Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins
Abstract
1. Introduction
2. Results and Discussion
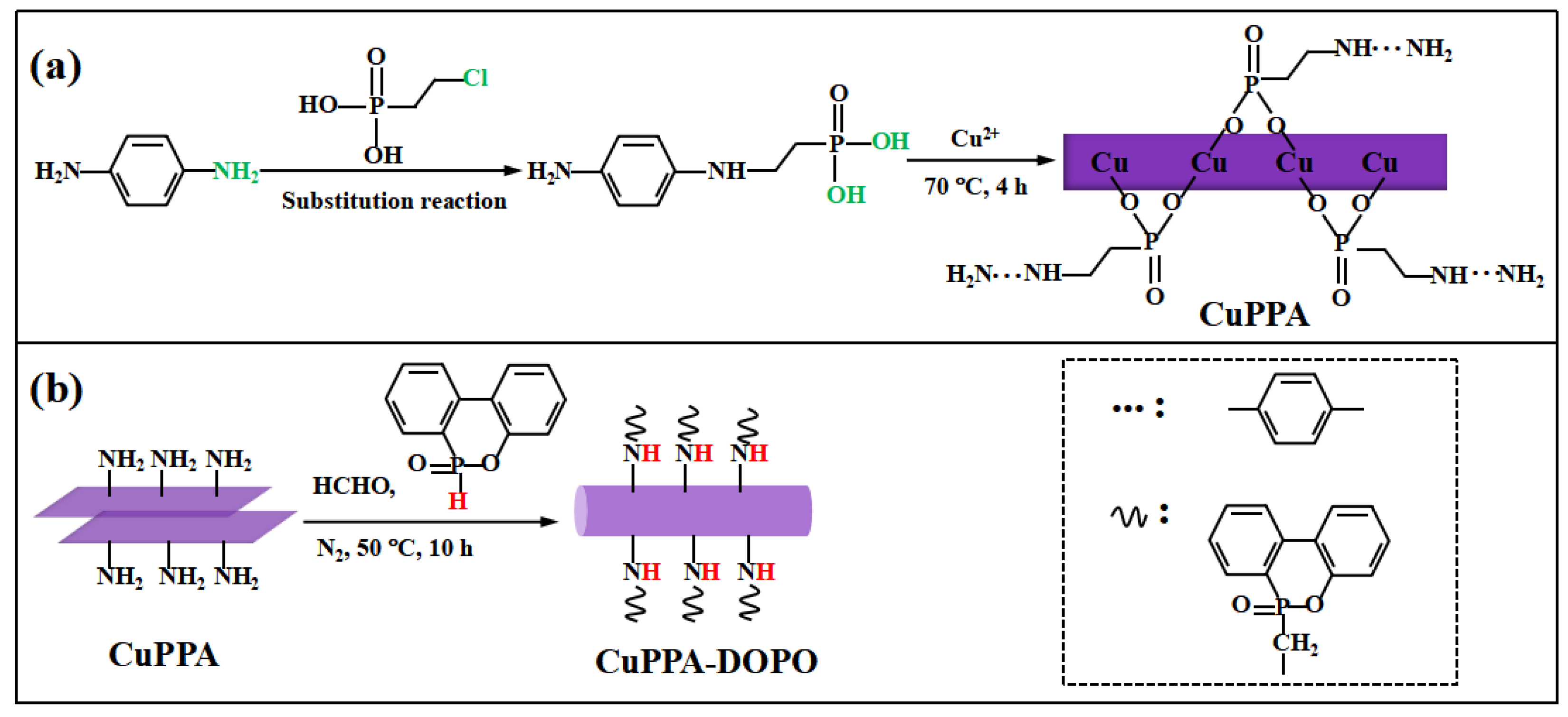
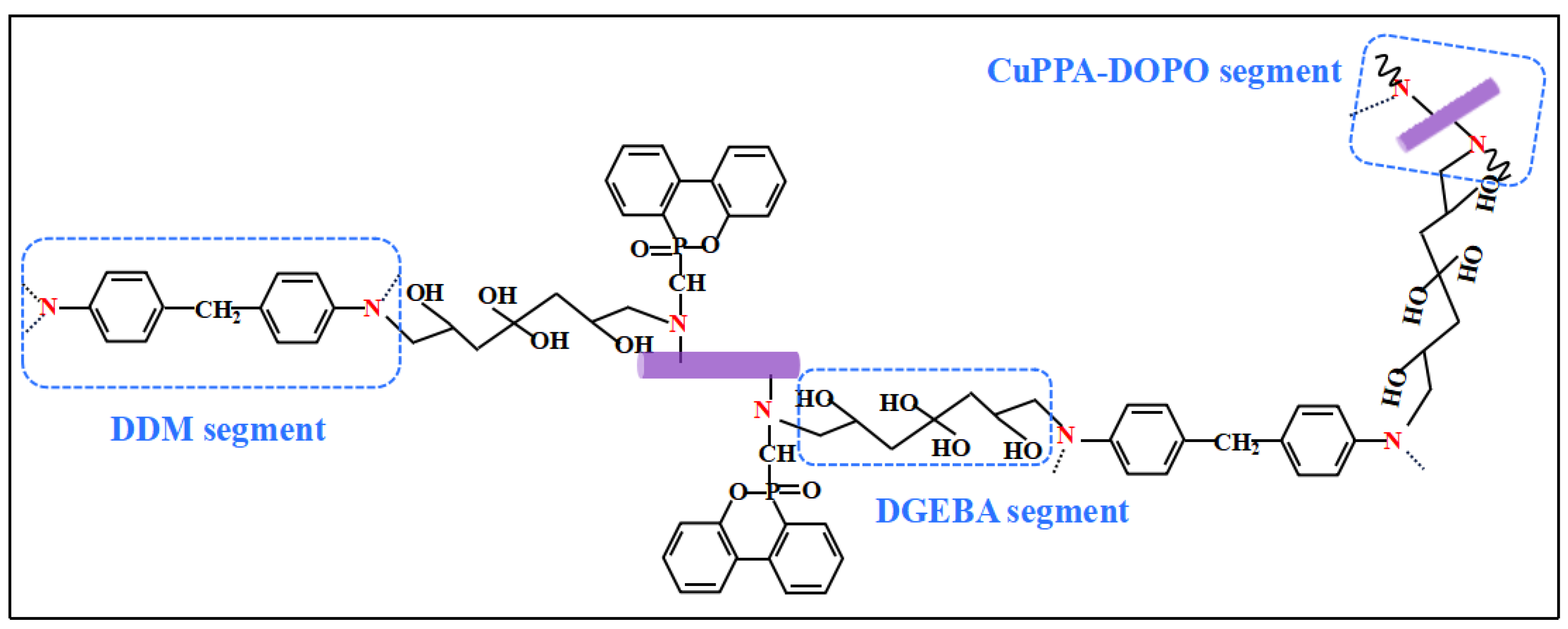
2.1. Structural Analysis of CuPPA-DOPO
2.2. Thermal Properties of EP Composites
2.3. Flame Retardancy of EP Composites
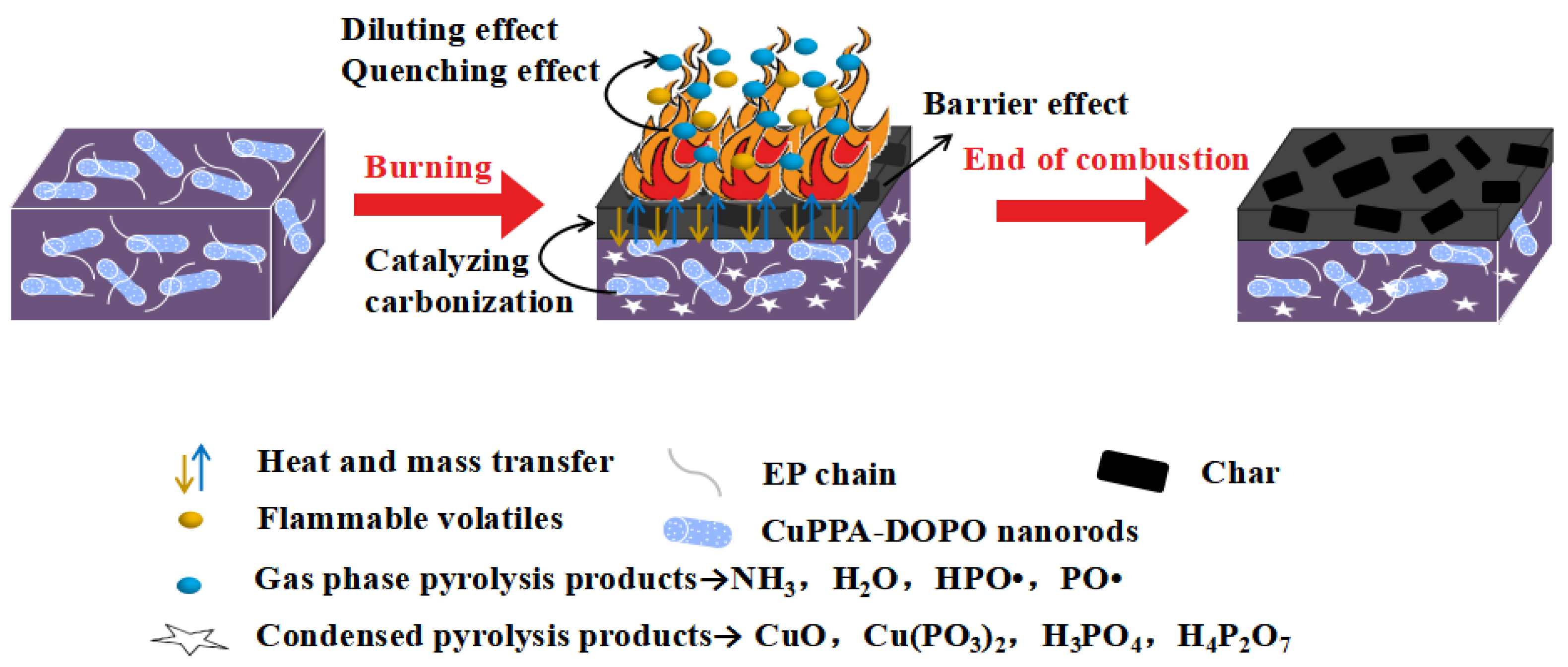
2.4. Flame-Retardance Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amino Phenyl Copper Phosphate
3.3. Preparation of DOPO-Amino Phenyl Copper Phosphate
3.4. Preparation of EP/CuPPA-DOPO Composites
3.5. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Farias, M.A.; Coelho, L.A.F.; Pezzin, S.H. Hybrid Nanocomposites Based on Epoxy/silsesquioxanes Matrices Reinforced with Multi-walled Carbon Nanotubes. Mater. Res. 2015, 18, 1304–1312. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, J.C.; Huang, C.M.; Jehng, J.M.; Chang, H.C.; Juang, T.Y.; Su, W.C. Side-chain phenol-functionalized poly (ether sulfone) and its contribution to high-performance and flexible epoxy thermosets. Polymer 2013, 54, 6936–6941. [Google Scholar] [CrossRef]
- Lv, Q.; Huang, J.-Q.; Chen, M.-J.; Zhao, J.; Tan, Y.; Chen, L.; Wang, Y.-Z. An Effective Flame Retardant and Smoke Suppression Oligomer for Epoxy Resin. Ind. Eng. Chem. Res. 2013, 52, 9397–9404. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.Y.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Kong, Q.; Zhu, H.; Fan, J.; Zheng, G.; Zhang, C.; Wang, Y.; Zhang, J. Boosting flame retardancy of epoxy resin composites through incorporating ultrathin nickel phenylphosphate nanosheets. J. Appl. Polym. Sci. 2021, 138, 50265. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.A.; Bekeshev, A.Z. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Ran, S.; Ye, R.; Cai, Y.; Shen, H.; He, Y.; Fang, Z.; Guo, Z. Synergistic Flame Retardant Mechanism of Lanthanum Phenylphosphonate and Decabromodiphenyl Oxide in Polycarbonate. Polym. Compos. 2019, 40, 986–999. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, T.; Zhang, J.; Wang, D. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through layered copper phenylphosphate. Compos. Sci. Technol. 2018, 154, 136–144. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, C.; Liu, L.; Hong, J.; Dong, M.; Deng, X. Synergistic flame retardant properties of a layered double hydroxide in combination with zirconium phosphonate in polypropylene. RSC Adv. 2016, 94, 91720–91727. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Huang, Z.; Xing, W.; Song, L.; Hu, Y. Effect of aluminum diethylphosphinate on the thermal stability and flame retardancy of flexible polyurethane foams. Fire Saf. J. 2019, 106, 72–79. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Huang, S.; Wang, Y.; Yang, R.; Chai, H.; Zhu, F.; Kong, Q.; Zhang, Y.; Zhang, J. Suppressing fire hazard of poly(vinyl alcohol) based on (NH4)2[VO(HPO4)]2(C2O4)·5H2O with layered structure. J. Appl. Polym. Sci. 2021, 138, 51345. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A review on metal-organic hybrids as flame retardants for enhancing fire safety of polymer composites. Compos. Part B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Zhang, X.; Kong, Q.; Zhang, J.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2020, 140, 149–156. [Google Scholar] [CrossRef]
- Ran, S.; Guo, Z.; Chen, C.; Zhao, L.; Fang, Z. Carbon nanotube bridged cerium phenylphosphonate hybrids, fabrication and their effects on the thermal stability and flame retardancy of the HDPE/BFR composite. J. Mater. Chem. A 2014, 2, 2999–3007. [Google Scholar] [CrossRef]
