Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses: A Review
Abstract
1. Introduction
2. Highlighting of Bitter Taste and Astringent Perception
2.1. Generalities on Bitterness and Astringency
- Bitterness
- Astringency
2.2. Sensory Identification of Bitterness and Astringency (In Vivo Test)
2.3. Activation of the Human Bitter Taste Receptors TAS2Rs (In Vitro)
2.4. Correlation between Sensorial and In Vitro Analyses of Bitterness
3. Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses
3.1. Saponins
3.2. Phenolic Compounds
3.2.1. Phenolic Acids and Derivates
3.2.2. Stilbenes
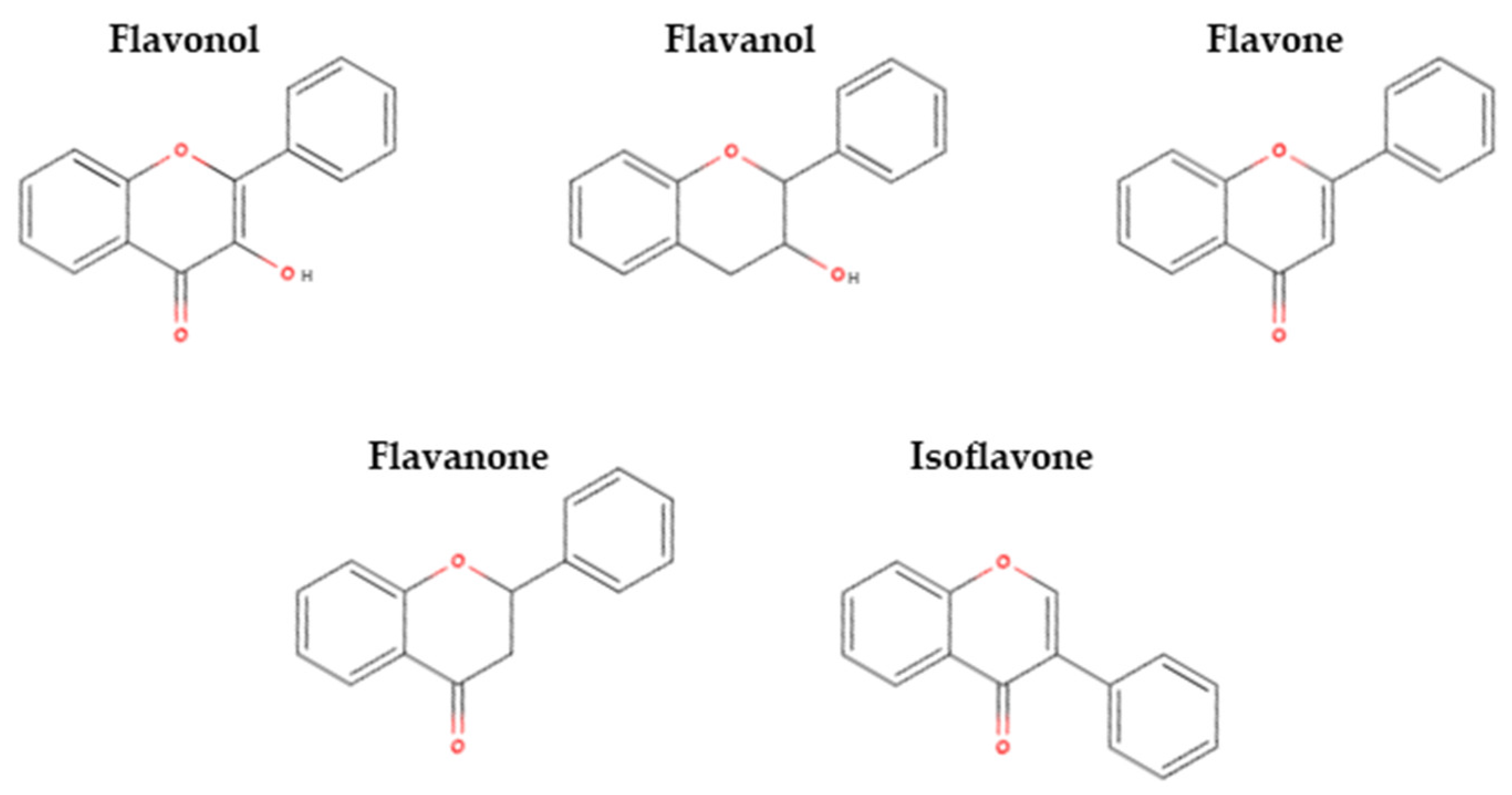
3.2.3. Flavonoids
- Flavonols
- Flavanols
| Phenolic Compounds | CAS * | M * (g/mol) | Pulses | Bitterness | Astringency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adzuki Beans | Beans ** | Chickpeas | Faba Beans | Lentils | Peas | Sensory Evaluation | TAS2R Evaluation | ||||
| PHENOLIC ACIDS | |||||||||||
| p-Hydroxybenzoic acid | 99-96-7 | 138.1 | 0.32–0.36 µg/g [94] 5.05 µg/g DM [103] 10.33 µg/g DM [104] 4.03–12.20 µg/g [108] | 2.1–44.4 µg/g DM [90] 1.6–56.6 µg/g DM [96] | 4.7 µg/g [111] 0.44–1.11 µg/g [88] | 0.94–1.00 µg/g DM [94] 3.75 µg/g DM [103] 73.46 µg/g [112] 3.25 µg/g DM [113] | 2.0 µg/g [15] 0.46–0.50 µg/g [94] 4.69–16.62 µg/g DM [114] | Slightly strong (2 g/L—water) [92] | DT: 665 µmol/L (wine) [50] | ||
| Protocatechuic acid | 99-50-3 | 154.1 | 67.6 µg/g [102] | 0.33–0.41 µg/g [94] 8.28 µg/g DM [103] 0.00–2.40 µg/g [108] | 28.3–48.0 µg/g DM [90] | D [71] 18.3 µg/g [111] 1.29–2.93 µg/g [88] | 0.49–0.52 µg/g DM [94] 4.27 µg/g DM [103] 1.45 µg/g DM [113] | D [15] 2.06–2.21 µg/g [94] 2.77–19.82 µg/g DM [114] | Moderate (2 g/L—water) [92] | DT: 206 µmol/L (wine) [50] | |
| p-Coumaric acid | 7400-08-0 | 164.2 | D [95] 31.3 µg/g [102] 0–180 µg/g DM [109] | D [95] 0.22 µg/g DM [103] | 17.6–99.4 µg/g DM [90] 0–4.1 µg/g DM [96] | D [95,115] 25.8 µg/g [111] 0.95–1.86 µg/g [88] | D [95] 3.22–3.42 µg/g DM [94] 38.84 µg/g [112] 6.47 µg/g DM [113] 37.3 µg/g DM [116] | D [15,95] 0.38–0.41 µg/g [94] 0.54–1.10 µg/g DM [114] | Moderate (2 g/L—water) [92] | Sensory detection [92] DT: 139 µmol/L (wine) [50] | |
| m-Coumaric acid | 588-30-7 | 164.2 | D [71] | D [95] | Moderate (2 g/L—water) [92] | DT: 292 µmol/L (wine) | |||||
| Gallic acid | 149-91-7 | 170.1 | 0–520 µg/g DM [109] | 0.0–213.0 µg/g DM [97] | 5 µg/g DM [13] 4.1–22.0 µg/g DM [90] 37.5–225.7 µg/g DM [96] 0.0–106.0 µg/g DM [97] | D [71] 26.9 µg/g [111] | 2.54 µg/g DM [103] 100.0 µg/g DM [97] | 0.016 µg/g [15] | Moderate (2 g/L—water) [92] | Sensory detection [92] | |
| Caffeic acid | 331-39-5 | 180.2 | 10.0–22.0 µg/g DM [13] | 17.7–103.3 µg/g DM [90] | D [95] 20 µg/g DM [13] 0.091 µg/g [15] | Moderate (2 g/L—water) [92] DT: 0.11 mM [117] Based on a model *** [15] | Sensory detection [92] Based on a model *** [15] | ||||
| STILBENES | |||||||||||
| Resveratrol | 501-36-0 | 228.2 | D [98] | Bitter (wine) [101] DT: 47 mg/L (water) [67] | TAS2R14: AT = 16 µM; EC50 = 30.3 µM [20] TAS2R39: AT = 63 µM; EC50 = 109 µM [20] | Astringent (wine) [101] | |||||
| FLAVONOLS | |||||||||||
| Kaempferol | 520-18-3 | 286.2 | D [95] 0–90 µg/g DM [109] | D [95] | 5.5–97.9 µg/g DM [90] 0–5.50 µg/g DM [96] | D [95] | D [95] 1.64 µg/g DM [103] | D [95] | TAS2R14: AT = 8 µM [20] TAS2R39: AT = 0.5 µM [20] | ||
| Quercetin | 117-39-5 | 302.2 | D [95] 36.2 µg/g [102] | D [95] 1.91 µg/g [108] | 7.0–104.9 µg/g DM [90] 0–14.5 µg/g DM [96] | D [95,98] | D [95] | D [95] 0–3 µg/g DM [97] | TAS2R14 (500 µM) [20] TAS2R39 (500 µM) [20] | ||
| Myricetin | 529-44-2 | 318.2 | 4.4–28.3 µg/g DM [90] 0–18.9 µg/g DM [96] | TAS2R14: AT = 250 µM [20] TAS2R39: AT = 1 µM [20] | |||||||
| Quercetin-3-O-glucoside | 482-35-9 | 464.4 | D [105] | 0.79 µg/g DM [104] | 1.0 µg/g DM [103] | 0.015 µg/g [15] | Based on a model *** [15] | ||||
| FLAVANOLS | |||||||||||
| (+)-Catechin | 154-23-4 | 290.3 | D [105] 0–160 µg/g DM [109] | 32.15 µg/g DM [104] 142.58 µg/g [108] 0.0–23.0 µg/g DM [97] 132.38 µg/g [110] | 4.7–92.4 µg/g DM [96] 0.0–26.0 µg/g DM [97] | D [98] 9.4 µg/g [111] 191–297 µg/g [88] 36.02 µg/g [112] | D [118] 0.1–0.3 µg/100 g DM [94] 0.53 µg/g DM [103] 0.77 µg/g DM [113] | D [15] | DT: 1000 µmol/L (water) [50] Weak (2 g/L—water) [92] Bitter (0.9 g/L—aqueous ethanol (1% v/v)) [106] | TAS2R14: AT = 500 µM [20] TAS2R39: AT = 250 µM [20] | DT: 410 µmol/L (water) [50,107] Astringent (0.9 g/L—aqueous ethanol (1% v/v)) [106] |
| (-)-Epicatechin | 490-46-0 | 290.3 | 25.7 µg/g [102] 0–90 µg/g DM [109] | D [98] 98.25 µg/g [112] | 70–97 µg/g DM [13] 4.17 µg/g DM [113] | DT: 930 µmol/L (water) [50] Moderate (2 g/L—water) [92] Bitter (0.9 g/L—aqueous ethanol (1% v/v)) [106] | TAS2R4: AT = 2000 µM; EC50 > 30151 µM [62] TAS2R5: AT = 1000 µM; EC50 = 3210 µM [62] TAS2R14: AT = 500 µM [20] TAS2R39: AT = 250–1000 µM; EC50 = 417.7–3800 µM [20,63,64] | DT: 930 µmol/L (water) [50,107] Astringent (0.9 g/L—aqueous ethanol (1% v/v)) [106] | |||
| (-)-Epigallocatechin | 970-74-1 | 306.3 | D [98] | 0.00–1.61 µg/g DM [114] | TAS2R39: AT = 500 µM; EC50 = 395.5 µM [20,63] | DT: 520 µmol/L (water) [107] | |||||
| (-)-Epicatechin gallate | 1257-08-5 | 442.4 | 363 µg/g [111] | TAS2R14: AT = 125 µM; EC50 = 70 µM [20,64] TAS2R39: AT = 32 µM; EC50 = 21.3–151 µM [20,63,64] | DT: 260 µmol/L (water) [107] | ||||||
