Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators
Abstract
1. Introduction
- -
- to conduct a comparative analysis of the patterns of the crystallization process of free water in samples of low-fat ice cream with a classic stabilization system and with β-glucan during low-temperature processing;
- -
- to investigate the dynamics of changes in the size of ice crystals in ice cream with various stabilizers during its storage;
- -
- to reveal the influence of β-glucan on the main physicochemical and organoleptic indicators of mixtures and ice cream.
2. Results and Discussion
2.1. Study of Physicochemical Indicators of Ice Cream
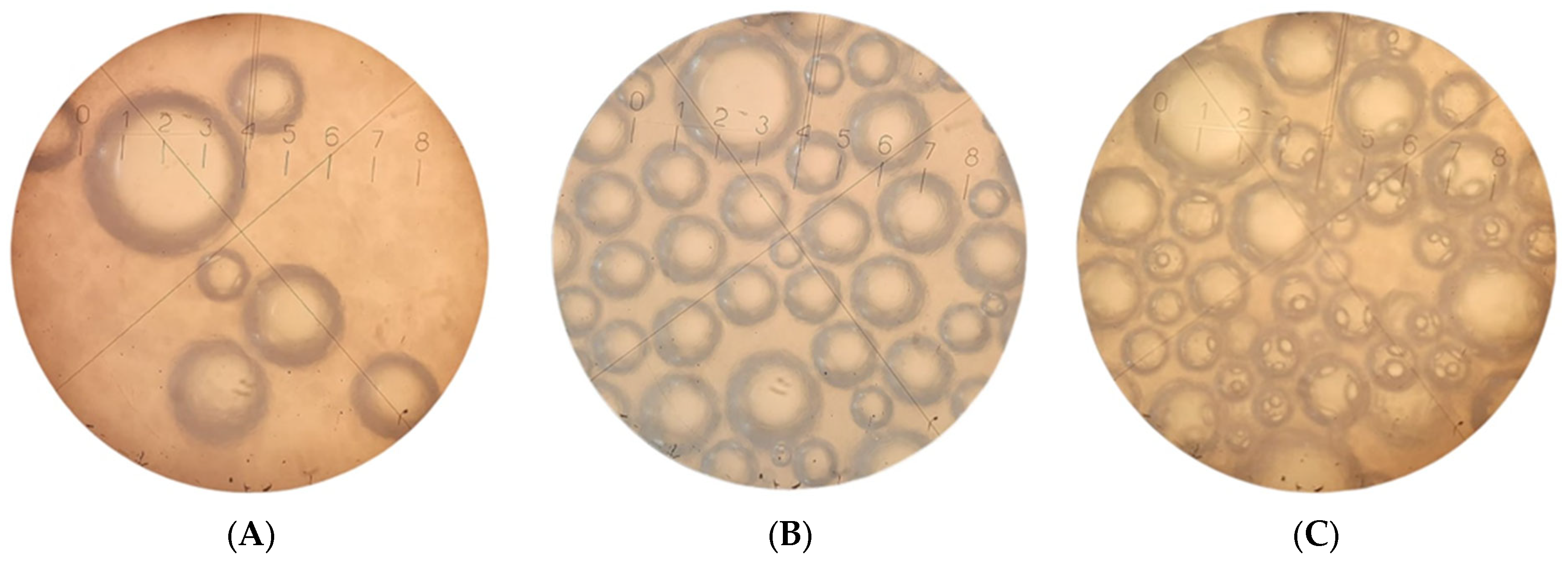
2.2. Analysis of the Microstructure of Low-Fat Ice Cream
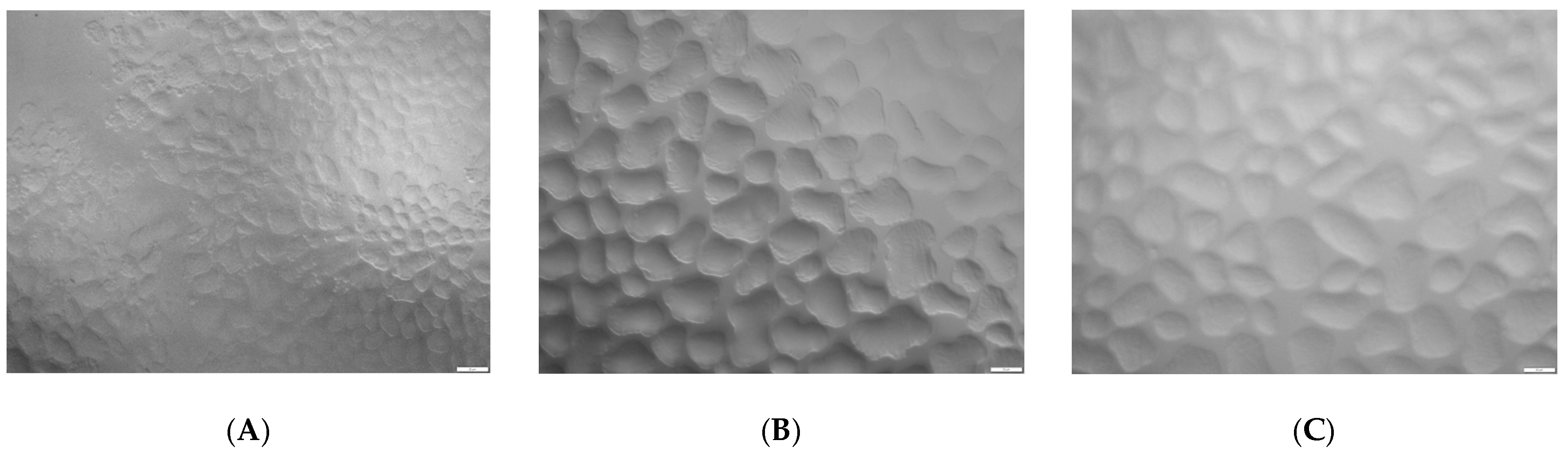
2.3. Microscopy Structure Analysis
2.4. Study of Cryoscopic Temperature and Content of Frozen Water
2.5. Sensory Evaluation
3. Materials and Methods
3.1. Materials
3.2. Ice Cream Production
- -
- at the first stage, the mix was cooled in a cooling cylinder (capacity—7 L) to a temperature of −1 °C at a rotation frequency of a scraper-type stirrer of 4.5 s−1 for 120 s;
- -
- at the second stage, the mix was frozen at a rotation frequency of 9 s−1 for 180 s to a temperature of −5.0 ± 0.5 °C.
