Acrylamide and 5-Hydroxymethylfurfural in Synthetic Sugar Cane Syrup: Mitigation by Additives
Abstract
1. Introduction
2. Results and Discussion
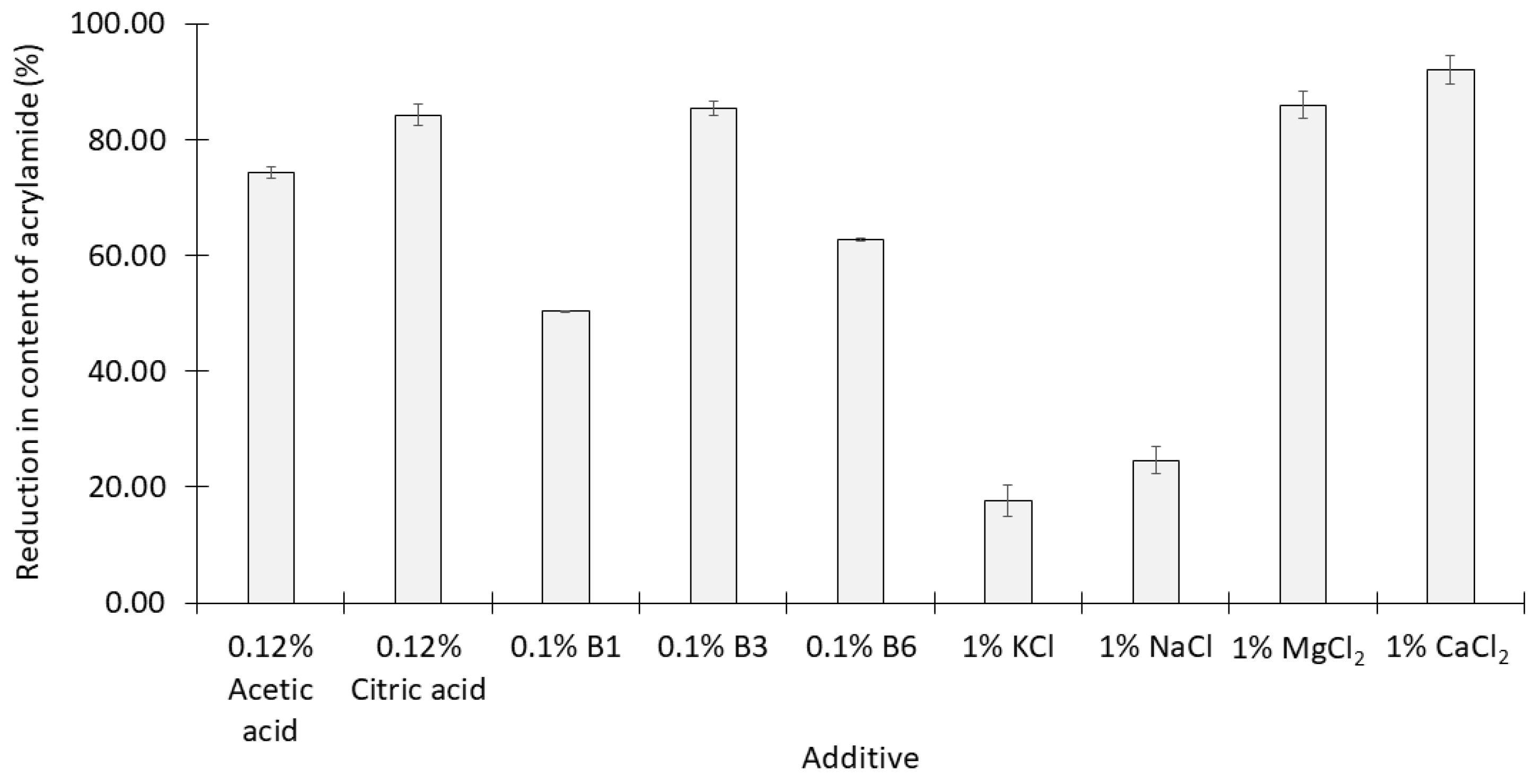
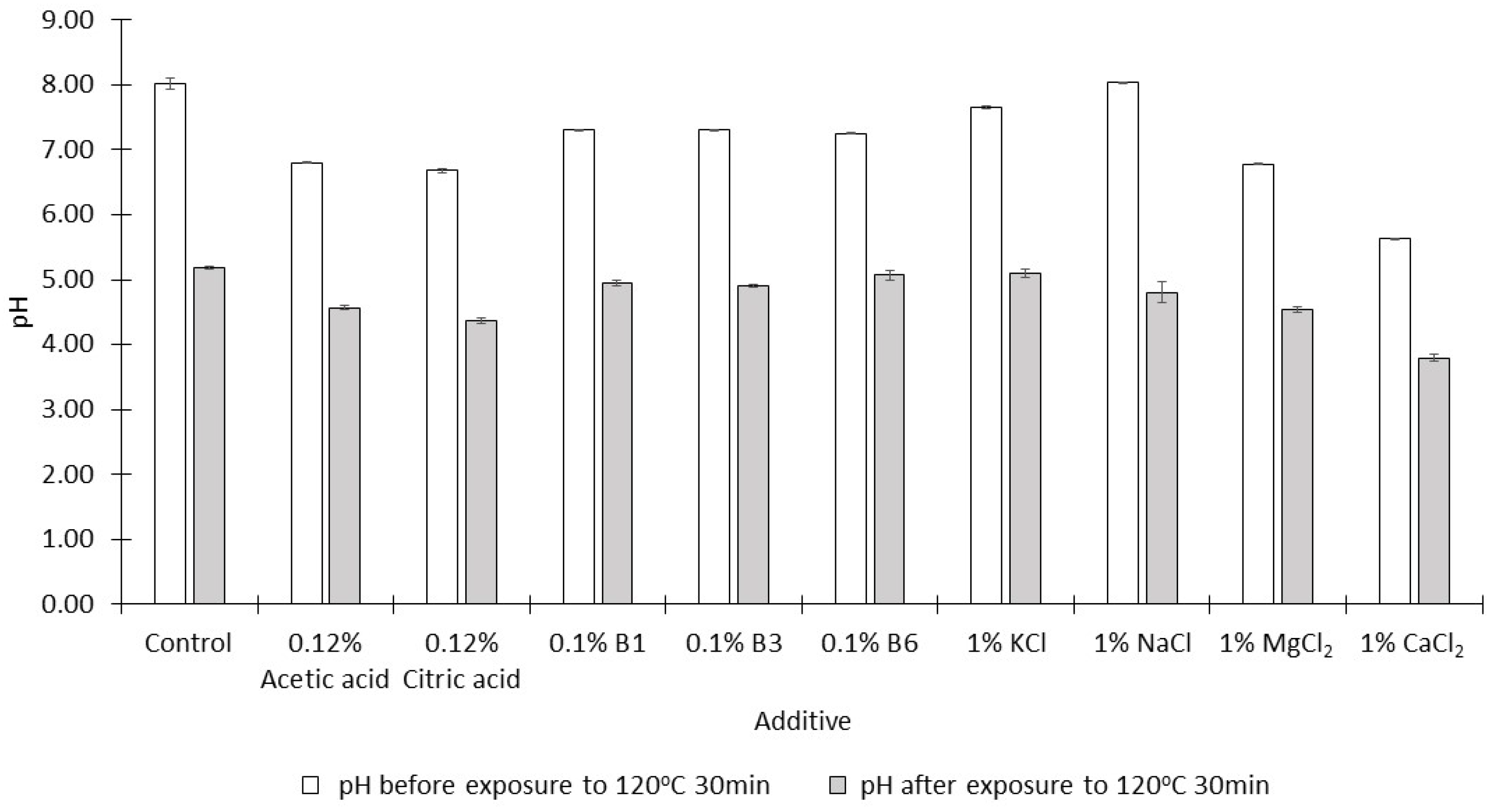
2.1. Effects of Various Single Acids, Salts, and B Vitamins on the Formation of Acrylamide
- The addition of 1% citric acid significantly reduced the acrylamide content of the syrup more effectively than 1% acetic acid (p < 0.05);
- Vitamin B3 was significantly more effective than the other two B vitamins at concentrations of 0.1% (p < 0.05);
- NaCl and KCl at concentrations of 1% were the least effective of all the additives examined while 1% CaCl2 was the most effective.
