Effect of Layered Aminovanadic Oxalate Phosphate on Flame Retardancy of Epoxy Resin
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analysis of AVOPh
2.2. Thermal Stability of EP Composites
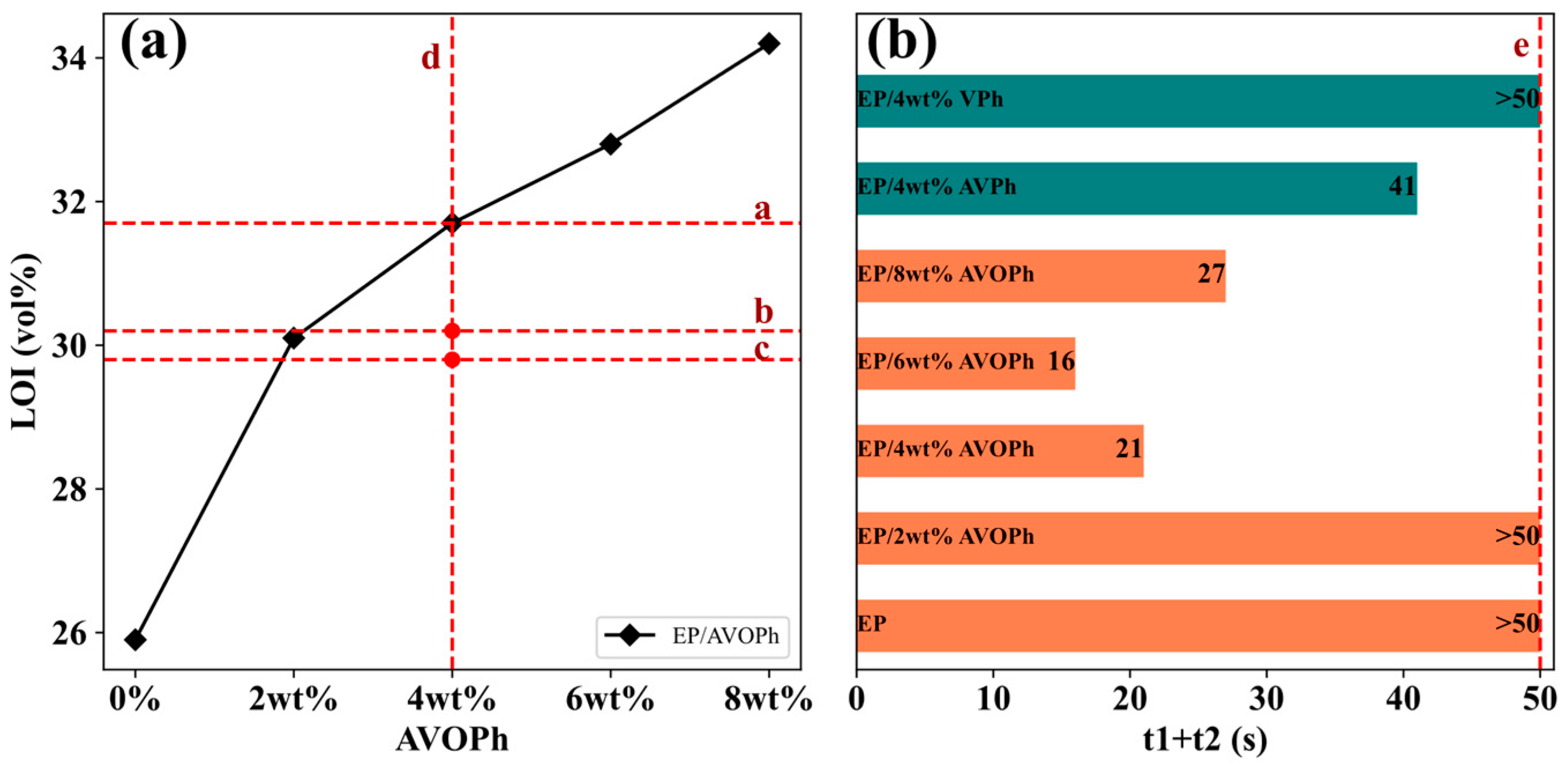
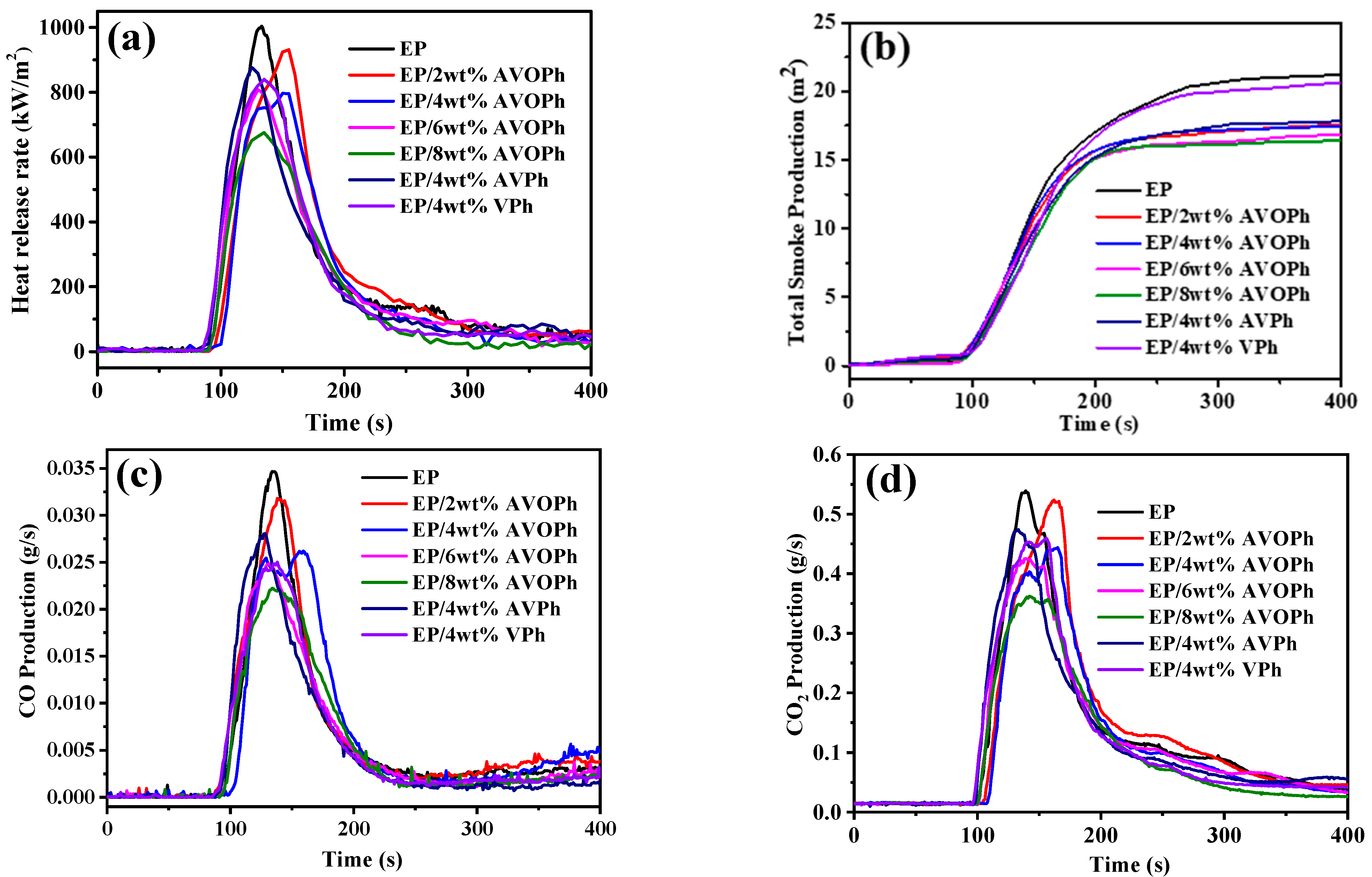
2.3. Flame Retardancy of EP Composites
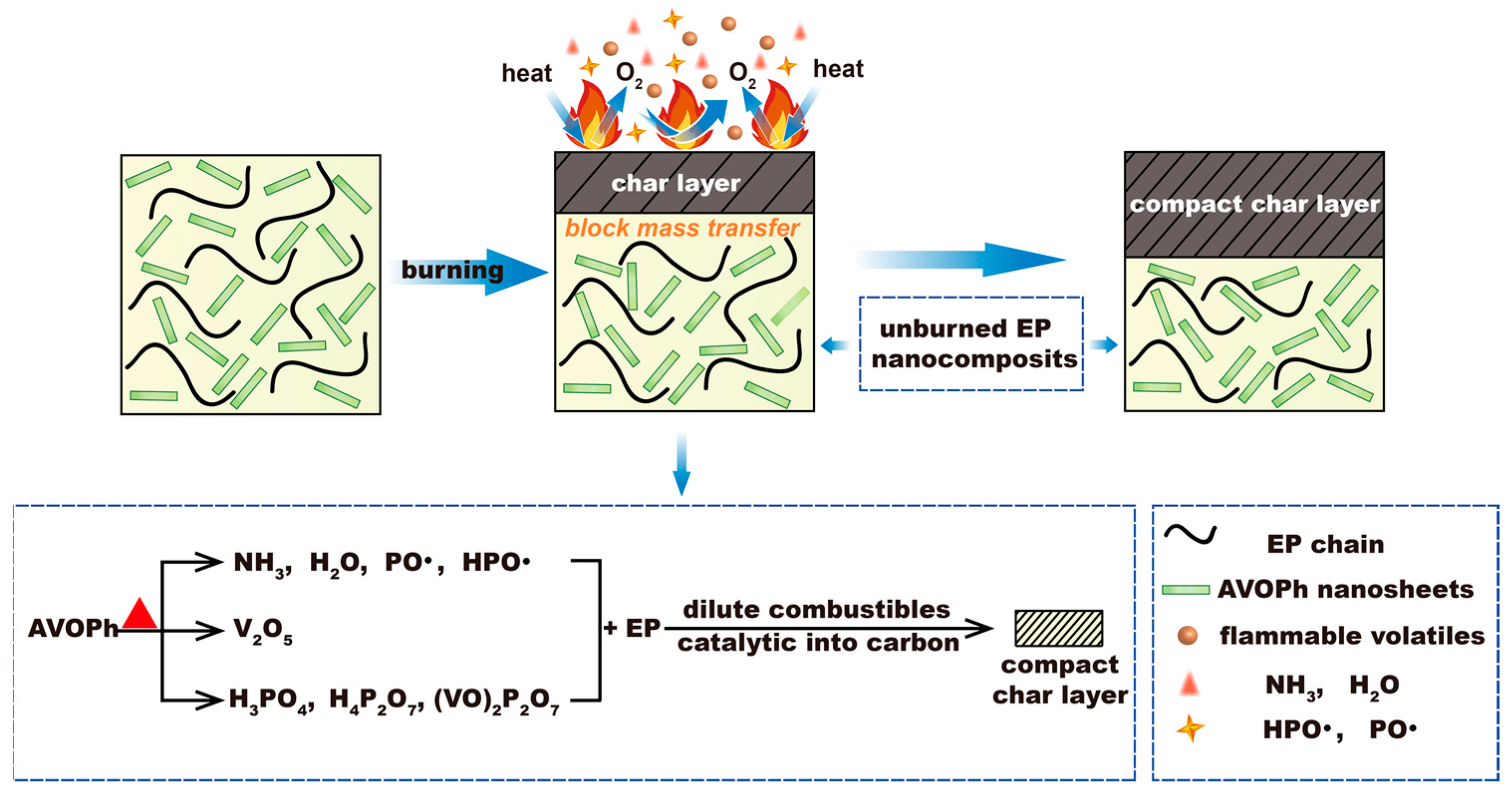
2.4. Flame Retardant Mechanism of AVOPh in EP
3. Experimental Section
3.1. Materials
3.2. Synthesis of AVOPh, AVPh, and VPh
3.3. Preparation of EP/AVOPh Composites
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Wang, M.; Zhang, J. The preparation of a composite flame retardant of layered double hydroxides and α-zirconium phosphate and its modification for epoxy resin. Mater. Today Commun. 2021, 28, 102711. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, Y.; Zhang, Z.; Zhang, W.; Li, X.; Yang, R. Nickle nanocrystals decorated on graphitic nanotubes with broad channels for fire hazard reduction of epoxy resin. J. Hazard. Mater. 2021, 402, 123880. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zhu, H.; Fan, J.; Zheng, G.; Zhang, C.; Wang, Y.; Zhang, J. Boosting flame retardancy of epoxy resin composites through incorporating ultrathin nickel phenylphosphate nanosheets. J. Appl. Polym. Sci. 2020, 138, 50265. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Zhang, X.; Kong, Q.; Zhang, J.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2020, 140, 149–156. [Google Scholar] [CrossRef]