| (-)-Epigallocatechin gallate | 989-51-5 | 458.4 | 0.1 µg/g [102] | 18.3 µg/g [111] | DT: 380 µM [66] | TAS2R4 [66] TAS2R5: EC50 = 12.30 [66] TAS2R14: AT = 250 µM; EC50 = 34 µM [20,64] TAS2R30 [66] TAS2R39: AT = 32–100 µM; EC50 = 8.50–181.6 µM [20,63,64,66,119] TAS2R43: EC50 = 16.72 [66] | DT: 190 µmol/L (water) [107] | ||||
| Theaflavin | 4670-05-7 | 564.5 | D [98] | TAS2R39: EC50 = 2.79 µM [64] | DT: 16 µmol/L (water) [107] | ||||||
| FLAVONES | |||||||||||
| Chrysin | 480-40-0 | 254.2 | 0–90 µg/g DM [109] | D [98,115] | TAS2R14: AT = 63 µM [20] TAS2R39: AT = 16 µM [20] | ||||||
| 7,4′-Dihydroxyflavone | 2196-14-7 | 254.2 | D [115] | TAS2R14: AT = 16 µM [20] TAS2R39: AT = 125 µM [20] | |||||||
| Luteolin | 491-70-3 | 286.2 | D [118] 0.33 µg/g DM [113] | TAS2R14: AT = 2 µM; EC50 = 6.0 µM [20] TAS2R39: AT = 0.5 µM; EC50 = 7.3 µM [20] | |||||||
| FLAVANONES | |||||||||||
| Pinocembrin | 480-39-7 | 256.2 | 1.26 µg/g [108] | D [98] | TAS2R14: AT = 8 µM; EC50 = 39.1 µM [20] TAS2R39: AT = 4 µM; EC50 = 48.9 µM [20] | ||||||
| Naringenin | 480-41-1 | 272.2 | D [118] | 0.082 µg/g [15] | TAS2R14: AT = 16 µM; EC50 = 36.2 µM [20] TAS2R39: AT = 8 µM; EC50 = 32.9 µM [20] | ||||||
| ISOFLAVONES | |||||||||||
| Daidzein | 486-66-8 | 254.2 | 0.209 µg/g DM [120] | 0.0–40.3 µg/g DM [90] 0.475 µg/g DM [120] | 0.59 µg/g DM [120] | 0.84 µg/g DM [120] | 0.41 µg/g DM [120] | Slightly (1 µM) [80] | TAS2R14: AT = 500 µM [21] TAS2R39: AT = 500 µM [21] | Astringent (0.1–1 µM) [80] | |
| Formomonetin | 485-72-3 | 268.3 | D [98] | TAS2R14: AT = 500 µM [21] TAS2R39: AT = 500 µM [21] | |||||||
| Genistein | 446-72-0 | 270.2 | 0.191 µg/g DM [120] | 0.7–33.8 µg/g DM [90] 0.766 µg/g DM [120] | D [98] 0.74 µg/g DM [120] | 0.139 µg/g DM [120] | 0.144 µg/g DM [120] | Slightly (1.5 µM) [80] | TAS2R14: AT = 4 µM; EC50 = 28.9 µM [21] TAS2R39: AT = 8 µM; EC50 = 49.4 µM [21] | Weakly (10 µM) [80] Astringent (1.5 µM) [80] | |
| PROCYANIDINS | |||||||||||
| Procyanidin B1 | 20315-25-7 | 578.5 | 213.0 µg/g [108] | D [98] | D [118] | DT: 400 µM [50] | TAS2R5: EC50 = 119.34 µM [66] TAS2R7: EC50 = 123.95 µM [66] | DT: 240 µM [50] | |||
| Procyanidin B2g (3-O-gallate) | 29106-49-8 | 578.5 | D [98,111] | 0.49 µg/g DM [103] | TAS2R5: EC50 = 6.29 µM [66] TAS2R39: EC50 = 9.11 µM [66] | ||||||
| Procyanidin B4 | 29106-51-2 | 578.5 | 16.0 µg/g [108] | D [98] | Bitter (0.9 g/L—aqueous ethanol (1% v/v)) [106] | TAS2R5 [66] | Astringent (0.9 g/L—aqueous ethanol (1% v/v)) [106] | ||||
| Procyanidin C2 | - | 866.8 | 42.4 µg/g [108] | D [98] | Bitter (0.9 g/L—aqueous ethanol (1% v/v)) [106] | TAS2R5: AT = 30.0 µM; EC50 = 35.6 µM [62] | Astringent (0.9 g/L—aqueous ethanol (1% v/v)) [106] | ||||
- Flavones
- Flavanones
- Isoflavones
3.2.4. Condensed Tannins
3.3. Alkaloids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guinet, M.; Nicolardot, B.; Voisin, A.-S. Provision of Contrasted Nitrogen-Related Ecosystem Services among Grain Legumes. Agron. Sustain. Dev. 2020, 40, 33. [Google Scholar] [CrossRef]
- Voisin, A.-S.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.-H.; Magrini, M.-B.; Meynard, J.-M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for Feed, Food, Biomaterials and Bioenergy in Europe: A Review. Agron. Sustain. Dev. 2014, 34, 361–380. [Google Scholar] [CrossRef]
- Lecerf, J.-M. Viande et santé humaine: Excès et défauts. Bull. Acad. Nat. Med. Paris 2011, 195, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile Compounds in Pulses: A Review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.; Salles, C. Taste Perception and Integration. In Flavor from Food to Behaviors, Wellbeing and Health; Elsevier Ltd.: Duxford, UK, 2016; pp. 101–119. ISBN 978-0-08-100295-7. [Google Scholar]
- Amarowicz, R.; Troszyńska, A.; Baryłko-Pikielna, N.; Shahidi, F. Polyphenolics Extracts from Legume Seeds: Correlations Between Total Antioxidant Activity, Total Phenolics Content, Tannins Content and Astringency. J. Food Lipids 2004, 11, 278–286. [Google Scholar] [CrossRef]
- Karolkowski, A.; Martin, C.; Bouzidi, E.; Albouy, J.-F.; Levavasseur, L.; Briand, L.; Salles, C. Heat Treatment, Cultivar and Formulation Modify the Sensory Properties and Consumer Acceptability of Gels Containing Faba Bean (Vicia Faba L. Minor) Protein Concentrates. Foods 2022, 11, 3018. [Google Scholar] [CrossRef]
- Gläser, P.; Dawid, C.; Meister, S.; Bader-Mittermaier, S.; Schott, M.; Eisner, P.; Hofmann, T. Molecularization of Bitter Off-Taste Compounds in Pea-Protein Isolates (Pisum Sativum L.). J. Agric. Food Chem. 2020, 68, 10374–10387. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of Environmental Biotic Factors on the Content of Saponins in Plants. Phytochem. Rev. 2011, 10, 493–502. [Google Scholar] [CrossRef]
- Oliete, B.; Lubbers, S.; Fournier, C.; Jeandroz, S.; Saurel, R. Effect of Biotic Stress on the Presence of Secondary Metabolites in Field Pea Grains. J. Sci. Food Agric. 2022, 102, 4942–4948. [Google Scholar] [CrossRef]