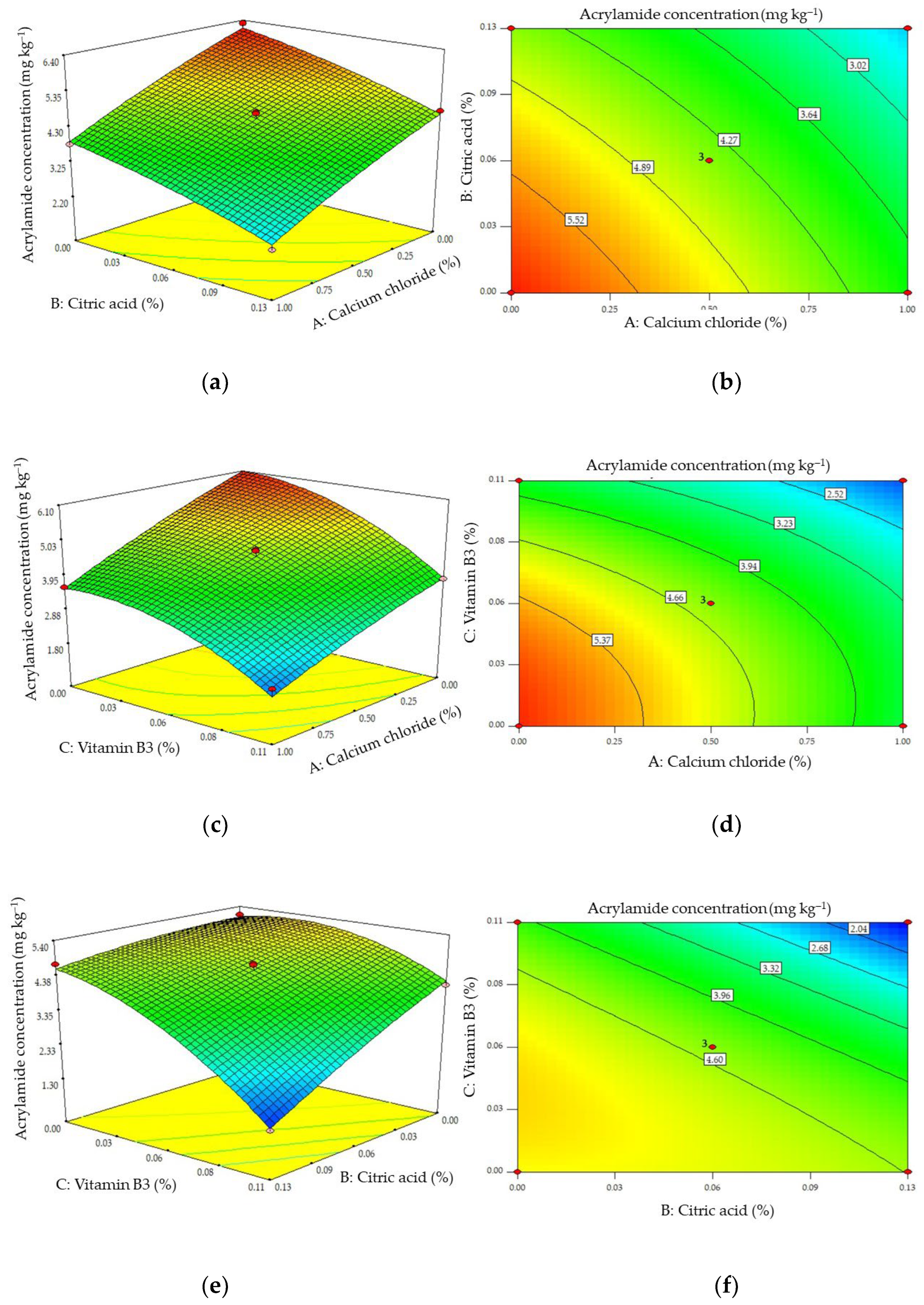
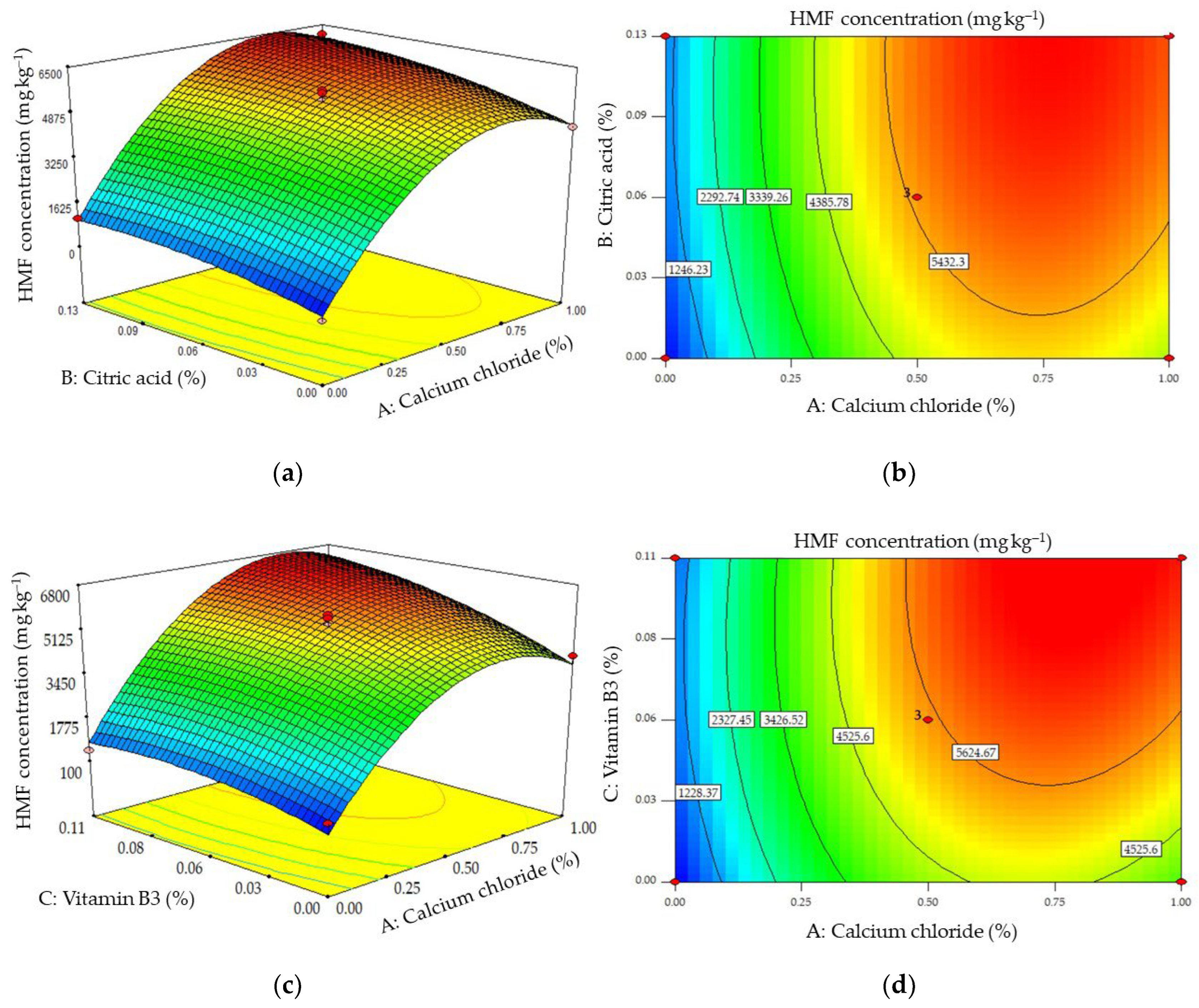
2.2. The Effects of Combinations of Additives on the Formation of Acrylamide and HMF
3. Materials and Methods
3.1. Materials
3.2. Preparation of Synthetic Thick Cane Juice
3.3. The Effects of Additives on Acrylamide Formation
3.4. Analyzing the Effects of Combined Additives: Response Surface Methodology
3.5. Analytical Methods
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Sachdev, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2020, 40, 545–561. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide Formation and Different Mitigation Strategies during Food Processing—A Review. Food Rev. Int. 2022, 38, 70–87. [Google Scholar] [CrossRef]
- Phaeon, N.; Chapanya, P.; Mueangmontri, R.; Pattamasuwan, A.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sriroth, K.; Nitayapat, N. Acrylamide in non-centrifugal sugars and syrups. J. Sci. Food Agric. 2021, 101, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, F.; Espitia, J.; Mendieta, O.; Escobar, S.; Rodríguez, J. Non-centrifugal cane sugar processing: A review on recent advances and the influence of process variables on qualities attributes of final products. J. Food Eng. 2019, 255, 32–40. [Google Scholar] [CrossRef]
- Augustine, D.; Bent, G.-A. Reducing Acrylamide Exposure: A Review of the Application of Sulfur-Containing Compounds—A Caribbean Outlook. Eur. J. Nutr. Food Saf. 2019, 9, 192–209. [Google Scholar] [CrossRef]
