3. Experimental
3.1. General Information
NMR spectra (
1H,
13C and
19F) were recorded on a Bruker AVANCE-III HD spectrometer (400, 101, and 376 MHz, respectively) equipped with a BBO probe in CDCl
3 or DMSO-
d6 using residual solvent signals as the internal standard. Spectral data are provided in the
Supplementary Materials (Figures S1–S75). High-resolution mass spectra were registered in MeCN solutions on a Bruker maXis impact (electrospray ionization, using HCO
2 Na–HCO
2H for calibration). IR spectra were measured on a FT-IR spectrometer Shimadzu IRAffinity-1 S equipped with an ATR sampling module. Reaction progress, the purity of isolated compounds, and R
f values were assessed by TLC on Silufol UV-254 plates. Column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Melting points were measured on Stuart SMP30 apparatus. Cyanoketones
14a–d,f,h,j,k [
37] and
14e [38] were synthesized according to the previously reported procedures and were identical to those described. All the reagents and solvents were purchased from commercial vendors and used as received.
3.2. General Procedure for Preparation of 4-(2-Aminophenyl)-2-aryl-4-oxo-butanenitriles 14g,i
These compounds were prepared in analogy to the method described in [
37]. A 25 mL round-bottom flask was charged with 3-aryl-2′-aminochalcone (2.00 mmol), acetic acid (120 mg, 0.114 mL, 2.00 mmol), and DMSO (6 mL). The mixture was vigorously stirred, and a solution of KCN (260 mg, 4.00 mmol) in water (0.5 mL) was added dropwise. Then, the reaction vessel was equipped with a reflux condenser, and the mixture was stirred at 50 °C for 1 h, while the reaction progress was monitored by TLC. Upon complete conversion, the mixture was diluted with water (30 mL) and extracted with dichloromethane (4 × 15 mL). Combined organic extracts were washed with water (4 × 15 mL), concentrated in vacuum, and purified by preparative column chromatography on silica gel eluting with 1:4 EtOAc/hexane.
4-(2-Aminophenyl)-2-(3-methoxyphenyl)-4-oxobutanenitrile (14g). Orange solid, mp (EtOH) 94.9–96.5 °C, Rf 0.63 (EtOAc/Hex, 1:2). Yield: 376 mg (1.40 mmol, 70%). 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 8.2 Hz, 1H), 7.37–7.31 (m, 2H), 7.30–7.24 (m, 1H), 6.95–6.86 (m, 2H), 6.70–6.57 (m, 2H), 6.31 (s, 2H), 4.47 (dd, J = 8.4, 5.7 Hz, 1H), 3.81 (s, 3H), 3.66 (dd, J = 17.5, 8.4 Hz, 1H), 3.44 (dd, J = 17.5, 5.9 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 196.4, 159.6, 150.8, 135.1, 130.6, 128.8 (2C), 127.5, 121.4, 117.6, 116.9, 116.0, 114.7 (2C), 55.5, 45.1, 31.4. IR, vmax/cm−1: 2939, 2255, 1676, 1646, 1238, 1539, 1622, 1236, 974. HRMS (ES TOF) calculated for [M + Na]+ C17H16 N2NaO2 303.1104, found 303.1098 (−2.0 ppm).
4-(2-Aminophenyl)-2-(3,4-dimethylphenyl)-4-oxobutanenitrile (14i). Yellow solid, mp (EtOH) 137.3–139.7 °C, Rf 0.39 (EtOAc/Hex, 1:2). Yield: 423 mg (1.52 mmol, 76%). 1H NMR (400 MHz, CDCl3) δ 7.59 (dd, J = 8.3, 1.5 Hz, 1H), 7.31–7.23 (m, 1H), 6.96 (dd, J = 8.2, 2.2 Hz, 1H), 6.91 (d, J = 2.2 Hz, 1H), 6.85 (d, J = 8.3 Hz, 1H), 6.66 (dd, J = 8.3, 1.1 Hz, 1H), 6.64–6.59 (m, 1H), 6.32 (s, 2H), 4.47 (dd, J = 8.4, 5.7 Hz, 1H), 3.90 (s, 3H), 3.87 (s, 3H), 3.67 (dd, J = 17.6, 8.4 Hz, 1H), 3.45 (dd, J = 17.5, 5.7 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 196.4, 150.8, 149.5, 149.0, 135.1, 130.6, 127.9, 121.3, 119.8, 117.6, 116.8, 116.0, 111.6, 110.6, 56.10, 56.08, 45.1, 31.8. IR, vmax/cm−1: 2931, 2247, 1684, 1507, 1254, 1248, 1018. HRMS (ES TOF) calculated for [M + Na]+ C18H18N2NaO 301.1311, found 301.1319 (2.7 ppm).
3.3. General Procedure for Preparation of 4-(2-Aminophenyl)-4-oxo-2-arylbutanoic Acids 15a–k
A 5 mL round-bottom flask equipped with a magnetic stirring bar was charged with the corresponding 4-(2-aminophenyl)-4-oxo-2-arylbutanenitrile 14a–k (2.00 mmol), concentrated aqueous HCl solution (2 mL), and refluxed for 2 h (TLC control). After the consumption of the starting material, the reaction mixture was cooled to room temperature, carefully neutralized with 10% solution of NaHCO3, and then washed with EtOAc (5 × 20 mL). The combined organic layer was concentrated and purified by column chromatography (EtOAc:Hex), followed by recrystallization from EtOAc.
4-(2-Aminophenyl)-4-oxo-2-phenylbutanoic acid (15a). Yellow solid, mp (EtOAc) 138.0–139.1 °C, Rf 0.40 (EtOAc/Hex, 1:1). Yield: 376 mg (1.40 mmol, 70%). 1H NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H), 7.84–7.81 (m, 1H), 7.39–7.32 (m, 4H), 7.29–7.21 (m, 2H), 7.18 (s, 2H), 6.77–6.72 (m, 1H), 6.55–6.48 (m, 1H), 4.06 (dd, J = 10.8, 3.7 Hz, 1H), 3.80 (dd, J = 17.9, 10.8 Hz, 1H), 3.20 (dd, J = 17.7, 3.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.5, 174.5, 151.1, 139.2, 134.2, 131.3, 128.6 (2C), 128.0 (2C), 127.1, 116.9, 116.2, 114.4, 46.2, 42.5. IR, vmax/cm−1: 3507, 3380, 2915, 2362, 1646, 1622, 1539, 1236, 974. HRMS (ES TOF) calculated for [M + Na]+ C16H15NNaO3 292.0944, found 292.0950 (2.1 ppm).
4-(2-Aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanoic acid (15b). Yellow solid, mp (EtOAc) 192.3–194.3 °C, Rf 0.27 (EtOAc/Hex, 1:1). Yield: 304 mg (1.02 mmol, 51%). 1H NMR (400 MHz, DMSO-d6) δ 12.24 (s, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.23 (t, J = 7.6 Hz, 1H), 7.18 (s, 2H), 6.90 (d, J = 8.2 Hz, 2H), 6.74 (d, J = 8.3 Hz, 1H), 6.56–6.47 (m, 1H), 4.00 (dd, J = 10.5, 3.7 Hz, 1H), 3.78 (dd, J = 17.7 Hz, 1H), 3.73 (s, 3H), 3.15 (dd, J = 17.7, 3.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.6, 174.8, 158.3, 151.1, 134.2, 131.3, 131.1, 129.0 (2C), 116.9, 116.3, 114.4, 114.0 (2C), 55.1, 45.3, 42.5. IR, vmax/cm−1: 3507, 3384, 2990, 2366, 1769, 1238, 827, 743. HRMS (ES TOF) calculated for [M + Na]+ C17H17NNaO4 322.1050, found 322.1059 (2.8 ppm).
