Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al3+ Ions
Abstract
1. Introduction
2. Results and Discussion
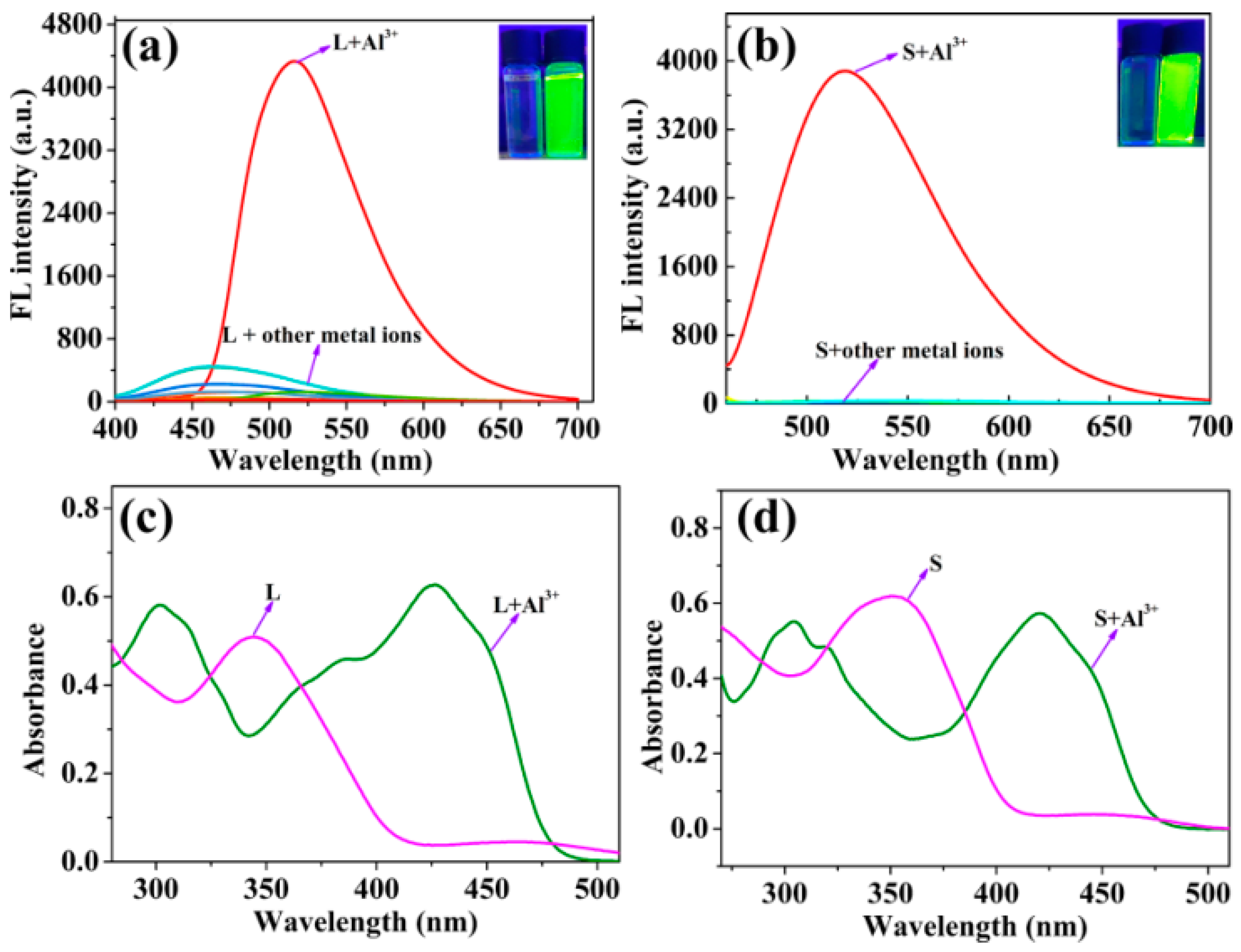
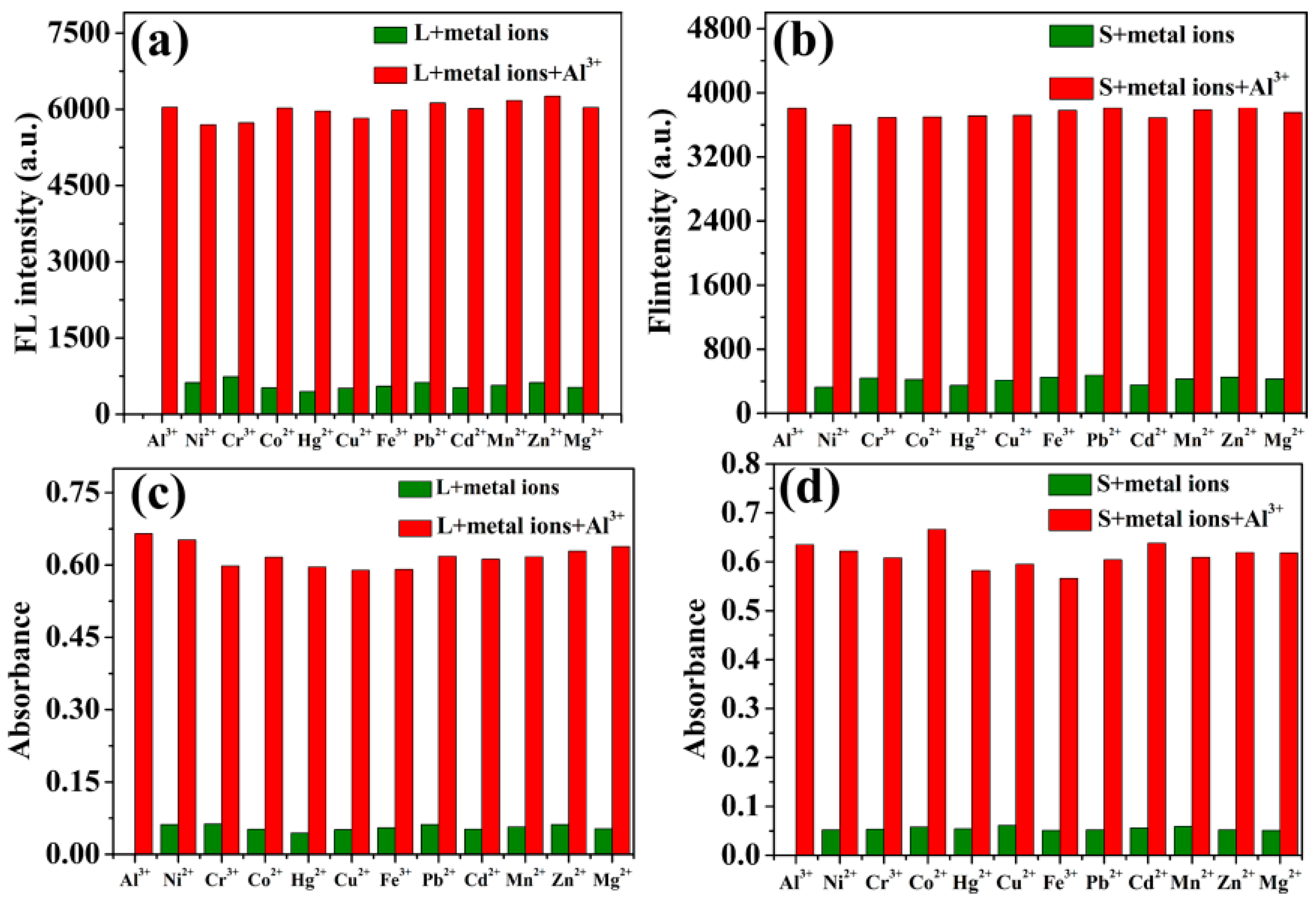
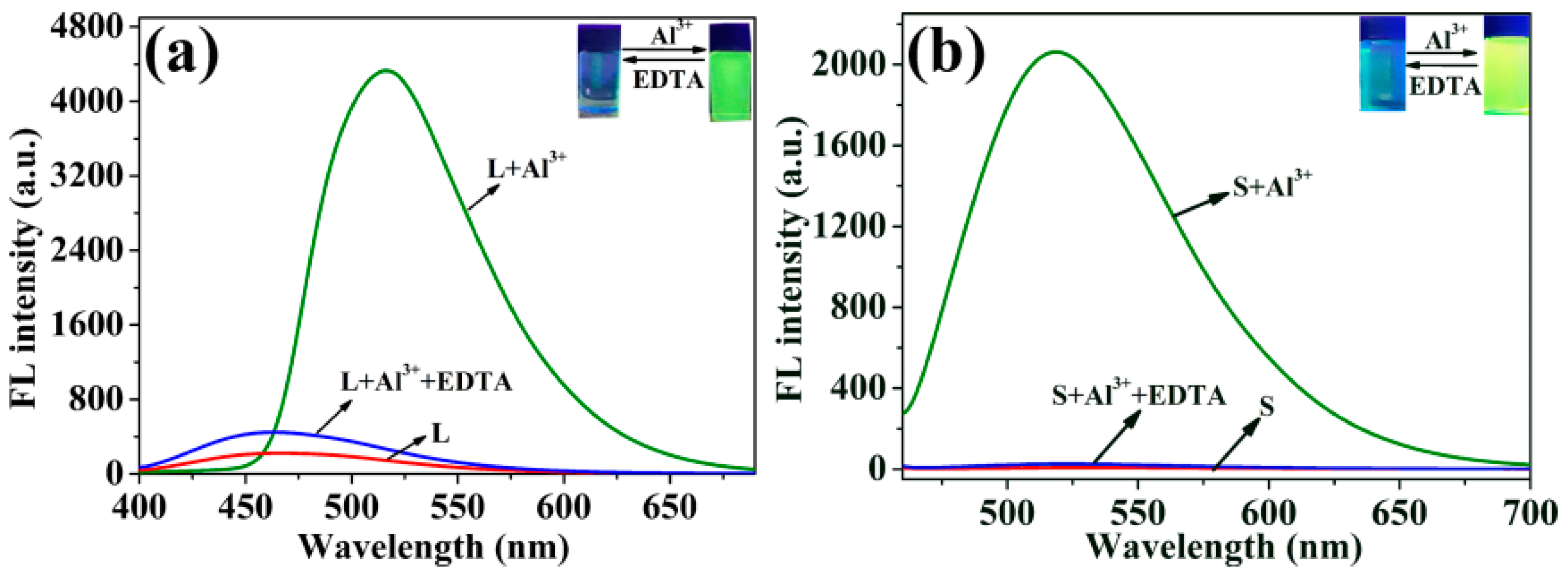
2.1. Fluorescence Emission Spectral Responses of Probes L and S
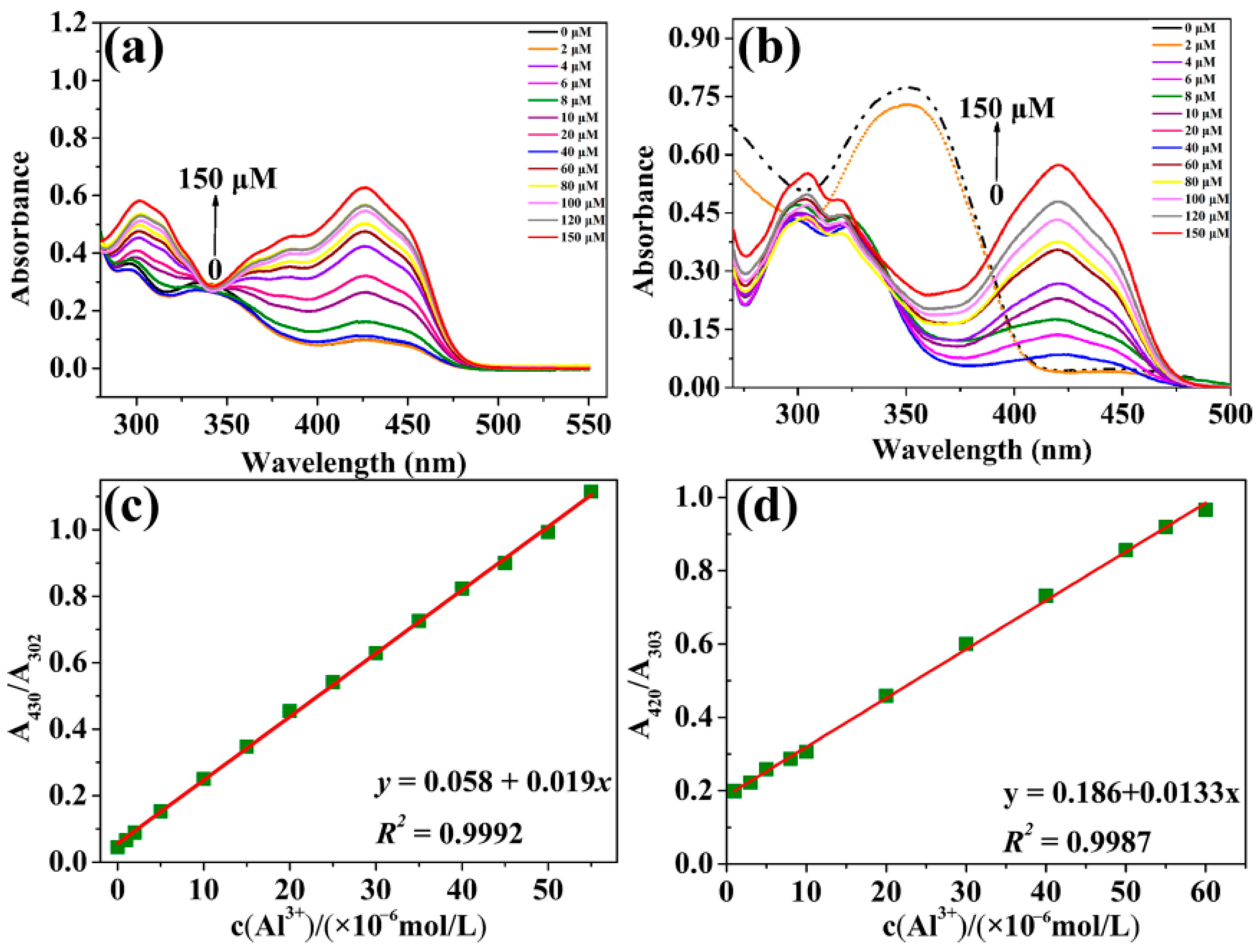
2.2. Quantitative Identification of Al3+ by Probes L and S
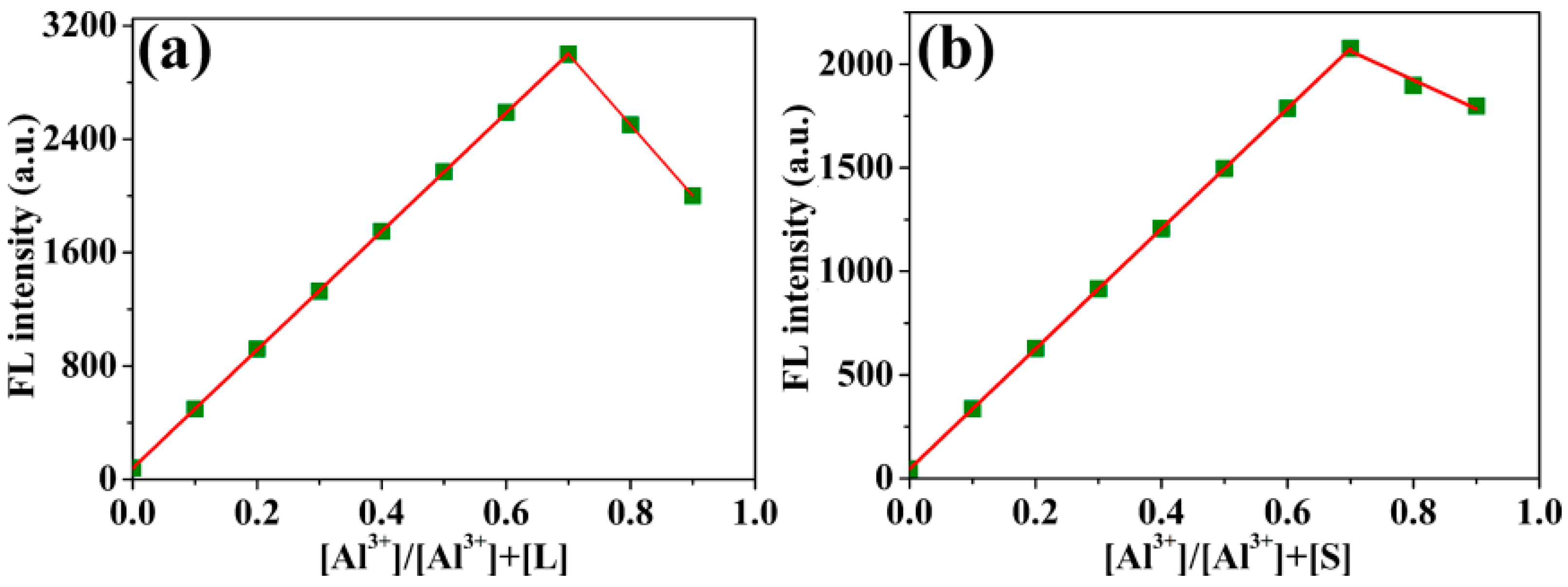
2.3. Complexation Mechanism of Probes L and S with Al3+
2.4. Reversibility Experiments
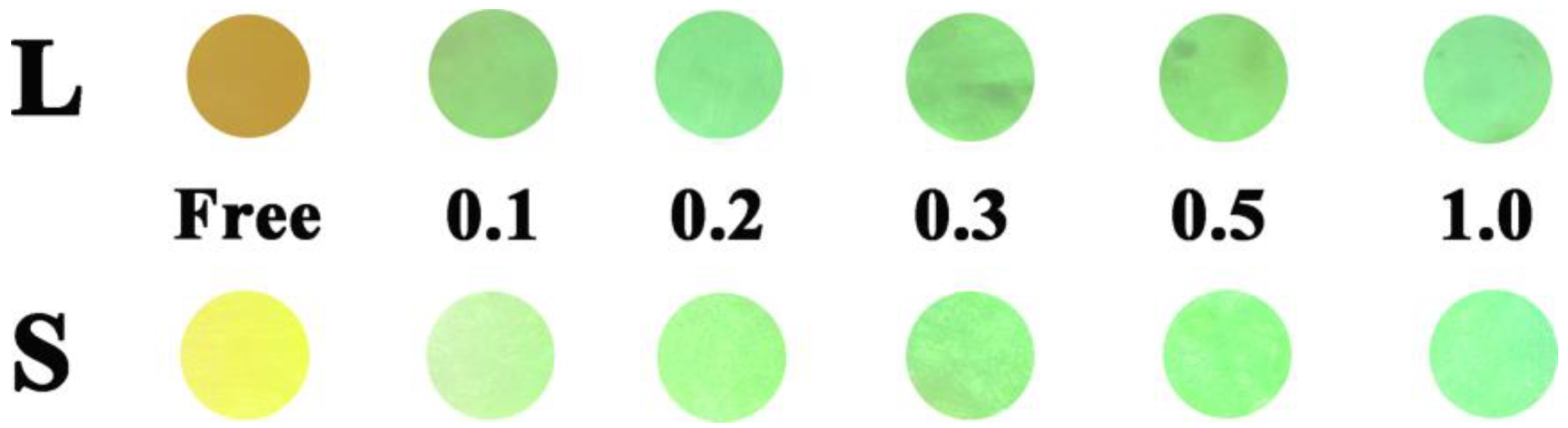
2.5. Filter Paper Strip Experiments
3. Experimental Procedure
3.1. Reagents and Apparatus
3.2. Synthesis
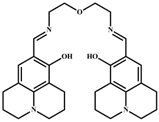