- Liu, W.; Nie, L.; Luo, L.; Yue, J.; Gan, L.; Lu, J.; Huang, J.; Liu, C. Enhanced dispersibility and uniform distribution of iron phosphonate to intensify its synergistic effect on polypropylene-based intumescent flame-retardant system. J. Appl. Polym. Sci. 2020, 137, 49552. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Z.; Ran, S.; Fang, Z. Synthesis of cerium phenylphosphonate and its synergistic flame retardant effect with decabromodiphenyl oxide in glass-fiber reinforced poly(ethylene terephthalate). Polym. Compos. 2014, 35, 539–547. [Google Scholar] [CrossRef]
- Zhang, F.; Bao, Y.; Ma, S.; Liu, L.; Shi, X. Hierarchical flower-like nickel phenylphosphonate microspheres and their calcined derivatives for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 7474–7481. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Wang, L.; Liu, H.; Wang, N.; Yang, B.; Guo, J.; Tian, L. Phosphorus-containing Salen-Ni metal complexes enhancing the flame retardancy and smoke suppression of epoxy resin composites. J. Appl. Polym. Sci. 2020, 137, 48734. [Google Scholar] [CrossRef]
- Guo, X.; Duan, M.; Zhang, J.; Xi, B.; Li, M.; Yin, R.; Zheng, X.; Liu, Y.; Cao, F.; An, X.; et al. A general self-assembly induced strategy for synthesizing two-dimensional ultrathin cobalt-based compounds toward optimizing hydrogen evolution catalysis. Adv. Funct. Mater. 2022, 32, 2209397. [Google Scholar] [CrossRef]
- He, Z.; Honeycutt, C.W.; Zhang, T.; Bertsch, P.M. Preparation and FT-IR characterization of metal phytate compounds. J. Environ. Qual. 2006, 35, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yu, B.; Yuan, Y.; Song, L.; Hu, Y. In situ preparation of reduced graphene oxide/DOPO-based phosphonamidate hybrids towards high-performance epoxy nanocomposites. Compos. Part B Eng. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, Z.; Chen, R.; Wang, J.; Zhou, H. Synthesis of DOPO-based pyrazine derivative and its effect on flame retardancy and thermal stability of epoxy resin. Polym. Adv. Technol. 2019, 30, 2331–2339. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J. Preparation and flame retardancy of a compounded epoxy resin system composed of phosphorus/nitrogen-containing active compounds. Polym. Degrad. Stab. 2015, 121, 398–406. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.; Gao, M.; Wang, J.; Sun, S.; Xue, K.; Zhang, S.; Liu, Y.; Zhang, J. Free-supporting dual-confined porous Si@c-ZIF@carbon nanofibersfor high-performance lithium-ion batteries. Chem. Commun. 2021, 57, 10580–10583. [Google Scholar] [CrossRef]
- Gnanasekar, P.; Feng, M.; Yan, N. Facile Synthesis of a Phosphorus-Containing Sustainable Biomolecular Platform from Vanillin for the Production of Mechanically Strong and Highly Flame-Retardant Resins. ACS Sustain. Chem. Eng. 2020, 8, 17417–17426. [Google Scholar] [CrossRef]
- Kong, Q.; Sun, Y.; Zhang, C.; Guan, H.; Zhang, J.; Wang, D.; Zhang, F. Ultrathin iron phenyl phosphonate nanosheets with appropriate thermal stability for improving fire safety in epoxy. Compos. Sci. Technol. 2019, 182, 107748. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Y.; Yu, B.; Li, C.; Song, J.; Tan, D.; Zhao, J. Synthesis of a novel reactive type flame retardant composed of phenophosphazine ring and maleimide for epoxy resin. Polym. Degrad. Stab. 2019, 165, 137–144. [Google Scholar] [CrossRef]