- Kong, Q.; Sun, Y.; Zhang, C.; Guan, H.; Zhang, J.; Wang, D.; Zhang, F. Ultrathin iron phenyl phosphonate nanosheets with appropriate thermal stability for improving fire safety in epoxy. Compos. Sci. Technol. 2019, 182, 107748. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Hong, J.; Luo, W.; Zeng, B.; Yuan, C.; Xu, Y.; Chen, G.; Dai, L. In-situ growth of metal-organophosphorus nanosheet/nanorod on graphene for enhancing flame retardancy and mechanical properties of epoxy resin. Compos. Part B Eng. 2020, 200, 108271. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, R.; Zhang, W.; Li, D.; Jiao, Q. Synergistic barrier effect of aluminum phosphate on flame retardant polypropylene based on ammonium polyphosphate/dipentaerythritol system. Mater. Des. 2019, 181, 107913. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Hu, Y. Preparation of layered organic-inorganic aluminum phosphonate for enhancing fire safety of polystyrene. Mater. Chem. Phys. 2017, 196, 109–117. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.-W.; Wang, P.-L.; Xing, W.; Song, L.; Hu, Y. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Sakai, E. Thermal stability and fire behavior of aluminum diethylphosphinate-epoxy resin nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 18–27. [Google Scholar] [CrossRef]
- Kong, Q.; Zhu, H.; Huang, S.; Wu, T.; Zhu, F.; Zhang, Y.; Wang, Y.; Zhang, J. Influence of multiply modified FeCu-montmorillonite on fire safety and mechanical performances of epoxy resin nanocomposites. Thermochim. Acta 2022, 707, 179112. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, P.; Sun, J.; Wen, P.; Zhang, S.; Kan, Y.; Liu, X.; Tang, G. Enhanced flame retardancy of rigid polyurethane foam via iron tailings and expandable graphite. J. Mater. Sci. 2022, 57, 18853–18873. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Wang, L.; Liu, H.; Wang, N.; Yang, B.; Guo, J.; Tian, L. Phosphorus-containing Salen-Ni metal complexes enhancing the flame retardancy and smoke suppression of epoxy resin composites. J. Appl. Polym. Sci. 2020, 137, 48734. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis. 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, T.; Zhang, J.; Wang, D.-Y. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through layered copper phenylphosphate. Compos. Sci. Technol. 2018, 154, 136–144. [Google Scholar] [CrossRef]
- Liu, J.; Qi, P.; Meng, D.; Li, L.; Sun, J.; Li, H.; Gu, X.; Jiang, S.; Zhang, S. Eco-friendly flame retardant and smoke suppression coating containing boron compounds and phytic acids for nylon/cotton blend fabrics. Ind. Crops Prod. 2022, 186, 115239. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.; Jiang, S.; Liu, M.; Liu, X.; Tang, G.; Cai, R.; Tan, W. Facile synthesis of zinc-containing mesoporous silicate from iron tailings and enhanced Fire retardancy of rigid polyurethane foam. Macromol. Mater. Eng. 2022. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Huang, S.; Wang, Y.; Yang, R.; Chai, H.; Zhu, F.; Kong, Q.; Zhang, Y.; Zhang, J. Suppressing fire hazard of poly(vinyl alcohol) based on (NH4)2[VO(HPO4)]2(C2O4)·5H2O with layered structure. J. Appl. Polym. Sci. 2021, 138, 51345. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Xiong, F.; Yu, R.; Wu, P.; Cui, L.; An, Q. VOPO4·2H2O as a new cathode material for rechargeable Ca-ion batteries. Chem. Commun. 2020, 56, 3805–3808. [Google Scholar] [CrossRef]