3.3. Methods
3.3.1. Active Acidity
3.3.2. Viscosity
3.3.3. Microstructure
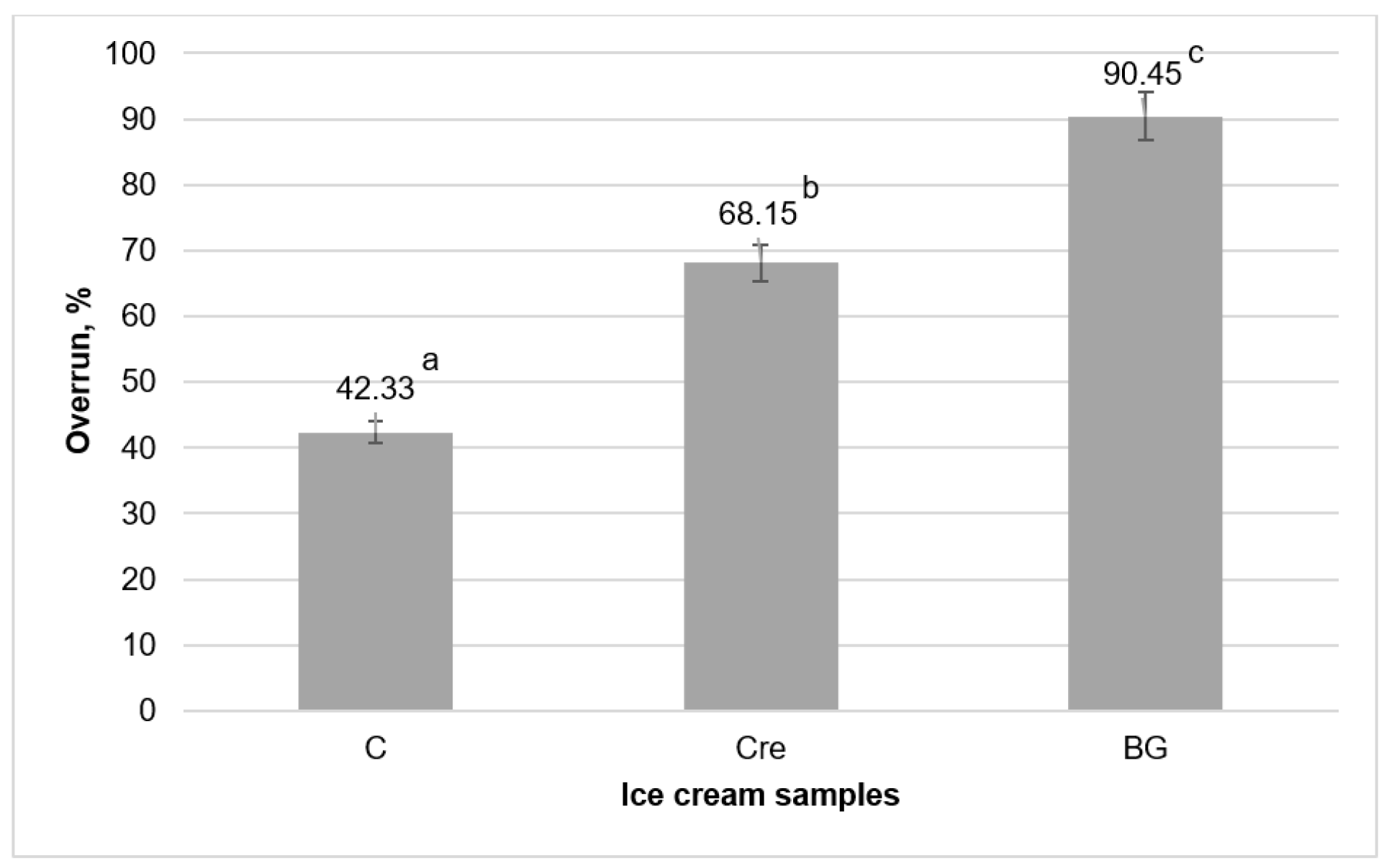
3.3.4. Overrun
of the glass with ice cream) × 100
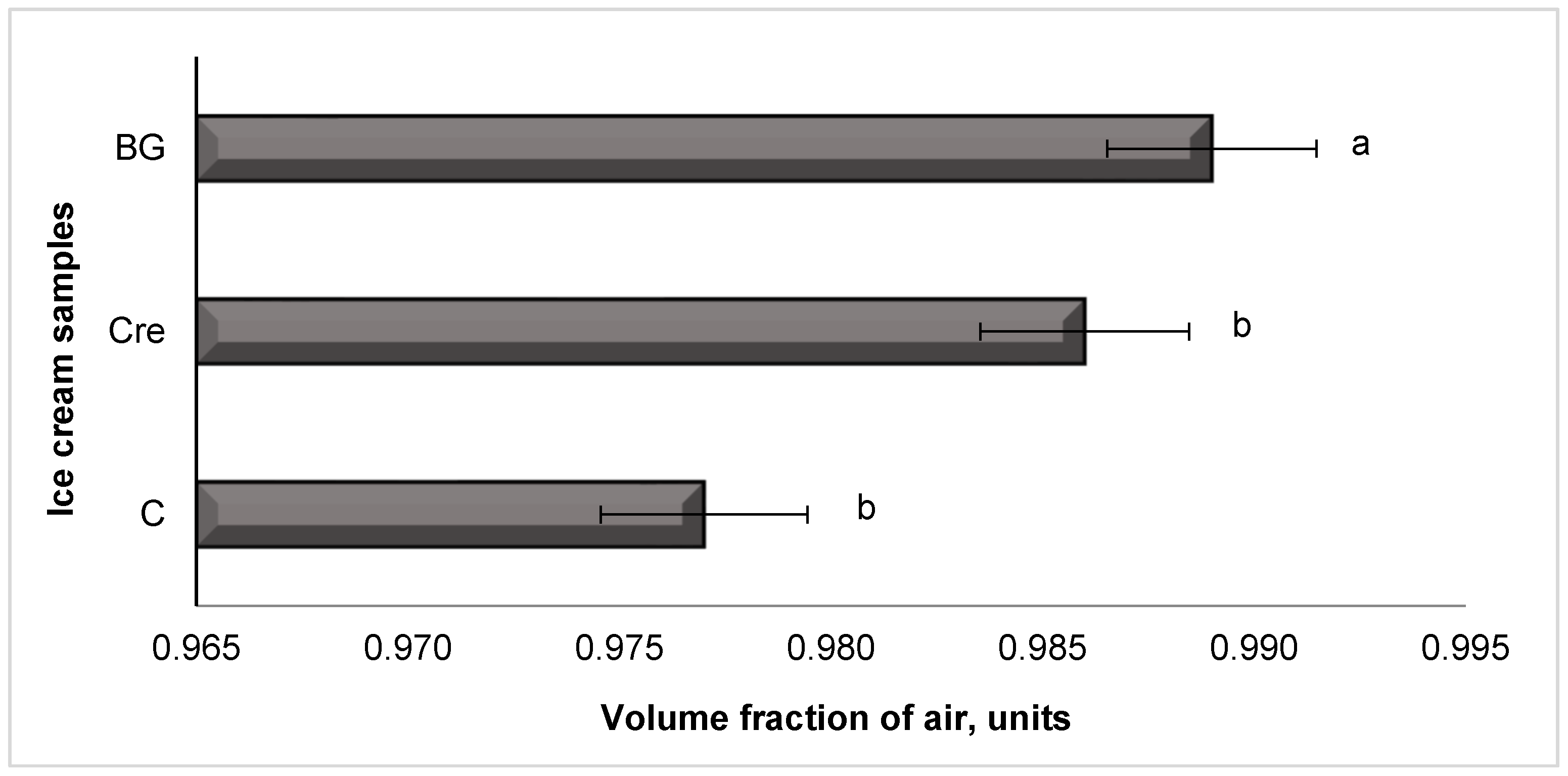
3.3.5. Volume Fraction of Air
3.3.6. Resistance to Melting
3.3.7. The Amount of Frozen Water
3.3.8. Microscopy Structure Analysis
3.3.9. Cryoscopic Temperature and Osmolality
3.3.10. Sensory Evaluation
3.3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ludvigsen, H.K. Application of emulsifiers in dairy and ice cream products. In Emulsifiers in Food Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 297–308. [Google Scholar] [CrossRef]
- Patel, M.R.; Baer, R.J.; Acharya, M.R. Increasing the protein content of ice cream. J. Dairy Sci. 2006, 89, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Palka, A.; Skotnicka, M. The Health-Promoting and Sensory Properties of Tropical Fruit Sorbets with Inulin. Molecules 2022, 27, 4239. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Ramana, K.V. Development of slow melting dietary fiber-enriched ice cream formulation using bacterial cellulose and inulin. J. Food Process. Preserv. 2022, 46, e15394. [Google Scholar] [CrossRef]
- Drewett, E.M.; Hartel, R.W. Ice crystallization in a scraped surface freezer. J. Food Eng. 2007, 78, 1060–1066. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A. Wpływ stabilizatorów na ograniczenie rekrystalizacji w lodach typu sorbet. Przemysł Spożywczy 2016, 1, 34–37. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Łaba, S.; Jakubczyk, E. The effects of selected stabilizers addition on physical properties and changes in crystal structure of whey ice cream. LWT 2022, 154, 112814. [Google Scholar] [CrossRef]
- Goff, H.D.; Hartel, R.W. Ice cream structure. In Ice Cream; Springer: Boston, MA, USA, 2013; pp. 313–352. [Google Scholar] [CrossRef]
- Gruneberg, A.K.; Graham, L.A.; Eves, R.; Agrawal, P.; Oleschuk, R.D.; Davies, P.L. Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology. 2021, 99, 28–39. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Antczak, A.; Samborska, K.; Lenart, A. Acid hydrolysis of kappa-carrageenan as a way of gaining new substances for freezing process modification and protection from excessive recrystallisation of ice. Int. J. Food Sci. 2015, 50, 1799–1806. [Google Scholar] [CrossRef]
