Novel Crown Ether Amino Acids as Fluorescent Reporters for Metal Ions
Abstract
1. Introduction
2. Results and Discussion
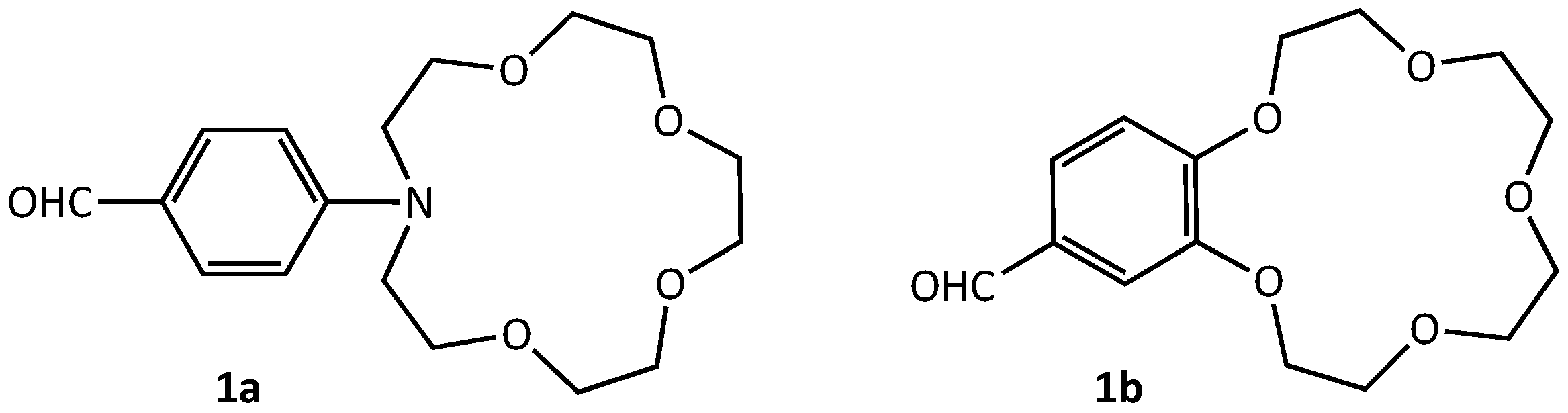
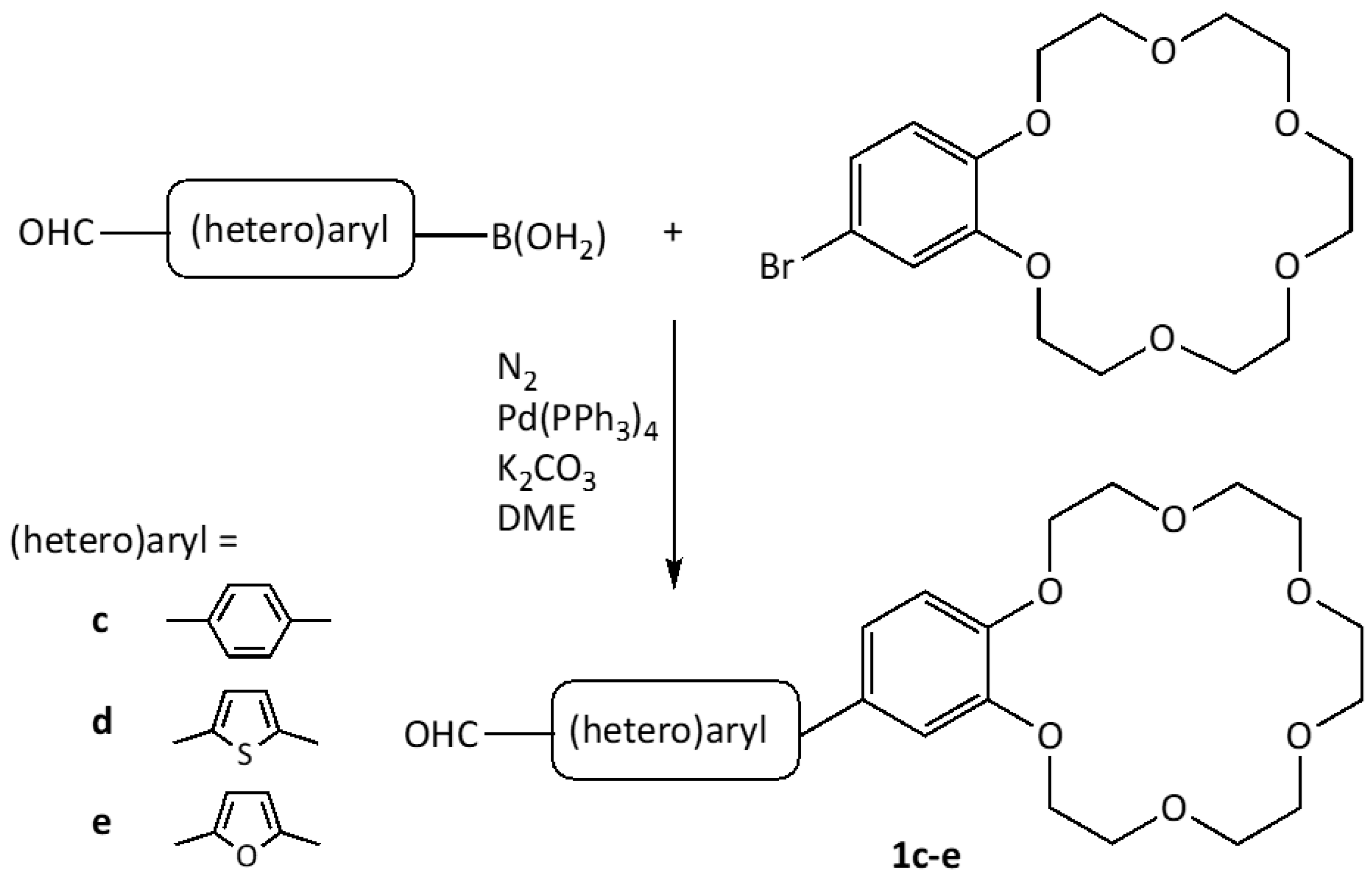
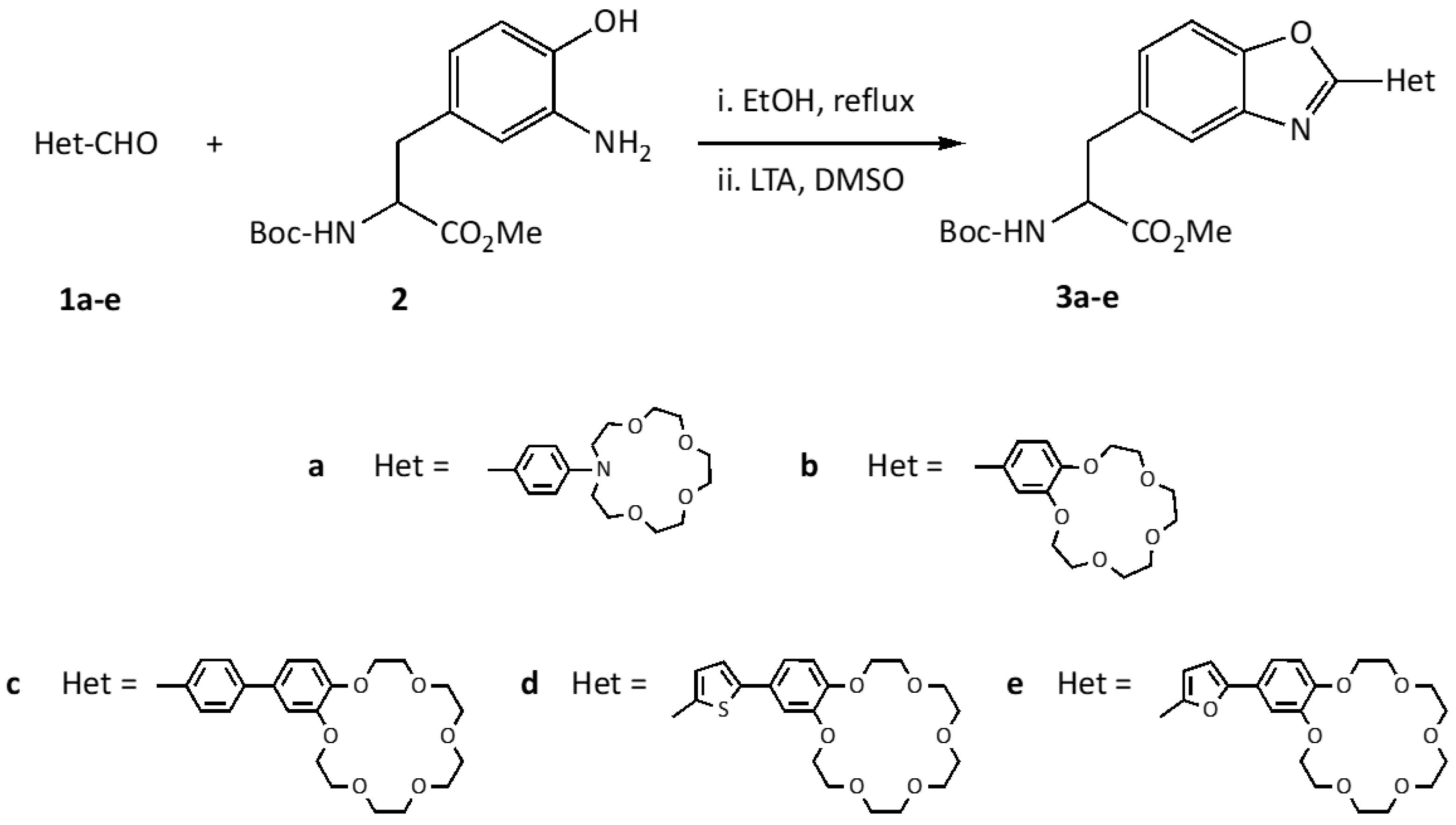
2.1. Synthesis
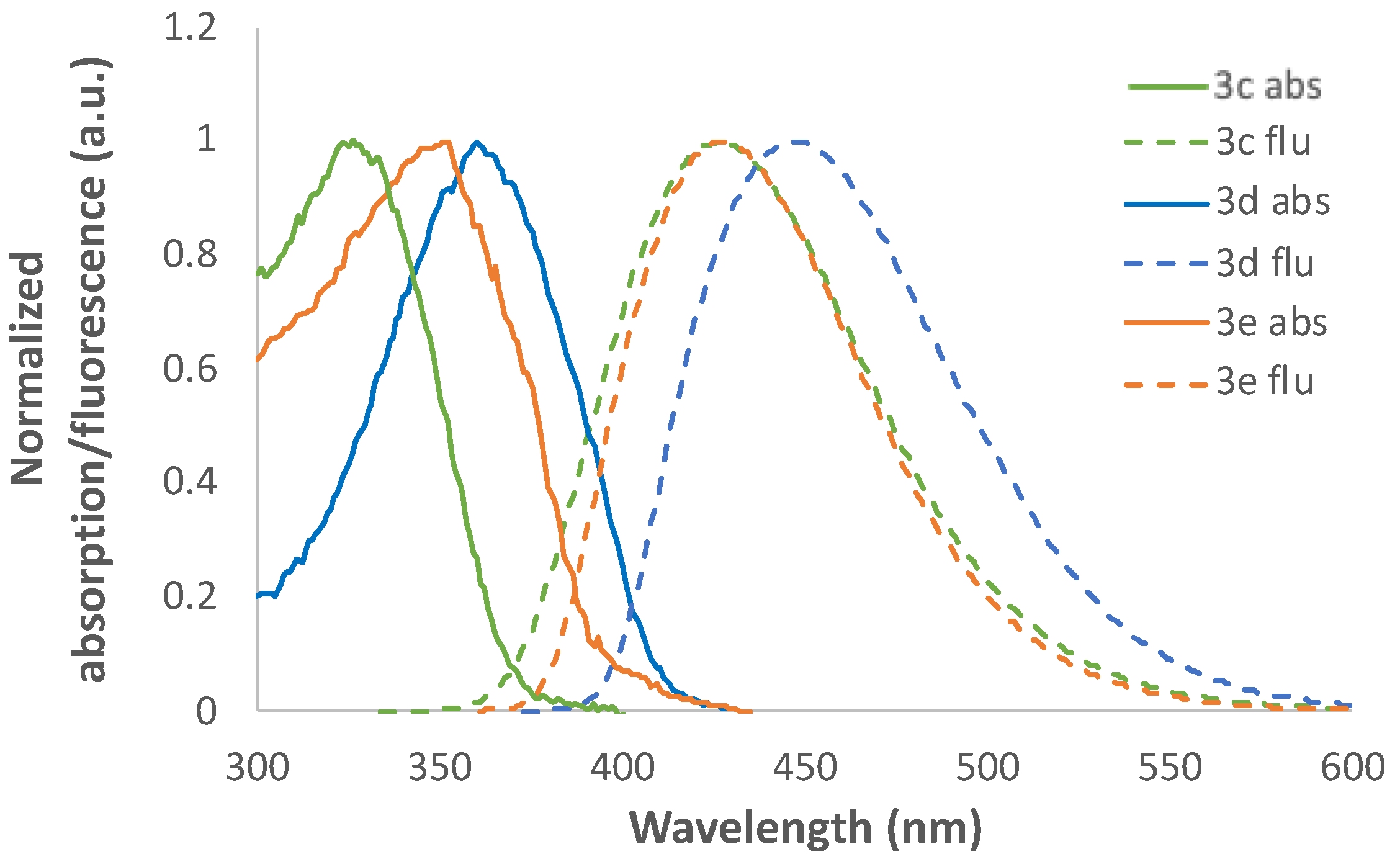
2.2. Photophysical Characterization
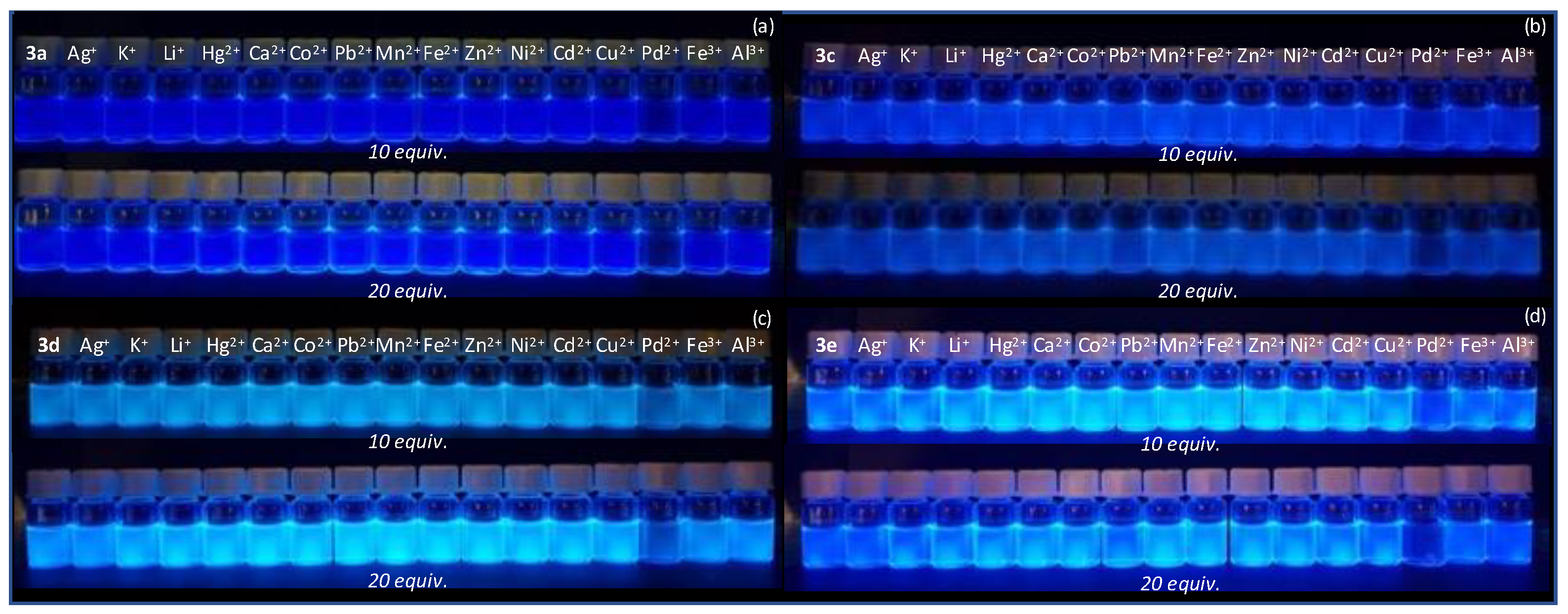
2.3. Preliminary Chemosensing Studies
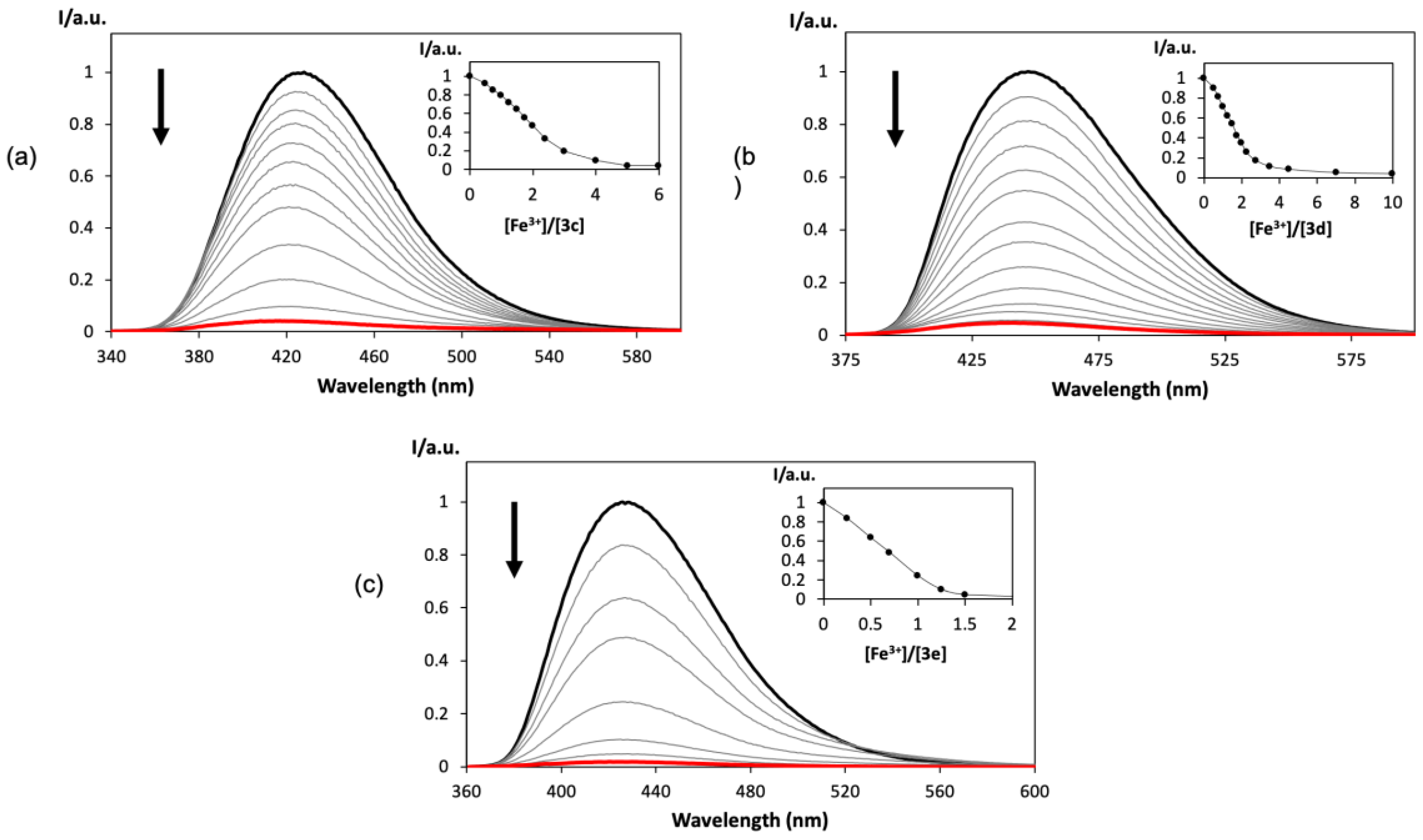
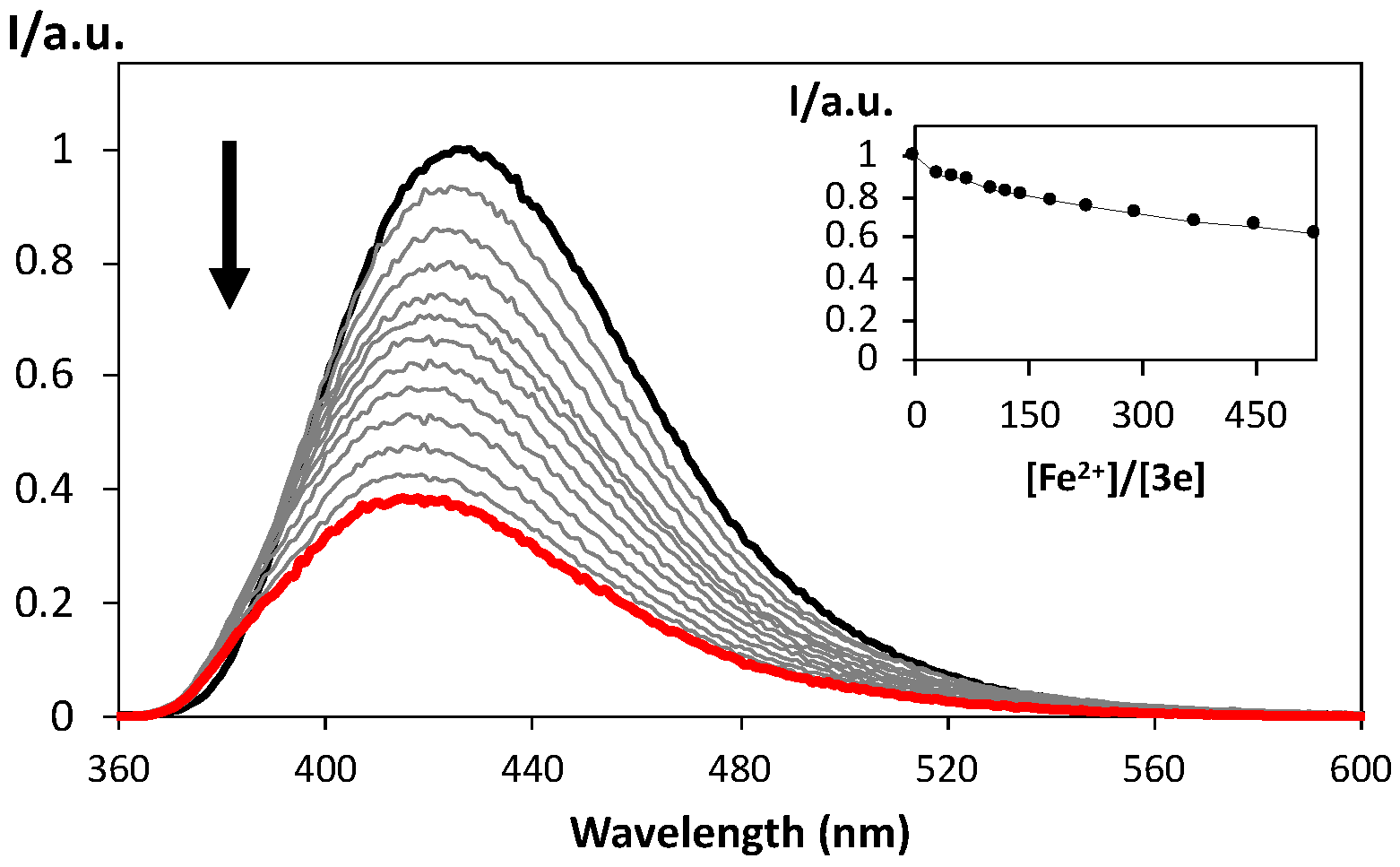
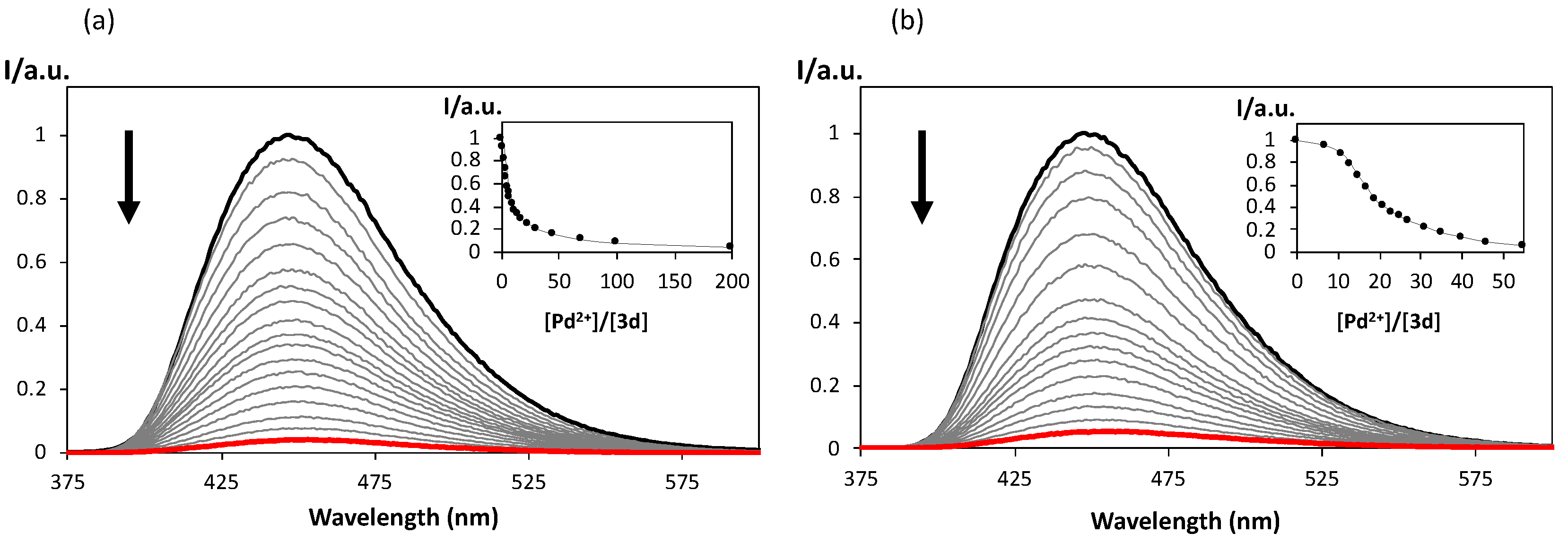
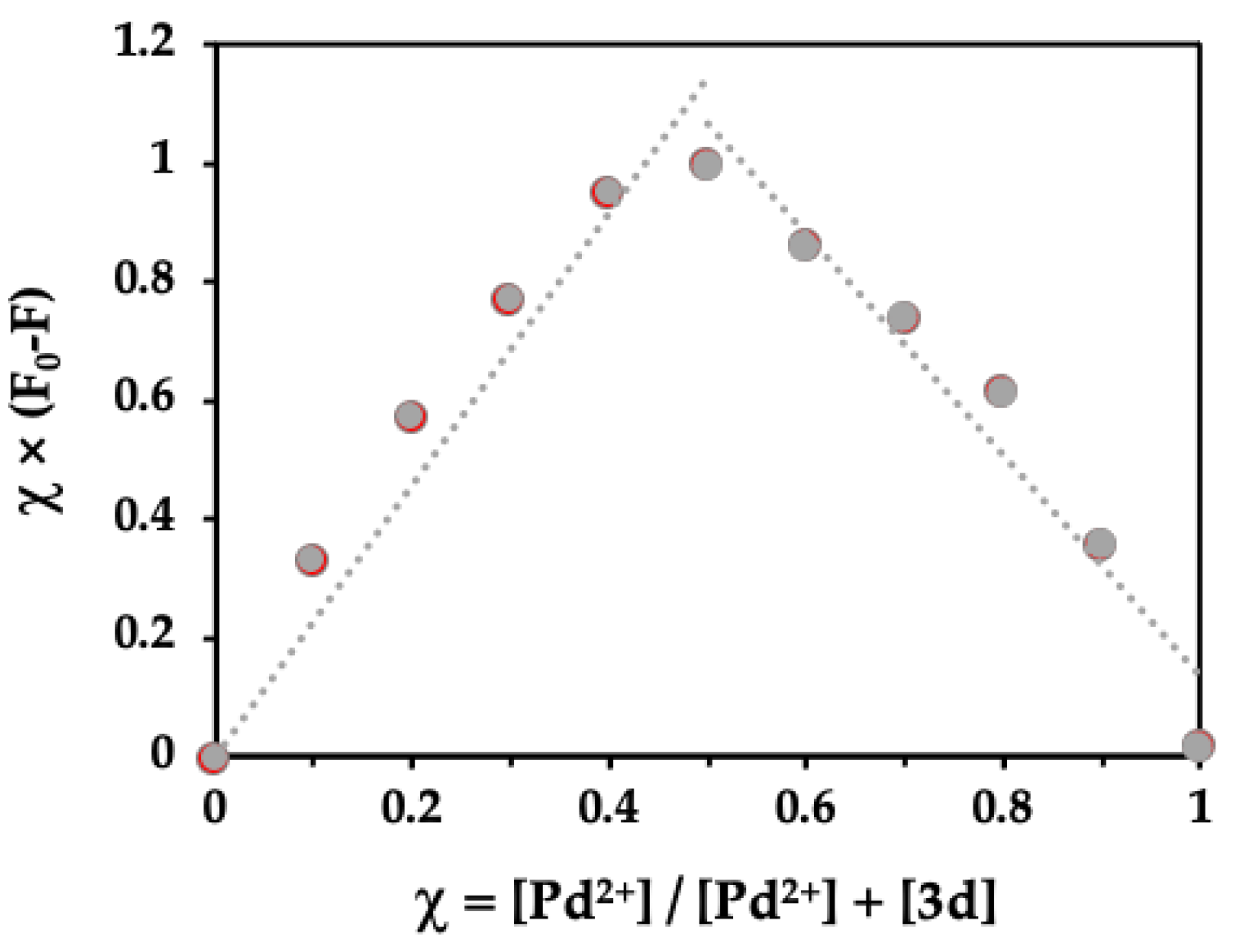
2.4. Spectrofluorimetric Titrations
3. Materials and Methods
3.1. Synthesis General
3.2. Synthesis of Formyl Crown Ethers 1c–e by Suzuki Coupling
3.2.1. Phenylbenzocrown 1c
3.2.2. Thienylbenzocrown 1d
3.2.3. Furylbenzocrown 1e
3.3. Synthesis of Crown Ether Benzoxazolyl-alanines 3a–e
3.3.1. Benzoxazolyl-alanine 3a
3.3.2. Benzoxazolyl-alanine 3b
3.3.3. Benzoxazolyl-alanine 3c
3.3.4. Benzoxazolyl-alanine 3d
3.3.5. Benzoxazolyl-alanine 3e
3.4. Sensing Studies General
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yu, H.; Feng, J.; Zhong, F.; Wu, Y. Chemical modification for the “Off-/On” regulation of enzyme activity. Macromol. Rap. Commun. 2022, 43, 2200195. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Yufu, T.; Kato, T. The effect of a peptide substrate containing an unnatural branched amino acid on chymotrypsin activity. Processes 2021, 9, 242. [Google Scholar] [CrossRef]