- Ulyanych, O.; Poltoretskyi, S.; Liubych, V.; Yatsenko, A.; Yatsenko, V.; Lazariev, O.; Kravchenko, V. Effect of Surface Drip Irrigation and Cultivars on Physiological State and Productivity of Faba Bean Crop. J. Agric. Sci. 2021, 32, 139–149. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Analysis of 17 Polyphenolic Compounds in Organic and Conventional Legumes by High-Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and Evaluation of Their Antioxidant Activity. Int. J. Food Sci. Nutr. 2018, 69, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant Breeding: Importance of Plant Secondary Metabolites for Protection against Pathogens and Herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Cosson, A.; Meudec, E.; Ginies, C.; Danel, A.; Lieben, P.; Descamps, N.; Cheynier, V.; Saint-Eve, A.; Souchon, I. Identification and Quantification of Key Phytochemicals in Peas—Linking Compounds with Sensory Attributes. Food Chem. 2022, 385, 132615. [Google Scholar] [CrossRef] [PubMed]
- Dupont, M.S.; Muzquiz, M.; Estrella, I.; Fenwick, G.R.; Price, K.R. Relationship between the Sensory Properties of Lupin Seed with Alkaloid and Tannin Content. J. Sci. Food Agric. 1994, 65, 95–100. [Google Scholar] [CrossRef]
- Heng, L.; Vincken, J.-P.; van Koningsveld, G.; Legger, A.; Gruppen, H.; van Boekel, T.; Roozen, J.; Voragen, F. Bitterness of Saponins and Their Content in Dry Peas. J. Sci. Food Agric. 2006, 86, 1225–1231. [Google Scholar] [CrossRef]
- Belitz, H.; Wieser, H. Bitter Compounds: Occurrence and Structure-activity Relationships. Food Rev. Int. 1985, 1, 271–354. [Google Scholar] [CrossRef]
- Huang, A.-S.; Hsieh, O.A.-L.; Chang, S.S. Characterization of the Nonvolatile Minor Constituents Responsible for the Objectionable Taste of Defatted Soybean Flour. J. Food Sci. 1982, 47, 19–23. [Google Scholar] [CrossRef]
- Roland, W.S.U.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.-P. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Vincken, J.-P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Humiski, L.M.; Aluko, R.E. Physicochemical and Bitterness Properties of Enzymatic Pea Protein Hydrolysates. J. Food Sci. 2007, 72, S605–S611. [Google Scholar] [CrossRef] [PubMed]
- Cosson, A.; Oliveira Correia, L.; Descamps, N.; Saint-Eve, A.; Souchon, I. Identification and Characterization of the Main Peptides in Pea Protein Isolates Using Ultra High-Performance Liquid Chromatography Coupled with Mass Spectrometry and Bioinformatics Tools. Food Chem. 2022, 367, 130747. [Google Scholar] [CrossRef] [PubMed]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter Taste, Phytonutrients, and the Consumer: A Review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W. Elucidation of Mammalian Bitter Taste. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–72. ISBN 978-3-540-32431-7. [Google Scholar]
- Behrens, M.; Meyerhof, W. The Vertebrate Gustatory System. In Flavour: From Food to Perception; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 57–78. ISBN 978-1-118-92938-4. [Google Scholar]
- Delompré, T.; Salles, C.; Briand, L. Saveur amère: De la molécule au comportement. Acad. Agric. Fr. 2020, 1–22. [Google Scholar]
- Jalševac, F.; Terra, X.; Rodríguez-Gallego, E.; Beltran-Debón, R.; Blay, M.T.; Pinent, M.; Ardévol, A. The Hidden One: What We Know About Bitter Taste Receptor 39. Front. Endocrinol. 2022, 13, 854718. [Google Scholar] [CrossRef]
- Dsamou, M. Protéome Salivaire et Sensibilité à l’amertume Chez l’Homme. Ph.D. Thesis, Université de Bourgogne, Dijon, France, 2012. [Google Scholar]
- Schwartz, M.; Brignot, H.; Feron, G.; Hummel, T.; Zhu, Y.; von Koskull, D.; Heydel, J.-M.; Lirussi, F.; Canon, F.; Neiers, F. Role of Human Salivary Enzymes in Bitter Taste Perception. Food Chem. 2022, 386, 132798. [Google Scholar] [CrossRef]
- Matsunami, H.; Montmayeur, J.-P.; Buck, L.B. A Family of Candidate Taste Receptors in Human and Mouse. Nature 2000, 404, 601–604. [Google Scholar] [CrossRef]
- Chamoun, E.; Liu, A.S.; Duizer, L.M.; Feng, Z.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W.L. Single Nucleotide Polymorphisms in Sweet, Fat, Umami, Salt, Bitter and Sour Taste Receptor Genes Are Associated with Gustatory Function and Taste Preferences in Young Adults. Nutr. Res. 2021, 85, 40–46. [Google Scholar] [CrossRef]
- Kim, U.; Wooding, S.; Ricci, D.; Jorde, L.B.; Drayna, D. Worldwide Haplotype Diversity and Coding Sequence Variation at Human Bitter Taste Receptor Loci. Hum. Mutat. 2005, 26, 199–204. [Google Scholar] [CrossRef]
- Kim, U.-K.; Breslin, P.A.S.; Reed, D.; Drayna, D. Genetics of Human Taste Perception. J. Dent. Res. 2004, 83, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Signaling in the Chemosensory Systems. Cell. Mol. Life Sci. 2006, 63, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Pronin, A.N.; Xu, H.; Tang, H.; Zhang, L.; Li, Q.; Li, X. Specific Alleles of Bitter Receptor Genes Influence Human Sensitivity to the Bitterness of Aloin and Saccharin. Curr. Biol. 2007, 17, 1403–1408. [Google Scholar] [CrossRef]
- Roudnitzky, N.; Behrens, M.; Engel, A.; Kohl, S.; Thalmann, S.; Hübner, S.; Lossow, K.; Wooding, S.P.; Meyerhof, W. Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception. PLOS Genet. 2015, 11, e1005530. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.P.; Ramirez, V.A. Global Population Genetics and Diversity in the TAS2R Bitter Taste Receptor Family. Front. Genet. 2022, 13, 952299. [Google Scholar] [CrossRef]
- Bufe, B.; Breslin, P.A.S.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.-K.; Drayna, D.; Meyerhof, W. The Molecular Basis of Individual Differences in Phenylthiocarbamide and Propylthiouracil Bitterness Perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef]
