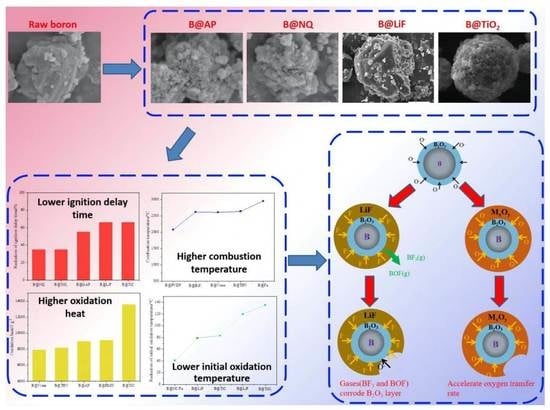
High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties
Abstract
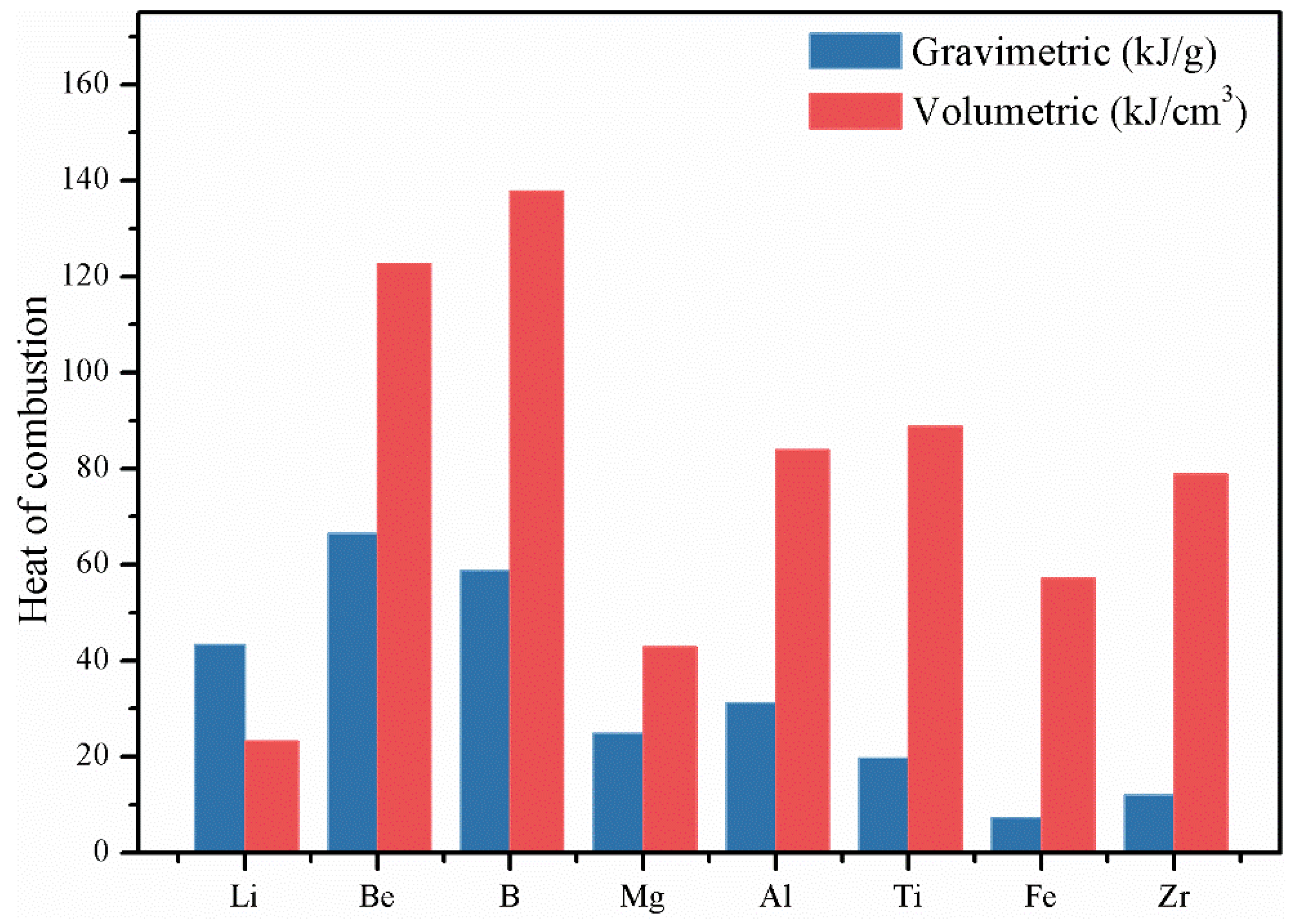
1. Introduction
2. Ignition and Combustion Mechanism of Boron Particles
2.1. Ignition Mechanism of Boron Particle
2.1.1. King’s Model
2.1.2. L-W Model
2.1.3. Y-K Model
2.1.4. B-D Model
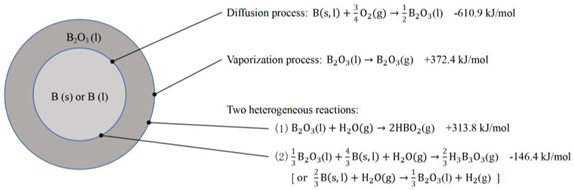
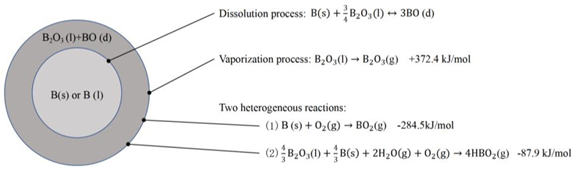
2.2. Combustion Mechanism of Boron Particles
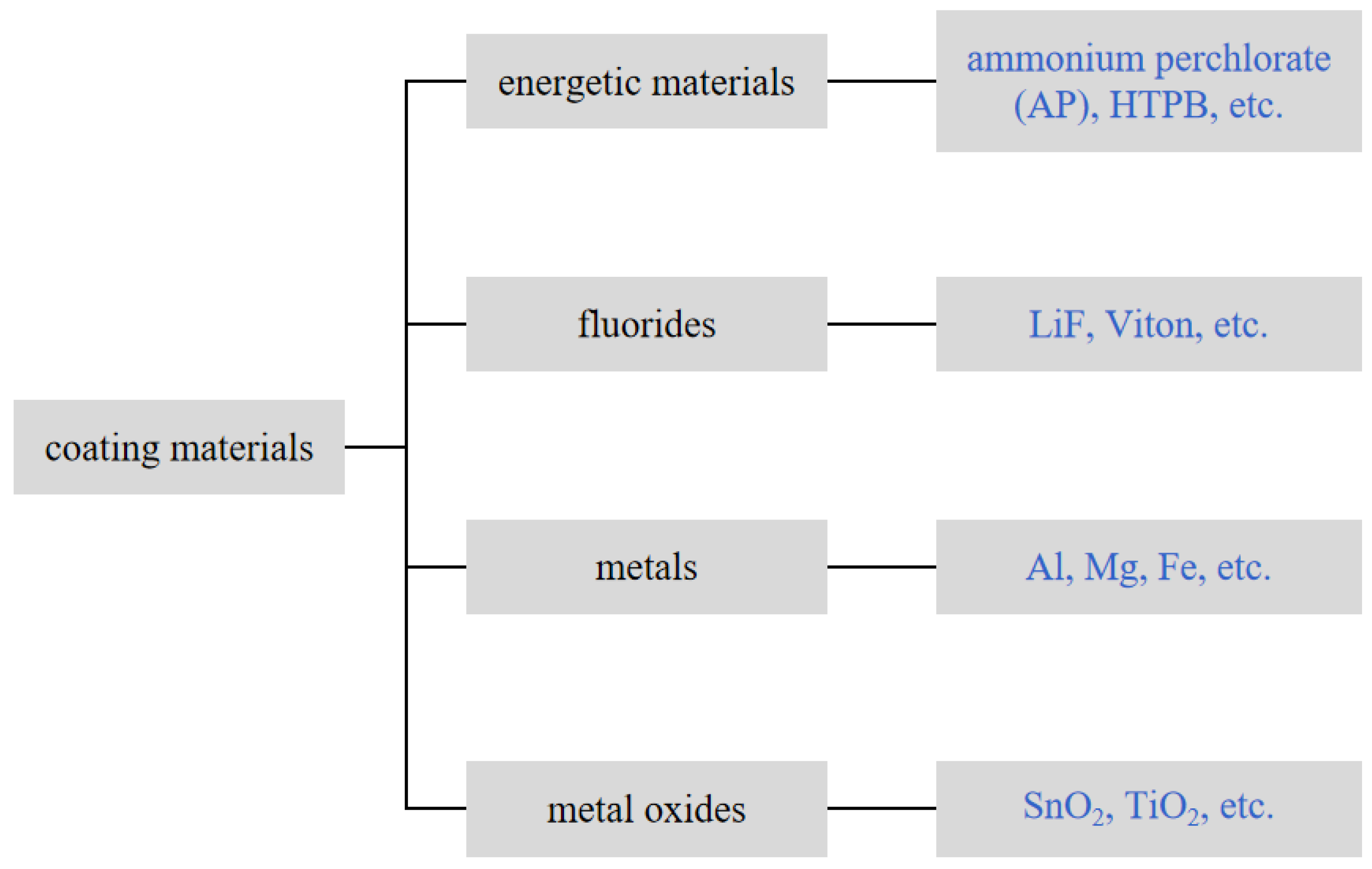
3. Surface Modification of Boron Powder
3.1. Boron Powder Surface Modification with Energetic Material
3.1.1. Boron Powder Coated with Oxidizer
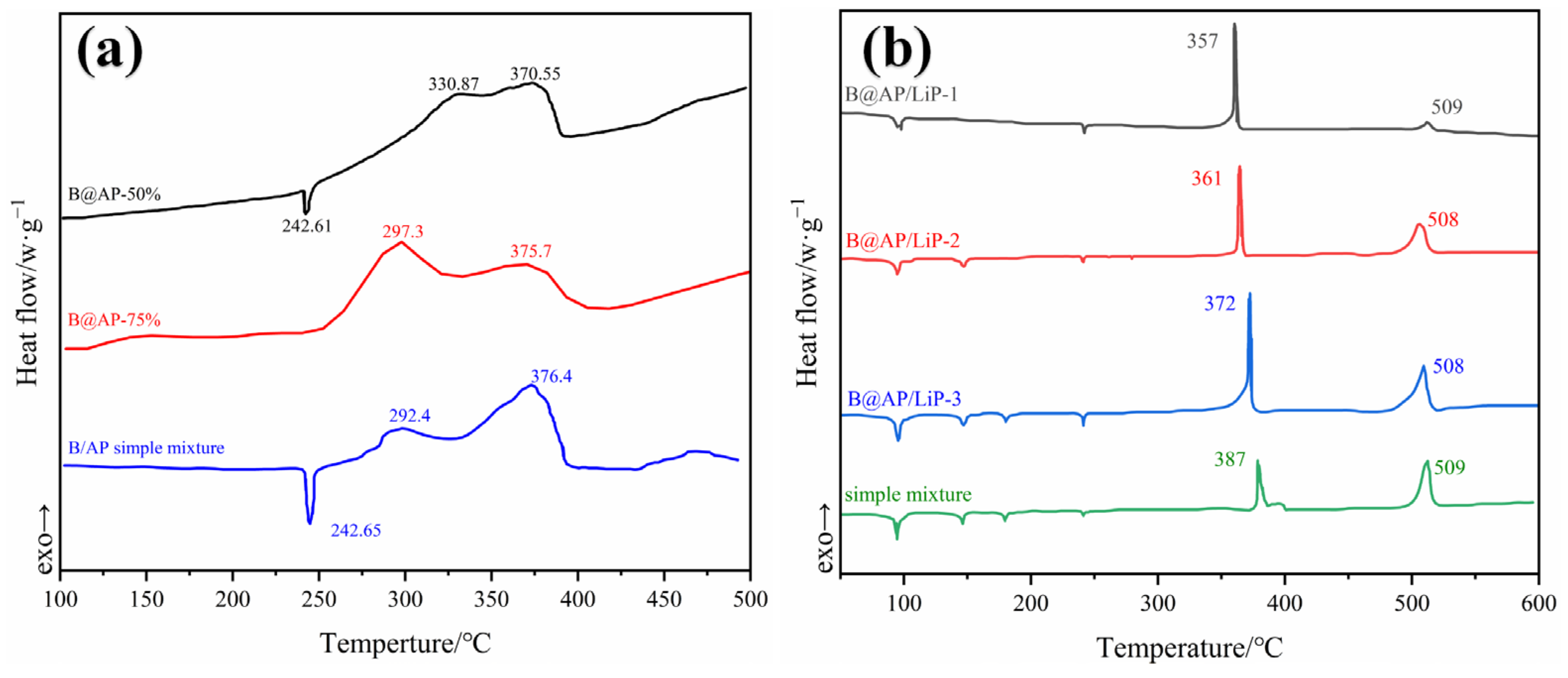
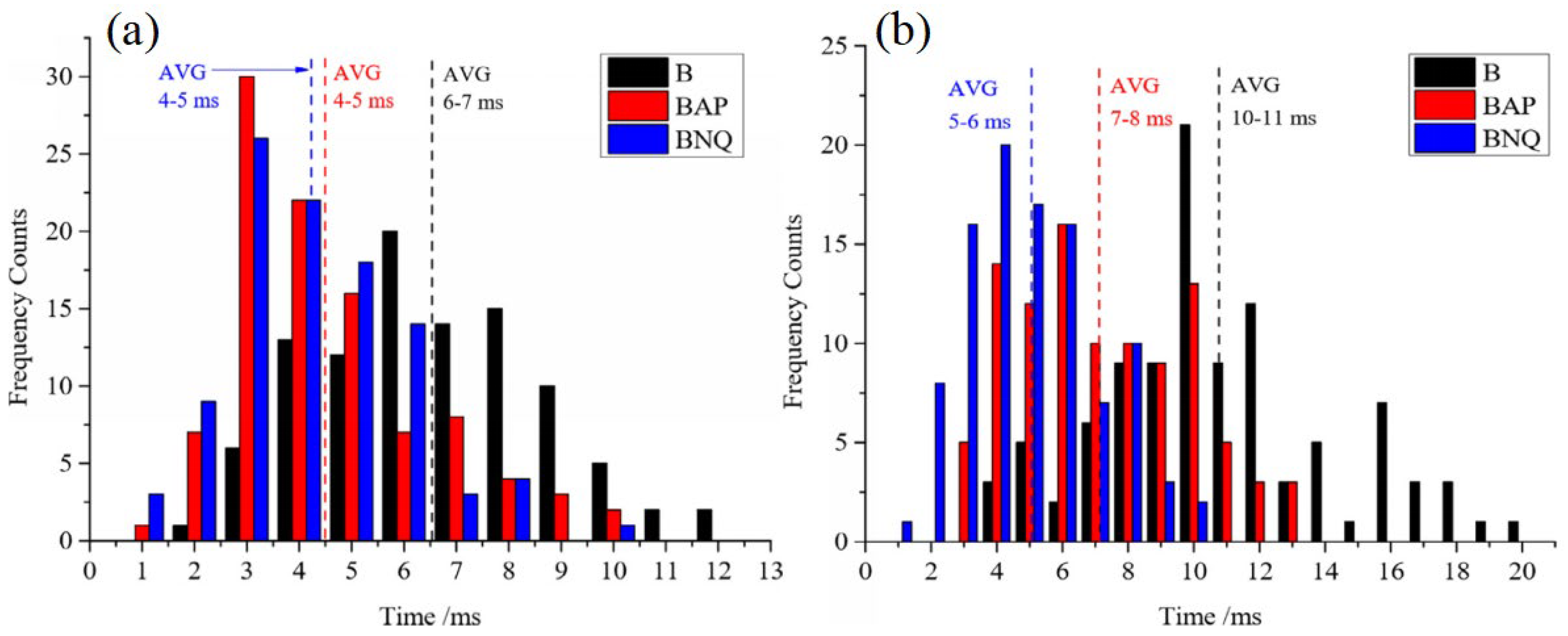
Boron Coated with Ammonium Perchlorate (AP)
Boron Coated with Octogen (HMX) and Nitroguanidine (NQ)
3.1.2. Boron Powder Coating with Binder
Boron Coated with PBT
Boron Coated with GAP
3.2. Boron Powder Surface Modification with Fluoride
3.2.1. Boron Powder Coated with Inorganic Fluoride
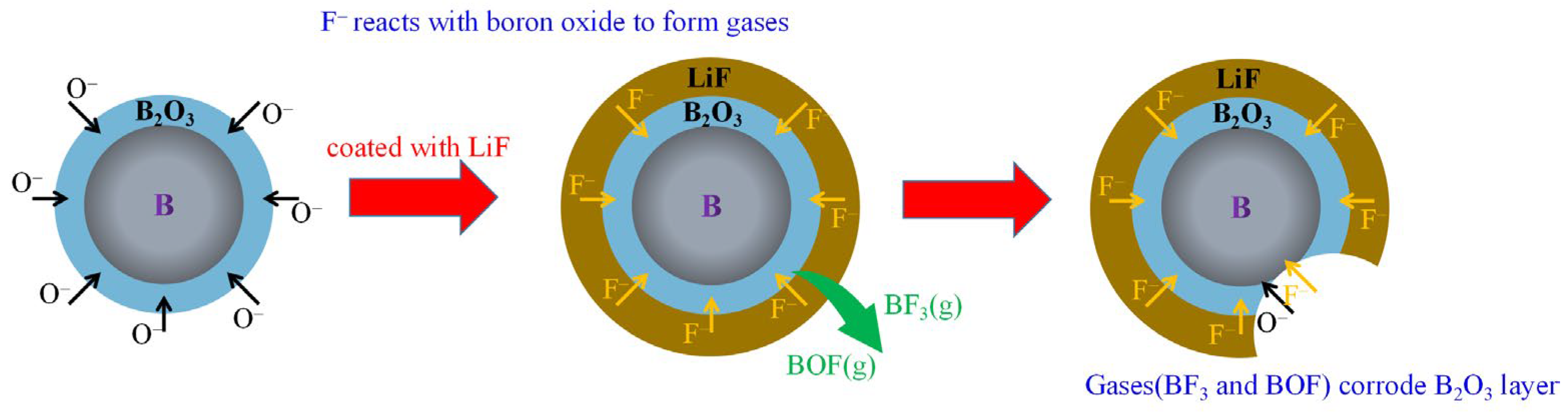
Boron Coated with LiF and BiF3
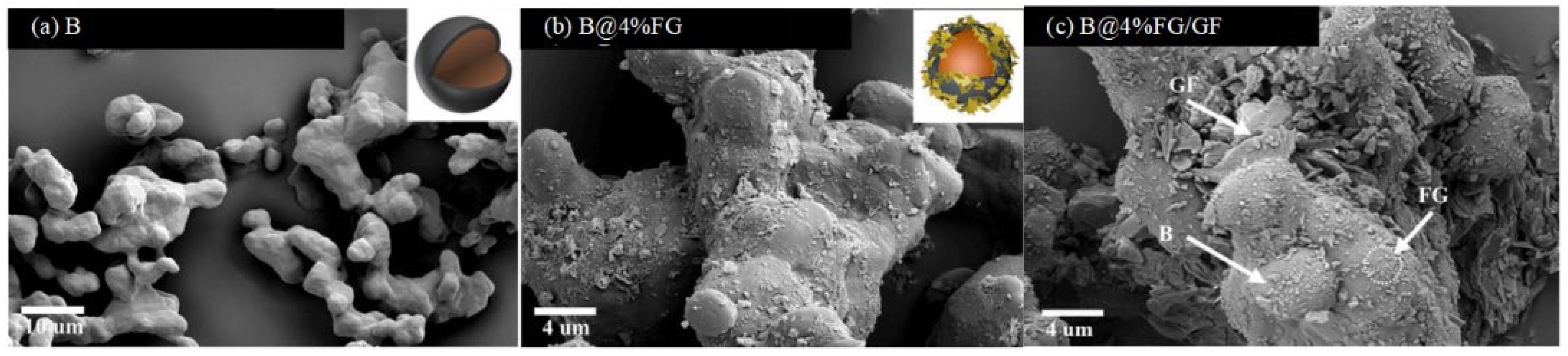
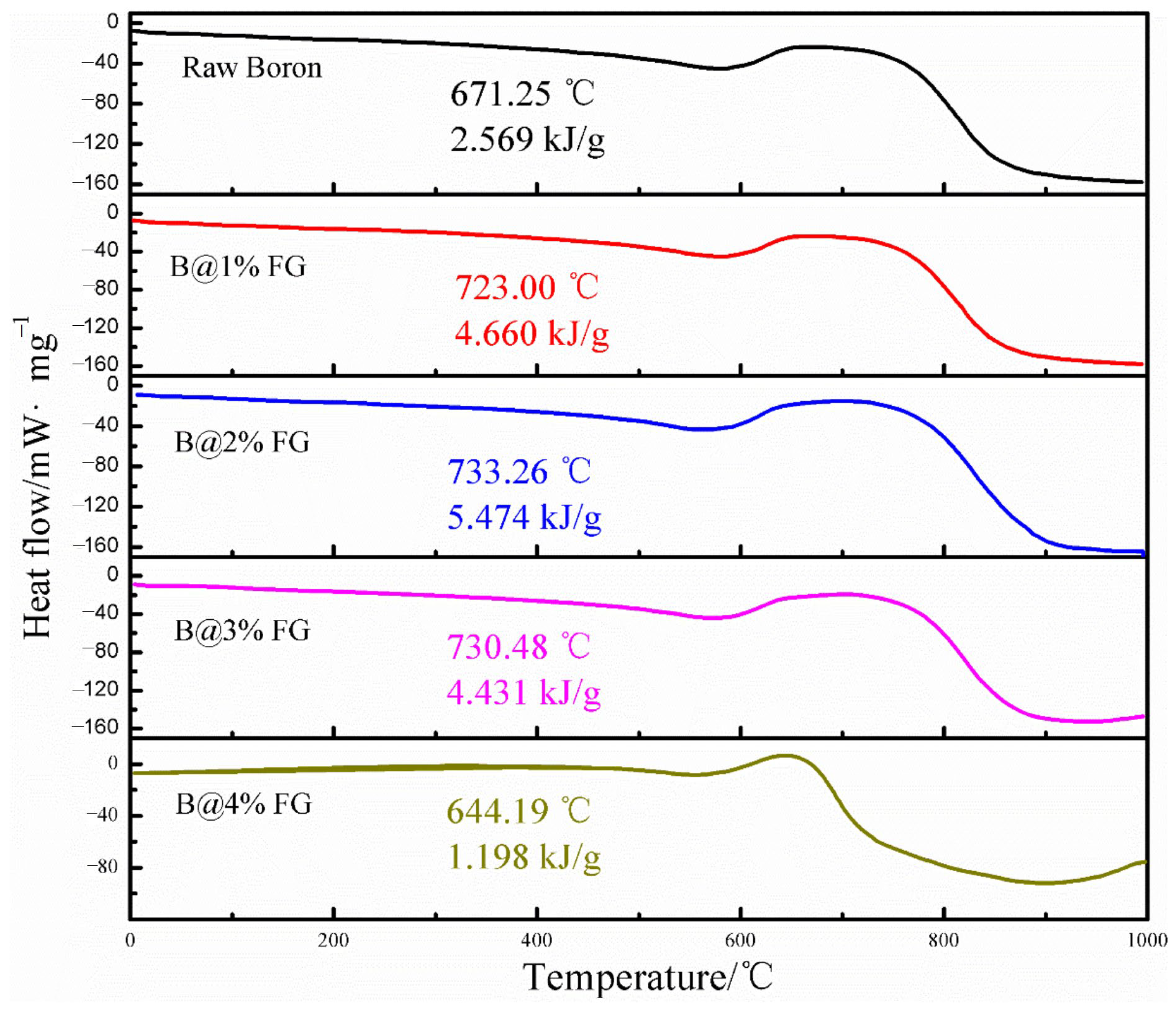
Boron Coated with Fluorographene (FG)
3.2.2. Boron Powder Coated with Fluoropolymer
3.3. Boron Powder Surface Modification with Metal and Metal Oxide
3.3.1. Boron Powder Coated with Metal
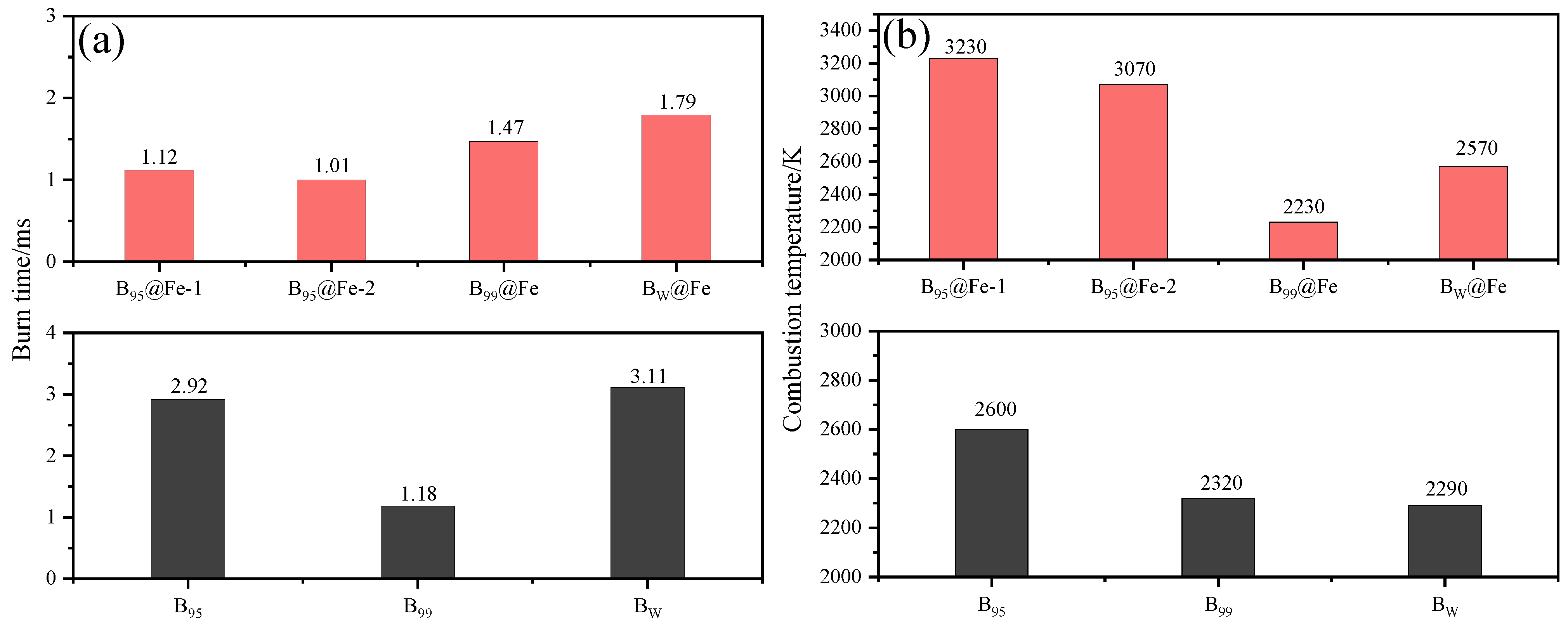
Boron Coated with Fe
Boron Coated with Mg
Boron Coated with Ti
3.3.2. Boron Powder Coated with Metal Oxide
Boron Coated with TiO2 or SnO2
Boron Coating with CeO2
4. Comparative Analysis
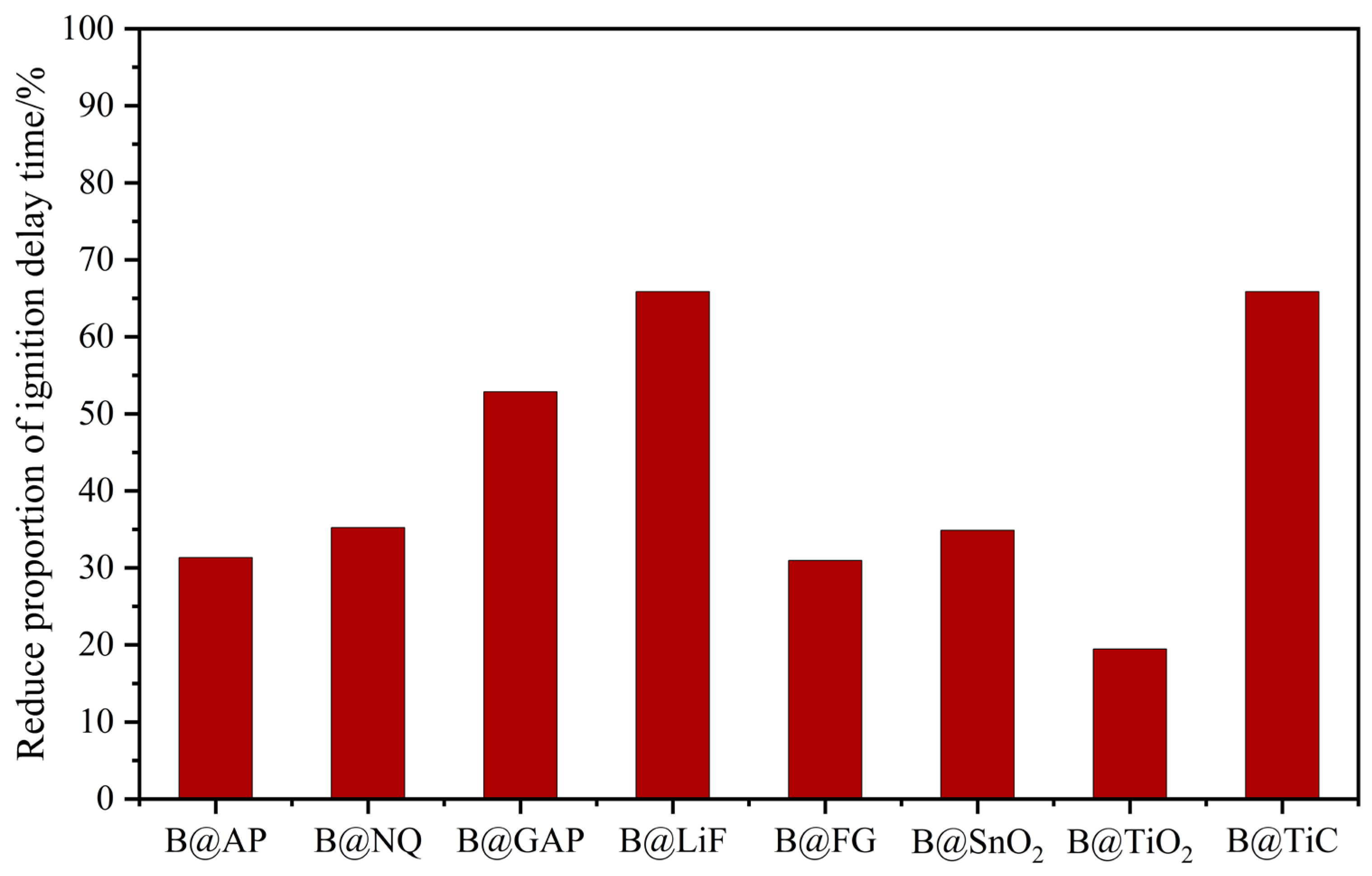
4.1. Ignition and Combustion Performances
4.2. Thermal Reaction Performance
4.3. Boron-Based Propellants and Explosives
5. Conclusions and Perspectives
- (1)
- Further research on the ignition and combustion behaviors of boron particles should been carried out, including the effects of the thermal mechanical properties, thickness, particle size, and crystal transformation of boron oxide shells on the ignition and combustion of boron particles. The effects of the coating layer, environmental atmosphere, and propellant additives on the behaviors of boron ignition and combustion should be determined, and the coating thickness and thickness control mechanism for fluoride, metal, and other coatings can be further explored through molecular dynamics simulations of the interactions between the coating and boron powder.
- (2)
- The development of new package-covering materials with more comprehensive performance, and new package-covering methods with controllable package-covering technology are urgently needed. In view of the performance defects of single-coating materials, the combustion performance of binary component coatings, or even ternary component coating materials should be studied.
- (3)
- To date, most of the research has been limited to basic studies on the ignition and combustion performance of boron powder coating modifications, which are limited to the laboratory. It is necessary to study the relationship between coating modification strategies and propellants. For example, the relationship between the performance parameters of propellants such as specific impulses, the critical pressure of propellant combustion chambers, pressure temperature coefficients, and the coating material of boron powder could be the focus of future research. Finally, more work is needed for the applications of coating boron powder at an industrial level.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Courty, L.; Gillard, P.; Ehrhardt, J.; Baschung, B. Experimental determination of ignition and combustion characteristics of insensitive gun propellants based on RDX and nitrocellulose. Combust. Flame 2021, 229, 111402. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Meshalikin, V.P.; Butusov, O.B.; Chistyakova, T.B.; Ferretti, M.; Cardinale, A.M.; Fabiano, B. Organic and inorganic biocidal energetic materials for agent defeat weapons: An overview and research perspectives. Energies 2023, 16, 675. [Google Scholar] [CrossRef]