- Bifulco, A.; Varganici, C.D.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives. Polym. Degrad. Stab. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Wang, P.; Fu, X.; Kan, Y.; Wang, X.; Hu, Y. Two high-efficient DOPO-based phosphonamidate flame retardants for transparent epoxy resin. High Perform. Polym. 2019, 31, 249–260. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Wang, P.; Qiu, S.; Cai, W.; Hu, Y. Ultra-low phosphorus loading to achieve the superior flame retardancy of epoxy resin. Polym. Degrad. Stab. 2018, 149, 119–128. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Zhao, H.; Yang, R.; He, Y.; Zhao, T.; Zhang, Y.; Kong, Q.; Sun, S.; Zhang, J. Influence of beta-cyclodextrin functionalized tin phenylphosphonate on the thermal stability and flame retardancy of epoxy composites. J. Renew. Mater. 2022, 10, 3121–3130. [Google Scholar] [CrossRef]
- Kong, Q.; Li, L.; Zhang, M.; Chai, H.; Li, W.; Zhu, F.; Zhang, J. Improving the thermal stability and flame retardancy of epoxy resin by lamellar cobalt potassium pyrophosphate. Polymers 2022, 14, 4927. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Klinkowski, C.; Wagner, S.; Ciesielski, M.; Doring, M. Bridged phosphorylated diamines: Synthesis, thermal stability and flame retarding properties in epoxy resins. Polym. Degrad. Stab. 2014, 106, 122–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.; Liew, K.M.; Song, L.; Wang, C.; Hu, Y. Highly effective P–P synergy of a novel DOPO-based flame retardant for epoxy resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, J.; Liu, Z.; Gu, Y.; Luan, G.; Sun, H.; Yu, Q.; Zhang, S.; Wang, Z. Effect of ethyl-bridged diphenylphosphine oxide on flame retardancy and thermal properties of epoxy resin. Polym. Adv. Technol. 2020, 31, 1426–1436. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- Xu, M.; Xu, G.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Fang, F.; Song, P.; Ran, S.; Guo, Z.; Wang, H.; Fang, Z. A facile way to prepare phosphorus-nitrogen-functionalized graphene oxide for enhancing the flame retardancy of epoxy resin. Compos. Commun. 2018, 10, 97–102. [Google Scholar] [CrossRef]