4-(2-Aminophenyl)-2-(4-fluorophenyl)-4-oxobutanoic acid (15c). Yellow solid, mp (EtOAc) 157.8–158.9 °C, Rf 0.31 (EtOAc/Hex, 1:1). Yield: 315 mg (1.10 mmol, 55%). 1H NMR (400 MHz, DMSO-d6) δ 12.38 (s, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.45–7.40 (m, 2H), 7.25–7.16 (m, 4H), 7.15 (s, 1H), 6.74 (d, J = 8.3 Hz, 1H), 6.52 (t, J = 7.5 Hz, 1H), 4.08 (dd, J = 10.6, 3.9 Hz, 1H), 3.80 (dd, J = 17.8, 10.6 Hz, 1H), 3.20 (dd, J = 17.8, 4.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.4, 174.4, 161.3 (d, J = 242.8 Hz), 151.1, 135.4 (d, J = 2.9 Hz), 134.3, 131.3, 129.9 (d, J = 8.1 Hz, 2C), 116.9, 116.2, 115.3 (d, J = 21.3 Hz, 2C), 114.4, 45.4, 42.4. 19F NMR (376 MHz, DMSO-d6) δ −115.9. IR, vmax/cm−1: 3460, 2919, 2358, 1765, 1507, 1236, 978, 739. HRMS (ES TOF) calculated for [M + Na]+ C16H14FNNaO3 310.0850, found 310.0842 (2.6 ppm).
4-(2-Aminophenyl)-2-(4-chlorophenyl)-4-oxobutanoic acid (15d). Yellow solid, mp (EtOAc) 191.1–192.4 °C, Rf 0.37 (EtOAc/Hex, 1:1). Yield: 315 mg (1.04 mmol, 52%). 1H NMR (400 MHz, DMSO-d6) δ 12.44 (s, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.40 (s, 4H), 7.26–7.22 (m, 1H), 7.18 (s, 2H), 6.74 (d, J = 8.2 Hz, 1H), 6.52 (t, J = 7.5 Hz, 1H), 4.08 (dd, J = 10.5, 4.0 Hz, 1H), 3.78 (dd, J = 17.9, 10.5 Hz, 1H), 3.22 (dd, J = 17.8, 4.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.3, 174.2, 151.1, 138.3, 134.3, 131.7, 131.3, 129.9 (2C), 128.5 (2C), 116.9, 116.2, 114.4, 45.6, 42.2. IR, vmax/cm−1: 3519, 3376, 3002, 2358, 1773, 1244, 820, 749. HRMS (ES TOF) calculated for [M + Na]+ C16H14ClNNaO3 326.0554, found 326.0562 (2.6 ppm).
4-(2-Aminophenyl)-2-(4-bromophenyl)-4-oxobutanoic acid (15e). Yellow solid, mp (EtOAc) 196.5–197.8 °C, Rf 0.33 (EtOAc/Hex, 1:1). Yield: 298 mg (0.86 mmol, 43%). 1H NMR (400 MHz, DMSO-d6) δ 12.43 (s, 1H), 7.83–7.81 (m, 1H), 7.56–7.52 (m, 2H), 7.36–7.33 (m, 2H), 7.26–7.22 (m, 1H), 7.18 (s, 2H), 6.75–6.73 (m, 1H), 6.54–6.50 (m, 1H), 4.06 (dd, J = 10.5, 4.0 Hz, 1H), 3.78 (dd, J = 17.8, 10.5 Hz, 1H), 3.22 (dd, J = 17.7, 4.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.3, 174.1, 151.1, 139.0, 134.2, 131.4 (2C), 131.3, 130.3 (2C), 120.1, 116.9, 116.2, 114.4, 45.9, 42.2. IR, vmax/cm−1: 3360, 2994, 2358, 1771, 1244, 1053, 819, 751. HRMS (ES TOF) calculated for [M + Na]+ C16H14BrNNaO3 370.0049, found 370.0038 (−3.0 ppm).
4-(2-Aminophenyl)-4-oxo-2-(p-tolyl)butanoic acid (15f). Colorless solid, mp (EtOAc) 153.7–155.2 °C, Rf 0.34 (EtOAc/Hex, 1:1). Yield: 350 mg (1.24 mmol, 62%). 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.1 Hz, 1H), 7.30–7.20 (m, 4H), 7.15 (d, J = 8.1 Hz, 2H), 6.66–6.57 (m, 2H), 4.21 (dd, J = 10.4, 3.9 Hz, 1H), 3.88 (dd, J = 17.8, 10.5 Hz, 1H), 3.27 (dd, J = 17.8, 4.0 Hz, 1H), 2.33 (s, 3H).13C NMR (101 MHz, CDCl3) δ 199.4, 178.8, 150.5, 137.6, 134.9, 134.7, 131.0, 129.7 (2C), 128.0 (2C), 117.5, 115.9, 46.1, 43.1, 21.2. IR, vmax/cm−1: 3380, 2994, 1773, 1684, 1656, 1507, 1475, 1437, 1240. HRMS (ES TOF) calculated for [M + Na]+ C17H17NNaO3 306.1101, found 306.1088 (4.3 ppm).
4-(2-Aminophenyl)-2-(3-methoxyphenyl)-4-oxobutanoic acid (15g). Colorless solid, mp (EtOAc) 163.9–165.1 °C, Rf 0.26 (EtOAc/Hex, 1:1). Yield: 316 mg (1.06 mmol, 53%). 1H NMR (400 MHz, DMSO-d6) δ 12.22 (s, 1H), 7.82 (dd, J = 8.3, 1.5 Hz, 1H), 7.32–7.25 (m, 2H), 7.28–7.19 (m, 1H), 7.16 (d, J = 13.6 Hz, 2H), 6.94–6.86 (m, 2H), 6.74 (dd, J = 8.4, 1.2 Hz, 1H), 6.52 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 4.00 (dd, J = 10.8, 3.9 Hz, 1H), 3.81–3.74 (m, 1H), 3.73 (s, 3H), 3.15 (dd, J = 17.8, 3.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 199.6, 174.7, 158.3, 151.1, 134.2, 131.3, 131.1, 129.0 (2C), 116.9, 116.2, 114.4, 114.0 (2C), 55.1, 45.3, 42.5. IR, vmax/cm−1: 3392, 2994, 1775, 1735, 1698, 1654, 1558, 1453, 1244. HRMS (ES TOF) calculated for [M + Na]+ C17H17NNaO4 322.1050, found 322.1055 (1.6 ppm).