- Pang, W.; Li, Y.; Deluca, L.T.; Liang, D.; Qin, Z.; Liu, X.; Xu, H.; Fan, X. Effect of metal nanopowders on the performance of solid rocket propellants: A review. Nanomaterials 2021, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, D.; Vigor, Y.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Eisen, N.E.; Gany, A. Examining metal additives in a marine hybrid-propellant, water-breathing ramjet. J. Mar. Sci. Eng. 2022, 10, 134. [Google Scholar] [CrossRef]
- Yadav, N.; Srivastava, P.K.; Varma, M. Recent advances in catalytic combustion of AP-based composite solid propellants. Def. Technol. 2021, 17, 1013–1031. [Google Scholar] [CrossRef]
- Vollath, D. Nanoparticles for energetic applications-formation and aggregation. FirePhysChem 2022, 2, 357–366. [Google Scholar] [CrossRef]
- Hashim, S.A.; Karmakar, S.; Roy, A. Combustion characteristics of boron-HTPB-based solid fuels for hybrid gas generator in ducted rocket applications. Combust. Sci. Technol. 2018, 191, 2082–2100. [Google Scholar] [CrossRef]
- Yen, N.H.; Wang, L.Y. Reactive metals in explosives. Propellants Explos. Pyrotech. 2012, 37, 143–155. [Google Scholar] [CrossRef]
- Nechiporenko, G.N.; Lempert, D.B. An analysis of energy potentialities of composite solid propellants containing beryllium or beryllium hydride as an energetic component. Chem. Phys. Rep. 1998, 17, 1927–1947. [Google Scholar]
- Yang, P.; Xia, Z.; Ma, L.; Chen, B.; Feng, Y.; Li, C.; Zhao, L. Direct-connect test of solid scramjet with symmetrical structure. Energies 2021, 14, 5589. [Google Scholar] [CrossRef]
- Dong, H.; Xia, M.; Wang, C.; Li, G.; Luo, Y. Al/NiO nanocomposites for enhanced energetic properties: Preparation by polymer assembly method. Mater. Des. 2019, 183, 108111. [Google Scholar] [CrossRef]
- Deluca, L.T. Overview of Al-based nanoenergetic ingredients for solid rocket propulsion. Def. Technol. 2018, 14, 357–365. [Google Scholar] [CrossRef]
- Mccollum, J.; Morey, A.M.; Iacono, S.T. Morphological and combustion study of interface effects in aluminum-poly (vinylidene fluoride) composites. Mater. Des. 2017, 134, 64–70. [Google Scholar] [CrossRef]
- Liu, X.; Schoenitz, M.; Dreizin, E.L. Combustion of Mg and composite Mg·S powders in different oxidizers. Combust. Flame 2018, 195, 292–302. [Google Scholar] [CrossRef]
- Xi, J.; Liu, J.; Wang, Y.; Li, H.; Zhang, Y.; Zhou, J.; Cen, K. Effects of coating agents on the ignition and combustion of boron particles. J. Solid Rocket Technol. 2013, 36, 654–659. [Google Scholar]
- Jiao, J.; Zhang, W.; Xia, Z.; Duan, J.; Chen, X.; Hu, J. Improvement of processing property of high energy fuel-rich HTPB propellant containing boron. J. Solid Rocket Technol. 2009, 32, 524–526. [Google Scholar]
- Yuan, J.F.; Liu, J.; Zhang, L.; Xu, P.; Chen, D.; Yang, W. Combustion and agglomeration characteristics of boron particles inboron-containing fuel-rich propellant. Combust. Flame 2021, 232, 111551. [Google Scholar] [CrossRef]
- Liu, L.L.; He, G.Q.; Wang, Y.H.; Hu, S.Q.; Liu, Y.M. Factors affecting the primary combustion products of boron-based fuel-rich propellants. J. Propuls. Power 2017, 33, 333–337. [Google Scholar] [CrossRef]