- Kong, Q.; Zhu, H.; Huang, S.; Wu, T.; Zhu, F.; Zhang, Y.; Wang, Y.; Zhang, J. Influence of multiply modified FeCu-montmorillonite on fire safety and mechanical performances of epoxy resin nanocomposites. Thermochim. Acta 2022, 707, 179112. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymers 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Wan, X.; Zhang, J.; Liu, Y.; Zheng, X.; Kong, Q.; Jin, Z. Controllable solid-phase fabrication of Fe2O3/Fe5C2/Fe-N-C electrocatalyst towards optimizing the oxygen reduction reaction in zinc-air batteries. Nano Lett. 2022, 22, 4879–4887. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Yuan, Y.; Liu, X.; Sun, T.; Wu, Z. Study of the epoxy/amine equivalent ratio on thermal properties, cryogenic mechanical properties, and liquid oxygen compatibility of the bisphenol A epoxy resin containing phosphorus. High Perform. Polym. 2019, 32, 429–430. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Yan, H.; Zhang, Y.; Song, P.; Zhang, T.; Wang, H.; Fang, Z. Deposition growth of Zr-based MOFs on cerium phenylphosphonate lamella towards enhanced thermal stability and fire safety of polycarbonate. Compos. Part B Eng. 2020, 197, 108064. [Google Scholar] [CrossRef]
- Gao, M.; Xue, Y.; Zhang, Y.; Zhu, C.; Yu, H.; Guo, X.; Sun, S.; Xiong, S.; Kong, Q.; Zhang, J. Growing Co-Ni-Se nanosheets on 3D carbon frameworks as advanced dualfunctional electrodes for supercapacitors and sodium ion batteries. Inorg. Chem. Front. 2022, 9, 3933–3942. [Google Scholar] [CrossRef]
