One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions
Abstract
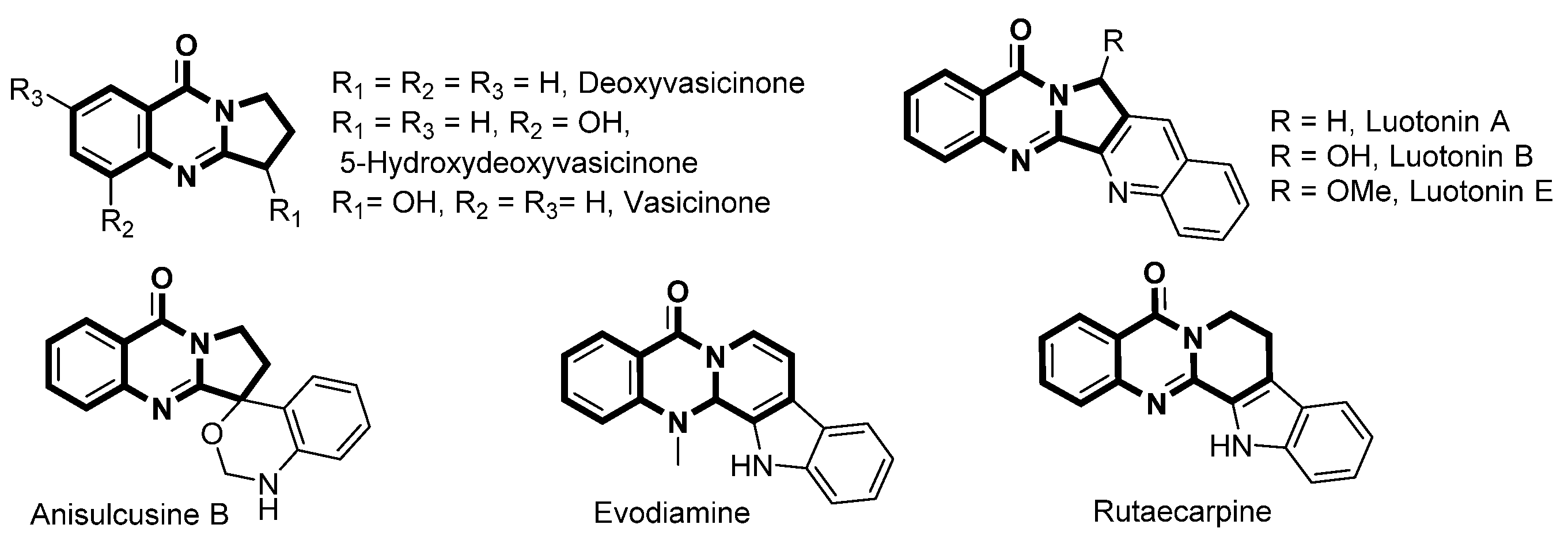
1. Introduction
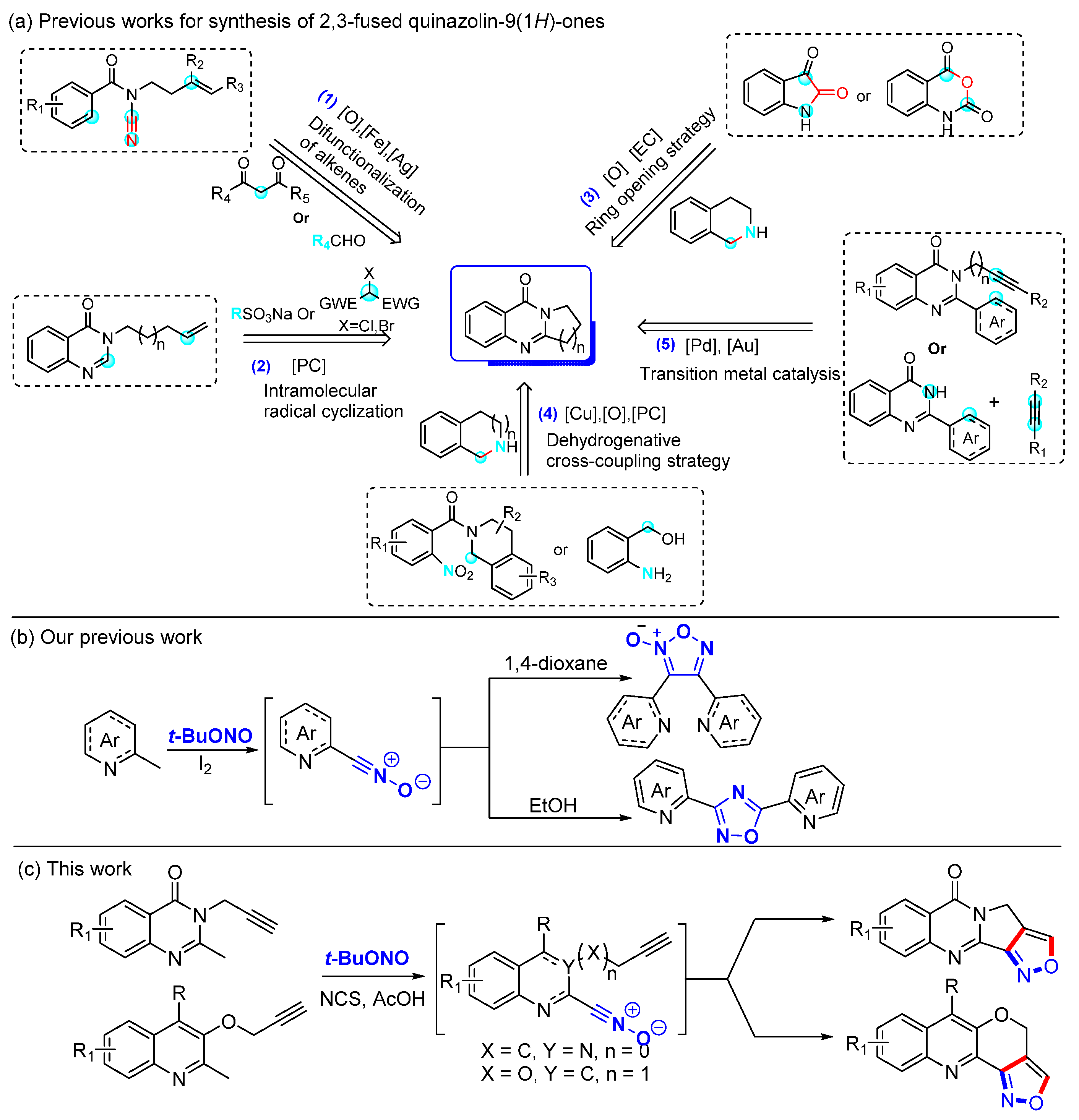
2. Results and Discussion
2.1. Reaction Optimization
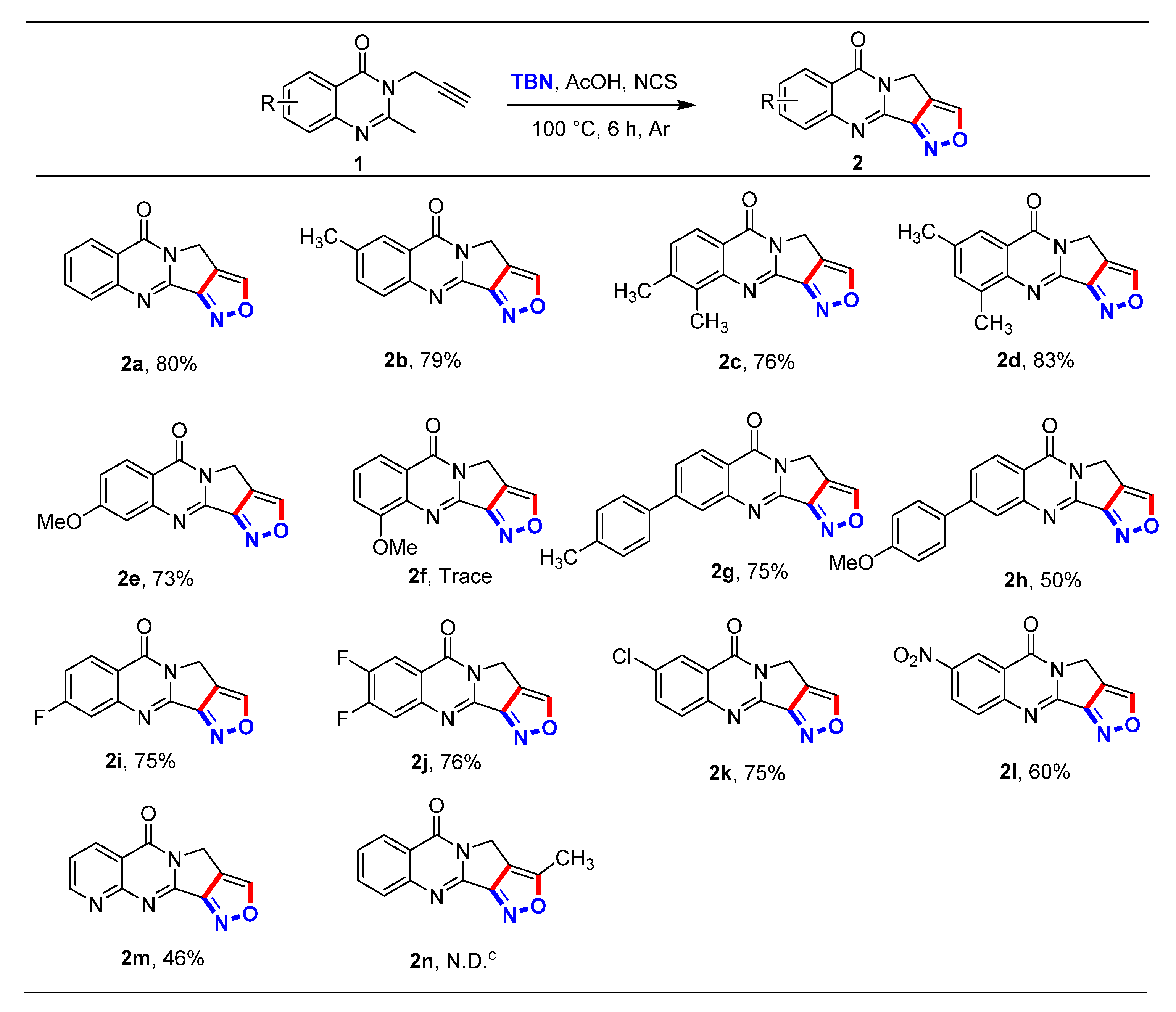
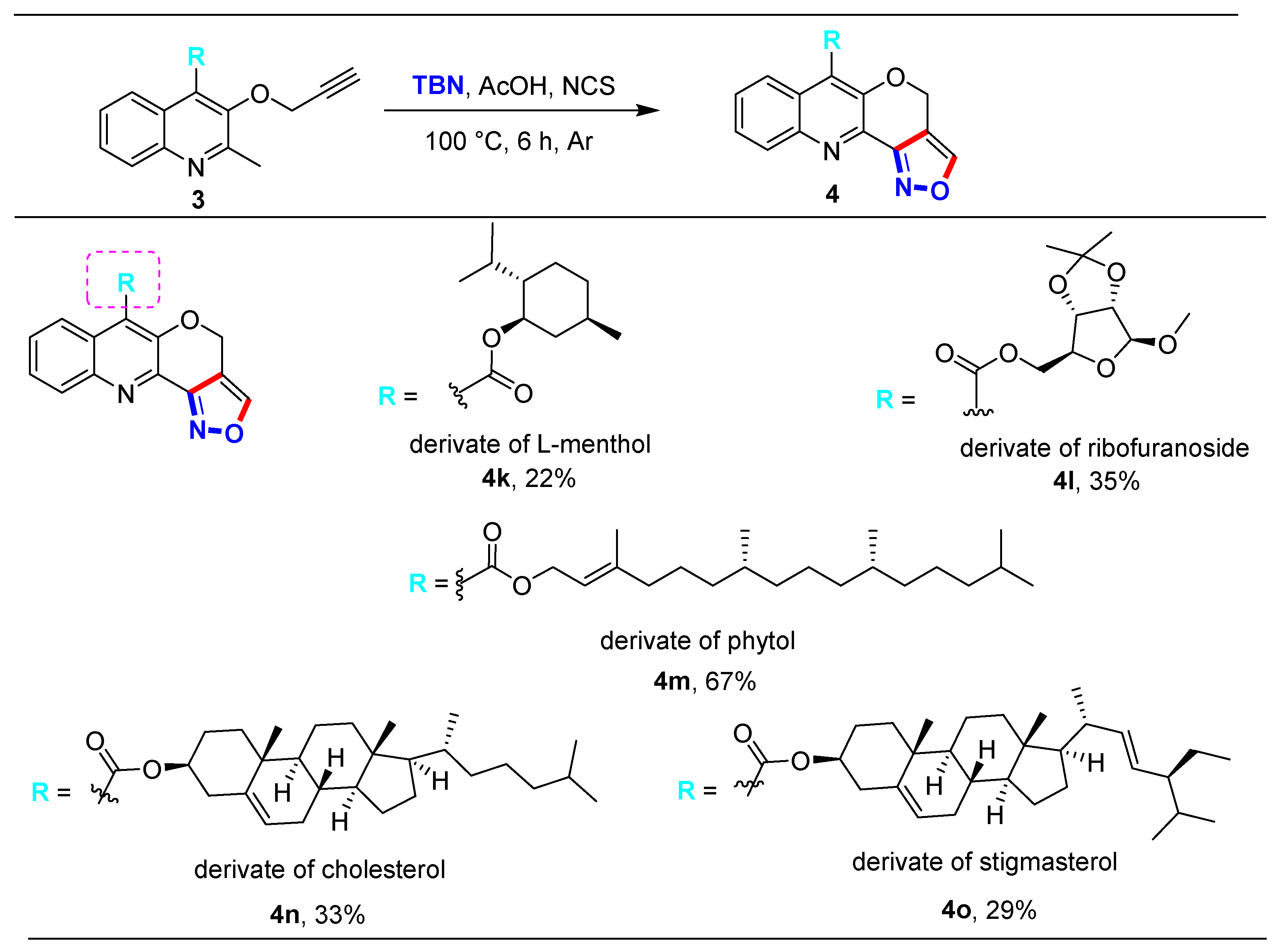
2.2. Substrate Scope
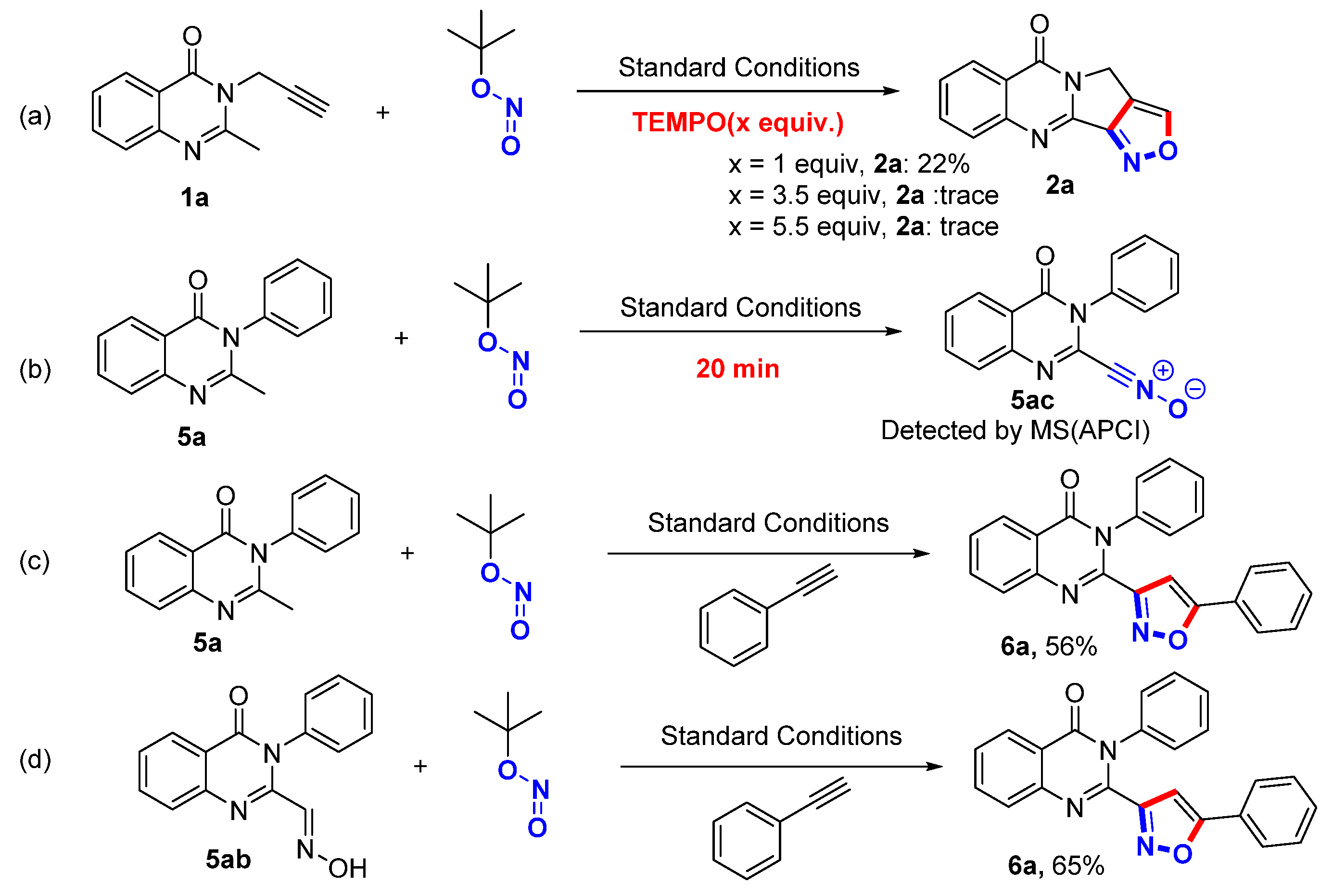
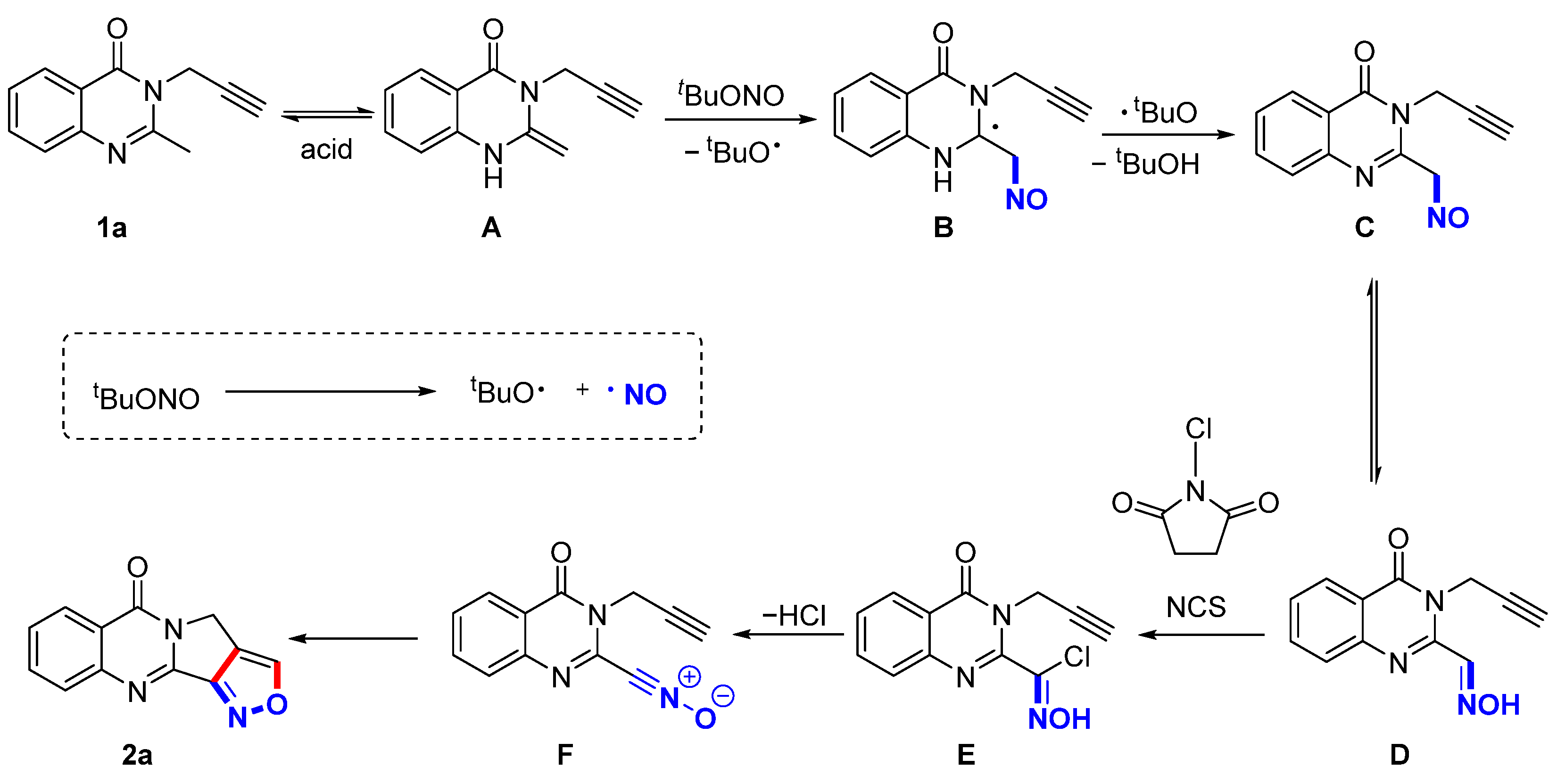
2.3. Mechanism Experiments
3. Materials and Methods
3.1. General Information
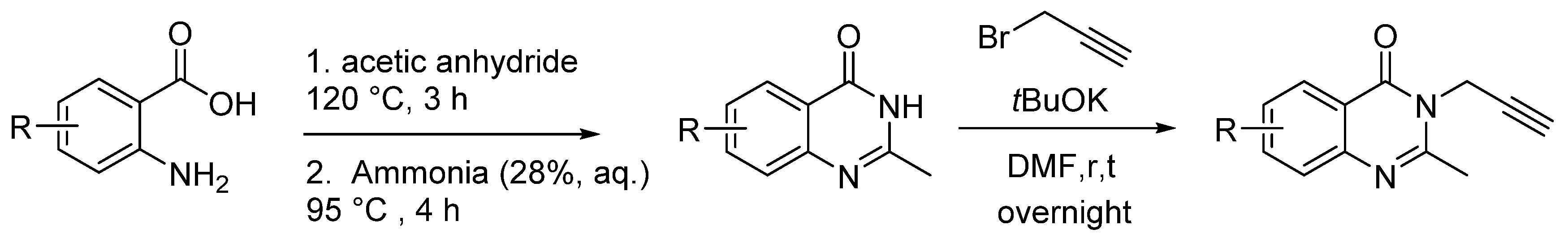
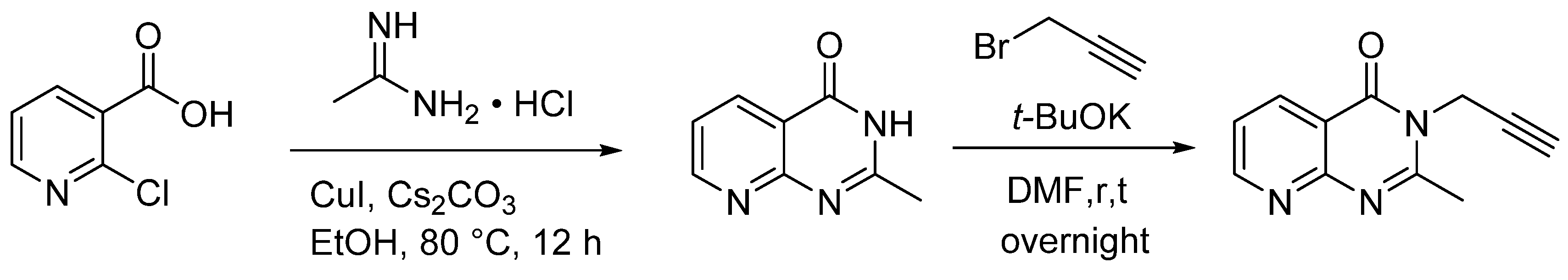
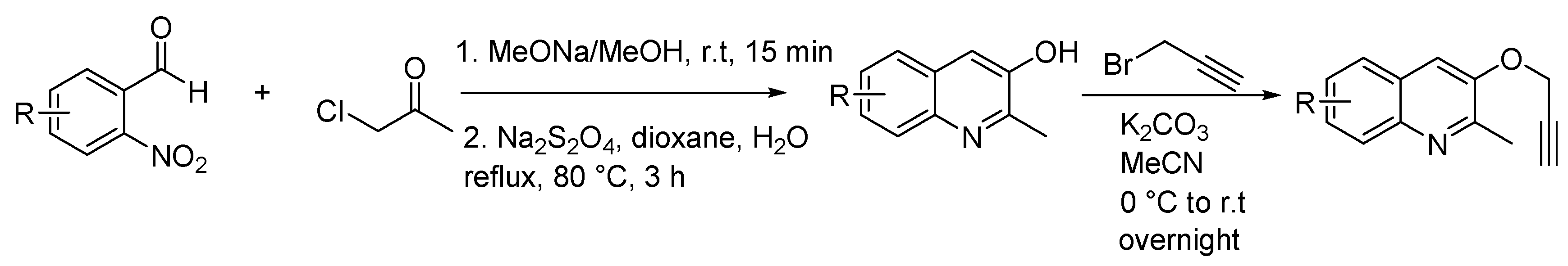
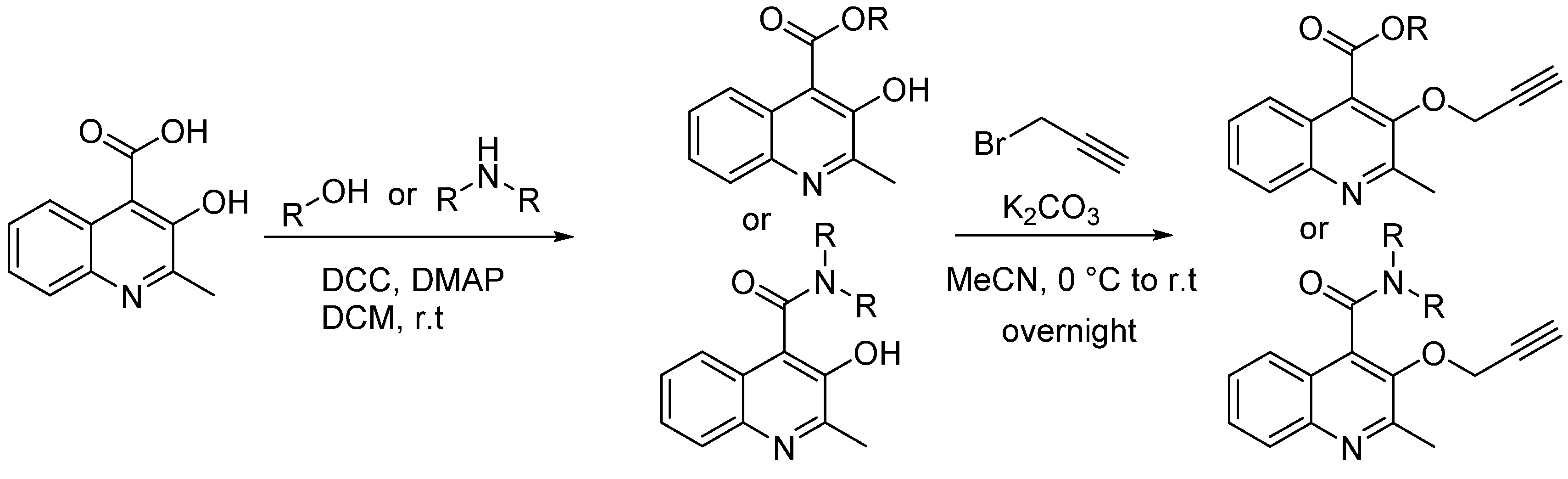
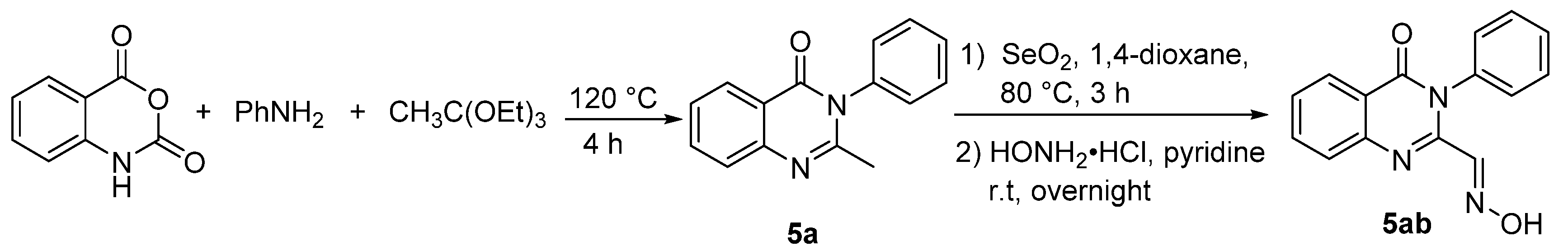
3.2. Synthetic Procedures
3.2.1. Typical Procedure (TP 1) for the Synthesis of 2 and 4 Taking 2a as an Example
3.2.2. Typical Procedure (TP 2) for the Synthesis of 6a
3.3. Characterization of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fan, Y.L.; Cheng, X.W.; Wu, J.B.; Liu, M.; Zhang, F.Z.; Xu, Z.; Feng, L.S. Antiplasmodial and antimalarial activities of quinolone derivatives: An overview. Eur. J. Med. Chem. 2018, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Wu, J.B.; Cheng, X.W.; Zhang, F.Z.; Feng, L.S. Fluoroquinolone derivatives and their anti-tubercular activities. Eur. J. Med. Chem. 2018, 146, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Gao, C.; Zhang, S.; Xu, L.; Xu, Z.; Feng, L.S.; Wu, X.; Zhao, F. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017, 139, 22–47. [Google Scholar] [CrossRef] [PubMed]
- Sekgota, K.C.; Majumder, S.; Isaacs, M.; Mnkandhla, D.; Hoppe, H.C.; Khanye, S.D.; Kriel, F.H.; Coates, J.; Kaye, P.T. Application of the Morita-Baylis-Hillman reaction in the synthesis of 3- (n-cycloalkylbenzamido)methyl -2-quinolones as potential hiv-1 integrase inhibitors. Bioorg. Chem. 2017, 75, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Zhang, S.; Pan, B.F.; Liu, X.F.; Feng, L.S. 4-Quinolone derivatives and their activities against gram positive pathogens. Eur. J. Med. Chem. 2018, 143, 710–723. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, C.; Ren, Q.C.; Chang, L.; Lv, Z.S.; Feng, L.S. Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 138, 501–513. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Liu, W.; Liang, L.L.; Gong, K.K.; Zhao, C.Y.; Sun, K.L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef]
- Swain, S.S.; Pati, S.; Hussain, T. Quinoline heterocyclic containing plant and marine candidates against drug-resistant Mycobacterium tuberculosis: A systematic drug-ability investigation. Eur. J. Med. Chem. 2022, 232, 21. [Google Scholar] [CrossRef]
- Yan, Y.M.; Li, X.; Zhang, C.H.; Lv, L.J.; Gao, B.; Li, M.H. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef] [PubMed]