- Kim, U.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional Cloning of the Human Quantitative Trait Locus Underlying Taste Sensitivity to Phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Age Modifies the Genotype-Phenotype Relationship for the Bitter Receptor TAS2R38. BMC Genet. 2010, 11, 60. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of Astringency: Structural Alteration of the Oral Mucosal Pellicle by Dietary Tannins and Protective Effect of BPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Canon, F.; Belloir, C.; Bourillot, E.; Brignot, H.; Briand, L.; Feron, G.; Lesniewska, E.; Nivet, C.; Septier, C.; Schwartz, M.; et al. Perspectives on Astringency Sensation: An Alternative Hypothesis on the Molecular Origin of Astringency. J. Agric. Food Chem. 2021, 69, 3822–3826. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency Is a Trigeminal Sensation That Involves the Activation of G Protein–Coupled Signaling by Phenolic Compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, C. An Overview of the Perception and Mitigation of Astringency Associated with Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Guerreiro, C.; Brandão, E.; de Jesus, M.; Gonçalves, L.; Pérez-Gregório, R.; Mateus, N.; de Freitas, V.; Soares, S. New Insights into the Oral Interactions of Different Families of Phenolic Compounds: Deepening the Astringency Mouthfeels. Food Chem. 2022, 375, 131642. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, Olfactory, and Food Texture Processing in the Brain, and the Control of Food Intake. Physiol. Behav. 2005, 85, 45–56. [Google Scholar] [CrossRef]
- Hufnagel, J.C.; Hofmann, T. Quantitative Reconstruction of the Nonvolatile Sensometabolome of a Red Wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef]
- Delompré, T.; Belloir, C.; Martin, C.; Salles, C.; Briand, L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients 2022, 14, 4141. [Google Scholar] [CrossRef]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus Lupulus L.) and Beer. Chem. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Bufe, B.; Hofmann, T.; Krautwurst, D.; Raguse, J.-D.; Meyerhof, W. The Human TAS2R16 Receptor Mediates Bitter Taste in Response to β-Glucopyranosides. Nat. Genet. 2002, 32, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Ottinger, H.; Hofmann, T. Characterization of an Intense Bitter-Tasting 1H,4H-Quinolizinium-7-Olate by Application of the Taste Dilution Analysis, a Novel Bioassay for the Screening and Identification of Taste-Active Compounds in Foods. J. Agric. Food Chem. 2001, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Aisala, H.; Manninen, H.; Laaksonen, T.; Linderborg, K.M.; Myoda, T.; Hopia, A.; Sandell, M. Linking Volatile and Non-Volatile Compounds to Sensory Profiles and Consumer Liking of Wild Edible Nordic Mushrooms. Food Chem. 2020, 304, 125403. [Google Scholar] [CrossRef] [PubMed]
- Troszyńska, A.; Estrella, I.; Lamparski, G.; Hernández, T.; Amarowicz, R.; Pegg, R.B. Relationship between the Sensory Quality of Lentil (Lens Culinaris) Sprouts and Their Phenolic Constituents. Food Res. Int. 2011, 44, 3195–3201. [Google Scholar] [CrossRef]
- Tuccillo, F.; Kantanen, K.; Wang, Y.; Martin Ramos Diaz, J.; Pulkkinen, M.; Edelmann, M.; Knaapila, A.; Jouppila, K.; Piironen, V.; Lampi, A.-M.; et al. The Flavor of Faba Bean Ingredients and Extrudates: Chemical and Sensory Properties. Food Res. Int. 2022, 162, 112036. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Akerboom, J.; Chen, T.-W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderón, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Tan, S.M.; Seetoh, W.-G. Construction of a Bioluminescence-Based Assay for Bitter Taste Receptors (TAS2Rs). Sci. Rep. 2022, 12, 17658. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the Bitterness of Green Tea Catechins by a Cell-Based Assay with the Human Bitter Taste Receptor HTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef]
- Yamazaki, T.; Sagisaka, M.; Ikeda, R.; Nakamura, T.; Matsuda, N.; Ishii, T.; Nakayama, T.; Watanabe, T. The Human Bitter Taste Receptor HTAS2R39 Is the Primary Receptor for the Bitterness of Theaflavins. Biosci. Biotechnol. Biochem. 2014, 78, 1753–1756. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Bitter Taste Receptor Research Comes of Age: From Characterization to Modulation of TAS2Rs. Semin. Cell Dev. Biol. 2013, 24, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Koga, C.C.; Becraft, A.R.; Lee, Y.; Lee, S.-Y. Taste Detection Thresholds of Resveratrol. J. Food Sci. 2015, 80, S2064–S2070. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–40. ISBN 9789811317453. [Google Scholar]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.-T.; Ko, J.-M.; Baek, I.-Y.; Lee, J.H. Rapid Characterisation and Comparison of Saponin Profiles in the Seeds of Korean Leguminous Species Using Ultra Performance Liquid Chromatography with Photodiode Array Detector and Electrospray Ionisation/Mass Spectrometry (UPLC–PDA–ESI/MS) Analysis. Food Chem. 2014, 146, 270–277. [Google Scholar] [CrossRef]
- Mekky, R.H.; Thabet, M.M.; Rodríguez-Pérez, C.; Elnaggar, D.M.Y.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Comparative Metabolite Profiling and Antioxidant Potentials of Seeds and Sprouts of Three Egyptian Cultivars of Vicia Faba L. Food Res. Int. 2020, 136, 109537. [Google Scholar] [CrossRef]