- Mestdagh, F.; Maertens, J.; Cucu, T.; Delporte, K.; Van Peteghem, C.; De Meulenaer, B. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chem. 2008, 107, 26–31. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Gómez-Narváez, F.; Mesías, M.; Delgado-Andrade, C.; Contreras-Calderón, J.; Ubillús, F.; Cruz, G.; Morales, F.J. Occurrence of acrylamide and other heat-induced compounds in panela: Relationship with physicochemical and antioxidant parameters. Food Chem. 2019, 301, 125256. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C.; Gómez-Narváez, F.; Contreras-Calderón, J.; Morales, F.J. Formation of Acrylamide and other Heat-Induced Compounds during Panela Production. Foods 2020, 9, 531. [Google Scholar] [CrossRef]
- Vargas Lasso, J.J.; Talero Pérez, Y.V.; Trujillo Suárez, F.A.; Camelo Caballero, L.R. Determinación de acrilamida en el procesamiento de la panela por cromatografía líquida. Cienc. En Desarro. 2014, 5, 99–105. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Lua, H.Y.; Naim, M.N.; Mohammed, M.A.P.; Hamidon, F.; Abu Bakar, N.F.; Vangnai, K.; Jittanit, W.; Teh, H.F. Inhibition of acrylamide formation in potato strip by ultrasonic-treated methylcellulose batter. Int. J. Food Sci. Technol. 2022, 57, 3292–3302. [Google Scholar] [CrossRef]
- Kolek, E.; Simko, P.; Simon, P.; Gatial, A. Confirmation of polymerisation effects of sodium chloride and its additives on acrylamide by infrared spectrometry. J. Food Nutr. Res. 2007, 46, 39–44. [Google Scholar]
- Zeng, X.; Cheng, K.-W.; Jiang, Y.; Lin, Z.-X.; Shi, J.-J.; Ou, S.-Y.; Chen, F.; Wang, M. Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem. 2009, 116, 34–39. [Google Scholar] [CrossRef]