4-(2-Aminophenyl)-2-(4-isopropylphenyl)-4-oxobutanoic acid (15h). Colorless solid, mp (EtOAc) 121.1–122.9 °C, Rf 0.6 (EtOAc/Hex, 1:1). Yield: 348 mg (1.12 mmol, 56%). 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 6.7 Hz, 1H), 7.29 (s, 2H), 7.24–7.16 (m, 5 H), 6.74 (d, J = 7.2 Hz, 1H), 6.51 (t, J = 7.6 Hz, 1H), 4.01 (dd, J = 10.8, 3.7 Hz, 1H), 3.78 (dd, J = 17.8, 10.8 Hz, 1H), 3.17 (dd, J = 17.8, 3.7 Hz, 1H), 2.86 (p, J = 6.9 Hz, 1H), 1.19 (d, J = 7.0 Hz, 6 H). 13C NMR (101 MHz, DMSO-d6) δ 199.6, 174.6, 151.06, 147.2, 136.6, 134.2, 131.3, 127.9 (2C), 126.5 (2C), 116.9, 116.2, 114.4, 45.8, 42.5, 33.1, 24.0 (2C). IR, vmax/cm−1: 3280, 2954, 1712, 1668, 1556, 1514, 1442, 1427, 1251, 1062. HRMS (ES TOF) calculated for [M + Na]+ C19H21NNaO3 334.1414, found 334.1422 (4.1 ppm).
4-(2-Aminophenyl)-2-(3,4-dimethylphenyl)-4-oxobutanoic acid (15i). Yellowish solid, mp (EtOAc) 138.0–139.8 °C, Rf 0.42 (EtOAc/Hex, 1:1). Yield: 439 mg (1.48 mmol, 74%). 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 7.82 (dd, J = 8.2, 1.6 Hz, 1H), 7.30–7.25 (m, 2H), 7.24–7.21 (m, 1H), 7.20–7.15 (m, 3H), 6.74 (dd, J = 8.5, 1.2 Hz, 1H), 6.51 (t, J = 7.5 Hz, 1H), 4.02 (dd, J = 10.7, 3.6 Hz, 1H), 3.78 (dd, J = 17.8, 10.8 Hz, 1H), 3.17 (dd, J = 17.8, 3.8 Hz, 1H), 2.57 (q, J = 7.6 Hz, 2H), 1.20–1.14 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 199.5, 174.6, 151.1, 142.6, 136.4, 134.2, 131.3, 128.0 (2C), 127.9 (2C), 116.9, 116.2, 114.4, 45.8, 42.5, 27.8, 15.7. IR, vmax/cm−1: 3380, 2974, 1702, 1688, 1650, 1558, 1503, 1455, 1234, 1189. HRMS (ES TOF) calculated for [M + Na]+ C18H19NNaO3 320.1257, found 320.1248 (−2.8 ppm).
4-(2-Aminophenyl)-2-(3-chlorophenyl)-4-oxobutanoic acid (15j). Colorless solid, mp (EtOAc) 164.0–165.3 °C, Rf 0.42 (EtOAc/Hex, 1:1). Yield: 339 mg (1.12 mmol, 56%). 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.1 Hz, 1H), 7.40 (s, 1H), 7.29 (d, J = 6.7 Hz, H), 6.65 (d, J = 7.4 Hz, 2H), 4.25 (dd, J = 10.4, 4.0 Hz, 1H), 3.89 (dd, J = 17.8, 10.3 Hz, 1H), 3.32 (dd, J = 17.7, 4.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) 198.8, 177.4, 150.6, 139.8, 134.9, 134.8, 131.0, 130.3, 128.3, 128.2, 126.5, 117.5, 117.3, 116.0, 46.1, 42.9. IR, vmax/cm−1: 3002, 1775, 1759, 1708, 1618, 1562, 1435, 1240, 1053. HRMS (ES TOF) calculated for [M + Na]+ C17H14ClNNaO3 326.0554, found 306.0541 (4.1 ppm).
4-(2-Aminophenyl)-2-(4-ethylphenyl)-4-oxobutanoic acid (15k). Yellowish solid, mp (EtOAc) 138.0–139.8 °C, Rf 0.42 (EtOAc/Hex, 1:1). Yield: 439 mg (1.48 mmol, 74%). 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 7.82 (dd, J = 8.2, 1.6 Hz, 1H), 7.30–7.25 (m, 2H), 7.24–7.21 (m, 1H), 7.20–7.15 (m, 3H), 6.74 (dd, J = 8.5, 1.2 Hz, 1H), 6.51 (t, J = 7.5 Hz, 1H), 4.02 (dd, J = 10.7, 3.6 Hz, 1H), 3.78 (dd, J = 17.8, 10.8 Hz, 1H), 3.17 (dd, J = 17.8, 3.8 Hz, 1H), 2.57 (q, J = 7.6 Hz, 2H), 1.20–1.14 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 199.5, 174.6, 151.1, 142.6, 136.4, 134.2, 131.3, 128.0 (2C), 127.9 (2C), 116.9, 116.2, 114.4, 45.8, 42.5, 27.8, 15.7. IR, vmax/cm−1: 3380, 2974, 1702, 1688, 1650, 1558, 1503, 1455, 1234, 1189. HRMS (ES TOF) calculated for [M + Na]+ C18H19NNaO3 320.1257, found 320.1248 (−2.8 ppm).
