold-catalyzed chemoselective reduction of α,β-unsaturated aldehydes using formic acid as hydrogen source. Asian J. Org. Chem. 2017, 6, 867–872. [Google Scholar] [CrossRef]
- Takale, B.S.; Wang, S.; Zhang, X.; Feng, X.; Yu, X.; Jin, T.; Bao, M.; Yamamoto, Y. Chemoselective reduction of α,β-unsaturated aldehydes using an unsupported nanoporous gold catalyst. Chem. Commun. 2014, 50, 14401–14404. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. HKUST-1 catalyzed room temperature hydrogenation of acetophenone by silanes. Catal. Commun. 2017, 97, 74–78. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Zhang, M.; Zhang, K.; Ma, J.; Xiao, S.; Wei, Z.; Deng, S. Highly efficient selective hydrogenation of levulinic acid to γ-valerolactone over Cu-Re/TiO2 bimetallic catalysts. RSC Adv. 2021, 12, 602–610. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, J.; Cheng, Y.; Chen, Z.; Kang, S.; Cai, Z.; Xu, Y.; Wei, J. Enhanced catalytic transfer hydrogenation of biomass-based furfural into 2-methylfuran over multifunctional Cu–Re bimetallic catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16624–16636. [Google Scholar] [CrossRef]
- Bazin, D.; Borko, L.; Koppany, Z.; Kovacs, I.; Stefler, G.; Sajo, L.I.; Schay, Z.; Guczi, L. Re±Co/NaY and Re±Co/Al2O3 bimetallic catalysts: In situ EXAFS study and catalytic activity. Catal. Lett. 2002, 84, 169–182. [Google Scholar] [CrossRef]
- Liu, C.; He, Y.; Wei, L.; Zhang, Y.; Zhao, Y.; Hong, J.; Chen, S.; Wang, L.; Li, J. Hydrothermal carbon-coated TiO2 as support for Co-based catalyst in Fischer–Tropsch synthesis. ACS Catal. 2018, 8, 1591–1600. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Rapid synthesis of unsaturated alcohols under mild conditions by highly selective hydrogenation. Chem. Commun. 2013, 49, 7034–7036. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ji, Y.; Wang, H.; Ao, Z.; Li, G.; An, T. Adsorption mechanisms of typical carbonyl-containing volatile organic compounds on anatase TiO2 (001) surface: A DFT investigation. J. Phys. Chem. C 2017, 121, 13717–13722. [Google Scholar] [CrossRef]
- Storsater, S.; Totdal, B.; Walmsley, J.; Tanem, B.; Holmen, A. Characterization of alumina-, silica-, and titania-supported cobalt Fischer–Tropsch catalysts. J. Catal. 2005, 236, 139–152. [Google Scholar] [CrossRef]
- Vít, Z.; Gulková, D.; Kaluža, L.; Kupčík, J. Pd–Pt catalysts on mesoporous SiO2–Al2O3 with superior activity for HDS of 4,6-dimethyldibenzothiophene: Effect of metal loading and support composition. Appl. Catal. B 2015, 179, 44–53. [Google Scholar] [CrossRef]