- Jain, A.; Joseph, K.; Anthonysamy, S.; Gupta, G.S. Kinetics of oxidation of boron powder. Thermochim. Acta 2011, 514, 67–73. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Oxidation kinetics and combustion of boron particles with modified surface. Combust. Flame 2016, 173, 288–295. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Sun, Y.; Schoenitz, M.; Dreizin, E.L. Heterogeneous reaction kinetics for oxidation and combustion of boron. Thermochim. Acta 2019, 682, 178415. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Wang, H.; DeLisio, J.B.; Yang, Y.; Zachariah, M.R. Boron ignition and combustion with doped δ-Bi2O3: Bond energy/oxygen vacancy relationships. Combust. Flame 2018, 197, 127–133. [Google Scholar] [CrossRef]
- Jiang, J.; Yilmaz, N.E.D.; Barker, K.P.; Baek, J.; Xia, Y.; Zheng, X.L. Enhancing mechanical and combustion performance of boron/polymer composites via boron particle functionalization. ACS Appl. Mater. Interfaces 2021, 13, 28908–28915. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Zhang, W.; Xu, H.; Li, J.; Li, Y.; Shi, X.; Li, Y. Application of amorphous boron granulated with hydroxyl-terminated polybutadiene in fuel-rich solid propellant. Propellants Explos. Pyrotech. 2011, 36, 360–366. [Google Scholar] [CrossRef]
- Wang, G.D.; Jing, S.M.; Liu, G.Q.; Gao, X.Y. Review on the Synthesis and Properties of the Energetic Compound Containing Boron. Curr. Org. Chem. 2020, 24, 1097–1107. [Google Scholar] [CrossRef]
- Mursalat, M.; Schoenitz, M.; Dreizin, E.L. Effect of particle morphology on reactivity, ignition and combustion of boron powders. Fuel 2022, 324, 124538. [Google Scholar] [CrossRef]
- Pivkina, A.E.; Meerov, D.B.; Monogarov, K.A.; Frolov, Y.V.; Muravyev, N.V. Prospects of using boron powders as fuel. II. Influence of aluminum and magnesium additives and their compounds on the thermal behavior of boron oxide. Combust. Explos. Shock Waves 2020, 56, 148–155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Hu, S.; Xiao, X. Effect of AP particle size and coating on combustion of boron. J. Solid Rocket Technol. 2004, 27, 50–52. [Google Scholar]
- Liu, J.; Liang, D.; Zhou, Y.; Zhou, J. Review on ignition and combustion characteristics of boron particles. J. Solid Rocket Technol. 2017, 40, 573–582. [Google Scholar]
- Zamostianu, A.; Yavor, Y. Burn rate of a novel boron-AN-water green solid propellant. FirePhysChem 2022, 2, 76–82. [Google Scholar] [CrossRef]
- Macek, A.; Semple, J.M. Combustion of boron particles at atmospheric pressure. Combust. Sci. Technol. 1969, 1, 181–191. [Google Scholar] [CrossRef]
- Macek, A.; Semple, J.M. Combustion of boron particles at elevated pressures. Symp. Int. Combust. 1971, 13, 859–868. [Google Scholar] [CrossRef]
- Macek, A. Combustion of boron particles: Experiment and theory. Symp. Int. Combust. 1973, 14, 1401–1411. [Google Scholar] [CrossRef]
- Uda, R.T. A Shock Tube Study of the Ignition Limit of Boron Particles. Master’s Thesis, Air Force Institute of Technology, Dayton, OH, USA, 1968. [Google Scholar]
- King, M.K. Boron ignition and combustion in air-augmented rocket afterburners. Combust. Sci. Technol. 1972, 5, 155–164. [Google Scholar] [CrossRef]
- King, M.K. Boron particle ignition in hot gas streams. Combust. Sci. Technol. 1973, 8, 255–273. [Google Scholar] [CrossRef]
- King, M.K. Modeling of single particle boron combustion. In Proceedings of the 19th JANNAF Combustion Meeting, Greenbelt, MD, USA, 4–7 October 1982; pp. 27–42. [Google Scholar]
- Mohan, G.; Williams, F.A. Ignition and combustion of boron in O2/inert atmospheres. AIAA J. 1972, 10, 776–783. [Google Scholar] [CrossRef]