- Jia, G.; Chen, Y.; Sun, A.; Orlien, V. Control of ice crystal nucleation and growth during the food freezing process. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2433–2454. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Song, Z.; Chen, X. A review of natural polysaccharides for food cryoprotection: Ice crystals inhibition and cryo-stabilization. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100291. [Google Scholar] [CrossRef]
- Cook, K.L.K.; Hartel, R.W. Mechanisms of ice crystallization in ice cream production. Compr. Rev. Food Sci. Food Saf. 2010, 9, 213–222. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, Y.J.; Chun, J.Y.; Hong, G.P.; Davaatseren, M.; Choi, M.J. Effect of frozen storage temperature on the quality of premium ice cream. Korean J. Food Sci. Anim. Resour. 2015, 35, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.J.; Schmidt, K.A. Ice cream: Foam formation and stabilization—A review. Food Rev. Int. 2010, 26, 122–137. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Janczewska-Dupczyk, A.; Kot, A.; Łaba, S.; Samborska, K. The impact of ι-and κ-carrageenan addition on freezing process and ice crystals structure of strawberry sorbet frozen by various methods. J. Food Sci. 2020, 85, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Osmak, T.; Mleko, S.; Bass, O.; Mykhalevych, A.; Kuzmyk, U. Enzymatic hydrolysis of lactose in concentrates of reconstituted demineralized whey, intended for ice cream production. Ukr. Food J. 2021, 10, 277–288. [Google Scholar] [CrossRef]
- Polischuk, G.; Bass, O.; Osmak, T.; Breus, N. Cryoprotective ability of starch syrup in the composition of aromatic and fruit-berry ice cream. Ukr. Food J. 2019, 8, 239–418. [Google Scholar] [CrossRef]
- Euston, S.R.; Goff, H.D. Emulsifiers in dairy products and dairy substitutes. In Food Emulsifiers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 217–254. [Google Scholar] [CrossRef]