- Du, H.T.; Liu, X.L.; Xie, J.S.; Ma, F. Novel deoxyvasicinone-donepezil hybrids as potential multitarget drug candidates for alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Elmuradov, B.Z.; Abdurazakov, A.S.; Shakhidoyatov, K.M. Synthesis and bactericidal activity of 6-h(nitro)-9-arylidenedeoxyvasicinones and their perchlorates. Chem. Nat. Compd. 2008, 44, 475–479. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, M.J.; Hillman, P.F.; Oh, D.C.; Fenical, W.; Nam, S.J.; Lim, K.M. Deoxyvasicinone with anti-melanogenic activity from marine-derived Streptomyces sp. CNQ-617. Mar. Drugs 2022, 20, 155. [Google Scholar] [CrossRef]
- Atta Ur, R.; Sultana, N.; Akhter, F.; Nighat, F.; Choudhary, M.I. Phytochemical studies onadhatoda vasicanees. Nat. Prod. Lett. 1997, 10, 249–256. [Google Scholar] [CrossRef]
- Liu, X.; Qian, P.; Wang, Y.; Pan, Y. Metal-free sequential decarbonylative annulation of N-cyanamides for the construction of 2,3-dihydropyrrolo[2,1-b]quinazolin-9(1H)-ones. Org. Chem. Front. 2017, 4, 2370–2374. [Google Scholar] [CrossRef]
- Mhaske, S.B.; Argade, N.P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 2006, 62, 9787–9826. [Google Scholar] [CrossRef]
- Wang, H.S.; Jiao, S.C.; Chen, K.R.; Zhang, X.; Zhao, L.X.; Liu, D.; Zhou, Y.; Liu, H. Direct access to pyrido/pyrrolo 2,1-b quinazolin-9(1H)-ones through silver-mediated intramolecular alkyne hydroamination reactions. Beilstein J. Org. Chem. 2015, 11, 416–424. [Google Scholar] [CrossRef]
- Cagir, A.; Jones, S.H.; Eisenhauer, B.M.; Gao, R.; Hecht, S.M. Synthesis and biochemical properties of E-ring modified luotonin A derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Cagir, A.; Jones, S.H.; Gao, R.; Eisenhauer, B.M.; Hecht, S.M.; Luotonin, A. A naturally occurring human dna topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Fayed, M.A.A.; Al-Saleem, M.S.M.; Al-Wahaibi, L.H.; Parvez, M.K.; Li, L.; Al-Dosari, M.S.; Sayed, H.M. Novel polycyclic pyrroloquinazoline alkaloids from Anisotes trisulcus and their biological activity. J. Asian. Nat. Prod. Res. 2020, 22, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Seo, Y.M.; Lee, S.K.; Bista, S.R.; Kang, M.J.; Jahng, Y.; Kim, E.; Kang, W.; Jeong, T.C. Effects of rutaecarpine on the metabolism and urinary excretion of caffeine in rats. Arch. Pharm. Res. 2011, 34, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Ganguly, B.; Das, S. C-H functionalization of quinazolinones by transition metal catalysis. Org. Biomol. Chem. 2020, 18, 4497–4518. [Google Scholar] [CrossRef]