- Pless, S.A.; Ahern, C.A. Unnatural amino acids as probes of ligand-receptor interactions and their conformational consequences. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 211–229. [Google Scholar] [CrossRef]
- Niu, W.; Guo, J. Expanding the chemistry of fluorescent protein biosensors through genetic incorporation of unnatural amino acids. Mol. BioSyst. 2013, 9, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, N.; Wang, W.; Xu, Z.; Wu, Y.; Luo, X. An electrochemical biosensor for alpha-fetoprotein detection in human serum based on peptides containing isomer D-amino acids with enhanced stability and antifouling property. Biosens. Bioelectron. 2021, 190, 113466. [Google Scholar] [CrossRef]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Elia, N. Using unnatural amino acids to selectively label proteins for cellular imaging: A cell biologist viewpoint. FEBS J. 2021, 288, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Khalily, M.P.; Soydan, M. Peptide-based diagnostic and therapeutic agents: Where we are and where we are heading? Chem. Biol. Drug Des. 2023, 101, 772–793. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, J.; Li, Q.; van Heel, A.J.; de Vries, M.P.; Broos, J.; Kuipers, O.P. Incorporation of tryptophan analogues into the lantibiotic nisin. Amino Acids 2016, 48, 1309–1318. [Google Scholar] [CrossRef]
- Ding, Y.; Ting, J.P.; Liu, J.; Al-Azzam, S.; Pandya, P.; Afshar, S. Impact of non-proteinogenic amino acids in the discovery and development of peptide therapeutics. Amino Acids 2020, 52, 1207–1226. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, W.; Zhang, W.; Konno, H.; Moriwaki, H.; Izawa, K.; Han, J.; Soloshonok, V.A. Tailor-made amino acid-derived pharmaceuticals approved by the FDA in 2019. Amino Acids 2020, 52, 1227–1261. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Wang, Q.; Meng, D. Unnatural amino acids: Promising implications for the development of new antimicrobial peptides. Crit. Rev. Microbiol. 2022, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Behera, L.M.; Ghosh, M.; Rana, S. Deciphering the conformational landscape of few selected aromatic noncoded amino acids (NCAAs) for applications in rational design of peptide therapeutics. Amino Acids 2022, 54, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Albert, L.; Vázquez, O. Photoswitchable peptides for spatiotemporal control of biological functions. Chem. Commun. 2019, 55, 10192–10213. [Google Scholar] [CrossRef]