- Prentice, J.L. Metal particle combustion progress report. In Metal Particle Combustion Progress Report; Naval Weapons Center China Lake: Ridgecrest, CA, USA, 1968; pp. 1–123. [Google Scholar]
- Meinkohn, D. The ignition of boron particles. Combust. Flame 1985, 59, 225–232. [Google Scholar] [CrossRef]
- Gaponenko, L.A.; Biunovskii, S.N.; Tulupov, Y.I.; Yakovleva, T.A. A model for the ignition of a single boron particle in a medium containing water. Combust. Explos. Shock Waves 1981, 17, 9–14. [Google Scholar] [CrossRef]
- Meese, R.A.; Skifstad, J.G. Ignition and global combustion models for clouds of boron particles. AIAA J. 1974, 12, 71–77. [Google Scholar] [CrossRef]
- Glassman, I.; Williams, F.A.; Antaki, P. A physical and chemical interpretation of boron particle combustion. Symp. Int. Combust. 1985, 20, 2057–2064. [Google Scholar] [CrossRef]
- Li, S.C.; Williams, F.A. Ignition and combustion of boron in wet and dry atmospheres. Symp. Int. Combust. 1991, 23, 1147–1154. [Google Scholar] [CrossRef]
- Li, S.C.; Williams, F.A. Ignition and combustion of boron particles. Int. J. Energetic Mater. Chem. Propuls. 1993, 2, 248–271. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, K.K. Ignition and combustion of boron particles. Prog. Energy Combust. Sci. 1996, 22, 511–541. [Google Scholar] [CrossRef]
- Ermolaev, G.V.; Zaitsev, A.V. Diffusion model of combustion of large boron particles. Combust. Explos. Shock. Waves 2018, 54, 442–449. [Google Scholar] [CrossRef]
- Bedarev, I.A.; Syrovaten, A.A. Simulation of the dynamics of ignition and combustion of boron particles in shook waves. J. Eng. Phys. Thermophys. 2022, 95, 1724–1731. [Google Scholar] [CrossRef]
- Ulas, A.; Kuo, K.K.; Gotzmer, C. Ignition and combustion of boron particles in fluorine-containing environments. Combust. Flame 2001, 127, 1935–1957. [Google Scholar] [CrossRef]
- Yetter, R.A.; Dryer, F.L.; Rabitz, H.; Brown, R.C.; Kolb, C.E. Effect of fluorine on the gasification rate of liquid boron oxide droplets. Combust. Flame 1998, 112, 387–403. [Google Scholar] [CrossRef]
- Chen, B.; Xia, X.; Huang, L.; Hu, J. Ignition and combustion model of a single boron particle. Fuel Process. Technol. 2017, 165, 34–43. [Google Scholar] [CrossRef]
- Ao, W.; Zhou, J.; Liu, J.; Yang, W.; Wang, Y.; Li, H. Kinetic model of single boron particle ignition based upon both oxygen and (BO)n diffusion mechanism. Combust. Explos. Shock Waves 2014, 50, 262–271. [Google Scholar] [CrossRef]
- Dreizin, E.L.; Calcote, H.F. A new mechanism of boron ignition: Through the formation of a saturated B-O solution. In Proceedings of the Technical Meeting: Eastern States Section of the Combustion Institute, Worcester, MA, USA, 16–18 October 1995; pp. 333–336. [Google Scholar]
- Dreizin, E.L.; Keil, D.G.; Felde, W.; Vicenzi, E.P. Phase changes in boron ignition and combustion. Combust. Flame 1999, 119, 272–290. [Google Scholar] [CrossRef]
- Dreizin, E.L. Effect of phase changes on Metal-particle combustion process. Combust. Explos. Shock Waves 2003, 39, 681–693. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Z.; Huan, L.; Ma, L. Review on combustion technology of boron-based soild ramjet afterburning chamber. Aero Weapon. 2018, 4, 3–20. [Google Scholar]
- King, K.M. Ignition and combustion of boron particles and clouds. J. Spacecr. Rocket 1982, 19, 294–306. [Google Scholar] [CrossRef]
- Li, S.C.; Williams, F.A.; Takahash, F. An investigation of combustion of boron suspensions. Symp. Int. Combust. 1989, 22, 1951–1960. [Google Scholar] [CrossRef]
- Hussmann, B.; Pfitzner, M. Extended combustion model for single boron particles-part I: Theory. Combust. Flame 2010, 157, 803–821. [Google Scholar] [CrossRef]