- He, L.; Li, H.Q.; Chen, J.B.; Wu, X.F. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Liu, J.C.; Xiao, X.; Lai, Y.T.; Zhang, Z.M. Recent advances in transition metal-catalyzed heteroannulative difunctionalization of alkenes via C-H activation for the synthesis of heterocycles. Org. Chem. Front. 2022, 9, 2256–2279. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhu, C.J.; Zhang, M.; Huang, S.J.; Lin, J.J.; Pan, X.D.; Su, W.P. Condensation of anthranilic acids with pyridines to furnish pyridoquinazolones via pyridine dearomatization. Chem. Commun. 2016, 52, 12869–12872. [Google Scholar] [CrossRef]
- Turner, O.J.; Hirst, D.J.; Murphy, J.A. hydrogen atom transfer-mediated domino cyclisation reaction to access (spiro)quinazolinones. Chem. Eur. J. 2020, 26, 3026–3029. [Google Scholar] [CrossRef]
- Turner, O.J.; Murphy, J.A.; Hirst, D.J.; Talbot, E.P.A. Hydrogen atom transfer-mediated cyclisations of nitriles. Chem. Eur. J. 2018, 24, 18658–18662. [Google Scholar] [CrossRef]
- Xu, G.; Tong, C.D.; Cui, S.L.; Dai, L.Y. A silver catalyzed domino reaction of N-cyanamide alkenes and 1,3-dicarbonyls for the synthesis of quinazolinones. Org. Biomol. Chem. 2018, 16, 5899–5906. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Z.X.; Huang, G.; Yu, J.T.; Pan, C.D. Cyanomethylative cyclization of unactivated alkenes with nitriles for the synthesis of cyano-containing ring-fused quinazolin-4(3H)-ones. New J. Chem. 2022, 46, 1347–1352. [Google Scholar] [CrossRef]
- Sun, B.; Huang, P.Y.; Yan, Z.Y.; Shi, X.Y.; Tang, X.L.; Yang, J.; Jin, C. Self-catalyzed phototandem perfluoroalkylation/cyclization of unactivated alkenes: Synthesis of perfluoroalkyl-substituted quinazolinones. Org. Lett. 2021, 23, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Shi, R.; Zhang, K.; Tang, X.; Shi, X.; Xu, J.; Yang, J.; Jin, C. Photoinduced homolytic decarboxylative acylation/cyclization of unactivated alkenes with α-keto acid under external oxidant and photocatalyst free conditions: Access to quinazolinone derivatives. Chem. Commun. 2021, 57, 6050–6053. [Google Scholar] [CrossRef]
- Yang, J.; Sun, B.; Ding, H.; Huang, P.Y.; Tang, X.L.; Shi, R.C.; Yan, Z.Y.; Yu, C.M.; Jin, C. Photo-triggered self-catalyzed fluoroalkylation/cyclization of unactivated alkenes: Synthesis of quinazolinones containing the CF2R group. Green Chem. 2021, 23, 575–581. [Google Scholar] [CrossRef]
- Chen, X.; Xia, F.; Zhao, Y.; Ma, J.; Ma, Y.; Zhang, D.; Yang, L.; Sun, P. TBHP-mediated oxidative decarboxylative cyclization in water: Direct and sustainable access to anti-malarial polycyclic fused quinazolinones and rutaecarpine. Chin. J. Chem. 2020, 38, 1239–1244. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Lu, S.; Sun, P. Electrosynthesis of polycyclic quinazolinones and rutaecarpine from isatoic anhydrides and cyclic amines. RSC Adv. 2020, 10, 44382–44386. [Google Scholar] [CrossRef]