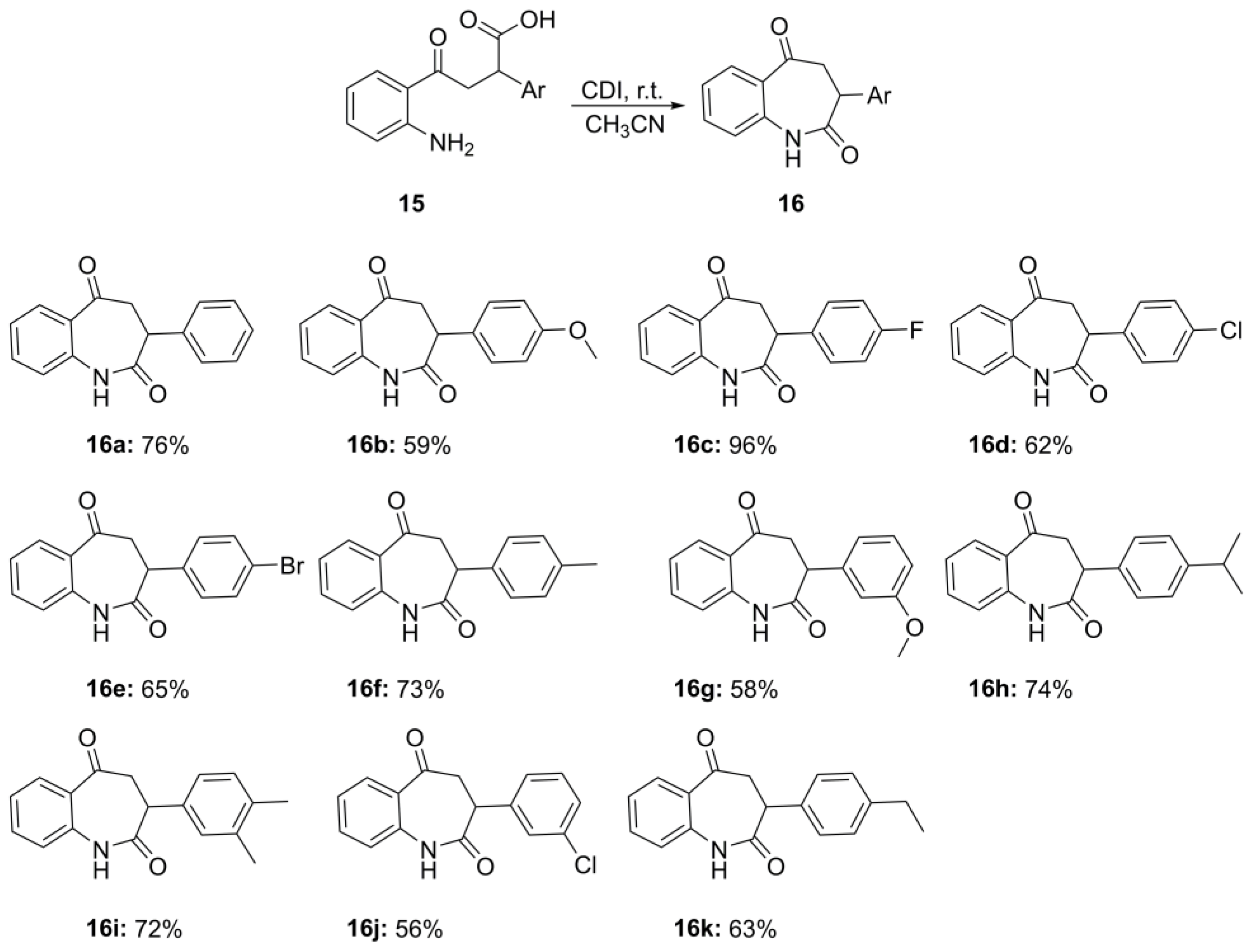
3.4. General Procedure for Preparation of 3-Aryl-3,4-dihydro-1H-benzo[b]azepine-2,5-diones 16a–k
A 5 mL round-bottom flask equipped with a magnetic stirring bar was charged with the corresponding 4-(2-aminophenyl)-4-oxo-2-arylbutanoic acid 15a–k (1.00 mmol), 1,1′-carbonyldiimidazole (162 mg, 1.00 mmol), and acetonitrile (0.1 mL). The reaction mixture was left stirring at room temperature for 4 h. After this period of time, 20 mL of water was poured into the reaction mixture and then, extracted with EtOAc (5 × 20 mL). The organic phase was concentrated on a rotary evaporator and residue was purified by column chromatography using mixture EtOAc/Hex.
3-Phenyl-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16a). Colorless solid, mp (EtOAc) 180.3–181.4 °C, Rf 0.48 (EtOAc/Hex, 1:1). Yield: 190 mg (0.76 mmol, 76%). 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 7.82–7.80 (m, 1H), 7.59–7.55 (m, 1H), 7.31–7.27 (m, 2H), 7.24–7.16 (m, 5 H), 4.29–4.25 (m, 1H), 3.60 (dd, J = 18.0, 12.0 Hz, 1H), 3.02–2.97 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 197.8, 173.5, 139.0, 138.3, 134.4, 130.0, 128.7 (2C), 128.1 (2C), 126.9, 126.7, 123.6, 121.9, 45.8, 43.2. IR, vmax/cm−1: 3312, 2919, 2366, 1670, 1485, 1250. HRMS (ES TOF) calculated for [M + Na]+ C16H13NNaO2 274.0838, found 274.0843 (1.8 ppm).
3-(4-Methoxyphenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16b). Colorless solid, mp (EtOAc) 174.8–176.2 °C, Rf 0.32 (EtOAc/Hex, 1:1). Yield: 166 mg (0.59 mmol, 59%). 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H), 7.99 (d, J = 7.9 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 7.12 (d, J = 8.7 Hz, 2H), 6.96 (d, J = 8.1 Hz, 1H), 6.87 (d, J = 8.1 Hz, 2H), 4.16 (d, J = 11.5 Hz, 1H), 3.79 (s, 3H), 3.53–3.45 (m, 1H), 3.20 (d, J = 18.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 197.8, 174.7, 159.0, 137.7, 134.8, 131.1, 129.5 (2C), 128.7, 127.5, 125.0, 121.7, 114.2 (2C), 55.4, 46.6, 43.4. IR, vmax/cm−1: 2923, 2374, 1771, 1662, 1514, 1248, 1027, 843, 767. HRMS (ES TOF) calculated for [M + Na]+ C17H15NNaO3 304.0944, found 304.0944 (−1.6 ppm).
3-(4-Fluorophenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16c). Colorless solid, mp (EtOAc) 212.5–214.6 °C, Rf 0.34 (EtOAc/Hex, 1:1). Yield: 258 mg (0.96 mmol, 96%). 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 7.83–7.80 (m, 1H), 7.60–7.56 (m, 1H), 7.30–7.25 (m, 2H), 7.22–7.19 (m, 2H), 7.16–7.10 (m, 2H), 4.34–4.30 (m, 1H), 3.61 (dd, J = 18.2, 12.4 Hz, 1H), 2.96–2.91 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 197.9, 173.3, 161.2 (d, J = 242.5 Hz), 138.9, 134.7 (d, J = 3.3 Hz), 134.4, 130.8 (d, J = 8.4 Hz, 2C), 130.1, 126.8, 123.7, 121.9, 114.8 (d, J = 21.3 Hz, 2C), 46.1, 42.2. 19F NMR (376 MHz, DMSO-d6) δ −116.1. IR, vmax/cm−1: 3285, 2915, 2334, 1779, 1652, 1376, 1246. HRMS (ES TOF) calculated for [M + Na]+ C16H12FNNaO2 292.0744, found 292.0747 (−1.1 ppm).
3-(4-Chlorophenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16d). Colorless solid, mp (EtOAc) 191.8–193.4 °C, Rf 0.33 (EtOAc/Hex, 1:1). Yield: 167 mg (0.62 mmol, 62%). 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H), 7.83–7.80 (m, 1H), 7.60–7.56 (m, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.6 Hz, 2H), 7.22–7.19 (m, 2H), 4.33 (d, J = 11.7 Hz, 1H), 3.62 (dd, J = 18.1, 12.5 Hz, 1H), 2.94 (d, J = 17.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 197.8, 173.1, 138.8, 137.6, 134.4, 131.6, 130.8 (2C), 130.1, 128.0 (2C), 126.8, 123.7, 121.9, 45.8, 42.3. IR, vmax/cm−1: 3297, 2994, 2370, 1771, 1383, 1242, 1057. HRMS (ES TOF) calculated for [M + Na]+ C16H12ClNNaO2 308.0449, found 308.0440 (−2.9 ppm).