3.2.1. Synthesis of o-Vanillin-p-aminoacetophenone Schiff Base (L)
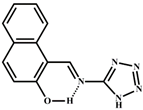
3.2.2. Synthesis of Salicylaldehyde-p-aminoacetophenone Schiff Base (S)
3.3. Spectrophotometric Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, M.M.; Lu, L.X.; Song, W.W.; Wang, X.Y.; Sun, T.M.; Zhu, J.L.; Wang, J. AIE-active Schiff base compounds as fluorescent probe for the highly sensitive and selective detection of Al3+ ions. J. Lumin. 2021, 233, 117911. [Google Scholar] [CrossRef]
- Kan, C.; Shao, X.T.; Wu, L.Y.; Zhang, Y.; Bao, X.F.; Zhu, J. A novel “OFF-ON-OFF” fluorescence chemosensor for hypersensitive detection and bioimaging of Al(Ⅲ) in living organisms and natural water environment. J. Photochem. Photobiol. A 2020, 398, 112618. [Google Scholar] [CrossRef]
- Zhu, G.H.; Huang, Y.; Wang, C.; Lu, L.X.; Sun, T.M.; Wang, M.; Tang, Y.F.; Shan, D.D.; Wen, S.J.; Zhu, J.L. A novel coumarin-based fluorescence chemosensor for Al3+ and its application in cell imaging. Spectrochim. Acta Part A 2019, 210, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.N.; Han, Y.J.; Lin, N.; Liu, H.H. Two pyridine-derived Schiff-bases as turn-on fluorescent sensor for detection of aluminium ion. Opt. Mater. 2019, 95, 109210. [Google Scholar] [CrossRef]
- Zhou, L.B.; Tan, Y.H.; Huang, L.M.; Fortin, C.; Campbell, P.G.C. Aluminum effects on marine phytoplankton: Implications for a revised iron hypothesis (iron–aluminum hypothesis). Biogeochem. 2018, 139, 123–137. [Google Scholar] [CrossRef]