- Jing, D.; Lu, C.; Chen, Z.; Jin, S.Y.; Xie, L.J.; Meng, Z.Y.; Su, Z.S.; Zheng, K. Light-driven intramolecular c-n cross-coupling via a long-lived photoactive photoisomer complex. Angew. Chem. Int. Edit. 2019, 58, 14666–14672. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Su, Z.S.; Jing, D.; Jin, S.Y.; Xie, L.J.; Li, L.R.; Zheng, K. Intramolecular reductive cyclization of o-nitroarenes via biradical recombination. Org. Lett. 2019, 21, 1438–1443. [Google Scholar] [CrossRef]
- Xie, F.; Chen, Q.H.; Xie, R.; Jiang, H.F.; Zhang, M. MOF-derived nanocobalt for oxidative functionalization of cyclic amines to quinazolinones with 2-aminoarylmethanols. ACS Catal. 2018, 8, 5869–5874. [Google Scholar] [CrossRef]
- Xie, L.; Lu, C.; Jing, D.; Ou, X.; Zheng, K. Metal-free synthesis of polycyclic quinazolinones enabled by a (nh4)2s2o8-promoted intramolecular oxidative cyclization. Eur. J. Org. Chem. 2019, 2019, 3649–3653. [Google Scholar] [CrossRef]
- Dabiri, M.; Lehi, N.F.; Movahed, S.K.; Khavasi, H.R. Palladium catalyzed cross-dehydrogenative coupling/annulation reaction: A practical and efficient approach to hydroxyisoindolo[1,2-b]quinazolinone. Eur. J. Org. Chem. 2019, 2019, 2933–2940. [Google Scholar] [CrossRef]
- Wang, W.; Zou, P.-S.; Pang, L.; Lei, Y.; Huang, Z.-Y.; Chen, N.-Y.; Mo, D.-L.; Pan, C.-X.; Su, G.-F. Synthesis of spiroindolenine-3,3′-pyrrolo[2,1-b]quinazolinones through gold(i)-catalyzed dearomative cyclization of N-alkynyl quinazolinone-tethered indoles. Org. Biomol. Chem. 2022, 20, 2069–2074. [Google Scholar] [CrossRef]
- Ali, I.; Lone, N.M.; Al-Othman, A.Z.; Al-Warthan, A.; Sanagi, M.M. Heterocyclic scaffolds: Centrality in anticancer drug development. Curr. Cancer Drug Tar. 2015, 16, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Giacalone, V.; Bonsignore, R.; Pace, A.; Gentile, C.; Pibiri, I.; Buscemi, S.; Lauria, A.; Piccionello, P.A. Heterocyclic scaffolds for the treatment of Alzheimer’s Disease. Curr. Pharm. Design 2016, 22, 3971–3995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mo, J.; Lin, H.-z.; Chen, Y.; Sun, H.-p. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef]
- Barmade, A.M.; Murumkar, R.P.; Sharma, K.M.; Yadav, R.M. Medicinal chemistry perspective of fused isoxazole derivatives. Curr. Top. Med. Chem. 2016, 16, 2863–2883. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Wakode, S. Contemporary advances of cyclic molecules proposed for inflammation. Eur. J. Med. Chem. 2021, 221, 113493. [Google Scholar] [CrossRef] [PubMed]
- Sysak, A.; Obmińska-Mrukowicz, B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur. J. Med. Chem. 2017, 137, 292–309. [Google Scholar] [CrossRef]