3-(4-Bromophenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16e). Colorless solid, mp (EtOAc) 191.8–193.4 °C, Rf 0.46 (EtOAc/Hex, 1:1). Yield: 214 mg (0.65 mmol, 65%). 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H), 7.83–7.80 (m, 1H), 7.60–7.56 (m, 1H), 7.51–7.48 (m, 2H), 7.22–7.19 (m, 4H), 4.32 (dd, J = 12.5, 1.9 Hz, 1H), 3.61 (dd, J = 18.0, 12.5 Hz, 1H), 2.93 (dd, J = 18.1, 2.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 197.8, 173.1, 138.8, 138.0, 134.4, 131.2 (2C), 130.9 (2C), 130.1, 126.8, 123.8, 121.9, 120.1, 45.8, 42.4. IR, vmax/cm−1: 2927, 2386, 1773, 1664, 1244, 1043, 769. HRMS (ES TOF) calculated for [M + Na]+ C16H12BrNNaO2 351.9944, found 351.9951 (2.0 ppm).
3-(p-Tolyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16f). Colorless solid, mp (EtOAc) 158.3–160.37 °C, Rf 0.34 (EtOAc/Hex, 1:2). Yield: 193 mg (0.73 mmol, 73%). 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 8.2 Hz, 2H), 7.08 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 8.2 Hz, 1H), 3.50 (dd, J = 18.1, 11.8 Hz, 1H), 3.21 (dd, J = 18.1, 2.0 Hz, 1H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 197.8, 174.9, 137.8, 137.4, 134.7, 133.6, 131.0, 129.5 (2C), 128.3 (2C), 127.4, 124.8, 121.8, 46.4, 43.8, 21.2. IR, vmax/cm−1: 3233, 2994, 1737, 1688, 1562, 1509, 1473, 1385, 1250, 1169. HRMS (ES TOF) calculated for [M + Na]+ C17H17NNaO4 288.0995, found 288.0985 (3.5 ppm).
3-(3-Methoxyphenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16g). Colorless solid, mp (EtOAc) 169.7–170.2 °C, Rf 0.47 (EtOAc/Hex, 1:1). Yield: 163 mg (0.58 mmol, 58%). 1H NMR (400 MHz, CDCl3) δ 8.76 (s, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 7.5 Hz, 1H), 7.11 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 8.1 Hz, 1H), 6.86 (d, J = 8.2 Hz, 2H), 4.19–4.11 (m, 1H), 3.78 (s, 3H), 3.48 (dd, J = 18.1, 11.7 Hz, 1H), 3.24–3.14 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 197.8, 175.1, 159.0, 137.8, 134.7, 131.0, 129.5 (2C), 128.7, 127.5, 124.9, 121.8, 114.2 (2C), 55.3, 46.6, 43.4. IR, vmax/cm−1: 3209, 2990, 1733, 1686, 1558, 1505, 1475, 1433, 1244. HRMS (ES TOF) calculated for [M + Na]+ C17H15NNaO3 304.0944, found 304.0939 (1.8 ppm).
3-(4-Isopropylphenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16h). Colorless solid, mp (EtOAc) 139.2–140.1 °C, Rf 0.44 (EtOAc/Hex, 1:2). Yield: 217 mg (0.74 mmol, 74%). 1H NMR (400 MHz, DMSO-d6) δ 10.32 (s, 1H), 7.81 (dd, J = 7.9, 1.7 Hz, 1H), 7.57 (ddd, J = 8.2, 7.2, 1.7 Hz, 1H), 7.24–7.17 (m, 2H), 7.16–7.09 (m, 4H), 4.22 (dd, J = 12.0, 1.9 Hz, 1H), 3.56 (dd, J = 17.9, 12.1 Hz, 1H), 2.98 (dd, J = 18.0, 1.9 Hz, 1H), 2.85 (hept, J = 6.9 Hz, 1H), 1.18 (d, J = 7.3 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 197.9, 173.6, 146.80, 139.0, 135.7, 134.3, 130.0, 128.6 (2C), 126.7, 126.0 (2C), 123.6, 121.8, 45.9, 42.8, 33.1, 24.0, 23.9. IR, vmax/cm−1: 3209, 2990, 1733, 1686, 1558, 1519, 1505, 1457, 1433, 1393, 1244. HRMS (ES TOF) calculated for [M + Na]+ C19H19NNaO2 316.1308, found 316.1316 (2.5 ppm).
3-(3,4-Dimethylphenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16i). Colorless solid, mp (EtOAc) 194.6–195.9 °C, Rf 0.28 (EtOAc/Hex, 1:1). Yield: 200 mg (0.72 mmol, 72%). 1H NMR (400 MHz, CDCl3) δ 7.99 (dd, J = 8.0, 1.7 Hz, 1H), 7.90 (s, 1H), 7.55–7.50 (m, 1H), 7.25–7.20 (m, 1H), 6.96 (d, J = 8.1 Hz, 1H), 6.82 (d, J = 8.2 Hz, 1H), 6.76–6.70 (m, 2H), 4.17 (dd, J = 11.8, 2.0 Hz, 1H), 3.85 (d, J = 6.5 Hz, 6 H), 3.50 (dd, J = 18.1, 11.8 Hz, 1H), 3.23 (dd, J = 18.1, 2.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 197.7, 174.4, 149.0, 148.6, 137.6, 134.8, 131.2, 129.0, 127.6, 125.1, 121.6, 120.6, 111.6, 111.3, 56.0 (2C), 46.6, 43.7. IR, vmax/cm−1: 3193, 2939, 1775, 1737, 1668, 1521, 1504, 1473, 1455, 1242, 1151. HRMS (ES TOF) calculated for [M + Na]+ C18H17NNaO2 302.1151, found 302.1143 (−2.6 ppm).
3-(3-Chlorophenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16j). Colorless solid, mp (EtOAc) 221.7–222.7 °C, Rf 0.4 (EtOAc/Hex, 1:2). Yield: 160 mg (0.56 mmol, 56%). 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 7.9 Hz, 1H), 7.89 (s, 1H), 7.59–7.50 (m, 1H), 7.33–7.25 (m, 3H), 7.21 (s, 1H), 7.10 (ddd, J = 5.7, 3.2, 1.9 Hz, 1H), 6.97 (d, J = 8.1 Hz, 1H), 4.17 (dd, J = 12.3, 2.0 Hz, 1H), 3.49 (dd, J = 18.2, 12.3 Hz, 1H), 3.18 (dd, J = 18.2, 2.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 197.3, 173.5, 138.7, 137.3, 134.9, 131.3, 130.1, 128.9, 128.1, 127.6, 126.8, 125.4, 121.8, 46.4, 43.7, 29.9. IR, vmax/cm−1: 3293, 2990, 1773, 1684, 1560, 1477, 1437, 1244. HRMS (ES TOF) calculated for [M + Na]+ C16H12ClNNaO2 308.0449, found 308.0456 (2.3 ppm).