- Mala, P.; Saraogi, I. Enhanced codon–anticodon interaction at in-frame UAG stop codon improves the efficiency of non-natural amino acid mutagenesis. ACS Chem. Biol. 2022, 9, 1051–1060. [Google Scholar] [CrossRef]
- Won, Y.; Pagar, A.D.; Patil, M.D.; Dawson, P.E.; Yun, H. Recent advances in enzyme engineering through incorporation of unnatural amino acids. Biotech. Bioproc. Eng. 2019, 24, 592–604. [Google Scholar] [CrossRef]
- de Gracia Retamosa, M.; Ruiz-Olalla, A.; Agirre, M.; de Cózar, A.; Bello, T.; Cossío, F.P. Additive and emergent catalytic properties of dimeric unnatural amino acid derivatives: Aldol and conjugate additions. Chem. Eur. J. 2021, 27, 15671–15687. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New fluorescent chemosensors for metal ions in solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. Probing biologically and environmentally important metal ions with fluorescent chemosensors: Thermodynamic versus optical response selectivity in some study cases. Coord. Chem. Rev. 2012, 256, 149–169. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. Novel highly emissive non proteinogenic amino acids: Synthesis of 1,3,4-thiadiazolyl asparagines and evaluation as fluorimetric chemosensors for biologically relevant transition metal cations. Amino Acids 2011, 40, 1065–1075. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. New 2,4,5-triarylimidazoles based on a phenylalanine core: Synthesis, photophysical characterization and evaluation as fluorimetric chemosensors for ion recognition. Dyes Pigment. 2016, 134, 358–368. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. Non-canonical amino acids bearing thiophene and bithiophene: Synthesis by an Ugi multicomponent reaction and studies on ion recognition ability. Amino Acids 2017, 49, 921–930. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New fluoroionophores for metal cations based on benzo[d]oxazol-5-yl-alanine bearing pyrrole and imidazole. Dyes Pigment. 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Heterocyclic amino acids as fluorescent reporters for transition metals: Synthesis and evaluation of novel furyl-benzoxazol-5-yl-L-alanines. New J. Chem. 2018, 42, 3483–3492. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Novel alanines bearing an heteroaromatic side chain: Synthesis and studies on fluorescent chemosensing of metals cations with biological relevance. Amino Acids 2018, 50, 671–684. [Google Scholar] [CrossRef]
- Oliveira, E.; Genovese, D.; Juris, R.; Zaccheroni, N.; Capelo, J.L.; Raposo, M.M.M.; Costa, S.P.G.; Prodi, L.; Lodeiro, C. Bioinspired systems for metal-ion sensing: New emissive peptide probes based on benzo[d]oxazole derivatives and their gold and silica nanoparticles. Inorg. Chem. 2011, 50, 8834–8849. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Balamurugan, R.; Liu, J.-H.; Liu, B.-T. A review of recent developments in fluorescent sensors for the selective detection of palladium ions. Coord. Chem. Rev. 2018, 376, 196–224. [Google Scholar] [CrossRef]
- Oliveira, E.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Lodeiro, C. Exploring the emissive properties of new azacrown compounds bearing aryl, furyl or thienyl moieties: A special case of Chelation Enhancement of Fluorescence upon interaction with Ca2+, Cu2+ or Ni2+. Inorg. Chem. 2010, 49, 10847–10857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.; Tang, H.; Wang, M.; Qin, Y. Click-immobilized K+-selective ionophore for potentiometric and optical sensors. Sens. Actuators B 2012, 171–172, 556–562. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Batista, P.M.R.; Raposo, M.M.M.; Costa, S.P.G. Crown ether benzoxazolyl-alanines as fluorimetric chemosensors for the detection of palladium in aqueous environment. Chem. Proc. 2021, 3, 5. [Google Scholar]
- Morris, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence quantum yield determinations. 9,10-Diphenylanthracene as a reference standard in different solvents. J. Phys. Chem. 1976, 80, 969–974. [Google Scholar] [CrossRef]