- Oomah, B.D.; Patras, A.; Rawson, A.; Singh, N.; Compos-Vega, R. 2-Chemistry of Pulses. In Pulse Foods; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 9–55. ISBN 978-0-12-382018-1. [Google Scholar]
- Krishnamurthy, P.; Tsukamoto, C.; Singh, R.J.; Lee, J.-D.; Kim, H.-S.; Yang, S.-H.; Chung, G. The Sg-6 Saponins, New Components in Wild Soybean (Glycine Soja Sieb. and Zucc.): Polymorphism, Geographical Distribution and Inheritance. Euphytica 2014, 198, 413–424. [Google Scholar] [CrossRef]
- Price, K.R.; Griffiths, N.M.; Curl, C.L.; Fenwick, G.R. Undesirable Sensory Properties of the Dried Pea (Pisum Sativum). The Rôle of Saponins. Food Chem. 1985, 17, 105–115. [Google Scholar] [CrossRef]
- Ikedo, S.; Shimoyamada, M.; Watanabe, K. Interaction between Bovine Serum Albumin and Saponin As Studied by Heat Stability and Protease Digestion. J. Agric. Food Chem. 1996, 44, 792–795. [Google Scholar] [CrossRef]
- Fenwick, D.E.; Oakenfull, D. Saponin Content of Food Plants and Some Prepared Foods. J. Sci. Food Agric. 1983, 34, 186–191. [Google Scholar] [CrossRef]
- Vernoud, V.; Lebeigle, L.; Munier, J.; Marais, J.; Sanchez, M.; Pertuit, D.; Rossin, N.; Darchy, B.; Aubert, G.; Le Signor, C.; et al. β-Amyrin Synthase1 Controls the Accumulation of the Major Saponins Present in Pea (Pisum Sativum). Plant Cell Physiol. 2021, 62, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Chitisankul, W.T.; Shimada, K.; Omizu, Y.; Uemoto, Y.; Varanyanond, W.; Tsukamoto, C. Mechanism of DDMP-Saponin Degradation and Maltol Production in Soymilk Preparation. LWT 2015, 64, 197–204. [Google Scholar] [CrossRef]
- Daveby, Y.D.; Åman, P.; Betz, J.M.; Musser, S.M. Effect of Storage and Extraction on Ratio of Soyasaponin I to 2,3-Dihydro-2,5-Dihydroxy-6-Methyl-4-Pyrone-Conjugated Soyasaponin I in Dehulled Peas (Pisum Sativum L). J. Sci. Food Agric. 1998, 78, 141–146. [Google Scholar] [CrossRef]
- Okubo, K.; Iijima, M.; Kobayashi, Y.; Yoshikoshi, M.; Uchida, T.; Kudou, S. Components Responsible for the Undesirable Taste of Soybean Seeds. Biosci. Biotechnol. Biochem. 1992, 56, 99–103. [Google Scholar] [CrossRef]
- Aldin, E.; Reitmeier, H.A.; Murphy, P. Bitterness of Soy Extracts Containing Isoflavones and Saponins. J. Food Sci. 2006, 71, S211–S215. [Google Scholar] [CrossRef]
- Donat, P.V.; Caprioli, G.; Conti, P.; Maggi, F.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Rapid Quantification of Soyasaponins I and Βg in Italian Lentils by High-Performance Liquid Chromatography (HPLC)–Tandem Mass Spectrometry (MS/MS). Food Anal. Methods 2014, 7, 1024–1031. [Google Scholar] [CrossRef]
- Heng, L. Flavour Aspects of Pea and Its Protein Preparations in Relation to Novel Protein Foods. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005. [Google Scholar]
- Heng, L.; Vincken, J.-P.; Hoppe, K.; van Koningsveld, G.A.; Decroos, K.; Gruppen, H.; van Boekel, M.A.J.S.; Voragen, A.G.J. Stability of Pea DDMP Saponin and the Mechanism of Its Decomposition. Food Chem. 2006, 99, 326–334. [Google Scholar] [CrossRef]
- Martínez Noguera, P.; Lantoine, J.; Roux, E.L.; Yang, S.; Jakobi, R.; Krause, S.; Saint-Eve, A.; Bonazzi, C.; Rega, B. Saponins from Pea Ingredients to Innovative Sponge Cakes and Their Association with Perceived Bitterness. Foods 2022, 11, 2919. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic Profiles of Ten Australian Faba Bean Varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef] [PubMed]
- Duc, G. Faba Bean (Vicia Faba L.). Field Crops Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Variability in the Distribution of Phenolic Compounds in Milled Fractions of Chickpea and Horse Gram: Evaluation of Their Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 8322–8330. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics Content and Antioxidant and Anti-Inflammatory Activities of Legume Fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef]
- Huang, C.J.; Zayas, J.F. Phenolic Acid Contributions to Taste Characteristics of Corn Germ Protein Flour Products. J. Food Sci. 1991, 56, 1308–1310. [Google Scholar] [CrossRef]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of Germination on Legume Phenolic Compounds and Their Antioxidant Activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Troszyńska, A.; Amarowicz, R.; Lamparski, G.; Wołejszo, A.; Baryłko-Pikielna, N. Investigation of Astringency of Extracts Obtained from Selected Tannins-Rich Legume Seeds. Food Qual. Prefer. 2006, 17, 31–35. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzon-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic Profiles and Their Contribution to the Antioxidant Activity of Selected Chickpea Genotypes from Mexico and ICRISAT Collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A Comprehensive Investigation of the Behaviour of Phenolic Compounds in Legumes during Domestic Cooking and in Vitro Digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef]