- López-López, A.; Beato, V.M.; Sánchez, A.H.; García-García, P.; Montaño, A. Effects of selected amino acids and water-soluble vitamins on acrylamide formation in a ripe olive model system. J. Food Eng. 2014, 120, 9–16. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L. Influence Factors on the Formation of Acrylamide in the Amino Acid/Sugar Chemical Model System. J. Food Nutr. Res. 2014, 2, 344–348. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, C.; Pei, K.; Cai, Y.; Zhang, G.; Hu, C.; Ou, S. Cysteine alone or in combination with glycine simultaneously reduced the contents of acrylamide and hydroxymethylfurfural. LWT Food Sci. Technol. 2015, 63, 275–280. [Google Scholar] [CrossRef]
- Khezerolou, A.; Alizadeh-Sani, M.; Zolfaghari Firouzsalari, N.; Ehsani, A. Formation, Properties, and Reduction Methods of Acrylamide in Foods: A Review Study. J. Nutr. Fasting Health 2018, 6, 52–59. [Google Scholar] [CrossRef]
- Elder, V.A.; Fulcher, J.G.; Leung, H.; Topor, M.G. Method for Reducing Acrylamide Formation in Thermally Processed Foods. U.S. Patent US20040058045A, 26 August 2004. [Google Scholar]
- Lindsay, R.C.; Jang, S. Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. Adv. Exp. Med. Biol. 2005, 561, 393–404. [Google Scholar] [CrossRef]
- Gökmen, V. Acrylamide formation is prevented by divalent cations during the Maillard reaction. Food Chem. 2007, 103, 196–203. [Google Scholar] [CrossRef]
- Andrews, L.S.; Godshall, M.A.; Moore, S. Sucrose Degradation Under Model Processing Conditions. J. Food Sci. 2002, 67, 1621–1624. [Google Scholar] [CrossRef]