- Szabo, A.G.; Rayner, D.M. Fluorescence decay of tryptophan conformers in aqueous solutions. J. Am. Chem. Soc. 1980, 102, 554–563. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, Y.; Chen, J.; Wu, F.S.; Luo, X.G.; Guo, Y.R.; Han, X.Y.; Wang, D.W. A colorimetric and fluorescence turn-on probe for the detection of palladium in aqueous solution and its application in vitro and in vivo. Spectrochim. Acta A 2020, 239, 118547. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhou, Y.; Ma, T.; Niu, J. A highly selective fluorescent probe for the detection of palladium(ii) ion in cells and aqueous media. Microchim. Acta 2013, 180, 211–217. [Google Scholar] [CrossRef]
- Molina, P.; Tárraga, A.; Otón, F. Imidazole derivatives: A comprehensive survey of their recognition properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Arduini, M.; Rampazzo, E.; Mancin, F.; Tecilla, P.; Tonellato, U. Template assisted self-organized chemosensors. Inorg. Chim. Acta 2007, 360, 721–727. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Imidazo-benzo-15-crown-5 ethers bearing arylthienyl and bithienyl moieties as novel fluorescent chemosensors for Pd2+ and Cu2+. Tetrahedron 2011, 67, 7106–7113. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]

| Compound | Absorption | Fluorescence | ||||
|---|---|---|---|---|---|---|
| λabs (nm) | log ε | λem (nm) | ΦF | Δλ (nm) (cm−1) | ||
| 3a | 334 | 4.04 | 395 | 0.82 | 61 | 4624 |
| 3b | 310 | 4.03 | 362 | 0.87 | 52 | 4634 |
| 3c | 324 | 3.99 | 428 | 0.62 | 104 | 7500 |
| 3d | 363 | 4.01 | 445 | 0.69 | 82 | 5076 |
| 3e | 351 | 4.04 | 426 | 0.65 | 75 | 5016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, P.M.R.; Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Novel Crown Ether Amino Acids as Fluorescent Reporters for Metal Ions. Molecules 2023, 28, 3326. https://doi.org/10.3390/molecules28083326
Batista PMR, Martins CDF, Raposo MMM, Costa SPG. Novel Crown Ether Amino Acids as Fluorescent Reporters for Metal Ions. Molecules. 2023; 28(8):3326. https://doi.org/10.3390/molecules28083326
Chicago/Turabian StyleBatista, Patrícia M. R., Cátia D. F. Martins, M. Manuela M. Raposo, and Susana P. G. Costa. 2023. "Novel Crown Ether Amino Acids as Fluorescent Reporters for Metal Ions" Molecules 28, no. 8: 3326. https://doi.org/10.3390/molecules28083326
APA StyleBatista, P. M. R., Martins, C. D. F., Raposo, M. M. M., & Costa, S. P. G. (2023). Novel Crown Ether Amino Acids as Fluorescent Reporters for Metal Ions. Molecules, 28(8), 3326. https://doi.org/10.3390/molecules28083326