- Abu-Reidah, I.; Contreras, M.d.M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura Carretero, A. UHPLC-ESI-QTOF-MS Based Metabolic Profiling of Vicia Faba L. Fabaceae) Seeds as a Key Strategy for Characterization in Foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Parajuli, P.; Shin, J.Y.; Lee, J.; Lee, S.; Hong, Y.-S.; Park, Y.I.; Kim, J.S.; Sohng, J.K. Enzymatic Biosynthesis of Novel Resveratrol Glucoside and Glycoside Derivatives. Appl. Environ. Microbiol. 2014, 80, 7235–7243. [Google Scholar] [CrossRef] [PubMed]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols Composition and Antioxidant Potential during ‘Blaufränkisch’ Grape Maceration and Red Wine Maturation, and the Effects of Trans-Resveratrol Addition. Food Chem. Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef] [PubMed]
- Sahar, A.; ur Rahman, U.; Ishaq, A.; Munir, M.S.; Aadil, R.M. 12-Health-Promoting Perspectives of Fruit-Based Functional Energy Beverages. In Sports and Energy Drinks; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 399–439. ISBN 978-0-12-815851-7. [Google Scholar]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Troszyńska, A. Antioxidant Activity of Extract of Adzuki Bean and Its Fractions. J. Food Lipids 2008, 15, 119–136. [Google Scholar] [CrossRef]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of Cooking and Germination on Phenolic Composition and Dietary Fibre Fractions in Dark Beans (Phaseolus Vulgaris L.) and Lentils (Lens Culinaris L.). LWT 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Dueñas, M.; Martínez-Villaluenga, C.; Limón, R.I.; Peñas, E.; Frias, J. Effect of Germination and Elicitation on Phenolic Composition and Bioactivity of Kidney Beans. Food Res. Int. 2015, 70, 55–63. [Google Scholar] [CrossRef]
- Liu, R.; Cai, Z.; Xu, B. Characterization and Quantification of Flavonoids and Saponins in Adzuki Bean (Vigna Angularis L.) by HPLC–DAD–ESI–MSn Analysis. Chem. Cent. J. 2017, 11, 93. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the Astringent Taste Compounds in Black Tea Infusions by Combining Instrumental Analysis and Human Bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Bioactive Phenolic Compounds and Functional Properties of Dehydrated Bean Flours. Food Res. Int. 2011, 44, 774–780. [Google Scholar] [CrossRef]
- Sangsukiam, T.; Duangmal, K. Changes in Bioactive Compounds and Health-Promoting Activities in Adzuki Bean: Effect of Cooking Conditions and in Vitro Simulated Gastrointestinal Digestion. Food Res. Int. 2022, 157, 111371. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In Vitro Anti-Inflammatory Activity of Phenolic Rich Extracts from White and Red Common Beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant Activity of Faba Bean Extract and Fractions Thereof. JFB 2018, 2, 112–118. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Dueñas, M.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Antioxidant Activity of a Red Lentil Extract and Its Fractions. Int. J. Mol. Sci. 2009, 10, 5513–5527. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Hernández, T.; Estrella, I. Changes in the Content of Bioactive Polyphenolic Compounds of Lentils by the Action of Exogenous Enzymes. Effect on Their Antioxidant Activity. Food Chem. 2007, 101, 90–97. [Google Scholar] [CrossRef]
- Dueñas, M.; Estrella, I.; Hernández, T. Occurrence of Phenolic Compounds in the Seed Coat and the Cotyledon of Peas (Pisum Sativum L.). Eur. Food Res. Technol. 2004, 219, 116–123. [Google Scholar] [CrossRef]
- Valente, I.M.; Cabrita, A.R.J.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Maia, M.R.G. Unravelling the Phytonutrients and Antioxidant Properties of European Vicia Faba L. Seeds. Food Res. Int. 2019, 116, 888–896. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Free Radical-Scavenging Capacity, Antioxidant Activity, and Phenolic Composition of Green Lentil (Lens Culinaris). Food Chem. 2010, 121, 705–711. [Google Scholar] [CrossRef]
- Glendinning, J.I. Is the Bitter Rejection Response Always Adaptive? Physiol. Behav. 1994, 56, 1217–1227. [Google Scholar] [CrossRef]
- Mirali, M.; Ambrose, S.J.; Wood, S.A.; Vandenberg, A.; Purves, R.W. Development of a Fast Extraction Method and Optimization of Liquid Chromatography–Mass Spectrometry for the Analysis of Phenolic Compounds in Lentil Seed Coats. J. Chromatogr. B 2014, 969, 149–161. [Google Scholar] [CrossRef]
- Bohin, M.C.; Roland, W.S.U.; Gruppen, H.; Gouka, R.J.; van der Hijden, H.T.W.M.; Dekker, P.; Smit, G.; Vincken, J.-P. Evaluation of the Bitter-Masking Potential of Food Proteins for EGCG by a Cell-Based Human Bitter Taste Receptor Assay and Binding Studies. J. Agric. Food Chem. 2013, 61, 10010–10017. [Google Scholar] [CrossRef] [PubMed]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and Genistein Contents of Vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Yang, S.-E.; Lien, J.-C.; Tsai, C.-W.; Wu, C.-R. Therapeutic Potential and Mechanisms of Novel Simple O-Substituted Isoflavones against Cerebral Ischemia Reperfusion. Int. J. Mol. Sci. 2022, 23, 10394. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Characterization and Quantification of Saponins and Flavonoids in Sprouts, Seed Coats and Cotyledons of Germinated Black Beans. Food Chem. 2012, 134, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Sarni-Manchado, P.; Cheynier, V. Les Polyphénols en Agroalimentaire; Éditions Tec & Doc: Paris, France, 2006; ISBN 978-2-7430-0805-5. [Google Scholar]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of Phytochemicals and Antinutritional Factors in Commercial Protein-Rich Plant Products. Food Qual. Prefer 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and Astringency of Grape and Wine Polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Fischer, U.; Noble, A. The Effect of Ethanol, Catechin Concentration, and PH on Sourness and Bitterness of Wine. Am. J. Enol. Vitic. 1994, 45, 6–10. [Google Scholar] [CrossRef]