- Tang, G.; Liu, X.; Zhou, L.; Zhang, P.; Deng, D.; Jiang, H. Steel slag waste combined with melamine pyrophosphate as a flame retardant for rigid polyurethane foams. Adv. Powder Technol. 2020, 31, 279–286. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Q.; Zhong, G.; Zhang, H.; Chen, M.; Liu, C. DOPO-based curing flame retardant of epoxy composite material for char formation and intumescent flame retardance. J. Appl. Polym. Sci. 2020, 138, 49918. [Google Scholar] [CrossRef]
- Tang, G.; Liu, X.; Yang, Y.; Chen, D.; Zhang, H.; Zhou, L.; Zhang, P.; Jiang, H.; Deng, D. Phosphorus-containing silane modified steel slag waste to reduce fire hazards of rigid polyurethane foams. Adv. Powder Technol. 2020, 31, 1420–1430. [Google Scholar] [CrossRef]
- Peng, W.; Xu, Y.; Nie, S.; Yang, W. A bio-based phosphaphenanthrene-containing derivative modified epoxy thermosets with good flame retardancy, high mechanical properties and transparency. RSC Adv. 2021, 11, 30943–30954. [Google Scholar] [CrossRef]
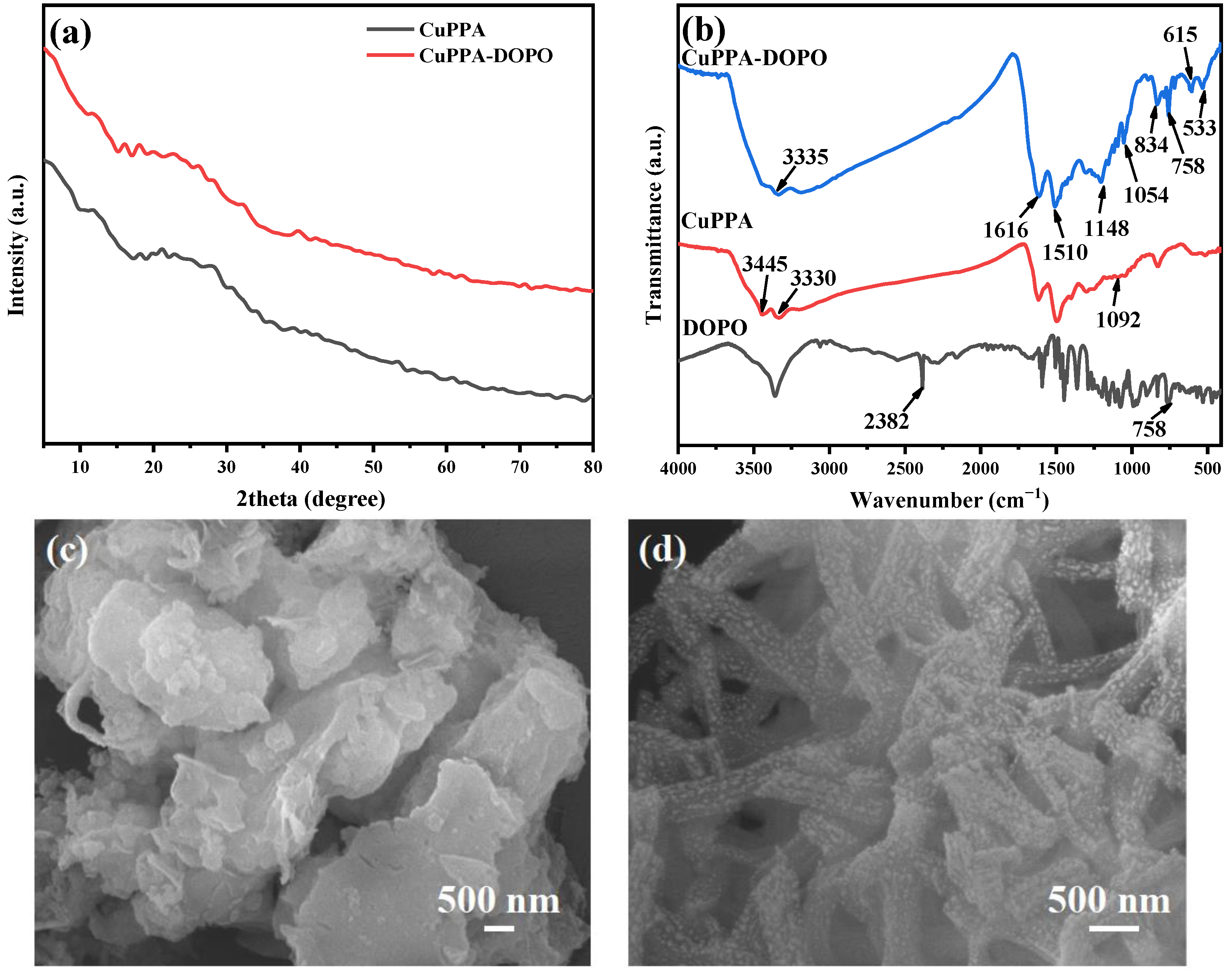
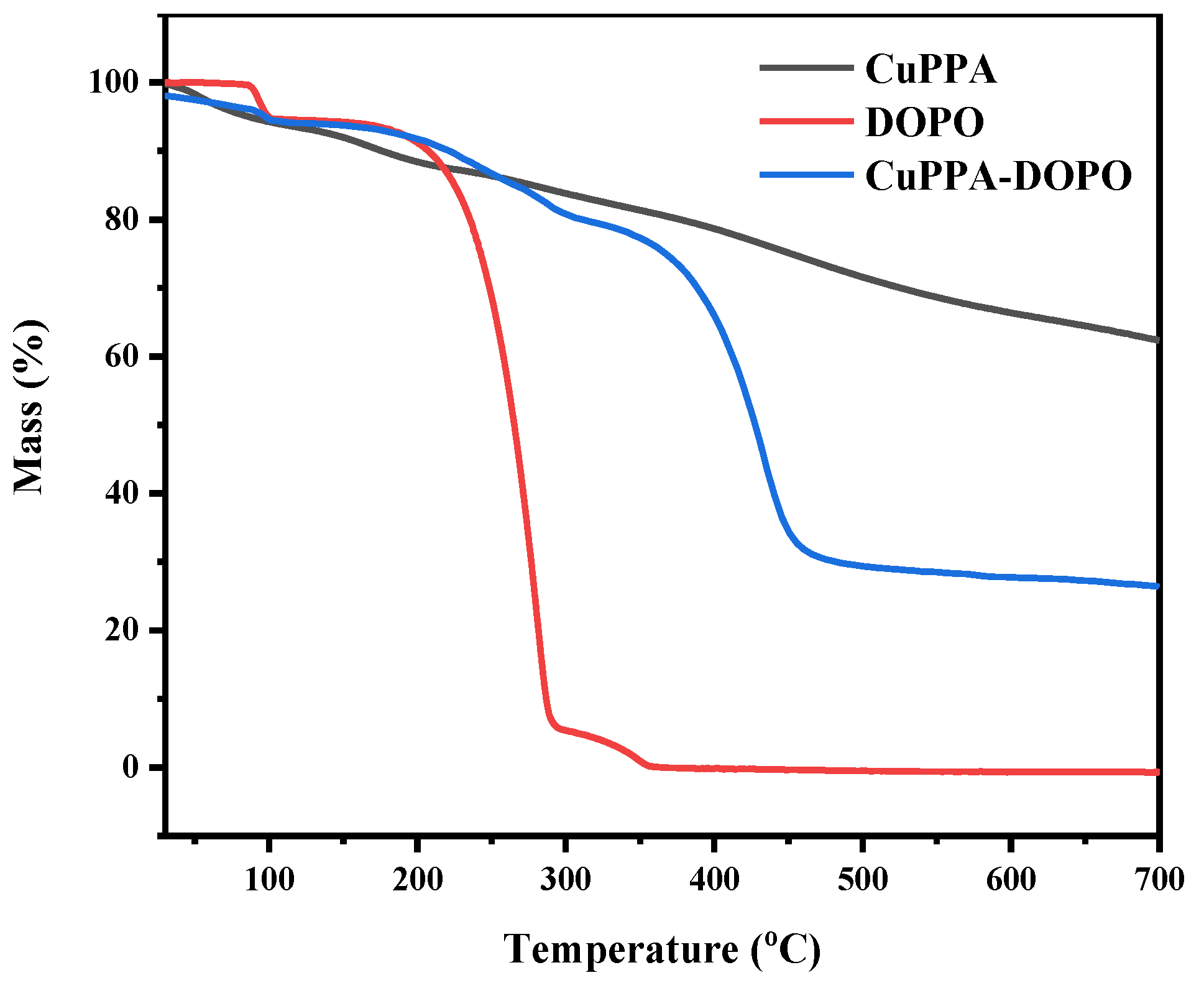
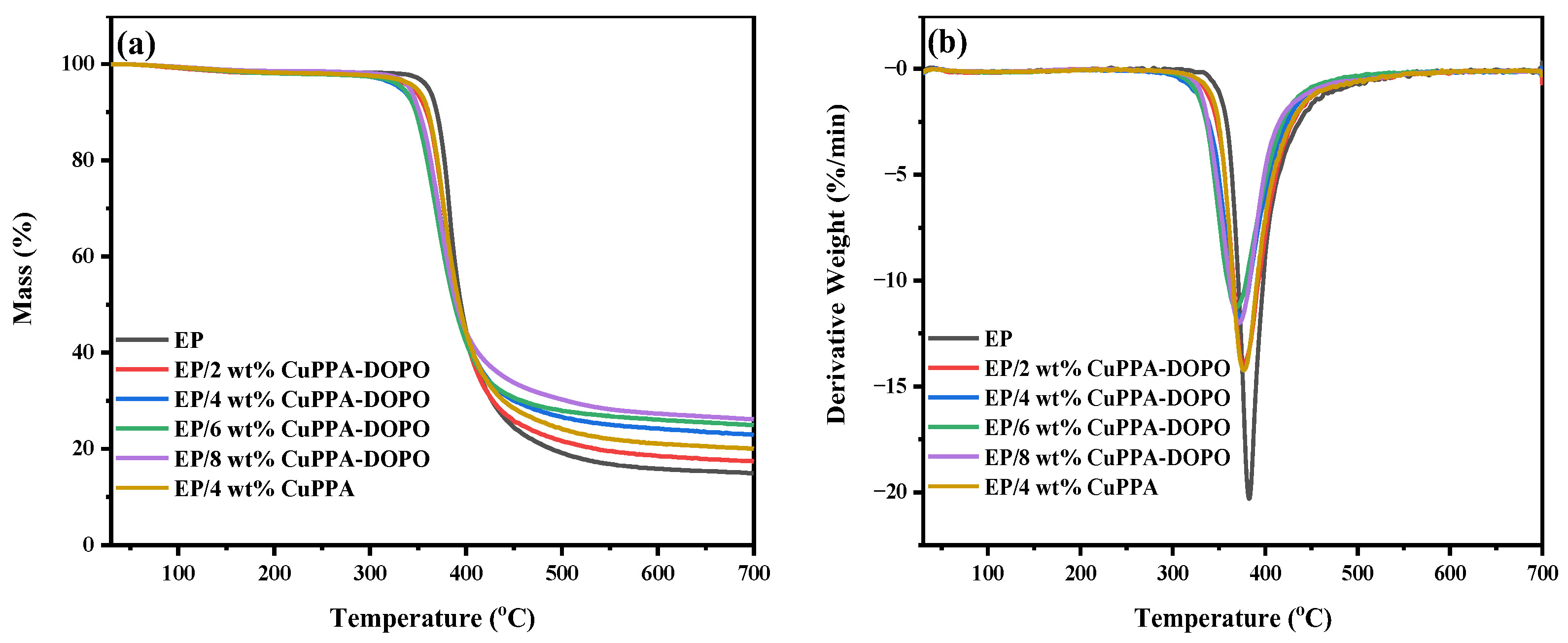

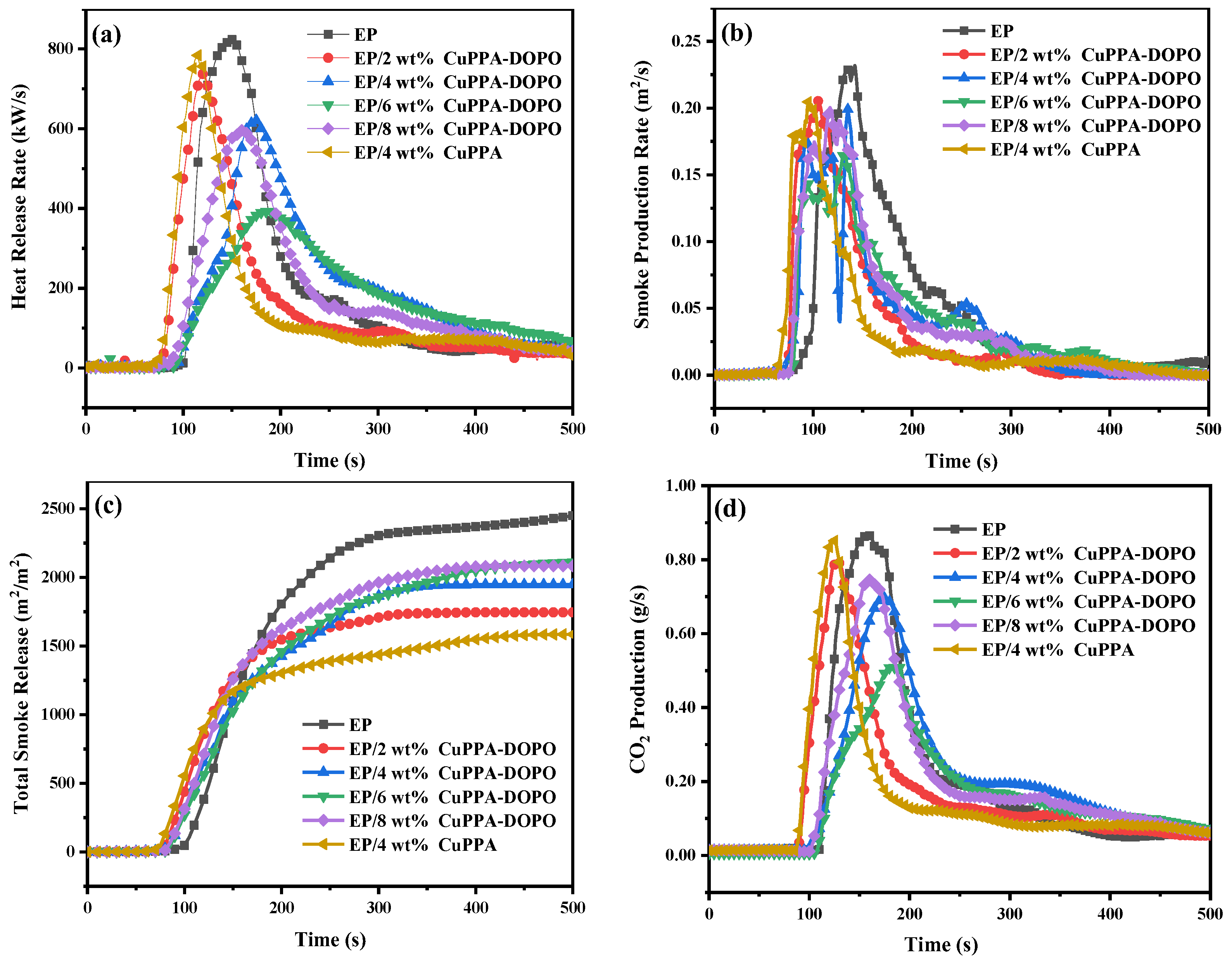
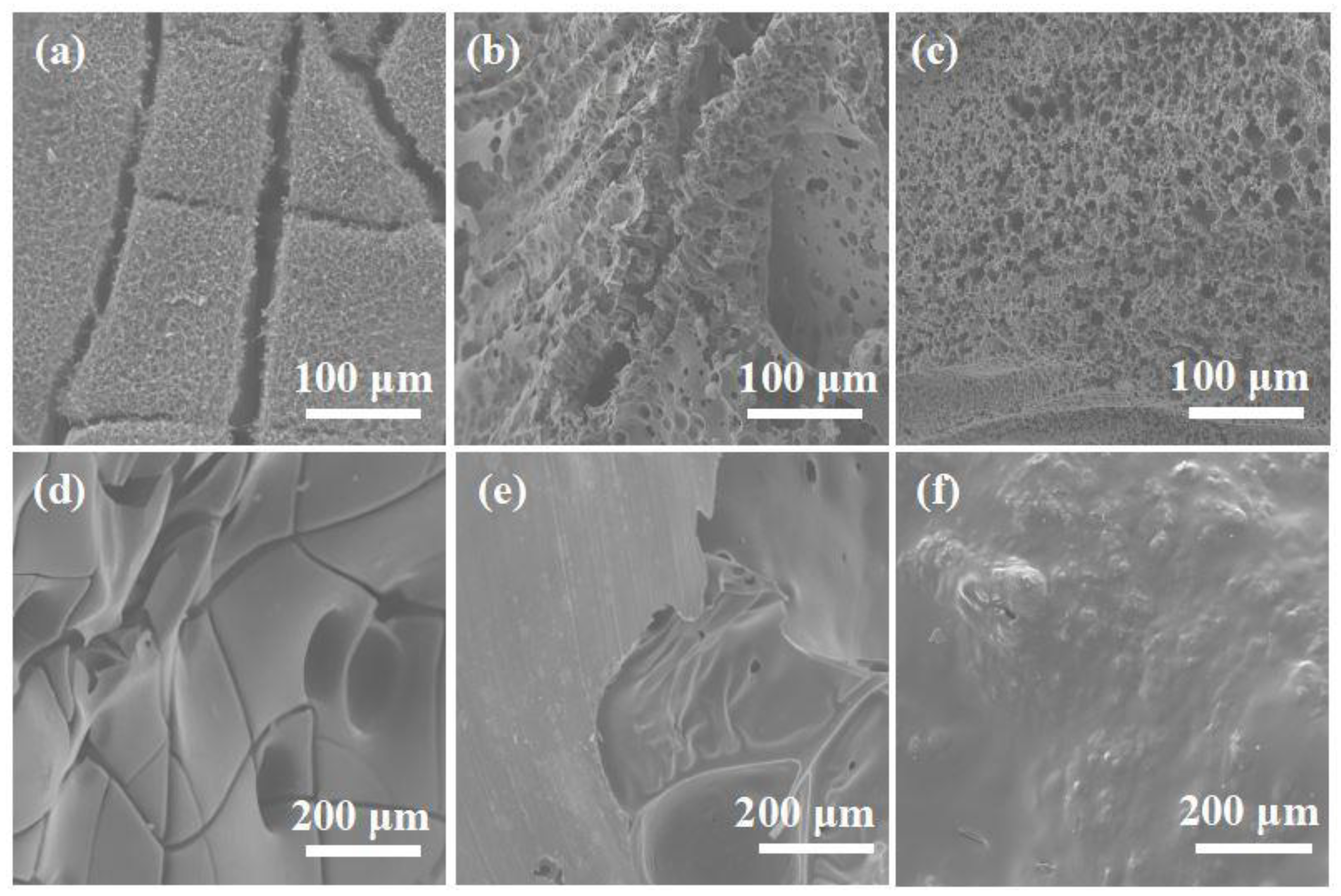
| Samples | T5% (°C) | Tmax (°C) | Residues (wt%, 700 °C) |
|---|---|---|---|
| EP | 360 | 382 | 14.9 |
| EP/2 wt% CuPPA-DOPO | 343 | 376 | 17.4 |
| EP/4 wt% CuPPA-DOPO | 333 | 373 | 23.0 |
| EP/6 wt% CuPPA-DOPO | 333 | 368 | 24.9 |
| EP/8 wt% CuPPA-DOPO | 342 | 372 | 26.1 |
| EP/4 wt% CuPPA | 350 | 376 | 19.8 |
| Samples | LOI (vol%) | UL-94 | |||
|---|---|---|---|---|---|
| t1 (s) | t2 (s) | t1 + t2 (s) | Rating | ||
| EP | 25.9 ± 0.2 | - | - | >50 | NR |
| EP/2 wt% CuPPA-DOPO | 28.8 ± 0.2 | 37.0 | 6.3 | 43.3 | V-1 |
| EP/4 wt% CuPPA-DOPO | 30.8 ± 0.3 | 20.5 | 7.0 | 27.5 | V-1 |
| EP/6 wt% CuPPA-DOPO | 32.6 ± 0.3 | 13.6 | 5.0 | 18.6 | V-1 |
| EP/8 wt% CuPPA-DOPO | 31.4 ± 0.3 | 14.5 | 9.0 | 23.5 | V-1 |