- Chen, F.; Huang, X.; Li, X.; Shen, T.; Zou, M.; Jiao, N. Dehydrogenative n-incorporation: A direct approach to quinoxaline n-oxides under mild conditions. Angew. Chem. Int. Edit. 2014, 53, 10495–10499. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Lin, F.; Liu, J.; Jiao, N. Chemoselective nitrosylation of anilines and alkynes via fragmentary or complete no incorporation. Chemistry 2018, 4, 1427–1442. [Google Scholar] [CrossRef]
- Chaudhary, P.; Gupta, S.; Muniyappan, N.; Sabiah, S.; Kandasamy, J. An efficient synthesis of N-nitrosamines under solvent, metal and acid free conditions using tert-butyl nitrite. Green Chem. 2016, 18, 2323–2330. [Google Scholar] [CrossRef]
- Ma, X.; Song, Q. Tert-Butyl nitrite mediated synthesis of fluorinated o-alkyloxime ether derivatives. Org. Lett. 2019, 21, 7375–7379. [Google Scholar] [CrossRef]
- Mir, B.A.; Singh, S.J.; Kumar, R.; Patel, B.K. tert-Butyl nitrite mediated different functionalizations of internal alkenes: Paths to furoxans and nitroalkenes. Adv. Synth. Catal. 2018, 360, 3801–3809. [Google Scholar] [CrossRef]
- Sau, P.; Rakshit, A.; Modi, A.; Behera, A.; Patel, B.K. Three sequential c-n bond formations: Tert-butyl nitrite as a N1 synthon in a three component reaction leading to imidazo 1,2-a quinolines and imidazo 2,1-a isoquinolines. J. Org. Chem. 2018, 83, 1056–1064. [Google Scholar] [CrossRef]
- Shu, Z.B.; Ye, Y.X.; Deng, Y.F.; Zhang, Y.; Wang, J.B. Palladium(II)-catalyzed direct conversion of methyl arenes into aromatic nitriles. Angew. Chem. Int. Edit. 2013, 52, 10573–10576. [Google Scholar] [CrossRef]
- Wan, L.; Qiao, K.; Yuan, X.; Zheng, M.W.; Fan, B.B.; Di, Z.C.; Zhang, D.; Fang, Z.; Guo, K. Nickel-catalyzed regioselective C-H Bond mono- and bis-nitration of aryloxazolines with tert-butyl nitrite as nitro source. Adv. Synth. Catal. 2017, 359, 2596–2604. [Google Scholar] [CrossRef]
- Yang, X.-H.; Song, R.-J.; Li, J.-H. Metal-free [4+2] annulation of arylalkynes with tert-butyl nitrite through C(sp2)-H oxidation to assemble benzo[e][1,2]oxazin-4-ones. Adv. Synth. Catal. 2015, 357, 3849–3856. [Google Scholar] [CrossRef]
- Dahiya, A.; Sahoo, A.K.; Alam, T.; Patel, B.K. tert-Butyl Nitrite (TBN), a multitasking reagent in organic synthesis. Chem. Asian J. 2019, 14, 4454–4492. [Google Scholar] [CrossRef]
- Gao, P.; Li, H.X.; Hao, X.H.; Jin, D.P.; Chen, D.Q.; Yan, X.B.; Wu, X.X.; Song, X.R.; Liu, X.Y.; Liang, Y.M. Facile synthesis of disubstituted isoxazoles from homopropargylic alcohol via C=N bond formation. Org. Lett. 2014, 16, 6298–6301. [Google Scholar] [CrossRef]