3-(4-Ethylphenyl)-3,4-dihydro-1H-benzo[b]azepine-2,5-dione (16k). Colorless solid, mp (EtOAc) 159.8–162.1 °C, Rf 0.28 (EtOAc/Hex, 1:1). Yield: 176 mg (0.63 mmol, 63%). 1H NMR (400 MHz, DMSO-d6) δ 10.32 (s, 1H), 7.80 (dd, J = 7.9, 1.7 Hz, 1H), 7.56 (ddd, J = 8.1, 7.2, 1.7 Hz, 1H), 7.22–7.16 (m, 2H), 7.12 (s, 4H), 4.21 (dd, J = 11.9, 1.9 Hz, 1H), 3.56 (dd, J = 17.9, 11.9 Hz, 1H), 2.99 (dd, J = 17.9, 1.9 Hz, 1H), 2.56 (d, J = 7.6 Hz, 2H), 1.16 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 197.8, 173.6, 142.2, 139.0, 135.4, 134.4, 130.0, 128.6 (2C), 127.5 (2C), 126.6, 123.6, 121.8, 45.8, 42.9, 27.8, 15.6. IR, vmax/cm−1: 3225, 2990, 1769, 1700, 1654, 1612, 1522, 1511, 1417, 1240. HRMS (ES TOF) calculated for [M + Na]+ C18H17NNaO2 302.1151, found 302.1160 (3.0 ppm).
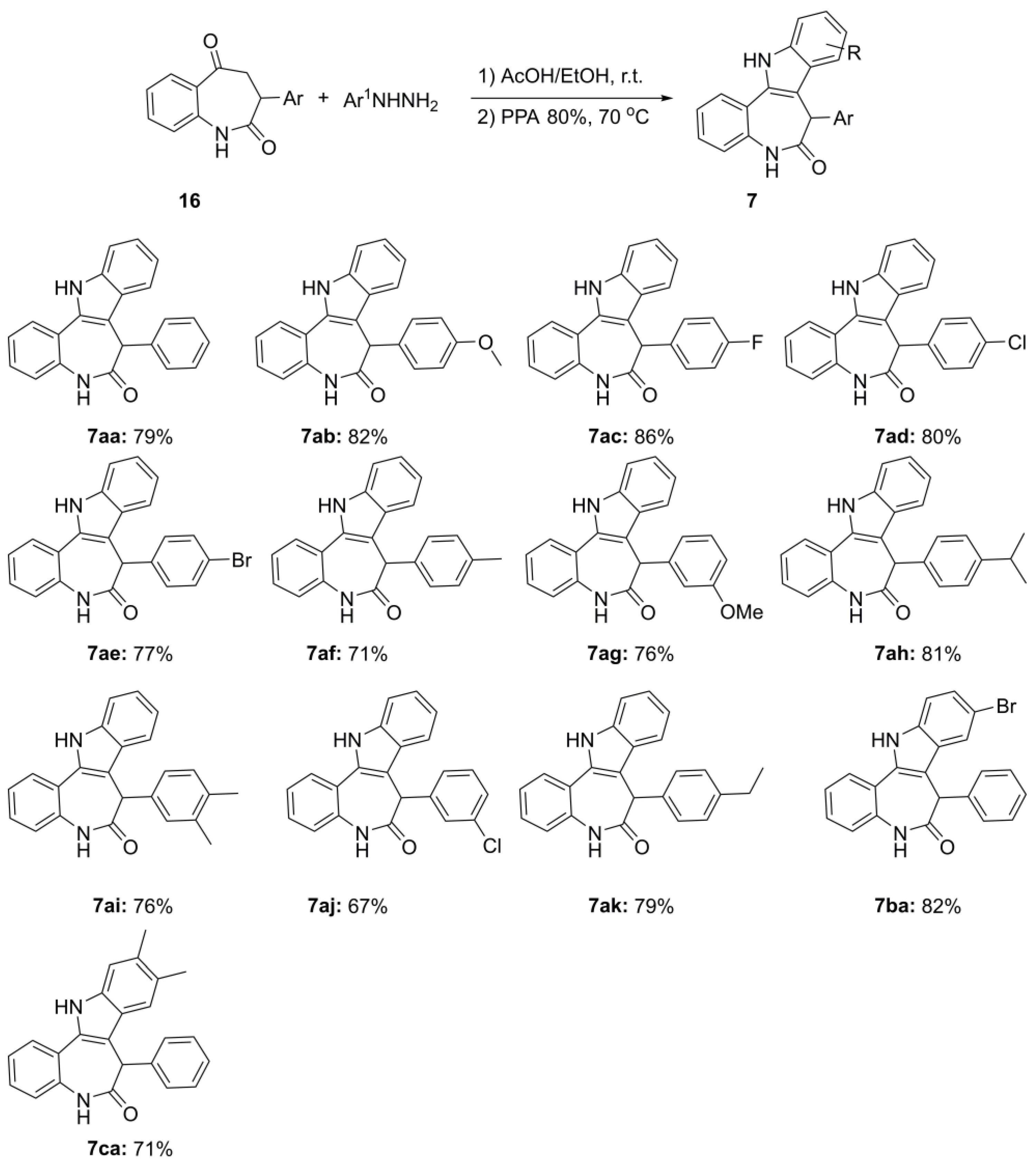
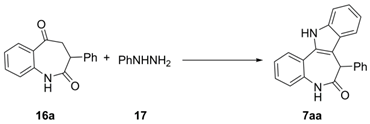
3.5. General Procedure for Preparation of 7-Arylpaullones (7aa–ak,ba,ca)
A 5 mL round-bottom flask equipped with magnetic stirring bar was charged with the corresponding keto-lactam 16a–k (0.50 mmol), phenylhydrazine (0.50 mmol), ethanol (0.25 mL), and AcOH (30 mg, 0.029 mL, 0.50 mmol). The reaction mixture was left stirring at room temperature for 30 min. After this, 0.5 g of 80% PPA was added and stirring continued at 70 °C for another 30 min. The reaction mixture was then poured into water (20 mL) and carefully neutralized with concentrated aqueous ammonia solution. The formed precipitate was filtered off, open-air dried, and purified by column chromatography using mixture EtOAc/Hex, followed by recrystallization from EtOAc.
7-Phenyl-7,12-dihydrobenzo [2,3]azepino[4,5-b]indol-6(5H)-one (7aa). Colorless solid, mp (EtOAc) 224.9–226.1 °C, Rf 0.67 (EtOAc/Hex, 1:1). Yield: 128 mg (0.40 mmol, 79%). 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 10.34 (s, 1H), 7.77–7.69 (m, 2H), 7.50 (d, J = 8.1 Hz, 1H), 7.25–7.17 (m, 3H), 7.15 (d, J = 7.4 Hz, 1H), 7.11–7.02 (m, 4H), 6.91 (d, J = 7.4 Hz, 2H), 5.52 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.6, 137.5, 137.4, 134.7, 132.6, 128.2 (2C), 128.1, 128.0, 127.6, 126.6, 126.4 (2C), 123.4, 122.6, 122.5, 121.3, 119.5, 117.8, 111.6, 110.1, 48.8. IR, vmax/cm−1: 3229, 2994, 1757, 1652, 1558, 1487, 1459, 1248, 1063. HRMS (ES TOF) calculated for [M + Na]+ C22H16N2NaO 347.1155, found 347.1149 (−1.7 ppm).