| EP/4 wt% CuPPA | 28.2 ± 0.2 | 36.0 | 9.3 | 45.3 | V-1 |
| Samples | PHRR (kW/m2) | PSPR (m2/s) | TSR (m2/m2) | CO2P (g/s) |
|---|---|---|---|---|
| EP | 822 | 0.23 | 2438 | 0.87 |
| EP/2 wt% CuPPA-DOPO | 739 | 0.21 | 1746 | 0.79 |
| EP/4 wt% CuPPA-DOPO | 617 | 0.20 | 1942 | 0.70 |
| EP/6 wt% CuPPA-DOPO | 390 | 0.17 | 2112 | 0.51 |
| EP/8 wt% CuPPA-DOPO | 597 | 0.20 | 2085 | 0.75 |
| EP/4 wt% CuPPA | 784 | 0.21 | 1585 | 0.85 |
| Samples | Components | |||
|---|---|---|---|---|
| EP (wt%) | DDM (wt%) | CuPPA-DOPO (wt%) | CuPPA (wt%) | |
| EP | 80.0 | 20.0 | 0 | 0 |
| EP/2 wt% CuPPA-DOPO | 78.4 | 19.1 | 2 | 0 |
| EP/4 wt% CuPPA-DOPO | 76.8 | 17.1 | 4 | 0 |
| EP/6 wt% CuPPA-DOPO | 75.2 | 15.7 | 6 | 0 |
| EP/8 wt% CuPPA-DOPO | 73.6 | 14.2 | 8 | 0 |
| EP/4 wt% CuPPA | 76.8 | 19.2 | 0 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, H.; Li, W.; Wan, S.; Liu, Z.; Zhang, Y.; Zhang, Y.; Zhang, J.; Kong, Q. Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins. Molecules 2023, 28, 623. https://doi.org/10.3390/molecules28020623
Chai H, Li W, Wan S, Liu Z, Zhang Y, Zhang Y, Zhang J, Kong Q. Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins. Molecules. 2023; 28(2):623. https://doi.org/10.3390/molecules28020623
Chicago/Turabian StyleChai, Huiyu, Weixi Li, Shengbing Wan, Zheng Liu, Yafen Zhang, Yunlong Zhang, Junhao Zhang, and Qinghong Kong. 2023. "Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins" Molecules 28, no. 2: 623. https://doi.org/10.3390/molecules28020623
APA StyleChai, H., Li, W., Wan, S., Liu, Z., Zhang, Y., Zhang, Y., Zhang, J., & Kong, Q. (2023). Amino Phenyl Copper Phosphate-Bridged Reactive Phosphaphenanthrene to Intensify Fire Safety of Epoxy Resins. Molecules, 28(2), 623. https://doi.org/10.3390/molecules28020623