- Hussmann, B.; Pfitzner, M. Extended combustion model for single boron particles-part II: Validation. Combust. Flame 2010, 157, 822–833. [Google Scholar] [CrossRef]
- Liu, S.; Ye, M.; Han, A.; Chen, X. Preparation and characterization of energetic materials coated superfine aluminum particles. Appl. Surf. Sci. 2014, 288, 349–355. [Google Scholar] [CrossRef]
- Zhang, K.L.; Alphonse, P.; Tenailleau, C. Nano energetic materials: Synthesis, characterization, modeling and application. In Energetic Materials: Chemistry, Hazards, and Environmental Aspects; Howell, J.R., Fletcher, T.E., Eds.; Nova Science Publishers, Inc: New York, NY, USA, 2010; pp. 141–164. ISBN 978-1-6087-6267-5. [Google Scholar]
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D. A review of energetic materials synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, D.; Liu, P.; Sun, X.L.; Yan, S.; Guo, X. Enhanced combustion behavior of TKX-50/B/NC composites viaelectrospray. J. Energetic Mater. 2022, 1–13. [Google Scholar] [CrossRef]
- Padwal, M.B.; Varma, M. Thermal decomposition and combustion characteristics of HTPB-coarse AP composite solid pro-pellants catalyzed with Fe2O3. Combust. Sci. Technol. 2018, 190, 1614–1629. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Singh, S.; Singh, G. Review on the catalytic effect of nanoparticles on the thermal decomposition of ammonium perchlorate. Energy Environ. Focus 2014, 3, 121–130. [Google Scholar] [CrossRef]
- Dennis, C.; Bojko, B. On the combustion of heterogeneous AP/HTPB composite propellants: A review. Fuel 2019, 254, 11564. [Google Scholar] [CrossRef]
- Liu, L.L.; He, G.Q.; Wang, Y.H. Effect of Oxidizer on the Combustion performance of boron-based fuel-rich propellant. J. Propuls. Power 2014, 30, 285–289. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, W.; Zhang, Q.; Su, L.; Yan, H.; Kou, K.; Guo, J. Improvement for AP coating superfine boron powder. Chin. J. Energetic Mater. 2007, 15, 382–386. [Google Scholar]
- Liang, D.; Liu, J.; Zhou, Y.; Zhou, J. Ignition and combustion characteristics of amorphous boron and coated boron particles in oxygen jet. Combust. Flame 2017, 185, 292–300. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, L.; Wang, H.; Zhao, K.; Luo, Y.; Zhang, H.L. Combustion performance of boron coated with AP. Acta Armament 2014, 35, 194–199. [Google Scholar]
- Gao, F.; Yang, K. Preparation and characterization of boron coated with double salt. J. Sichuan Ordnance 2015, 36, 142–144. [Google Scholar]
- Liu, Y.; Chen, Y.; Shi, L.; Yao, W. Preparation of BAP composite particles and their Effects on rheological roperties of HTPB/B/AP slurries. J. Energetic Mater. 2014, 32, 71–79. [Google Scholar]
- Hu, Y.; Wang, X.; Zhang, J.; Zhu, Z.; Ren, X.; Yang, Y.; Lin, K.; Pang, A.; Yong, S. Encapsulated boron-based energetic spherical composites with improved reaction efficiency and combustion performance. Chem. Eng. J. 2022, 433, 134478. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Chen, B.; Zhou, J.; Cen, K. Improvement in energy release properties of boron-based propellant by oxidant coating. Thermochim. Acta 2016, 638, 58–68. [Google Scholar] [CrossRef]
- Yoon, J.K.; Thakre, P.; Yang, V. Modeling of RDX/GAP/BTTN pseudo-propellant combustion. Combust. Flame 2006, 145, 300–315. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Kuibida, L.V.; Volkov, E.N.; Shmakov, A.G. Mass spectrometric study of combustion and thermal decomposition of GAP. Combust. Flame 2002, 129, 136–150. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Hajimirsadeghi, S.S.; Atifeh, S.M. Effect of functional group on thermal stability of cellulose derivative energetic polymers. Fuel 2012, 95, 394–399. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Guo, J.; Pang, W.; Kou, K. Research of the surface coating of superfine boron particles with PBT. Chin. J. Energ. Mater. 2005, 13, 185–188. [Google Scholar]