- Do, J.; Bontchev, R.P.; Jacobson, A.J. A Hydrothermal Investigation of the 1/2V2O5−H2C2O4/H3PO4/NH4OH System: Synthesis and Structures of (NH4)VOPO4·1.5H2O, (NH4)0.5VOPO4·1.5H2O, (NH4)2[VO(H2O)3]2[VO(H2O)][VO(PO4)2]2·3H2O, and (NH4)2[VO(HPO4)]2(C2O4)·5H2O. Inorg. Chem. 2000, 39, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Bircsk, Z.; Harrison, W.T.A. NH4VOPO4·H2O, a New One-Dimensional Ammonium Vanadium(IV) Phosphate Hydrate. Inorg. Chem. 1998, 37, 5387–5389. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Dian, Y.; Chen, L.; Zhang, X.; Feng, X.; Chen, W.; Zhao, Y. Stable cross-linked gel terpolymer electrolyte containing methyl phosphonate for sodium ion batteries. J. Membr. Sci. 2019, 583, 163–170. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef]
- Ou, M.; Lian, R.; Cui, J.; Guan, H.; Liu, L.; Jiao, C.; Chen, X. Co-curing preparation of flame retardant and smoke-suppressive epoxy resin with a novel phosphorus-containing ionic liquid. Chemosphere 2023, 311, 137061. [Google Scholar] [CrossRef]
- Chai, H.; Li, W.; Wan, S.; Liu, Z.; Zhang, Y.; Zhang, Y.; Zhang, J.; Kong, Q. Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins. Molecules 2023, 28, 623. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schoenberger, F.; Doering, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3764. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Li, L.; Zhang, M.; Chai, H.; Li, W.; Zhu, F.; Zhang, J. Improving the Thermal Stability and Flame Retardancy of Epoxy Resins by Lamellar Cobalt Potassium Pyrophosphate. Polymers 2022, 14, 4927. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zou, B.; Xiao, Y.; Qiu, S.; Xu, Z.; Yang, Y.; Jiang, G.; Zhang, Z.; Song, L.; Hu, Y. Targeted modification of black phosphorus by MIL-53(Al) inspired by “Cannikin’s Law” to achieve high thermal stability of flame retardant polycarbonate at ultra-low additions. Compos. Part B Eng. 2022, 238, 109943. [Google Scholar] [CrossRef]
- Li, L.; Hua, F.; Xi, H.; Yang, J.; Xiao, T.; Zuo, R.; Xu, X.; Yang, Z.; Lei, Z. Synthesis of Phosphorous Phenanthrene/L-Tryptophan Flame Retardant for Enhanced Flame Retardancy of Epoxy Resins. Macromol. Res. 2022, 30, 937–946. [Google Scholar] [CrossRef]
- He, T.; Guo, J.; Qi, C.; Feng, R.; Yang, B.; Zhou, Y.; Tian, L.; Cui, J. Core-shell structure flame retardant Salen-PZN-Cu@Ni-Mof microspheres enhancing fire safety of epoxy resin through the synergistic effect. J. Polym. Res. 2022, 29, 27. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Wang, X.; Wen, P.; Cai, W.; Hu, Y.; Liew, K.M. Molybdenum disulfide nanosheets as barrier enhancing nanofillers in thermal decomposition of polypropylene composites. Chem. Eng. J. 2016, 295, 278–287. [Google Scholar] [CrossRef]
- Sang, L.; Cheng, Y.; Yang, R.; Li, J.; Kong, Q.; Zhang, J. Polyphosphazene-wrapped Fe-MOF for improving flame retardancy and smoke suppression of epoxy resins. J. Therm. Anal. Calorim. 2021, 144, 51–59. [Google Scholar] [CrossRef]