- Barros, E.L.D.S.; Silva, C.C.; Canella, M.H.M.; Verruck, S.; Prestes, A.A.; Vargas, M.O.; Maran, B.M.; Esmerino, M.A.; Silva, R.; Balthazar, C.F.; et al. Effect of replacement of milk by block freeze concentrated whey in physicochemical and rheological properties of ice cream. Food Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Burkus, Z.; Temelli, F. Stabilization of emulsions and foams using barley β-glucan. Food Res. Int. 2000, 33, 27–33. [Google Scholar] [CrossRef]
- Nakov, G.; Jukić, M.; Šimić, G.; Šumanovac, F.; Komlenić, D.K.; Lukinac, J. Effect of the Addition of Hulless Barley Flour on the Quality of Short-Dough Cookies. Foods 2022, 11, 2428. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent advances of cereal β-glucan on immunity with gut microbiota regulation functions and its intelligent gelling application. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Jang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef] [PubMed]
- Yazar, G.; Rosell, C.M. Fat replacers in baked products: Their impact on rheological properties and final product quality. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Arsenyeva, T.P. Handbook of a dairy production technologist. In Ice Cream; GIORD: St. Petersburg, Russia, 2002; Volume 4, pp. 116–124. (In Russian) [Google Scholar]
- Sapiga, V.; Polischuk, G.; Buniowska, M.; Shevchenko, I.; Osmak, T. Polyfunctional properties of oat β-glucan in the composition of milk-vegetable ice cream. Ukr. Food J. 2021, 10, 691–706. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of barley non-starchy polysaccharides on selected quality attributes of sponge cakes. LWT-Food Sci. Technol. 2017, 85, 252–261. [Google Scholar] [CrossRef]
- Sulejmani, E.; Demiri, M. The effect of stevia, emulsifier and milk powder on melting rate, hardness and overrun of ice cream formulations during storage. Mljekarstvo 2020, 70, 120–130. [Google Scholar] [CrossRef]
- Specialized Equipment LLC. Available online: https://www.specializedequip.com/overrun (accessed on 10 January 2023).