- Shyu, I.M.; Liu, T.K. Combustion characteristics of GAP-coated boron particles and the fuel-rich solid propellant. Combust. Flame 1995, 100, 634–644. [Google Scholar] [CrossRef]
- Shin, W.G.; Han, D.; Park, Y.; Hyun, H.S.; Sung, H.G.; Sohn, Y. Combustion of boron particles coated with an energetic polymer material. Korean J. Chem. Eng. 2016, 33, 3016–3020. [Google Scholar] [CrossRef]
- Tang, C.; Lee, Y.J.; Litzinger, T.A. Simultaneous temperature and species measurements of the glycidyl azide polymer (GAP) propellant during laser-induced decomposition. Combust. Flame 1999, 117, 244–256. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, J.; Hu, C.; Zhang, L.; Li, C.; Wei, X. Ignition and combustion characteristics of boron-based powder fuel under different binder systems. J. Solid Rocket Technol. 2021, 44, 454–460. [Google Scholar]
- Fan, H.; Wang, N.; Guan, D. Effect of GAP coating boron on the ignition performance and combustion residues for boron-based propellants. J. Propul. Technol. 2002, 23, 262–264. [Google Scholar]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E.L. Fluorine-containing oxidizers for metal fuels in energetic formulations. Def. Technol. 2019, 15, 1–22. [Google Scholar] [CrossRef]
- Xu, H.; Feng, X.; Tian, X.; Feng, B. Effect of fluorine-binder on the under-water explosion energy of boron-containing explosive. Initiat. Pyrotech. 2015, 4, 38–41. [Google Scholar]
- Zhang, J.; Zhang, Q.; Guo, J.; Pang, W.; Kou, K. Surface coating of superfine boron particles with lithium fluoride. Chin. J. Explos. Propellants 2005, 28, 8–11. [Google Scholar]
- Zhou, W.; Yetter, R.A.; Dryer, F.L.; Rabitz, H.; Brown, R.C.; Kolb, C.E. Effect of fluorine on the combustion of “clean” surface boron particles. Combust. Flame 1998, 112, 507–521. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Q.; Zhao, C. Liquid phase in-situ synthesis of LiF coated boron powder composite and performance study. Def. Technol. 2020, 16, 635–641. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Wang, Y.; Huang, L.; Xiao, J. Effect of LiF coating on the thermal oxidation characteristics for boron powder. Chin. J. Energetic Mater. 2013, 21, 57–60. [Google Scholar]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E.L. Bismuth fluoride-coated boron powders as enhanced fuels. Combust. Flame 2020, 221, 1–10. [Google Scholar] [CrossRef]
- Taqieddin, A.; Heiranian, M.; Aluru, N.R. Interfacial properties of water on hydrogenated and fluorinated graphene surfaces: Parametrization of nonbonded interactions. J. Phys. Chem. C 2020, 124, 21467–21475. [Google Scholar] [CrossRef]
- Belenkov, M.E.; Chernov, V.M.; Belenkov, E.A. Structure of fluorographene and its polymorphous varieties. J. Phys. Conf. Ser. 2018, 1124, 022010. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Y.; Chen, J.; Li, Z.; Wang, J.; Nie, F. Surface engineering boron/graphite fluoride composite with enhanced ignition and combustion performances. Fuel 2022, 323, 124374. [Google Scholar] [CrossRef]
- Liu, T.; Shyu, I.M.; Hsia, Y.S. Effect of fluorinated graphite on combustion of boron and boron-based fuel-rich propellants. J. Propuls. Power 1996, 12, 26–33. [Google Scholar] [CrossRef]
- Keerthi, V.; Nie, H.Q.; Pisharath, S.; Hng, H.H. Combustion characteristics of fluoropolymer coated boron powders. Combust. Sci. Technol. 2020, 194, 1183–1198. [Google Scholar] [CrossRef]