- Yu, C.; Wu, T.; Yang, F.; Wang, H.; Rao, W.; Zhao, H.-B. Interfacial engineering to construct P-loaded hollow nanohybrids for flame-retardant and high-performance epoxy resins. J. Colloid Interface Sci. 2022, 628, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fang, G.; Tao, Y.; Meng, X.; Lin, Y.; Bhagia, S.; Wu, X.; Yong, Q.; Ragauskas, A.J. Nacre-inspired hemicelluloses paper with fire retardant and gas barrier properties by self-assembly with bentonite nanosheets. Carbohydr. Polym. 2019, 225, 115219. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Yu, H. Phosphorylated Boron Nitride Nanosheets as Highly Effective Barrier Property Enhancers. Ind. Eng. Chem. Res. 2018, 57, 14096–14105. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Suparanon, T.; Phetwarotai, W. Fire-extinguishing characteristics and flame retardant mechanism of polylactide foams: Influence of tricresyl phosphate combined with natural flame retardant. Int. J. Biol. Macromol. 2020, 158, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lee, M.; Kim, J. Relationship between structures of phosphorus compounds and flame retardancies of the mixtures with acrylonitrile–butadiene–styrene and ethylene–vinyl acetate copolymer. Polym. Adv. Technol. 2011, 22, 512–519. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Yang, L.; Wang, D. Few layered Co(OH)2 ultrathin nanosheet-based polyurethane nanocomposites with reduced fire hazard: From eco-friendly flame retardance to sustainable recycling. Green. Chem. 2016, 18, 3066–3074. [Google Scholar] [CrossRef]