- Ateteallah, H.A.; Abd-Alla, A.A.; Ateteallah, A.H.; Hassan, N.A. Physicochemical and Sensory Properties of Low-Fat Ice Cream Made with Inulin and Maltodextrin as Fat Replacers. J. Food Dairy Sci. 2020, 11, 151–156. [Google Scholar] [CrossRef]
- Goff, H.D. Formation and stabilisation of structure in ice-cream and related products. Curr. Opin. Colloid. Interface Sci. 2002, 7, 432–437. [Google Scholar] [CrossRef]
- Aljewicz, M.; Florczuk, A.; Dabrowska, A. Influence of β-glucan structures and contents on the functional properties of low-fat ice cream during storage. Pol. J. Food Nutr. Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.; Awad, R.A. Some quality attributes of low fat ice cream substituted with hulless barley flour and barley ß-glucan. J. Food Sci. Technol. 2015, 52, 6425–6434. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, J.; Zhao, Q.; Zheng, J. Structure and characteristic of β-glucan in cereal: A review. J. Food Process. Preserv. 2015, 39, 3145–3153. [Google Scholar] [CrossRef]
- Voronin, G.L.; Ning, G.; Coupland, J.N.; Roberts, R.; Harte, F.M. Freezing kinetics and microstructure of ice cream from high-pressure-jet processing of ice cream mix. J. Dairy Sci. 2021, 104, 2843–2854. [Google Scholar] [CrossRef]
- Flores, A.; Goff, H. Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. J. Dairy Sci. 1999, 82, 1399–1407. [Google Scholar] [CrossRef]
- Lomolino, G.; Zannoni, S.; Zabara, A.; Da Lio, M.; De Iseppi, A. Ice recrystallisation and melting in ice cream with different proteins levels and subjected to thermal fluctuation. Int. Dairy J. 2020, 100, 104557. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva, H.L.; Celeguini, R.M.; Santos, R.; Pastore, G.M.; Junior, C.A.; Cruz, A.G. Effect of galactooligosaccharide addition on the physical, optical, and sensory acceptance of vanilla ice cream. J. Dairy Sci. 2015, 98, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.; Gonzalez, J.E.; Alvarez, G.; Benkhelifa, H.; Flick, D.; Leducq, D. Online ice crystal size measurements by the focused beam reflectance method (FBRM) during sorbet freezing. Procedia Food Sci. 2011, 1, 1256–1264. [Google Scholar] [CrossRef]
- Góral, M.; Kozłowicz, K.; Pankiewicz, U.; Góral, D.; Kluza, F.; Wójtowicz, A. Impact of stabilizers on the freezing process, and physicochemical and organoleptic properties of coconut milk-based ice cream. LWT 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Kot, A.; Kamińska-Dwórznicka, A.; Antczak, A.; Jakubczyk, E.; Matwijczuk, A. Effect of ι-carrageenan and its acidic and enzymatic hydrolysates on ice crystal structure changes in model sucrose solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128744. [Google Scholar] [CrossRef]
- Muzammil, H.S.; Rasco, B.; Sablani, S. Effect of inulin and glycerol supplementation on physicochemical properties of probiotic frozen yogurt. Food Nutr. Res. 2017, 61, 1290314. [Google Scholar] [CrossRef]
- Sei, T.; Gonda, T.; Arima, Y. Growth rate and morphology of ice crystals growing in a solution of trehalose and water. J. Cryst. Growth. 2002, 240, 218–229. [Google Scholar] [CrossRef]
- Whelan, A.P.; Regand, A.; Vega, C.; Kerry, J.P.; Goff, H.D. Effect of trehalose on the glass transition and ice crystal growth in ice cream. Int. J. Food Sci. 2008, 43, 510–516. [Google Scholar] [CrossRef]
- Kaleda, A.; Tsanev, R.; Klesment, T.; Vilu, R.; Laos, K. Ice cream structure modification by ice-binding proteins. Food Chem. 2018, 246, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Marella, C.; Muthukumarappan, K. Food freezing technology. In Handbook of Farm, Dairy and Food Machinery Engineering; Academic Press: Cambridge, MA, USA, 2013; pp. 355–378. [Google Scholar] [CrossRef]