- Cheng, S.; Lu, S.; Liu, X.; Li, G.; Wang, F. Enhanced activity of alkali-treated ZSM-5 zeolite-supported Pt-Co catalyst for selective hydrogenation of cinnamaldehyde. Molecules 2023, 28, 1730. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, Q.; Cheng, Y.; Dong, M.; Zhang, Z.; Zhu, X.; Liu, Y.; Sun, Y. Low loading of CoRe/TiO2 for efficient hydrodeoxygenation of levulinic acid to γ-valerolactone. ACS Sustain. Chem. Eng. 2021, 9, 10882–10891. [Google Scholar] [CrossRef]
- Huck-Iriart, C.; Soler, L.; Casanovas, A.; Marini, C.; Prat, J.; Llorca, J.; Escudero, C. Unraveling the chemical state of Cobalt in Co-based catalysts during ethanol steam reforming: An in situ study by near ambient pressure XPS and XANES. ACS Catal. 2018, 8, 9625–9636. [Google Scholar] [CrossRef]
- Duke, A.S.; Galhenage, R.P.; Tenney, S.A.; Ammal, S.C.; Heyden, A.; Sutter, P.; Chen, D.A. In situ ambient pressure X-ray photoelectron spectroscopy studies of methanol oxidation on Pt(111) and Pt–Re alloys. J. Phys. Chem. C 2015, 119, 23082–23093. [Google Scholar] [CrossRef]
- Rozmysłowicz, B.; Kirilin, A.; Aho, A.; Manyar, H.; Hardacre, C.; Wärnå, J.; Salmi, T.; Murzin, D.Y. Selective hydrogenation of fatty acids to alcohols over highly dispersed ReOx/TiO2 catalyst. J. Catal. 2015, 328, 197–207. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High efficient conversion of furfural to 2-methylfuran over Ni-Cu/Al2O3 catalyst with formic acid as a hydrogen donor. Appl. Catal. A-Gen. 2017, 547, 248–255. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.; Cui, K.; Xia, Y.; Yuan, G.; Feng, J.; Xiong, W. Chemoselective hydrogenation of cinnamaldehyde over amorphous coordination polymer supported Pt-Co bimetallic nanocatalyst. Chem. Phys. Lett. 2022, 801, 139683. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, Z.; Wang, Y.; Lv, J.; Wang, Y. Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over graphene supported Pt–Co bimetallic catalysts. Catal. Lett. 2014, 144, 980–986. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, D.; Yuan, F.; Han, Q.; Dong, Y.; Liu, Y.; Niu, X.; Zhu, Y. An effective Co-promoted platinum of Co–Pt/SBA-15 catalyst for selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. Catal. Lett. 2016, 146, 1535–1543. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.G.; Zhou, R.X. Bimetallic Pt-Co catalysis on carbon nanotubes for the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol: Preparation and characterization. J. Mol. Catal. A Chem. 2008, 279, 140–146. [Google Scholar] [CrossRef]