- Sau, P.; Santra, S.K.; Rakshit, A.; Patel, B.K. tert-Butyl nitrite-mediated domino synthesis of isoxazolines and isoxazoles from terminal aryl alkenes and alkynes. J. Org. Chem. 2017, 82, 6358–6365. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Zhao, Y.W.; Fang, S.W.; Long, W.H.; Sun, H.M.; Wan, X.B. Coupling reaction of cu-based carbene and nitroso radical: A tandem reaction to construct isoxazolines. Org. Lett. 2017, 19, 5896–5899. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Ogunlana, A.A.; Fang, S.W.; Long, W.H.; Sun, H.M.; Bao, X.G.; Wan, X.B. In situ generation of nitrile oxides from copper carbene and tert-butyl nitrite: Synthesis of fully substituted isoxazoles. Org. Biomol. Chem. 2018, 16, 4683–4687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Hu, W.L.; Chen, S.; Hu, X.G. Cu-catalyzed synthesis of fluoroalkylated isoxazoles from commercially available amines and alkynes. Org. Lett. 2018, 20, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Zhu, L.-H.; Liu, P.; Wang, X.-Y.; Yuan, H.-Y.; Zhao, Y.-L. Copper-catalyzed cascade cyclization reactions of diazo compounds with tert-butyl nitrite and alkynes: One-pot synthesis of isoxazoles. J. Org. Chem. 2019, 84, 16214–16221. [Google Scholar] [CrossRef]
- Zhang, X.-W.; He, X.-L.; Yan, N.; Zheng, H.-X.; Hu, X.-G. Oxidize Amines to nitrile oxides: One type of amine oxidation and its application to directly construct isoxazoles and isoxazolines. J. Org. Chem. 2020, 85, 15726–15735. [Google Scholar] [CrossRef]
- Yue, X.; Hu, M.; He, X.; Wu, S.; Li, J.-H. A radical-mediated 1,3,4-trifunctionalization cascade of 1,3-enynes with sulfinates and tert-butyl nitrite: Facile access to sulfonyl isoxazoles. Chem. Commun. 2020, 56, 6253–6256. [Google Scholar] [CrossRef]
- Ma, L.; Jin, F.; Cheng, X.; Tao, S.Y.; Jiang, G.Z.; Li, X.X.; Yang, J.W.; Bao, X.G.; Wan, X.B. 2+2+1 Cycloaddition of N-tosylhydrazones, tert-butyl nitrite and alkenes: A general and practical access to isoxazolines. Chem. Sci. 2021, 12, 9823–9830. [Google Scholar] [CrossRef]
- Ma, L.; Kou, L.Y.; Jin, F.; Cheng, X.L.; Tao, S.Y.; Jiang, G.Z.; Bao, X.G.; Wan, X.B. Acyclic nitronate olefin cycloaddition (ANOC): Regio- and stereospecific synthesis of isoxazolines. Chem. Sci. 2021, 12, 774–779. [Google Scholar] [CrossRef]
- Dai, P.; Tan, X.; Luo, Q.; Yu, X.; Zhang, S.; Liu, F.; Zhang, W.-H. Synthesis of 3-acyl-isoxazoles and Δ2-isoxazolines from methyl ketones, alkynes or alkenes, and tert-butyl nitrite via a Csp3-H radical functionalization/cycloaddition cascade. Org. Lett. 2019, 21, 5096–5100. [Google Scholar] [CrossRef]