- Herrera, M.L.; M’Cann, J.I.; Ferrero, C.; Hagiwara, T.; Zaritzky, N.E.; Hartel, R.W. Thermal, mechanical, and molecular relaxation properties of frozen sucrose and fructose solutions containing hydrocolloids. Food Biophys. 2007, 2, 20–28. [Google Scholar] [CrossRef]
- Buyong, N.; Fennema, O. Amount and size of ice crystals in frozen samples as influenced by hydrocolloids. J. Dairy Sci. 1998, 71, 2630–2639. [Google Scholar] [CrossRef]
- Marshall, R.T.; Goff, H.D.; Hartel, R.W. The ice cream industry. In Ice Cream; Springer: Boston, MA, USA, 2003. [Google Scholar] [CrossRef]
- Wildmoser, H.; Scheiwiller, J.; Windhab, E.J. Impact of disperse microstructure on rheology and quality aspects of ice cream. LWT-Food Sci. Technol. 2004, 37, 881–891. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, rheological and chemical characteristics during storage of set type full fat yoghurt fortified with barley β-glucan. J. Food Sci. Technol. 2020, 57, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef]
- Qu, X.; Nazarenko, Y.; Yang, W.; Nie, Y.; Zhang, Y.; Li, B. Effect of Oat β-Glucan on the Rheological Characteristics and Microstructure of Set-Type Yogurt. Molecules 2021, 26, 4752. [Google Scholar] [CrossRef]
- Wu, J.; Deng, X.; Tian, B.; Wang, L.; Xie, B. Interactions between oat β-glucan and calcofluor characterized by spectroscopic method. J. Agric. Food Chem. 2008, 56, 1131–1137. [Google Scholar] [CrossRef]
- Yangılar, F. Production and evaluation of mineral and nutrient contents, chemical composition, and sensory properties of ice creams fortified with laboratory-prepared peach fibre. Food Nutr. Res. 2016, 60, 31882. [Google Scholar] [CrossRef]
- Fabro, M.A.; Milanesio, H.V.; Robert, L.M.; Speranza, J.L.; Murphy, M.; Rodríguez, G.; Castañeda, R. Determination of acidity in whole raw milk: Comparison of results obtained by two different analytical methods. J. Dairy Sci. 2006, 89, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Hegedušić, V.; Rimac, S.; Herceg, Z.; Škreblin, M. Influence of stabilizers addition on the rheological properties of the model ice cream mixtures. Mljekarstvo 1998, 48, 87–96. [Google Scholar]
- Mykhalevych, A.; Sapiga, V.; Polischuk, G.; Osmak, T. Functional and technological properties of oat beta-glucan in acidophilic-whey ice cream. Food Environ. Saf. 2022, 21, 116–128. [Google Scholar] [CrossRef]
- Sapiga, V.; Polischuk, G.; Osmak, T.; Mykhalevych, A.; Maslikov, M. Scientific explanation of the composition and technological modes of manufacture of dairy ice cream with vegetable puree. Ukr. J. Food Sci. 2019, 7, 83–91. [Google Scholar] [CrossRef]
- Daw, E.; Hartel, R.W. Fat destabilization and melt-down of ice creams with increased protein content. Int. Dairy J. 2015, 43, 33–41. [Google Scholar] [CrossRef]
- Polischuk, G.; Kochubey-Lytvynenko, O.; Osmak, T.; Kuzmik, U.; Bass, O.; Mykhalevych, A.; Sapiga, V. Scientific explanation of composition of acidophilic-whey ice cream, enriched with protein. Food Environ. Saf. 2021, 20, 13–20. [Google Scholar] [CrossRef]
- Polishchuk, G.E.; Gudz, I.S. Ice Cream Technology; Firma Inkos: Kyiv, Ukraine, 2008; p. 44. (In Ukrainian) [Google Scholar]
- Polischuk, G.; Sharahmatova, T.; Breus, N.; Bass, O.; Shevchenko, I. Studies of water freezing features in ice cream with starch syrup. Food Sci. Technol. 2019, 13, 71–77. [Google Scholar] [CrossRef]