- Gong, K.; Cai, L.; Shi, C.; Gao, F.; Yin, L.; Qian, X.; Zhou, K. Organic-inorganic hybrid engineering MXene derivatives for fire resistant epoxy resins with superior smoke suppression. Compos. Part A Appl. Sci. Manuf. 2022, 161, 107109. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Su, F.; Xie, J.; Xin, Y.; Zhang, W.; Liu, C.; Yao, D.; Zheng, Y. Core-Shell ZIF67@ZIF8 Modified with Phytic Acid as an Effective Flame Retardant for Improving the Fire Safety of Epoxy Resins. ACS Omega 2022, 7, 21664–21674. [Google Scholar] [CrossRef] [PubMed]


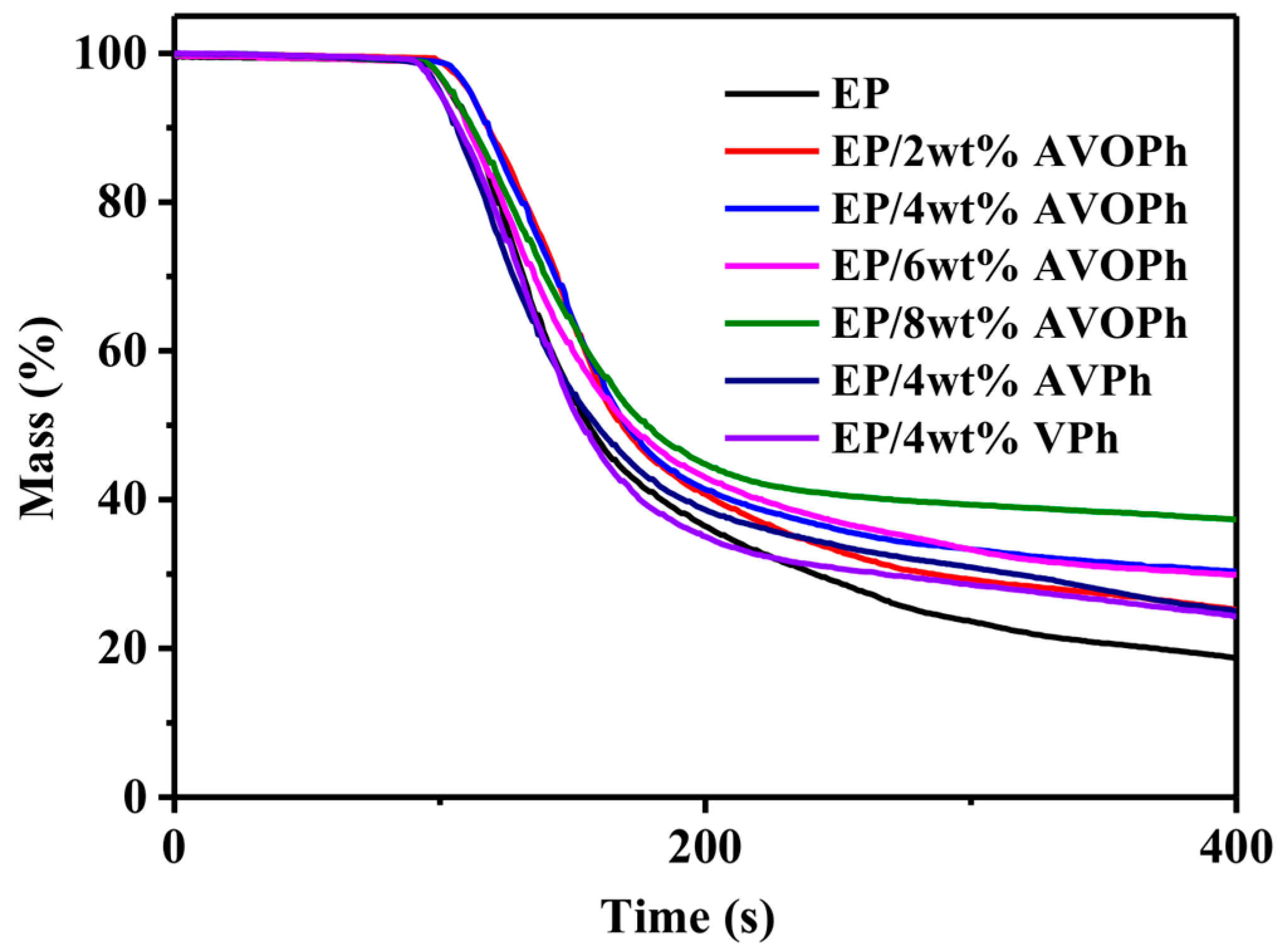

| Samples | T5% (°C) | T50% (°C) | Tmax (°C) | Residues (wt%, 700 °C) |
|---|---|---|---|---|
| EP | 360 | 394 | 380 | 15.3 |
| EP/2 wt% AVOPh | 349 | 391 | 374 | 19.8 |
| EP/4 wt% AVOPh | 343 | 390 | 369 | 21.2 |
| EP/6 wt% AVOPh | 340 | 389 | 368 | 21.8 |
| EP/8 wt% AVOPh | 339 | 391 | 369 | 23.0 |
| EP/4 wt% AVPh | 355 | 389 | 370 | 17.8 |
| EP/4 wt% VPh | 334 | 388 | 366 | 22.6 |
| Samples | LOI (vol%) | UL-94 | |
|---|---|---|---|
| t1 + t2 (s) | Rating | ||
| EP | 25.9 | >50 | NR * |
| EP/2 wt% AVOPh | 30.1 | >50 | NR |
| EP/4 wt% AVOPh | 31.7 | 21 | V1 ** |
| EP/6 wt% AVOPh | 32.8 | 16 | V1 |
| EP/8 wt% AVOPh | 34.2 | 27 | V1 |
| EP/4 wt% AVPh | 30.2 | 41 | V1 |
| EP/4 wt% VPh | 29.8 | >50 | NR |
| Samples | PHRR (kW/m2) | TSP (m2) | PCOP (g/s) | PCO2P (g/s) | Mass Residue (%) |
|---|---|---|---|---|---|
| EP | 1004 | 22.4 | 0.035 | 0.54 | 18.7 |
| EP/2 wt% AVOPh | 931 | 17.6 | 0.031 | 0.52 | 25.2 |
| EP/4 wt% AVOPh | 796 | 17.4 | 0.026 | 0.44 | 30.3 |