- Qin, Z.; Yi, J.; Pang, W.; Wang, C.; Li, H.; Xu, H.; Qu, B.; Zhao, F.; Hao, N.; Huang, X.F. Effect of spherical Al-Mg-Zr on the combustion characteristics of composite propellants. FirePhysChem 2022, 2, 14–19. [Google Scholar] [CrossRef]
- Song, N.; Liu, J.; Zhang, G.; Yan, Z.; Gao, H.; Yang, L. Catalytic action of submicrometer spherical Ta/Ph-Fe on combustion of AP/HTPB propellant. Propellants. Explos. Pyrotech. 2018, 43, 637–641. [Google Scholar] [CrossRef]
- Huang, S.; Deng, S.; Jiang, Y.; Zheng, X. Experimental effective metal oxides to enhance boron combustion. Combust. Flame 2019, 205, 278–285. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Y.; Liu, J.; Ao, W.; Li, H.; Zhou, J. Effect of metal oxides on thermal reaction characteristics of boron powder. In Proceedings of the Aeronautics & Astronautics Science & Technology Doctoral Forum of China, Hefei, China, 3 June 2013; pp. 54–59. [Google Scholar]
- Gu, X.; Li, S.; Tang, Y.; Zhao, K.; Bai, S.X. Recent advances in super-thermite. Mater. Rep. 2023, 37, 1060211. [Google Scholar]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Effect of purity, surface modification and iron coating on ignition and combustion of boron in air. Combust. Sci. Technol. 2021, 193, 1567–1586. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Combustion of boron and boron-iron composite particles in different oxidizers. Combust. Flame 2018, 192, 44–58. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Boron doped with iron: Preparation and combustion in air. Combust. Flame 2019, 200, 286–295. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and stabilization of monodisperse Fe nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Paravan, C.; Colombo, G.; Deluca, L.T.; Shen, R.; Ye, Y. Ignition temperature of metal fuel in different atmosphere. Initiat. Pyrotech. 2014, 4, 24–27. [Google Scholar]
- Pace, K.K.; Jmymowycz, T.A.; Yang, V. Effect of magnesium-coated boron particles on burning characteristics fuels in high-speed crossnows. Int. J. Energetic Mater. Chem. Propuls. 1993, 2, 332–347. [Google Scholar] [CrossRef]
- Rosenband, V.; Natan, B.; Gany, A. Ignition of boron particles coated by a thin titanium film. J. Propuls. Power 1995, 11, 1125–1131. [Google Scholar] [CrossRef]
- Xi, J.; Liu, J.; Wang, Y.; Hu, Y.; Zhang, Y.; Zhou, J.H. Metal oxides as catalysts for boron oxidation. J. Propuls. Power 2014, 30, 47–53. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Xu, H.; Han, A.; Ye, M.; Pan, G. Preparation and properties of boron-based Nano-B/NiO thermite. Propellants Explos. Pyrotech. 2016, 40, 873–879. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Han, A.J.; Ye, M.Q.; Zhang, S.T. Preparation and properties of boron-based Nano-B/CuO thermite. KnE Mater. Sci. 2016, 2016, 95–102. [Google Scholar] [CrossRef]
- Lee, H.; Deshmukh, P.R.; Kim, J.H.; Hyun, H.S.; Sohn, Y.; Shin, W.G. Spray drying formation of metal oxide (TiO2 or SnO2) nanoparticle coated boron particles in the form of microspheres and their physicochemical properties. J. Alloys Compd. 2019, 810, 151923. [Google Scholar] [CrossRef]
- Devener, B.V.; Perez, J.P.; Jankovich, J.; Anderson, S.L. Oxide-free, catalyst-coated, fuel-soluble, air-stable boron nanopowder as combined combustion catalyst and high energy density fuel. Energy Fuels 2015, 23, 6111–6120. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Lee, H.; Kim, Y.; Shin, W.G. Ignition and oxidation performance of SnO2 coated boron particles: A solid fuel for energetic applications. J. Alloys Compd. 2021, 886, 161123. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Kim, Y.; Shin, W.G. Ignition performance of TiO2 coated boron particles using a shock tube. Ceram. Int. 2022, 48, 6166–6176. [Google Scholar] [CrossRef]