- Kharenko, E.N.; Arkhipov, L.O.; Yarychevskaya, N.N. Establishing the functional dependence of the amount of frozen water on the individual cryoscopic temperatures of fish. Trudy VNYRO. 2019, 176, 81–94. (In Russian) [Google Scholar] [CrossRef]
- Dibirasulaev, M.A.; Belozerov, G.A.; Arkhipov, L.O.; Dibirasulaev, D.M.; Donetskikh, A.G. Towards the development of scientifically based modes of refrigeration storage of meat of various quality groups at subcryoscopic temperatures. Birds Poult. Prod. 2017, 1, 29–32. (In Russian) [Google Scholar]
- Kamińska-Dwórznicka, A.; Matusiak, M.; Samborska, K.; Witrowa-Rajchert, D.; Gondek, E.; Jakubczyk, E.; Antczak, A. The influence of kappa carrageenan and its hydrolysates on the recrystallization process in sorbet. J. Food Eng. 2015, 167, 162–165. [Google Scholar] [CrossRef]
- Cherevychna, N.; Haponceva, O. Modern white quality assessment systems of wine. Young Sci. J. 2019, 5, 281–286. [Google Scholar] [CrossRef]
- Shabanova, I.A.; Kh, T.L.; Tsugkieva, V.B.; Doev, D.N.; Datieva, B.A. The effect of beet fiber on the evaluation of the quality of gingerbread. Int. J. Res. 2022, 2, 180–186. [Google Scholar] [CrossRef]
- Shibani, F.; Asadollahi, S.; Eshaghi, M. The effect of beta-glucan as a fat substitute on the sensory and physico-chemical properties of low-fat ice cream. J. Food Saf. Process. 2021, 1, 71–84. [Google Scholar]
- Şimşek, B.; İlhan, G.Ü.N. Some physicochemical, rheological and sensory properties of flavored ice cream. Ömer Halisdemir Üniv. Mühendis. Bilim. Derg. 2021, 10, 598–605. [Google Scholar] [CrossRef]



| Sample | pH | Titrated Acidity, °T | Coefficient of Dynamic Viscosity, mPa·s |
|---|---|---|---|
| C | 6.62 ± 0.01 c | 19.0 ± 0.1 a | 135.5 ± 1.7 a |
| Cre | 6.54 ± 0.05 b | 20.0 ± 0.1 b | 154.5 ± 1.9 b |
| BG | 6.39 ± 0.02 a | 22.5 ± 0.5 c | 202.4 ± 3.5 c |
| Sample | Time of Storage | Minimal Size of Ice Crystals (µm) | MaximalSize of Ice Crystals (µm) | Average Diameter DA in the Class with the Highest Frequency (µm) |
|---|---|---|---|---|
| C | 24 h C 1 W EC 1 M F | 7.31 ± 0.20 d 11.43 ± 0.48 e 13.62 ± 0.18 f | 23.56 ± 1.15 c 30.91 ± 1.27 e 34.75 ± 1.89 f | 15.57 ± 3.04 b 20.19 ± 2.96 c 22.32 ± 2.28 c |
| Cre | 24 h B 1 W C 1 M D | 6.65 ± 0.31 c 6.77 ± 0.24 c 7.66 ± 0.17 d | 18.35 ± 0.54 b 26.84 ± 1.01 d 35.46 ± 1.45 f | 10.54 ± 1.42 a 15.67 ± 2.07 b 17.69 ± 1.98 bc |
| BG | 24 h A 1 W AB 1 M B | 1.42 ± 0.08 a 1.54 ± 0.05 a 2.13 ± 0.07 b | 14.53 ± 0.66 a 18.33 ± 0.54 b 19.36 ± 0.87 b | 10.15 ± 1.42 a 12.62 ± 1.70 a 12.96 ± 1.92 a |
| Sample | Cryoscopic Temperature, °C | Osmotic Pressure, mOsm/kg |
|---|---|---|
| C | −2.058 ± 0.01 a | 1108 ± 0.01 a |
| Cre | −2.136 ± 0.02 b | 1150 ± 0.01 b |
| BG | −2.224 ± 0.01 c | 1197 ± 0.01 c |
| Processing Temperature, °C | Amount of Frozen Water, % | ||
|---|---|---|---|
| C | Cre | BG | |
| −5 | 58.84 ± 0.25 b | 57.28 ± 1.87 ab | 55.52 ± 1.52 a |
| −10 | 79.42 ± 1.12 a | 78.64 ± 2.36 a | 77.76 ± 1.69 a |
| −15 | 86.28 ± 2.08 a | 85.76 ± 2.87 a | 85.17 ± 2.58 a |
| −20 | 89.71 ± 1.54 a | 89.32 ± 1.49 a | 88.88 ± 2.79 a |
| −25 | 91.77 ± 1.65 a | 91.46 ± 0.98 a | 91.1 ± 1.55 a |
| −30 | 93.14 ± 1.88 a | 92.88 ± 1.75 a | 92.59 ± 2.01 a |
| −35 | 94.12 ± 1.13 a | 93.90 ± 1.07 a | 93.65 ± 1.12 a |
| −40 | 94.86 ± 0.89 a | 94.66 ± 1.73 a | 94.44 ± 0.56 a |
| Descriptor | Overall Score, Points | ||
|---|---|---|---|
| C | Cre | BG | |
| Criterion 1. Appearance | |||
| Low-dispersed air bubbles | 4.51 a ± 0.11 | 4.94 b ± 0.05 | 4.98 b ± 0.01 |
| Homogeneity of mass | 4.72 a ± 0.15 | 4.85 b ± 0.04 | 4.94 c ± 0.05 |
| Foaming | 3.84 a ± 0.21 | 4.45 b ± 0.22 | 4.80 c ± 0.17 |