7-(4-Methoxyphenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ab). Colorless solid, mp (EtOAc) 284.2–287.2 °C, Rf 0.38 (EtOAc/Hex, 1:1). Yield: 145 mg (0.41 mmol, 82%) 1H NMR (400 MHz, DMSO-d6) δ 11.73 (s, 1H), 10.31 (s, 1H), 7.72 (t, J = 6.6 Hz, 2H), 7.49 (d, J = 8.2 Hz, 1H), 7.23–7.13 (m, 3H), 7.08 (t, J = 7.5 Hz, 2H), 6.82 (d, J = 8.8 Hz, 2H), 6.64 (d, J = 8.8 Hz, 2H), 5.45 (s, 1H), 3.58 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.8, 157.9, 137.5, 134.8, 132.5, 129.2, 128.0, 127.53, 127.50 (2C), 126.4, 123.4, 122.6, 122.5, 121.3, 119.4, 117.8, 113.6 (2C), 111.5, 110.4, 54.9, 48.1. IR, vmax/cm−1: 3297, 2978, 2366, 1773, 1638, 1240, 1039. HRMS (ES TOF) calculated for [M + Na]+ C23H18N2NaO2 377.1260, found 377.1251 (−2.4 ppm).
7-(4-Fluorophenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ac). Colorless solid, mp (EtOAc) 298.8–303.1 °C, Rf 0.36 (EtOAc/Hex, 1:1). Yield: 147 mg (0.43 mmol, 86%). 1H NMR (400 MHz, DMSO-d6) δ 11.77 (s, 1H), 10.37 (s, 1H), 7.76–7.71 (m, 2H), 7.50 (d, J = 8.2 Hz, 1H), 7.24–7.19 (m, 2H), 7.17–7.13 (m, 1H), 7.11–7.05 (m, 2H), 6.92 (d, J = 7.2 Hz, 4H), 5.51 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.4, 160.8 (d, J = 242.8 Hz), 137.5, 134.6, 133.5 (d, J = 2.9 Hz), 132.6, 128.3 (d, J = 8.4 Hz, 2C), 128.1, 127.4, 126.4, 123.5, 122.6, 122.5, 121.4, 119.5, 117.8, 115.0 (d, J = 21.3 Hz, 2C), 111.6, 110.1, 48.0. 19F NMR (376 MHz, DMSO-d6) δ -116.3. IR, vmax/cm−1: 3297, 2919, 1726, 1640, 1499, 1220, 1029. HRMS (ES TOF) calculated for [M + Na]+ C22H15FN2NaO 365.1061, found 365.1072 (3.0 ppm).
7-(4-Chlorophenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ad). Colorless solid, mp (EtOAc) 291.0–293.0 °C, Rf 0.33 (EtOAc/Hex, 1:1). Yield: 143 mg (0.40 mmol, 80%). 1H NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H), 10.40 (s, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.72–7.70 (m, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.25–7.19 (m, 2H), 7.17–7.13 (m, 3H), 7.11–7.05 (m, 2H), 6.90–6.88 (m, 2H), 5.52 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 137.5, 136.4, 134.5, 132.6, 131.2, 128.24 (2C), 128.23 (2C), 128.1, 127.5, 126.4, 123.6, 122.7, 122.4, 121.4, 119.6, 117.9, 111.6, 109.8, 48.1. IR, vmax/cm−1: 3281, 3010, 2362, 1771, 1238, 1051. HRMS (ES TOF) calculated for [M + Na]+ C22H15ClN2NaO 381.0765, found 381.0770 (1.3 ppm).
7-(4-Bromophenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ae). Colorless solid, mp (EtOAc) 273.2–274.5 °C, Rf 0.47 (EtOAc/Hex, 1:1). Yield: 155 mg (0.39 mmol, 77%). 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 1H), 10.40 (s, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.72 (d, J = 6.5 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 7.29 (d, J = 8.6 Hz, 2H), 7.25–7.19 (m, 2H), 7.17–7.13 (m, 1H), 7.11–7.06 (m, 2H), 6.83 (d, J = 8.6 Hz, 2H), 5.50 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.1, 137.5, 136.9, 134.5, 132.6, 131.1 (2C), 128.6 (2C), 128.2, 127.5, 126.5, 123.6, 122.7, 122.4, 121.4, 119.8, 119.6, 117.9, 111.6, 109.7, 48.1. IR, vmax/cm−1: 2994, 2362, 1773, 1248, 1053. HRMS (ES TOF) calculated for [M + Na]+ C22H15BrN2NaO 425.0260, found 425.0272 (2.8 ppm).
7-(p-Tolyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7af). Yellowish solid, mp (EtOAc) 252.3–255.1 °C, Rf 0.55 (EtOAc/Hex, 1:1). Yield: 125 mg (0.36 mmol, 71%). 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 10.31 (s, 1H), 7.75–7.67 (m, 2H), 7.49 (d, J = 8.2 Hz, 1H), 7.24–7.17 (m, 2H), 7.16–7.11 (m, 1H), 7.10–7.03 (m, 2H), 6.88 (d, J = 7.9 Hz, 2H), 6.78 (d, J = 8.3 Hz, 2H), 5.46 (s, 1H), 2.10 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.7, 137.5, 135.7, 134.7, 134.4, 132.5, 128.8 (2C), 128.0, 127.6, 126.4, 126.3 (2C), 123.4, 122.6, 122.5, 121.3, 119.4, 117.8, 111.5, 110.2, 48.4, 20.5. IR, vmax/cm−1: 3225, 2991, 1749, 1655, 1555, 1482, 1452, 1248. HRMS (ES TOF) calculated for [M + Na]+ C23H18N2NaO 361.1311, found 361.1305 (−1.7 ppm).
7-(3-Methoxyphenyl-7,12-dihydrobenzo [2,3]azepino [4,5-b]indol-6(5H)-one (7ag). Colorless solid, mp (EtOAc) 268.6–270.9 °C, Rf 0.45 (EtOAc/Hex, 1:1). Yield: 135 mg (0.38 mmol, 76%). 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 10.30 (s, 1H), 7.72 (t, J = 6.9 Hz, 2H), 7.49 (d, J = 8.1 Hz, 1H), 7.18 (dt, J = 24.3, 7.2 Hz, 3H), 7.07 (d, J = 7.8 Hz, 2H), 6.81 (d, J = 8.6 Hz, 2H), 6.64 (d, J = 8.6 Hz, 2H), 5.44 (s, 1H), 3.58 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.8, 157.9, 137.5, 134.7, 132.5, 129.2, 128.0, 127.52, 127.50 (2C), 126.4, 123.4, 122.6, 122.5, 121.3, 119.4, 117.8, 113.6 (2C), 111.6, 110.4, 54.9, 48.1. IR, vmax/cm−1: 3015, 2895, 1773, 1759, 1654, 1556, 1506, 1451, 1363, 1159. HRMS (ES TOF) calculated for [M + Na]+ C23H18N2NaO2 377.1260, found 377.1256 (−1.1 ppm).