- Wang, G.-W.; Cheng, M.-X.; Ma, R.-S.; Yang, S.-D. Cu-catalyzed selective cascade sp3 C–H bond oxidative functionalization towards isoxazoline derivatives. Chem. Commun. 2015, 51, 6308–6311. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W.; Li, S.-X.; Wu, Q.-X.; Yang, S.-D. Cu-catalyzed sp3 C–H bond oxidative functionalization of alkylazaarenes and substituted ethanones: An efficient approach to isoxazoline derivatives. Org. Chem. Front. 2015, 2, 569–573. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, Y.; Song, Q. Synthesis of furoxans and isoxazoles via divergent [2 + 1 + 1 + 1] annulations of sulfoxonium ylides and t-buONO. Org. Lett. 2019, 21, 5273–5276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Cao, J.-K.; Ren, J.-J.; Hong, L.; Liang, R.-J.; Hao, K.-Y.; Wei, K.-L.; Mi, B.-J.; Liu, Y.; Zhu, Y.-P. Generation of azaarene nitrile oxides from methyl azaarenes and t-BuONO enabling the synthesis of furoxans and 1,2,4-oxadiazoles. Org. Chem. Front. 2022, 9, 1121–1126. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Y.; Gao, P.; Zhang, S.; Jia, X.; Yuan, Y. Direct transformation of nitrogen-containing methylheteroarenes to heteroaryl nitrile by sodium nitrite. Org. Lett. 2022, 24, 6341–6345. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Xiao, F.; Deng, G.-J. A three-component approach to isoxazolines and isoxazoles under metal-free conditions. Org. Biomol. Chem. 2019, 17, 9163–9168. [Google Scholar] [CrossRef]
- Yu, J.; Lu, M. Copper(ii)-promoted direct conversion of methylarenes into aromatic oximes. Org. Biomol. Chem. 2015, 13, 7397–7401. [Google Scholar] [CrossRef]
- Jones, M.L. Heterocyclic Compounds as Antibiotic Potentiators. U.S. Patent Application No 14/965,759, 12 December 2014. [Google Scholar]
- Ouahrouch, A.; Taourirte, M.; Engels, J.W.; Benjelloun, S.; Lazrek, H.B. Synthesis of new 1,2,3-triazol-4-yl-quinazoline nucleoside and acyclonucleoside analogues. Molecules 2014, 19, 3638–3653. [Google Scholar] [CrossRef]
- Liu, X.; Fu, H.; Jiang, Y.; Zhao, Y. A Simple and efficient approach to quinazolinones under mild copper-catalyzed conditions. Angew. Chem. Int. Edit. 2009, 48, 348–351. [Google Scholar] [CrossRef]
- An, S.-Z. Compound as WNT Signaling Inhibitor, Composition, and Use Thereof. U.S. Patent No 9,556,144, 29 January 2015. [Google Scholar]
- Mamedov, V.A.; Mamedova, V.L.; Syakaev, V.V.; Korshin, D.E.; Khikmatova, G.n.Z.; Mironova, E.V.; Bazanova, O.B.; Rizvanov, I.d.K.; Latypov, S.K. Simple synthesis of 3-hydroxyquinolines via Na2S2O4-mediated reductive cyclization of (2-(2-nitrophenyl)oxiran-1-yl)(aryl)methanones(o-nitrobenzalacetophenone oxides). Tetrahedron 2017, 73, 5082–5090. [Google Scholar] [CrossRef]
- Choi, J.-K. Protein tyrosine phosphatase 1b inhibitors: Heterocyclic carboxylic acids. ChemInform 2004, 35, 1455–1464. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef]
- Aspée, A.; García, O.; Maretti, L.; Sastre, R.; Scaiano, J.C. Free radical reactions in poly(methyl methacrylate) films monitored using a prefluorescent quinoline−TEMPO sensor. Macromolecules 2003, 36, 3550–3556. [Google Scholar] [CrossRef]