| Small ice crystals | 3.21 a ± 0.14 | 4.09 b ± 0.17 | 4.52 c ± 0.18 |
| Form stability | 4.33 b ± 0.19 | 4.62 c ± 0.11 | 4.14 a ± 0.14 |
| Criterion 2. Smell and aroma | |||
| Sweet | 4.30 b ± 0.05 | 4.38 b ± 0.21 | 4.13 a ± 0.12 |
| Pleasant | 3.12 a ± 0.11 | 3.55 b ± 0.10 | 4.70 c ± 0.15 |
| Milky | 3.74 a ± 0.15 | 4.52 b ± 0.23 | 4.79 b ± 0.03 |
| Creamy | 2.25 a ± 0.10 | 3.07 b ± 0.09 | 4.14 c ± 0.21 |
| Absence of extraneous odors | 4.63 a ± 0.22 | 4.98 b ± 0.14 | 4.81 b ± 0.08 |
| Criterion 3. Color | |||
| White | 4.07 b ± 0.05 | 4.01 b ± 0.10 | 3.15 a ± 0.13 |
| With a yellow tint | 4.84 b ± 0.02 | 4.70 b ± 0.22 | 4.34 a ± 0.11 |
| Creamy | 4.76 b ± 0.21 | 4.67 b ± 0.02 | 4.59 a ± 0.21 |
| Intense | 4.07 a ± 0.15 | 4.04 a ± 0.21 | 4.82 b ± 0.09 |
| Homogeneous | 3.53 a ± 0.17 | 3.87 b ± 0.06 | 4.52 c ± 0.11 |
| Criterion 4. Consistency | |||
| Overrun | 3.58 a ± 0.01 | 3.97 b ± 0.10 | 4.64 c ± 0.22 |
| A mass that does not melt quickly | 2.90 a ± 0.09 | 4.06 b ± 0.21 | 4.56 c ± 0.18 |
| Without sandiness | 2.92 a ± 0.05 | 3.24 b ± 0.11 | 4.01 c ± 0.13 |
| Homogeneous | 2.88 a ± 0.15 | 3.03 b ± 0.11 | 3.80 c ± 0.15 |
| Small ice crystals | 3.43 a ± 0.02 | 3.87 b ± 0.18 | 4.50 c ± 0.14 |
| Criterion 5. Nature of melting | |||
| Watery mass | 4.90 c ± 0.05 | 4.41 b ± 0.18 | 4.02 a ± 0.21 |
| Spongy mass | 2.00 a ± 0.18 | 2.64 b ± 0.21 | 3.09 c ± 0.08 |
| Mass that melts quickly | 4.73 c ± 0.23 | 4.24 b ± 0.17 | 3.81 a ± 0.15 |
| Homogeneous | 3.63 a ± 0.16 | 3.93 b ± 0.15 | 4.08 c ± 0.14 |
| Without curdling | 4.72 a ± 0.01 | 4.85 b ± 0.02 | 4.94 c ± 0.03 |
| Criterion 6. Taste and aftertaste | |||
| Creamy | 3.28 a ± 0.07 | 4.35 b ± 0.28 | 4.56 c ± 0.01 |
| Pleasant | 3.51 a ± 0.04 | 4.09 b ± 0.12 | 4.80 c ± 0.02 |
| Milky | 3.33 a ± 0.11 | 3.54 ab ± 0.14 | 3.93 b ± 0.17 |
| Sweet | 2.04 a ± 0.15 | 2.21 a ± 0.18 | 3.48 b ± 0.05 |
| Without a sweet aftertaste | 4.40 a ± 0.12 | 4.56 a ± 0.22 | 4.84 b ± 0.07 |
| Integral score | 74.78 a ± 1.37 | 80.60 b ± 2.05 | 88.20 c ± 1.25 |
| Ingredients | Weight, kg, per 1000.0 kg (Excluding Losses) | ||
|---|---|---|---|
| C | Cre | BG | |
| Milk (3.2% fat) | 625.0 | 625.0 | 625.0 |
| Sugar | 150.0 | 150.0 | 150.0 |
| Skimmed milk powder | 46.3 | 46.3 | 46.3 |
| Stabilization system Cremodan® SI 320 | - | 5.0 | - |
| Oat β-glucan | - | - | 5.0 |
| Vanillin | 0.1 | 0.1 | 0.1 |
| Water | 178.6 | 173.6 | 173.6 |
| Total | 1000.0 | 1000.0 | 1000.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buniowska-Olejnik, M.; Mykhalevych, A.; Polishchuk, G.; Sapiga, V.; Znamirowska-Piotrowska, A.; Kot, A.; Kamińska-Dwórznicka, A. Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators. Molecules 2023, 28, 2924. https://doi.org/10.3390/molecules28072924
Buniowska-Olejnik M, Mykhalevych A, Polishchuk G, Sapiga V, Znamirowska-Piotrowska A, Kot A, Kamińska-Dwórznicka A. Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators. Molecules. 2023; 28(7):2924. https://doi.org/10.3390/molecules28072924
Chicago/Turabian StyleBuniowska-Olejnik, Magdalena, Artur Mykhalevych, Galyna Polishchuk, Victoria Sapiga, Agata Znamirowska-Piotrowska, Anna Kot, and Anna Kamińska-Dwórznicka. 2023. "Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators" Molecules 28, no. 7: 2924. https://doi.org/10.3390/molecules28072924
APA StyleBuniowska-Olejnik, M., Mykhalevych, A., Polishchuk, G., Sapiga, V., Znamirowska-Piotrowska, A., Kot, A., & Kamińska-Dwórznicka, A. (2023). Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators. Molecules, 28(7), 2924. https://doi.org/10.3390/molecules28072924