- Lv, Y.; Han, M.; Gong, W.; Wang, D.; Chen, C.; Wang, G.; Zhang, H.; Zhao, H. Fe-Co alloyed nanoparticles catalyzing efficient hydrogenation of cinnamaldehyde to cinnamyl alcohol in water. Angew. Chem. Int. Ed. 2020, 59, 23521–23526. [Google Scholar] [CrossRef]
- Zhao, J.; Malgras, V.; Na, J.; Liang, R.; Cai, Y.; Kang, Y.; Alshehri, A.A.; Alzahrani, K.A.; Alghamdi, Y.G.; Asahi, T.; et al. Magnetically induced synthesis of mesoporous amorphous CoB nanochains for efficient selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. Chem. Eng. J. 2020, 398, 125564. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, L.; Li, X.; Chen, L.; Zhang, Y.; Duan, C. NH2-MIL-125(Ti)-derived porous cages of titanium oxides to support Pt-Co alloys for chemoselective hydrogenation reactions. Chem. Sci. 2019, 10, 2111–2117. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, Y.; Park, J.; Liang, X. Atomic layer deposited Pt-Co bimetallic catalysts for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols. J. Catal. 2018, 366, 61–69. [Google Scholar] [CrossRef]
- Shi, J.; Nie, R.; Zhang, M.; Zhao, M.; Hou, Z. Microwave-assisted fast fabrication of a nanosized Pt3Co alloy on reduced graphene oxides. Chin. J. Catal. 2014, 35, 2029–2037. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, C.; Li, Q.; Hou, J.; Li, Y.; Ai, S. Nitrogen- and oxygen-functionalized carbon nanotubes supported Pt-based catalyst for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A-Gen. 2015, 506, 134–142. [Google Scholar] [CrossRef]
- Plessers, E.; De Vos, D.E.; Roeffaers, M.B.J. Chemoselective reduction of α,β-unsaturated carbonyl compounds with UiO-66 materials. J. Catal. 2016, 340, 136–143. [Google Scholar] [CrossRef]
- He, S.; Xie, L.; Che, M.; Chan, H.C.; Yang, L.; Shi, Z.; Tang, Y.; Gao, Q. Chemoselective hydrogenation of α,β-unsaturated aldehydes on hydrogenated MoOx nanorods supported iridium nanoparticles. J. Mol. Catal. A Chem. 2016, 425, 248–254. [Google Scholar] [CrossRef]
- Perdomo, I.C.; Gianolio, S.; Pinto, A.; Romano, D.; Contente, M.L.; Paradisi, F.; Molinari, F. Efficient enzymatic preparation of flavor esters in water. J. Agric. Food Chem. 2019, 67, 6517–6522. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Du, X.L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.J.; Ould Hamou, C.A.; Réocreux, R.; Michel, C.; Giorgi, J.B. Adsorption and decomposition of formic acid on Cobalt(0001). J. Phys. Chem. C 2018, 122, 20279–20288. [Google Scholar] [CrossRef]