- Jung, M.Y.; Choi, D.S.; Ju, J.W. A Novel Technique for Limitation of Acrylamide Formation in Fried and Baked Corn Chips and in French Fries. J. Food Sci. 2003, 68, 1287–1290. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-hydroxymethyl-2-furaldehyde formation in honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chen, K.-T.; Lin, J.-A.; Chen, Y.-T.; Chen, Y.-A.; Wu, J.-T.; Hsieh, C.-W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Risner, C.H.; Kiser, M.J.; Dube, M.F. An Aqueous High-Performance Liquid Chromatographic Procedure for the Determination of 5-Hydroxymethylfurfural in Honey and Other Sugar-containing Materials. J. Food Sci. 2006, 71, C179–C184. [Google Scholar] [CrossRef]
- Vázquez Araújo, L.; Verdú, A.; Miquel, A.; Burló, F.; Carbonell-Barrachina, A. Changes in physico-chemical properties, hydroxymethylfurfural and volatile compounds during concentration of honey and sugars in Alicante and Jijona Turrón. Eur. Food Res. Technol. 2007, 225, 757–767. [Google Scholar] [CrossRef]

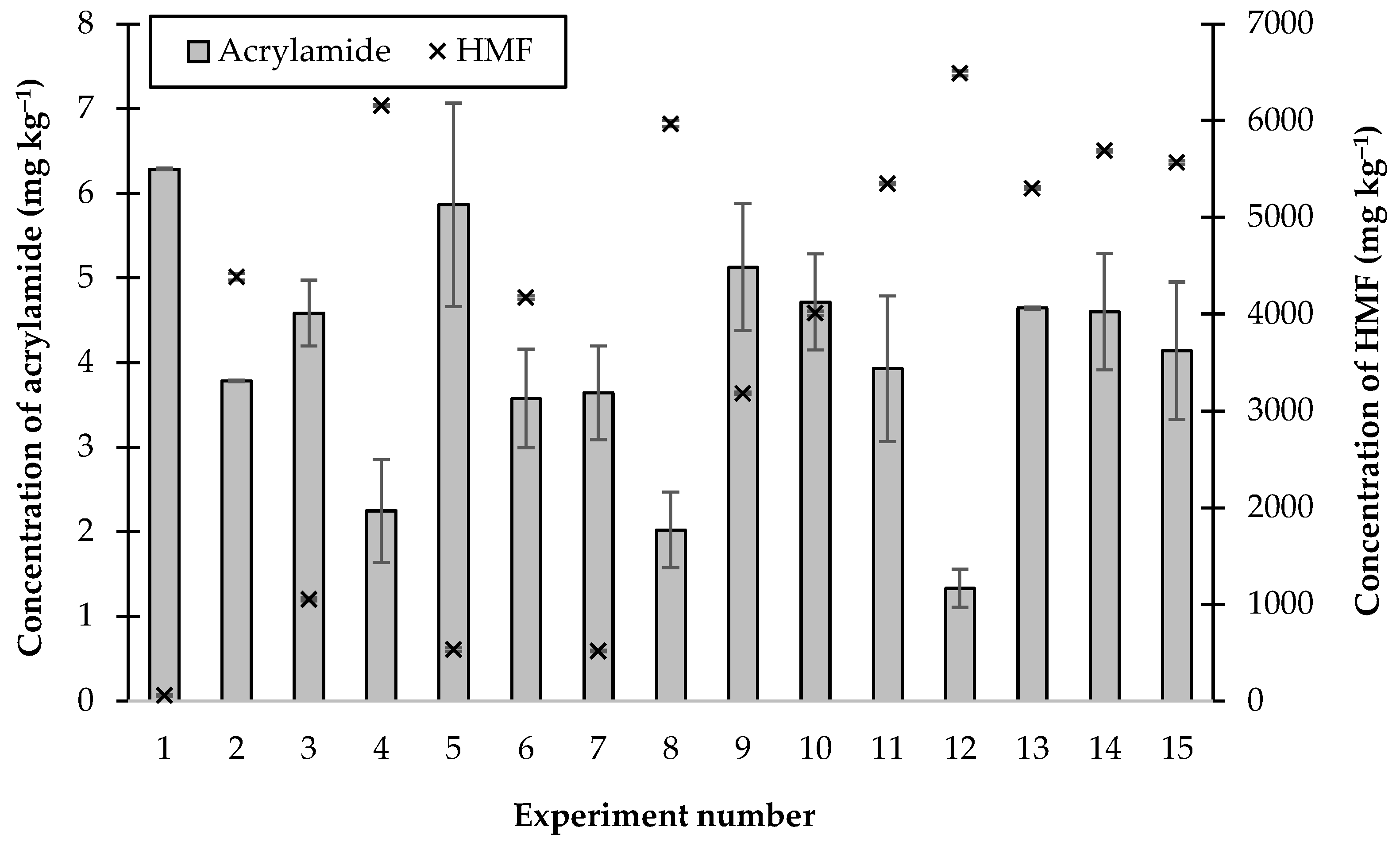
| Experiment Number | Additive Factors | ||
|---|---|---|---|
| [X1 Coding Level] CaCl2 (% w/w) | [X2 Coding Level] Citric Acid (% w/w) | [X3 Coding Level] Vitamin B3 (%w/w) | |
| 1 | [−1] 0 | [−1] 0 | [0] 0.0562 |
| 2 | [1] 1.0000 | [−1] 0 | [0] 0.0562 |
| 3 | [−1] 0 | [1] 0.1250 | [0] 0.0562 |
| 4 | [1] 1.0000 | [1] 0.1250 | [0] 0.0562 |
| 5 | [−1] 0 | [0] 0.0625 | [−1] 0 |
| 6 | [1] 1.0000 | [0] 0.0625 | [−1] 0 |
| 7 | [−1] 0 | [0] 0.0625 | [1] 0.1125 |
| 8 | [1] 1.0000 | [0] 0.0625 | [1] 0.1125 |
| 9 | [0] 0.5000 | [−1] 0 | [−1] 0 |
| 10 | [0] 0.5000 | [1] 0.1250 | [−1] 0 |
| 11 | [0] 0.5000 | [−1] 0 | [1] 0.1125 |
| 12 | [0] 0.5000 | [1] 0.1250 | [1] 0.1125 |