- Kumar, D.; Jadhavar, P.S.; Nautiyal, M.; Sharma, H.; Meena, P.K.; Adane, L.; Pancholia, S.; Chakraborti, A.K. Convenient synthesis of 2,3-disubstituted quinazolin-4(3H)-ones and 2-styryl-3-substituted quinazolin-4(3H)-ones: Applications towards the synthesis of drugs. RSC Adv. 2015, 5, 30819–30825. [Google Scholar] [CrossRef]
- Farghaly, A.M.; Mohsen, A.; Omar, M.E.; Khalil, M.A.; Gaber, M.A. Synthesis of 3-aryl-2-substituted-4(3H)-quinazolines as potential antimicrobial agents. Farmaco 1990, 454, 431–438. [Google Scholar]
- Rai, B.K.; Thakur, R.K.; Kumari, M. Synthesis and characterisation of Co(II) complexes with some new quinazolone oximes. Asian J. Chem. 2001, 13, 651–655. [Google Scholar]


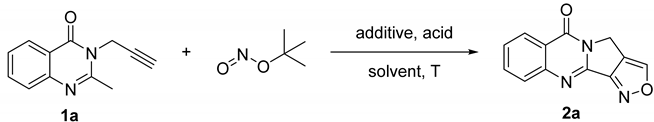 | |||||
| Entry | Solvent | Acid | Additive | Temp (°C) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | DMSO | 80 | 45 | ||
| 2 | DMF | 80 | 62 | ||
| 3 | 1,4-Dioxane | 80 | 56 | ||
| 4 | MeCN | 80 | 64 | ||
| 5 | MeCN | 60 | 55 | ||
| 6 | MeCN | 100 | 65 | ||
| 7 c | MeCN | 100 | 72 | ||
| 8 c,d | MeCN | 100 | 75 | ||
| 9 c,d | MeCN | NCS | 100 | 76 | |
| 10 c,d | MeCN | TFA | NCS | 100 | 46 |
| 11 c,d | MeCN | AcOH | NCS | 100 | 80 |
| 12 c,d | MeCN | Propanoic acid | NCS | 100 | 68 |
| 13 c,d | MeCN | Propanedioic acid | NCS | 100 | 58 |
| 14 c,d | MeCN | 4-Nitrobenzoic acid | NCS | 100 | 68 |
| 15 c,d | MeCN | 2-Nitrobenzoic acid | NCS | 100 | 73 |
| 16 c,d | MeCN | 4-Chlorobenzoic acid | NCS | 100 | 71 |
| 17 c,d | MeCN | PTA e | NCS | 100 | 55 |
| 18 c,d | MeCN | AcOH | 100 | 66 | |
| 19 c,d | MeCN | 4-Chlorobenzoic acid | 100 | 42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, Y.; Chen, J.; Chen, M.; Li, X.; Jiang, T.; Liu, F.; Yang, X.; Sun, Y.; Zhu, Y. One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions. Molecules 2023, 28, 2787. https://doi.org/10.3390/molecules28062787
Wang Z, Zhao Y, Chen J, Chen M, Li X, Jiang T, Liu F, Yang X, Sun Y, Zhu Y. One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions. Molecules. 2023; 28(6):2787. https://doi.org/10.3390/molecules28062787
Chicago/Turabian StyleWang, Zhuo, Yuhan Zhao, Jiaxin Chen, Mengyao Chen, Xuehan Li, Ting Jiang, Fang Liu, Xi Yang, Yuanyuan Sun, and Yanping Zhu. 2023. "One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions" Molecules 28, no. 6: 2787. https://doi.org/10.3390/molecules28062787
APA StyleWang, Z., Zhao, Y., Chen, J., Chen, M., Li, X., Jiang, T., Liu, F., Yang, X., Sun, Y., & Zhu, Y. (2023). One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions. Molecules, 28(6), 2787. https://doi.org/10.3390/molecules28062787