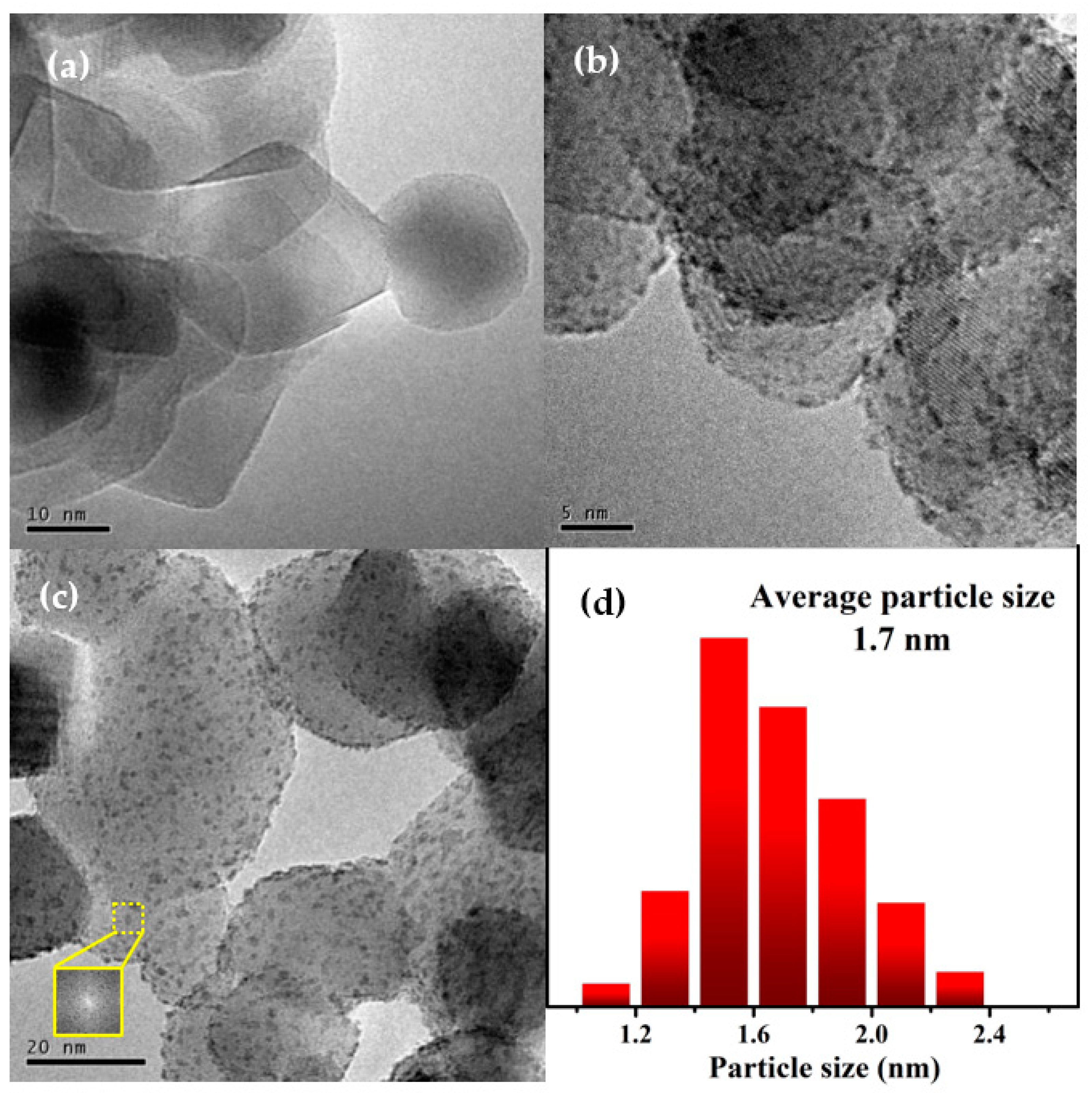


| Sample | dTEM (nm) | dCo a (nm) | Dispersion of Co a (%) | SBET (m2·g−1) | Dp b (nm) | Vp b (cm3·g−1) |
|---|---|---|---|---|---|---|
| TiO2 | / | / | / | 52.8 | 11.3 | 0.15 |
| Co/TiO2 | / | 8.1 | 12.3 | 52.9 | 28.9 | 0.41 |
| Co1Re1/TiO2 | 1.7 | 1.8 | 55.7 | 48.5 | 24.5 | 0.33 |
| Re/TiO2 | / | / | / | 50.7 | 27.5 | 0.37 |
| Entry | Hydrogen Donor | Molar Ratio (FA:NEt3) | Conv. (%) | Sel. (%) | |||
|---|---|---|---|---|---|---|---|
| COL | HCAL | HCOL | Others | ||||
| 1 | FA | / | 64 | 29 | 4 | 3 | 64 |
| 2 | FA | 1:1 | 99 | 89 | / | 10 | 1 |
| 3 | FA | 2:5 | 99 | 72 | / | 28 | / |
| 4 | FA | 5:2 | 99 | 80 | / | 20 | / |
| 5 a | Hydrogen | / | 57 | 17 | 42 | 25 | 16 |
| Entry | Molar Ratio (CAL:FA) | Conv. (%) | Sel. (%) | |||
|---|---|---|---|---|---|---|
| COL | HCAL | HCOL | Others | |||
| 1 | 1:1.5 | 92 | 89 | / | 11 | / |
| 2 | 1:2 | 99 | 89 | / | 10 | 1 |
| 3 | 1:3 | 94 | 60 | / | 7 | 33 |
| Entry | Substrate | Time (h) | Conv. (%) | Sel. (%) | |
|---|---|---|---|---|---|
| 1 |  | 5 | 99 | 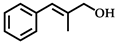 (86) (86) | 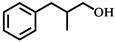 (14) (14) |
| 2 | 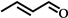 | 8 | 93 |  (74) (74) |  (26) (26) |
| 3 | 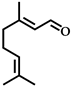 | 8 | 92 |  (96) (96) | 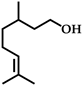 (5) (5) |
| 4 | 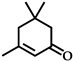 | 12 | 99 | 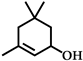 (84) (84) | 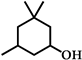 (16) (16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, Y.; Jiang, L.; Cheng, Y.; Liu, Y.; Wei, Z. Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst. Molecules 2023, 28, 3336. https://doi.org/10.3390/molecules28083336
Chen M, Wang Y, Jiang L, Cheng Y, Liu Y, Wei Z. Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst. Molecules. 2023; 28(8):3336. https://doi.org/10.3390/molecules28083336
Chicago/Turabian StyleChen, Mengting, Yun Wang, Limin Jiang, Yuran Cheng, Yingxin Liu, and Zuojun Wei. 2023. "Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst" Molecules 28, no. 8: 3336. https://doi.org/10.3390/molecules28083336
APA StyleChen, M., Wang, Y., Jiang, L., Cheng, Y., Liu, Y., & Wei, Z. (2023). Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst. Molecules, 28(8), 3336. https://doi.org/10.3390/molecules28083336