- Cristian, J.-M.; Rosalva, M.-E.; Anaberta, C.M.; Mercedes, M.; Mercedes, M.P.; Gloria, D.-O. Effect of Aqueous, Acid, and Alkaline Thermal Treatments on Antinutritional Factors Content and Protein Quality in Lupinus Campestris Seed Flour. J. Agric. Food Chem. 2010, 58, 1741–1745. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. Alkaloids in the Valorization of European Lupinus Spp. Seeds Crop. Ind. Crops Prod. 2017, 95, 286–295. [Google Scholar] [CrossRef]
- Frick, K.M.; Foley, R.C.; Kamphuis, L.G.; Siddique, K.H.M.; Garg, G.; Singh, K.B. Characterization of the Genetic Factors Affecting Quinolizidine Alkaloid Biosynthesis and Its Response to Abiotic Stress in Narrow-Leafed Lupin (Lupinus Angustifolius L.). Plant Cell Environ. 2018, 41, 2155–2168. [Google Scholar] [CrossRef]
- Sbihi, H.M.; Nehdi, I.A.; Tan, C.P.; Al-Resayes, S.I. Bitter and Sweet Lupin (Lupinus Albus L.) Seeds and Seed Oils: A Comparison Study of Their Compositions and Physicochemical Properties. Ind. Crops Prod. 2013, 49, 573–579. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Gautam, M.; Lampi, A.-M.; Ollilainen, V.; Stoddard, F.; Sontag-Strohm, T.; Salovaara, H.; Piironen, V. Determination of Vicine and Convicine from Faba Bean with an Optimized High-Performance Liquid Chromatographic Method. Food Res. Int. 2015, 76, 168–177. [Google Scholar] [CrossRef]
- Duc, G.; Marget, P.; Esnault, R.; Guen, J.L.; Bastianelli, D. Genetic Variability for Feeding Value of Faba Bean Seeds (Vicia Faba): Comparative Chemical Composition of Isogenics Involving Zero-Tannin and Zero-Vicine Genes. J. Agric. Sci. 1999, 133, 185–196. [Google Scholar] [CrossRef]
- Purves, R.W.; Khazaei, H.; Vandenberg, A. Quantification of Vicine and Convicine in Faba Bean Seeds Using Hydrophilic Interaction Liquid Chromatography. Food Chem. 2018, 240, 1137–1145. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Wink, M. Chemical Defense of Lupins. Mollusc-Repellent Properties of Quinolizidine Alkaloids. Z. Naturforsch. C J. Biosci. 1984, 39, 553–558. [Google Scholar] [CrossRef]
- Jiménez-Martínez, C.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Diminution of Quinolizidine Alkaloids, Oligosaccharides and Phenolic Compounds from Two Species of Lupinus and Soybean Seeds by the Effect of Rhizopus Oligosporus. J. Sci. Food Agric. 2007, 87, 1315–1322. [Google Scholar] [CrossRef]
- Abd Allah, M.A.; Foda, Y.H.; Abu Salem, F.M.; Abd Allah, Z.S. Treatments for Reducing Total Vicine in Egyptian Faba Bean (Giza 2 Variety). Plant Food Hum. Nutr. 1988, 38, 201–210. [Google Scholar] [CrossRef]
- Wang, Y.; Tuccillo, F.; Lampi, A.-M.; Knaapila, A.; Pulkkinen, M.; Kariluoto, S.; Coda, R.; Edelmann, M.; Jouppila, K.; Sandell, M.; et al. Flavor Challenges in Extruded Plant-Based Meat Alternatives: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2898–2929. [Google Scholar] [CrossRef]
- Kim, I.M.-R.; Kawamura, Y.; Lee, C.-H. Isolation and Identification of Bitter Peptides of Tryptic Hydrolysate of Soybean 11S Glycinin by Reverse-Phase High-Performance Liquid Chromatography. J. Food Sci. 2003, 68, 2416–2422. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Eisner, P. Soy Protein Hydrolysates Fermentation: Effect of Debittering and Degradation of Major Soy Allergens. LWT 2016, 71, 202–212. [Google Scholar] [CrossRef]
- Thomas-Danguin, T.; Barba, C.; Salles, C.; Guichard, E. Perception of Mixtures of Odorants and Tastants: Sensory and Analytical Points of View. In Flavour; John Wiley & Sons, Ltd: Chichester, West Sussex, UK, 2016; pp. 319–340. ISBN 978-1-118-92938-4. [Google Scholar]
- Thomas-Danguin, T.; Sinding, C.; Tournier, C.; Saint-Eve, A. 5-Multimodal Interactions. In Flavor From Food to Behaviors, Wellbeing and Health; Etiévant, P., Guichard, E., Salles, C., Voilley, A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing; Elsevier Ltd.: Duxford, UK, 2016; pp. 121–141. ISBN 978-0-08-100295-7. [Google Scholar]
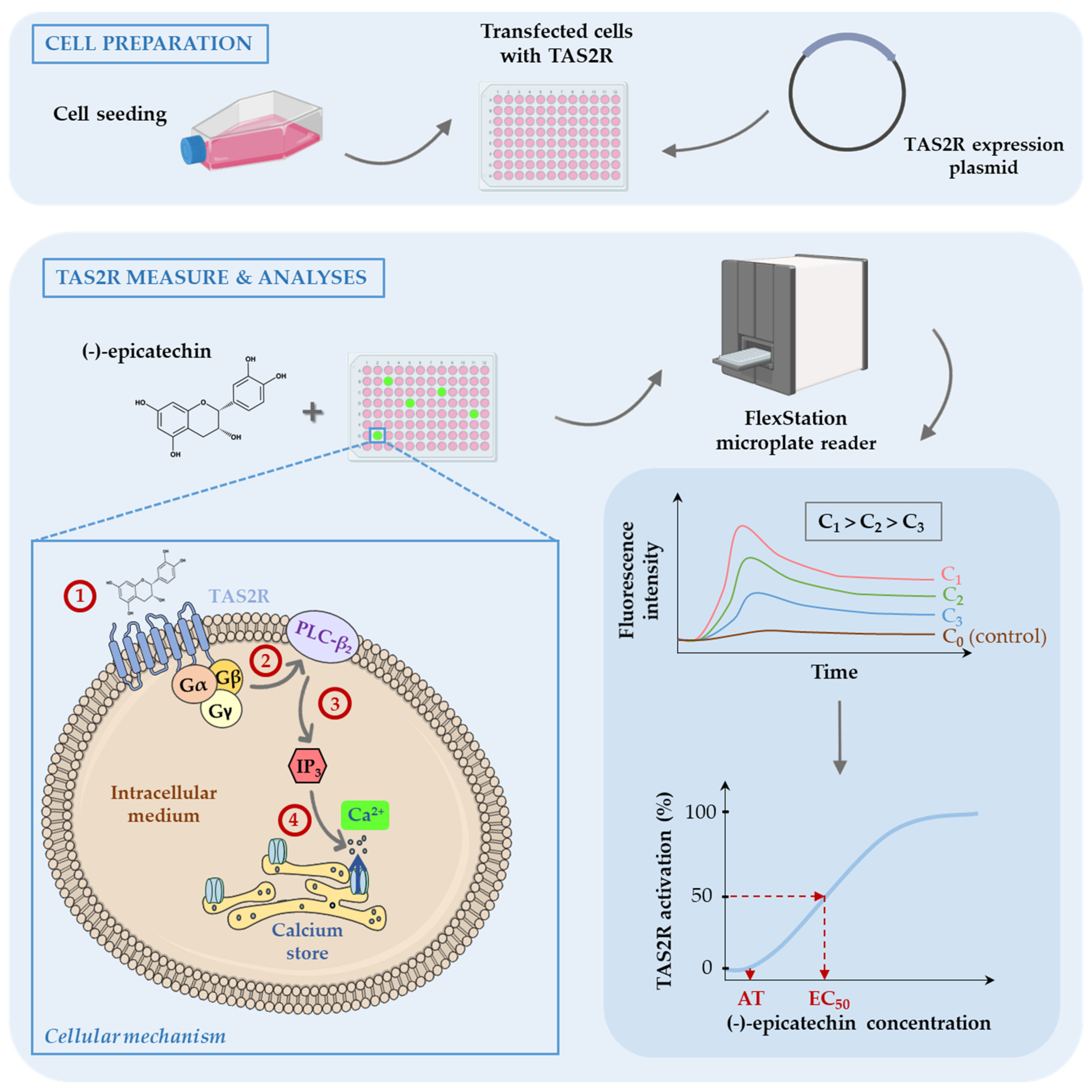


| Bitter Compounds | Human Bitter Characteristics | Cellular Bitter Characteristics | Ratio (Human/Cellular) | References | ||||
|---|---|---|---|---|---|---|---|---|
| DT (µM) | EC50 (µM) | TAS2R | AT (µM) | EC50 (µM) | Threshold | EC50 | ||
| phenyl-β-D-glucopyranose | 100 | 700 | TAS2R16 | 70 | 1100 | 1.4 | 0.6 | [53] |
| salicilin | 200 | 1100 | TAS2R16 | 70 | 1400 | 2.9 | 0.8 | [53] |
| helicin | 400 | 2200 | TAS2R16 | 300 | 2300 | 1.3 | 1.0 | [53] |
| arbutin | 900 | 5400 | TAS2R16 | 500 | 5800 | 1.8 | 0.9 | [53] |
| 2-nitrophenyl-β-D-glucopyranose | ND | - | TAS2R16 | 1500 | ND | NC | NC | [53] |
| naphthyl-β-D-glucopyranose | 200 | 1400 | TAS2R16 | 400 | 1000 | 0.5 | 1.4 | [53] |
| methyl-β-D-glucopyranose | 32,000 | 320,000 | TAS2R16 | 15,000 | ND | 2.1 | NC | [53] |
| amygdalin | ND | - | TAS2R16 | 2300 | 20,000 | NC | NC | [53] |
| esculin | 4000 | ND | TAS2R16 | 4000 | ND | 1 | NC | [53] |
| phenyl-β-D-galactopyranose | 40,000 | ND | TAS2R16 | ND | - | NC | NC | [53] |