| 13 | [0] 0.5000 | [0] 0.0625 | [0] 0.0562 |
| 14 | [0] 0.5000 | [0] 0.0625 | [0] 0.0562 |
| 15 | [0] 0.5000 | [0] 0.0625 | [0] 0.0562 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model (Equation (1)) | 25.74 | 9 | 2.86 | 40.06 | 0.0004 |
| A—CaCl2 | 9.58 | 1 | 9.58 | 134.22 | <0.0001 |
| B—citric acid | 4.88 | 1 | 4.88 | 68.43 | 0.0004 |
| C—vitamin B3 | 8.73 | 1 | 8.73 | 122.37 | 0.0001 |
| AB | 7.014 × 10−3 | 1 | 7.014 × 10−3 | 0.098 | 0.7666 |
| AC | 0.11 | 1 | 0.11 | 1.57 | 0.2660 |
| BC | 1.19 | 1 | 1.19 | 16.69 | 0.0095 |
| A2 | 0.052 | 1 | 0.052 | 0.73 | 0.4333 |
| B2 | 0.051 | 1 | 0.051 | 0.72 | 0.4353 |
| C2 | 1.19 | 1 | 1.19 | 16.64 | 0.0096 |
| Residual | 0.36 | 5 | 0.071 | ||
| Lack of fit | 0.20 | 3 | 0.067 | 0.85 | 0.5796 |
| Pure error | 0.16 | 2 | 0.078 | ||
| Cor total | 26.09 | 14 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model (Equation (2)) | 7.143 × 107 | 8 | 8.929 × 106 | 43.77 | <0.0001 |
| A—CaCl2 | 4.292 × 107 | 1 | 4.292 × 107 | 210.39 | <0.0001 |
| B—citric acid | 2.800 × 106 | 1 | 2.800 × 106 | 13.72 | 0.0100 |
| C—vitamin B3 | 5.169 × 106 | 1 | 5.169 × 106 | 25.34 | 0.0024 |
| AB | 1.495 × 105 | 1 | 1.495 × 105 | 0.73 | 0.4248 |
| AC | 8.165 × 105 | 1 | 8.165 × 105 | 4.00 | 0.0924 |
| A2 | 1.925 × 107 | 1 | 1.925 × 107 | 94.37 | <0.0001 |
| B2 | 3.864 × 105 | 1 | 3.864 × 105 | 1.89 | 0.2179 |
| C2 | 7.153 × 105 | 1 | 7.153 × 105 | 3.51 | 0.1103 |
| Residual | 1.224 × 106 | 6 | 2.040 × 105 | ||
| Lack of fit | 1.146 × 106 | 4 | 2.864 × 105 | 7.30 | 0.1241 |
| Pure error | 78,490.83 | 2 | 39,245.42 | ||
| Cor total | 7.266 × 107 | 14 |