7-(4-Isopropylphenyl)-7,12-dihydrobenzo [2,3]azepino [4,5-b]indol-6(5H)-one (7ah). Colorless solid, mp (EtOAc) 234.6–235.9 °C, Rf 0.51 (EtOAc/Hex, 1:1). Yield: 148 mg (0.40 mmol, 81%). 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 10.33 (d, J = 1.7 Hz, 1H), 7.78–7.68 (m, 2H), 7.48 (d, J = 8.1 Hz, 1H), 7.24–7.18 (m, 2H), 7.18–7.13 (m, 1H), 7.11–7.06 (m, 2H), 6.96 (d, J = 8.3 Hz, 2H), 6.84 (d, J = 8.1 Hz, 2H), 5.47 (s, 1H), 2.69 (p, J = 6.9 Hz, 1H), 1.04 (dd, J = 6.9, 3.3 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 171.7, 146.7, 137.5, 134.9, 134.7, 132.4, 128.1, 127.5, 126.4 (3 C), 126.2 (2C), 123.4, 122.6, 122.3, 121.3, 119.4, 117.8, 111.5, 110.1, 48.63, 32.8, 23.8, 23.7. IR, vmax/cm−1: 2986, 2891, 1777, 1700, 1638, 1521, 1457, 1419, 1244, 1049. HRMS (ES TOF) calculated for [M + Na]+ C25H22N2NaO 389.1624, found 389.1630 (1.5 ppm).
7-(3,4-Dimethylphenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ai). Colorless solid, mp (EtOAc) 192.8–195.4 °C, Rf 0.55 (EtOAc). Yield: 134 mg (0.38 mmol, 76%). 1H NMR (400 MHz, DMSO-d6) δ 11.73 (s, 1H), 10.26 (s, 1H), 7.74 (dd, J = 18.3, 7.8 Hz, 2H), 7.52–7.45 (m, 1H), 7.28–7.15 (m, 3H), 7.08 (t, J = 7.1 Hz, 2H), 6.69–6.63 (m, 1H), 6.51–6.45 (m, 2H), 5.45 (s, 1H), 3.58 (d, J = 2.3 Hz, 3H), 3.38 (d, J = 2.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.8, 148.1, 147.5, 137.5, 134.9, 132.5, 129.6, 128.1, 127.4, 126.4, 123.5, 122.7, 122.6, 121.3, 119.4, 119.0, 117.8, 111.6, 111.5, 110.3, 110.2, 55.3, 55.0, 48.6. IR, vmax/cm−1: 2990, 1773, 1759, 1634, 1560, 1507, 1457, 1378, 1246, 1135, 1055. HRMS (ES TOF) calculated for [M + Na]+ C24H20N2NaO 375.1468, found 375.1475 (1.8 ppm).
7-(3-Chlorophenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7aj). Colorless solid, mp (EtOAc) 243.9–245.9 °C, Rf 0.5 (EtOAc/Hex, 1:1). Yield: 120 mg (0.34 mmol, 67%). 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 1H), 10.41 (s, 1H), 7.78 (d, J = 7.9 Hz, 1H), 7.73 (dd, J = 7.7, 1.7 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.25–7.20 (m, 3H), 7.18 (dd, J = 7.5, 1.4 Hz, 1H), 7.15–7.11 (m, 2H), 7.11–7.04 (m, 1H), 6.90–6.86 (m, 1H), 6.85 (d, J = 1.7 Hz, 1H), 5.57 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.0, 139.9, 137.5, 134.5, 132.8, 132.7, 130.2, 128.2, 127.4, 126.7, 126.5, 126.2, 125.2, 123.7, 122.7, 122.4, 121.4, 119.6, 117.9, 111.6, 109.5, 48.3. IR, vmax/cm−1: 3002, 1773, 1759, 1638, 1556, 1509, 1451, 1378, 1242, 1053. HRMS (ES TOF) calculated for [M + Na]+ C22H15ClN2NaO 381.0765, found 381.0773 (2.1 ppm).
7-(4-Ethylphenyl)-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ak). Colorless solid, mp (EtOAc) 241.0–243.2 °C, Rf 0.49 (EtOAc). Yield: 139 mg (0.4 mmol, 79%). 1H NMR (400 MHz, DMSO-d6) δ 11.73 (s, 1H), 10.32 (d, J = 1.7 Hz, 1H), 7.76–7.69 (m, 2H), 7.49 (d, J = 8.2 Hz, 1H), 7.25–7.17 (m, 2H), 7.17–7.12 (m, 1H), 7.11–7.05 (m, 2H), 6.92 (d, J = 8.3 Hz, 2H), 6.82 (d, J = 8.3 Hz, 2H), 5.47 (s, 1H), 2.40 (q, J = 7.5 Hz, 2H), 1.02 (t, J = 7.6 Hz, 3H).13C NMR (101 MHz, DMSO-d6) δ 171.7, 142.0, 137.5, 134.7 (2C), 132.5, 128.0, 127.7 (2C), 127.6, 126.4, 126.3 (2C), 123.4, 122.5, 122.4, 121.3, 119.4, 117.8, 111.5, 110.2, 48.5, 27.6, 15.4. IR, vmax/cm−1: 3283, 3007, 2365, 1773, 1652, 1555, 1481, 1229, 1055. HRMS (ES TOF) calculated for [M + Na]+ C24H20N2NaO 375.1468, found 375.1457 (−2.9 ppm).
9-Bromo-7-phenyl-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ba). Yellowish solid, mp (EtOAc) 295.2–297.4 °C, Rf 0.39 (EtOAc/Hex, 1:1). Yield: 165 mg (0.41 mmol, 82%). 1H NMR (400 MHz, DMSO-d6) δ 11.97 (s, 1H), 10.36 (s, 1H), 8.03 (s, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.33 (t, J = 9.7 Hz, 1H), 7.22 (t, J = 7.6 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 7.11–6.99 (m, 3H), 6.89 (d, J = 7.4 Hz, 2H), 5.58 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.5, 137.2, 136.1, 134.9, 134.1, 129.4, 128.5, 128.2 (2C), 126.7, 126.5, 126.4 (2C), 125.0, 123.5, 122.0, 121.4, 120.4, 113.5, 112.1, 109.8, 48.4. IR, vmax/cm−1: 3277, 2994, 1769, 1757, 1638, 1558, 1455, 1401, 1240, 1045. HRMS (ES TOF) calculated for [M + Na]+ C22H15BrN2NaO 425.0260, found 425.0271 (2.6 ppm).
9,10-Dimethyl-7-phenyl-7,12-dihydrobenzo[2,3]azepino[4,5-b]indol-6(5H)-one (7ca). Yellowish solid, mp (EtOAc) 252.3–255.1 °C, Rf 0.55 (EtOAc/Hex, 1:1). Yield: 125 mg (0.36 mmol, 71%). 1H NMR (400 MHz, DMSO-d6) δ (s, 1H), 10.29 (s, 1H), 7.68 (d, J = 9.5 Hz, 1H), 7.48 (s, 1H), 7.26 (s, 1H), 7.17–7.11 (m, 2H), 7.10–7.02 (m, 4H), 6.89 (d, J = 7.3 Hz, 2H), 5.44 (s, 1H), 2.35 (s, 3H), 2.30 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.5, 137.5, 136.5, 134.4, 131.6, 131.3, 128.2 (2C), 127.7, 127.6, 126.6, 126.4 (2C), 126.2, 126.1, 123.4, 122.7, 121.3, 117.8, 111.8, 109.7, 48.9, 20.3, 19.9. IR, vmax/cm−1: 2986, 2893, 1777, 1710, 1635, 1522, 1505, 1451, 1363, 1149. HRMS (ES TOF) calculated for [M + Na]+ C24H20N2NaO 375.1468, found 375.1472 (−1.6 ppm).