| phenyl-α-D-glucopyranose | 9000 | 50,000 | TAS2R16 | ND | - | NC | NC | [53] |
| phenylthiocarbamide | PAV: 3.28 AVI: 1360 | - | TAS2R38-PAV TASR238-AVI | 0.02 ND | 1.1 - | NC-164 | NC | [40] |
| propylthiouracil | PAV: 10.7 AVI: 413 | - | TAS2R38-PAV TASR238-AVI | 0.06 ND | 2.1 - | NC-178.3 | NC | [40] |
| trans-isocohumulone | 19 | 300 | TAS2R1 TAS2R14 | 1 1 | 10.6 14.5 | 19 | 20.7–28.3 | [25] |
| trans-isohumulone | 20 | 200 | TAS2R1 | 0.3 1 | 9.0 11.2 | 20–66.7 | 17.8–22.2 | [25] |
| trans-isoadhumulone | 13 | 130 | TAS2R14 | 0.3 1 | 6.7 9.0 | 13–43.3 | 14.4–19.4 | [25] |
| cis-isocohumulone | 7 | 180 | TAS2R1 | 1 1 | 7.4 9.4 | 7 | 19.1–24.3 | [25] |
| cis-isohumulone | 10 | 110 | TAS2R14 | 0.3 0.3 | 3.3 2.6 | 33.3 | 33.3– 42.3 | [25] |
| cis-isoadhumulone | 8 | 100 | TAS2R1 | 0.3 1 | 2.5 2.8 | 8–26.7 | 35.7–40 | [25] |
| cohumulone | 17 | >500 | TAS2R1 TAS2R40 | 0.03 0.003 | 0.2 0.04 | 566.7–5667.7 | NC | [25] |
| humulone | 21 | ND | TAS2R1 TAS2R40 | 0.1 0.1 | 1.4 0.4 | 210 | NC | [25] |
| adhumulone | 21 | ND | TAS2R1 TAS2R40 | 0.1 0.03 | 0.7 0.2 | 210–700 | NC | [25] |
| colupulone | 39 | >500 | TAS2R1 TAS2R40 | 0.1 0.03 | 0.7 0.2 | 390–1300 | NC | [25] |
| lupulone | 35 | ND | TAS2R1 | 0.1 3 | 3.0 1.3 | 11.7–350 | NC | [25] |
| adlupulone | 37 | ND | TAS2R14 | 1 3 | 2.2 4.1 | 12.3–37 | NC | [25] |
| isoxanthohumol | 16 | >500 | TAS2R1 TAS2R14 TAS2R40 | 3 3 10 | ND ND ND | 1.6–5.3 | NC | [25] |
| xanthohumol | 10 | 140 | TAS2R1 TAS2R14 TAS2R40 | 1 3 3 | ND ND ND | 3.3–10 | NC | [25] |
| 8-prenylnaringenin | 8 | ND | TAS2R14 | 0.3 | 1.5 | 26.7 | NC | [25] |
| vitamin B1 | 1100 | - | TAS2R1 | 100 | - | 11.0 | - | [51] |
| vitamin B2 | 650 | - | - | - | - | - | - | [51] |
| vitamin B3 | 5500 | - | - | - | - | - | - | [51] |
| vitamin B6 | 5200 | - | TAS2R7 TAS2R14 | 1000 1000 | 25,520 10,520 | 5.2 | NC | [51] |
| vitamin A | ND | - | TAS2R38 | 50 | 290 | NC | NC | [51] |
| vitamin D | ND | - | TAS2R10 | 50 | 250 | NC | NC | [51] |
| resveratrol | 206 | - | TAS2R14 TAS2R39 | 16 63 | 30.3 109 | 3.3–12.9 | NC | [20,67] |
| (+)-catechin | 1000 | - | TAS2R14 TAS2R39 | 500 250 | ND | 2–4 | NC | [20,50] |
| (-)-epicatechin | 930 | - | TAS2R4 TAS2R5 TAS2R14 TAS2R39 | 2000 1000 500 250–1000 | >30,151 3210 500 418–3800 | 0.5–3.7 | NC | [20,50,62,63,64] |
| (-)-epigallocatechin gallate | 380 | - | TAS2R14 TAS2R39 | 250 32–100 | - | 1.5–11.9 | - | [20,63,64,66] |
| Pulses | Concentration (mg/g DM) | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Seed | Cotyledon | Hull | Embryonic Axe | ||||||||||
| PC | F | T | PC | F | T | PC | F | T | PC | F | T | ||
| Chickpeas | - | - | - | 15.2 | 7.5 | 5.2 | 75.9 | 12.6 | 32.4 | 46.1 | 9.3 | 11.4 | [90] |
| Faba beans | 39.69 | - | 6.85 | 39.17 | - | 7.22 | 22.30 | - | 16.23 | - | - | - | [91] |
| Lentils | 6.30 | - | 1.27 | 4.27 | - | 0.40 | 57.19 | - | 46.27 | - | - | - | [91] |
| Flavanols | TAS2R4 | TAS2R5 | TAS2R14 | TAS2R30 | TAS2R39 | TAS2R43 | Reference |
|---|---|---|---|---|---|---|---|
| (+)-catechin | + (500; ND) | + (250; ND) | [20] | ||||
| (-)-epicatechin | + (2000; ≥30,151) | + (1000; 3210) | + (500; ND) − (≤100) | − (≤100) | + (250; ND) + (1000; 3800) + (ND; 417.7) | − (≤100) | [20] [62] [63] |
| (-)-epigallocatechin | − (≤500) | + (500; ND) + (ND; 395.5) | [20] [63] | ||||
| (-)-epicatechin gallate | + (125; ND) + (ND; 70) | + (32; 151) + (ND; 21.3) + (ND; 88.2) | [20] [64] [63] | ||||
| (-)-epigallocatechin gallate | + (≤100; ND) | + (≤100; 12.30) | + (250; ND) − (≤100) + (ND; 34) | + (≤100; ND) | + (32; 161) + (≤100; 8.50) + (ND; 112) + (ND; 181.6) | + (≤100; 16.72) | [20] [66] [64] [63] |
| Theaflavin | − (ND) | + (ND; 2.79) | [64] |
| Legumes | Genistein (µg/kg DM) | Daidzein (µg/kg DM) |
|---|---|---|
| broad beans (raw) | 74 | 59 |
| chickpeas (dried, raw) | 475 | 766 |
| lentils (dried, raw) | 139 | 84 |
| red kidney beans (raw) | 191 | 209 |
| soybeans (dried, raw) | 583.103 | 838.103 |
| peas (dried, raw) | 41 | 144 |
| Procyanidins | TAS2R5 | TAS2R7 | TAS2R39 | Reference | |
|---|---|---|---|---|---|
| dimers | B1 | + (≤67; 119.34) | + (≤67; 123.95) | − (≤67) | [66] |
| B2 | − (≤67) | − (≤67) | − (≤67) | [66] | |
| B2g (3-O-gallate) | + (≤100; 6.29) | − (≤100) | + (≤100; 9.11) | [66] | |
| B3 | − (≤67) | − (≤67) | − (≤67) | [66] | |
| B4 | + (≤133; ND) | − (≤133) | − (≤133) | [66] | |
| trimers | C1 | − (≤150) | − (≤150) | − (≤150) | [66] |
| C2 | + (30.0; 35.6) | − (≤300) | − (≤300) | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karolkowski, A.; Belloir, C.; Briand, L.; Salles, C. Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses: A Review. Molecules 2023, 28, 3298. https://doi.org/10.3390/molecules28083298
Karolkowski A, Belloir C, Briand L, Salles C. Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses: A Review. Molecules. 2023; 28(8):3298. https://doi.org/10.3390/molecules28083298
Chicago/Turabian StyleKarolkowski, Adeline, Christine Belloir, Loïc Briand, and Christian Salles. 2023. "Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses: A Review" Molecules 28, no. 8: 3298. https://doi.org/10.3390/molecules28083298
APA StyleKarolkowski, A., Belloir, C., Briand, L., & Salles, C. (2023). Non-Volatile Compounds Involved in Bitterness and Astringency of Pulses: A Review. Molecules, 28(8), 3298. https://doi.org/10.3390/molecules28083298