| EP/6 wt% AVOPh | 795 | 16.8 | 0.024 | 0.42 | 29.9 |
| EP/8 wt% AVOPh | 675 | 16.4 | 0.022 | 0.36 | 37.3 |
| EP/4 wt% AVPh | 877 | 17.8 | 0.028 | 0.47 | 25.0 |
| EP/4 wt% VPh | 840 | 19.7 | 0.025 | 0.45 | 24.3 |
| Samples | Components | |||
|---|---|---|---|---|
| EP (wt%) | AVOPh (wt%) | AVPh (wt%) | VPh (wt%) | |
| EP | 100 | 0 | 0 | 0 |
| EP/2 wt% AVOPh | 98 | 2 | 0 | 0 |
| EP/4 wt% AVOPh | 96 | 4 | 0 | 0 |
| EP/6 wt% AVOPh | 94 | 6 | 0 | 0 |
| EP/8 wt% AVOPh | 92 | 8 | 0 | 0 |
| EP/4 wt% AVPh | 96 | 0 | 4 | 0 |
| EP/4 wt% VPh | 96 | 0 | 0 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Li, W.; Huang, S.; Zhang, Z.; Liu, H.; Zhan, W.; Chen, M.; Kong, Q. Effect of Layered Aminovanadic Oxalate Phosphate on Flame Retardancy of Epoxy Resin. Molecules 2023, 28, 3322. https://doi.org/10.3390/molecules28083322
Hu P, Li W, Huang S, Zhang Z, Liu H, Zhan W, Chen M, Kong Q. Effect of Layered Aminovanadic Oxalate Phosphate on Flame Retardancy of Epoxy Resin. Molecules. 2023; 28(8):3322. https://doi.org/10.3390/molecules28083322
Chicago/Turabian StyleHu, Po, Weixi Li, Shuai Huang, Zongmian Zhang, Hong Liu, Wang Zhan, Mingyi Chen, and Qinghong Kong. 2023. "Effect of Layered Aminovanadic Oxalate Phosphate on Flame Retardancy of Epoxy Resin" Molecules 28, no. 8: 3322. https://doi.org/10.3390/molecules28083322
APA StyleHu, P., Li, W., Huang, S., Zhang, Z., Liu, H., Zhan, W., Chen, M., & Kong, Q. (2023). Effect of Layered Aminovanadic Oxalate Phosphate on Flame Retardancy of Epoxy Resin. Molecules, 28(8), 3322. https://doi.org/10.3390/molecules28083322