| Additive | Code | Coding Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| CaCl2 (% w/w) | X1 | 0 | 0.5000 | 1.0000 |
| Citric acid (% w/w) | X2 | 0 | 0.0625 | 0.1250 |
| Vitamin B3 (% w/w) | X3 | 0 | 0.0562 | 0.1125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phaeon, N.; Chapanya, P.; Pattamasuwan, A.; Issa-Issa, H.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sendra, E.; Sriroth, K.; Uan-on, T.; Nitayapat, N. Acrylamide and 5-Hydroxymethylfurfural in Synthetic Sugar Cane Syrup: Mitigation by Additives. Molecules 2023, 28, 3212. https://doi.org/10.3390/molecules28073212
Phaeon N, Chapanya P, Pattamasuwan A, Issa-Issa H, Lipan L, Carbonell-Barrachina ÁA, Sendra E, Sriroth K, Uan-on T, Nitayapat N. Acrylamide and 5-Hydroxymethylfurfural in Synthetic Sugar Cane Syrup: Mitigation by Additives. Molecules. 2023; 28(7):3212. https://doi.org/10.3390/molecules28073212
Chicago/Turabian StylePhaeon, Nuchnicha, Pisittinee Chapanya, Anutin Pattamasuwan, Hanán Issa-Issa, Leontina Lipan, Ángel Antonio Carbonell-Barrachina, Esther Sendra, Klanarong Sriroth, Tanat Uan-on, and Nuttakan Nitayapat. 2023. "Acrylamide and 5-Hydroxymethylfurfural in Synthetic Sugar Cane Syrup: Mitigation by Additives" Molecules 28, no. 7: 3212. https://doi.org/10.3390/molecules28073212
APA StylePhaeon, N., Chapanya, P., Pattamasuwan, A., Issa-Issa, H., Lipan, L., Carbonell-Barrachina, Á. A., Sendra, E., Sriroth, K., Uan-on, T., & Nitayapat, N. (2023). Acrylamide and 5-Hydroxymethylfurfural in Synthetic Sugar Cane Syrup: Mitigation by Additives. Molecules, 28(7), 3212. https://doi.org/10.3390/molecules28073212