- Sung, J.; Shin, M.; Deshmukh, P.R.; Hyun, H.S.; Sohn, Y.; Shin, W.G. Preparation of ultrathin TiO2 coating on boron particles by thermal chemical vapor deposition and their oxidation-resistance performance. J. Alloys Compd. 2018, 767, 924–931. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Qiu, Q. Nano carbides-mediated acceleration of energy release behavior of amorphous boron during ignition and combustion. Energy Rep. 2020, 6, 1160–1169. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, H.; Yang, Y.; Li, Y.; Meng, Y.; Li, Y.; Song, D.; Chen, H.; Artiaga, R. Preparation of B/Nitrocellulose/Fe particles and their effect on the performance of an ammonium perchlorate propellant. Combust. Flame 2020, 211, 456–464. [Google Scholar] [CrossRef]






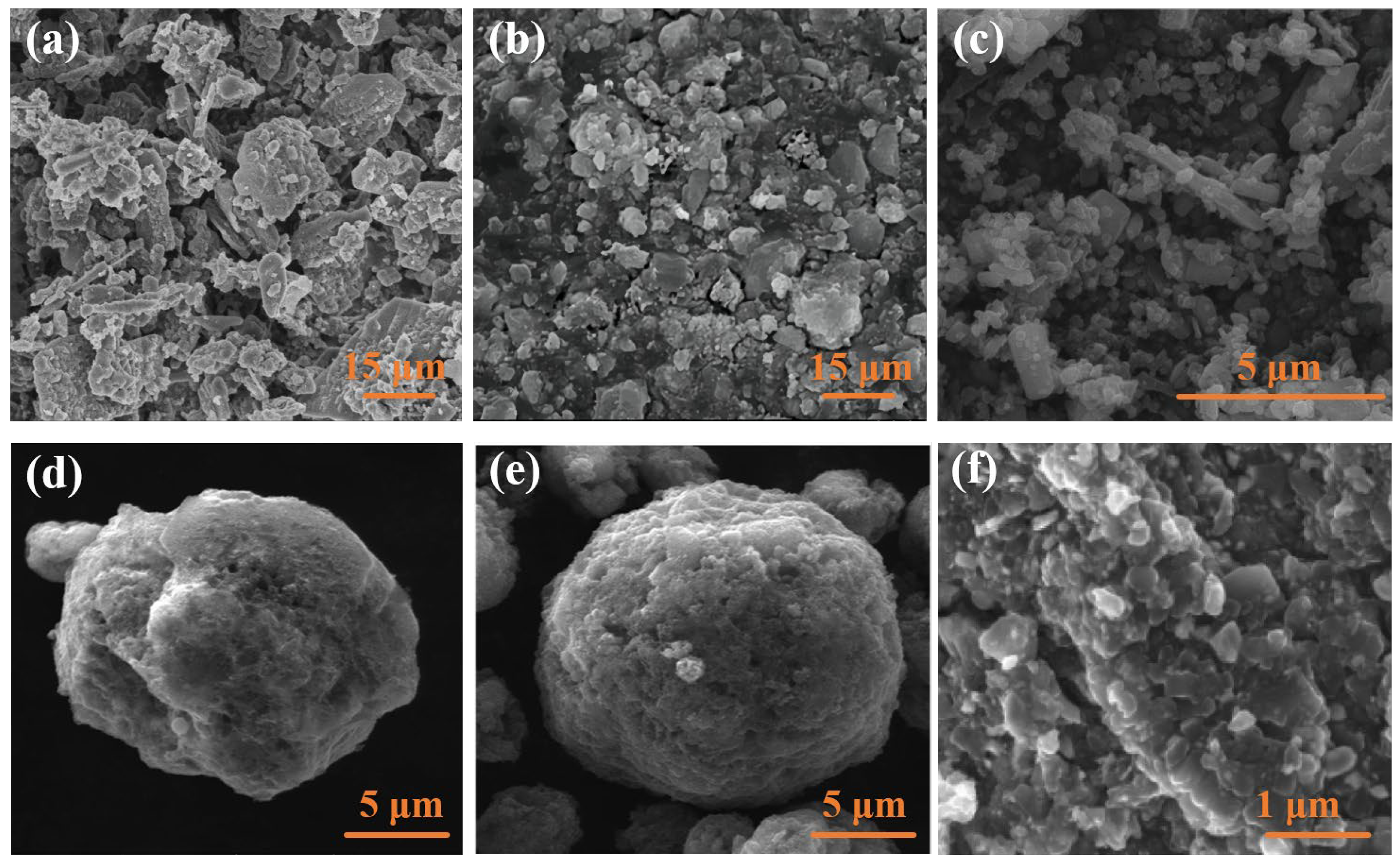

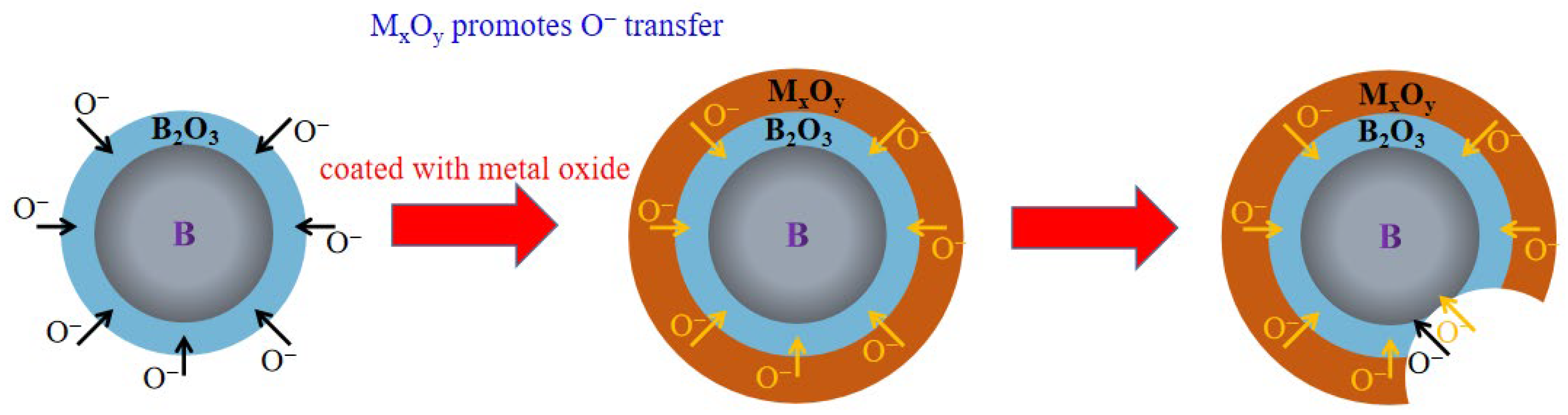

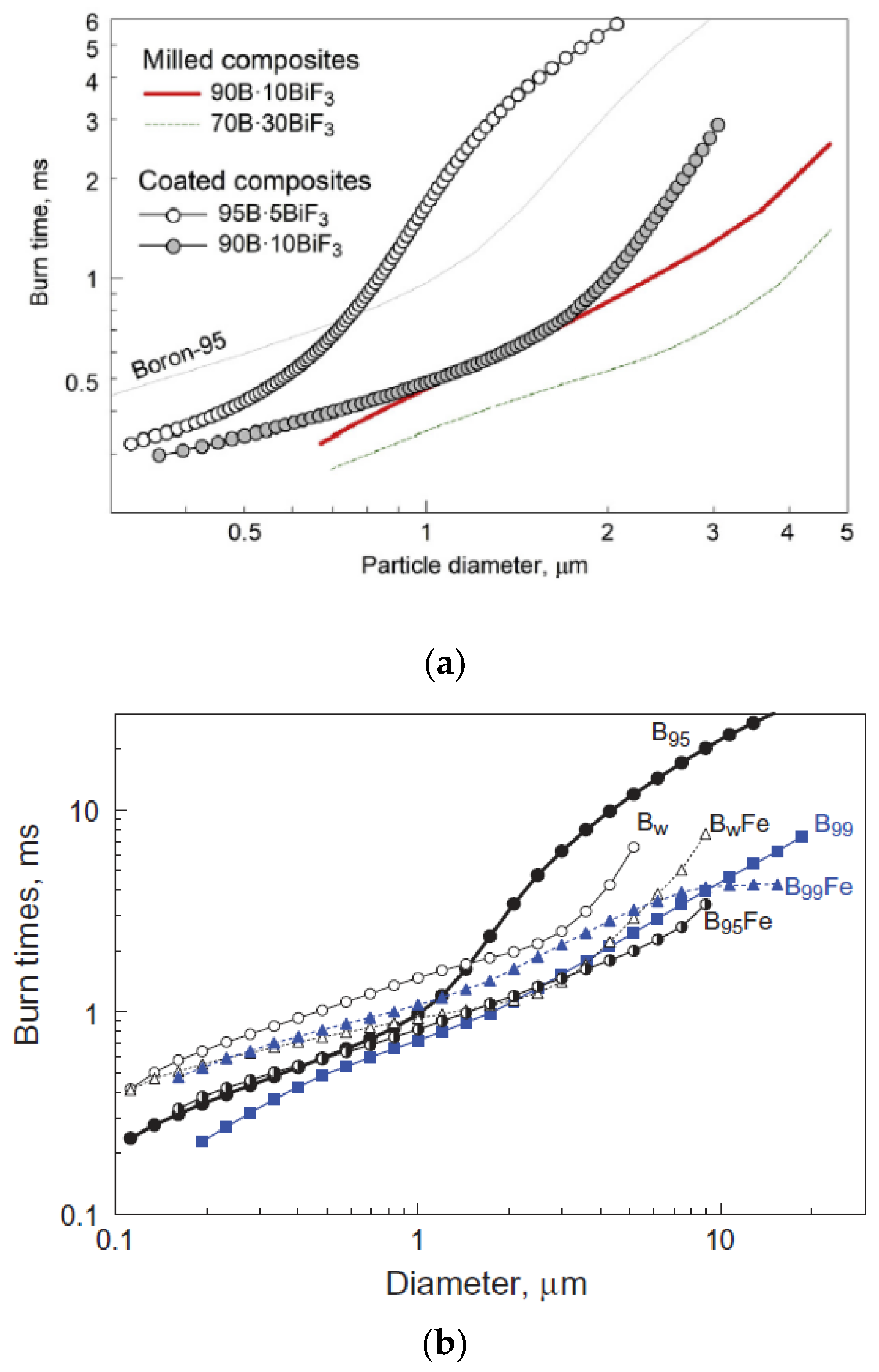
| Viscosity/Pa·s | Stir Time/Min | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | |
| HTPB + B | 4000 | 9000 | 13,200 | 16,400 | 19,000 | 22,600 | 25,000 | 28,000 |
| HTPB + B@PBT | 26 | 835 | 530 | 800 | 960 | 1225 | 1364 | 1389 |
| Samples | Combustion Temperature/°C | Burning Time/ms | ||
|---|---|---|---|---|
| Before Coating | After Coating | Before Coating | After Coating | |
| B@AP [76] | 960.27 | 1116.24 | – | – |
| B@LiP [76] | 960.27 | 901.02 | – | – |
| B@KNO3 [76] | 960.27 | 826.87 | – | – |
| B@HMX [76] | 960.27 | 1163.92 | – | – |
| B@GAP [81] | – | – | 47.1 | 31.5 |
| B@BiF3 [92] | – | – | 3.4 | 1.09 |
| B@PVDF [97] | 1817 | 2077 | – | – |
| B@Viton [97] | 1817 | 2607 | – | – |
| B@THV [97] | 1817 | 2637 | – | – |
| B@Fe [105] | 2327 | 2797 | 2.92 | 1.01 |
| Samples | CC | FM | Ton/°C | ΔT/°C | Oxidation Heat/J·g−1 | Δm/% |
|---|---|---|---|---|---|---|
| B@AP [76] | 10 | recrystallization method | 704.1 | 15.7 | 8966 | 35.37 |
| B@LiP [76] | 10 | recrystallization method | 640.4 | 79.4 | 8865 | 32.27 |
| B@HMX [76] | 10 | recrystallization method | 707.7 | 12.1 | 9110 | 34.27 |
| B@KNO3 [76] | 10 | recrystallization method | 659.6 | 60.2 | 8618 | 33.23 |
| B@LiF [91] | 10 | neutral precipitation method | 599 | 120 | – | 80.6 |
| B@LiF [90] | 10 | neutral precipitation method | 599 | 114 | – | 205.6 |
| B@PVDF [97] | 4 | recrystallization method | 747 | 7 | 7500 | 89.2 |
| B@Viton [97] | 4 | recrystallization method | 741 | 13 | 7900 | 91.2 |
| B@THV [97] | 4 | recrystallization method | 745 | 9 | 8200 | 92.9 |
| B@TiO2 [116] | wet-chemistry method | 665.4 | 135.7 | – | 94.2 | |
| B@TiO2 [113] | 0.2 | spray-drying method | 580.6 | 96.1 | – | 76.7 |
| B@SnO2 [113] | 0.2 | spray-drying method | 570.6 | 106.1 | – | 50.7 |
| B@SnO2 [115] | wet-chemistry method | 400 | 50 | – | 99.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Liu, Y.; Zhao, B.; Liu, W.; Yan, Q.; Fu, X. High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties. Molecules 2023, 28, 3209. https://doi.org/10.3390/molecules28073209
Liu Y, Wang Y, Liu Y, Zhao B, Liu W, Yan Q, Fu X. High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties. Molecules. 2023; 28(7):3209. https://doi.org/10.3390/molecules28073209
Chicago/Turabian StyleLiu, Yang, Yinglei Wang, Yuezhou Liu, Baodong Zhao, Weixiao Liu, Qilong Yan, and Xiaolong Fu. 2023. "High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties" Molecules 28, no. 7: 3209. https://doi.org/10.3390/molecules28073209
APA StyleLiu, Y., Wang, Y., Liu, Y., Zhao, B., Liu, W., Yan, Q., & Fu, X. (2023). High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties. Molecules, 28(7), 3209. https://doi.org/10.3390/molecules28073209