- Farhat, S.M.; Mahboo, A.; Iqbal, G.; Ahmed, T. Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol. Trace Elem. Res. 2017, 177, 115–121. [Google Scholar] [CrossRef]
- Virk, S.A.; Eslick, G.D. Meta-analysis of antacid use and Alzheimer’s disease implications for the aluminum hypothesis. Epidemiology 2015, 26, 769–773. [Google Scholar] [CrossRef]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Parkinson’s disease: Mechanisms and biochemical processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martín, Á.; Palacios, R.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Miguel, M.; et al. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: A concerted action of NAD(P)H oxidase and COX-2. Toxicology 2017, 390, 10–21. [Google Scholar] [CrossRef]
- Wang, P.F.; Liu, L.J.; Meng, F.D.; Khan, M.A.; Li, H. “Turn-On” fluorescent biosensors for high selective and sensitive detection of Al3+ ion. Front. Chem. 2020, 8, 607614. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wang, F.X.; Mu, S.Y.; Sun, X.; Li, Q.Z.; Xie, C.Z.; Liu, H.B. Highly selective and sensitive chemosensor for Al(III) based on isoquinoline Schiff base. Spectrochim. Acta Part A 2020, 243, 118754. [Google Scholar] [CrossRef] [PubMed]
- Coedo, A.G.; Dorado, T.; Padilla, I.; Farinas, J.C. Study of heterogeneities in steels and determination of soluble and total aluminium and titanium concentration by laser ablation inductively coupled plasma mass spectrometry. Talanta 2007, 71, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.K.; Cheung, N.H. Minimally destructive and multi-element analysis of aluminium alloys by ArF laser-induced atomic emissions. J. Anal. At. Spectrom. 2007, 22, 292–297. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zhang, Y.H.; Chen, Y.H.; Sun, T.M.; Tang, Y.F.; Huang, Y.; Yang, Q.Q.; Ma, D.Y.; Wang, Y.P.; Wang, M. A Schiff base fluorescence probe for highly selective turn-on recognition of Zn2+. Tetrahedron Lett. 2017, 58, 365–370. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, L.; Sun, C.Y.; Li, W.J.; Chang, Z.D.; Qi, D.D. A new fluorescent-colorimetric chemosensor based on a Schiff base for detecting Cr3+, Cu2+, Fe3+ and Al3+ ions. Spectrochim. Acta Part A 2019, 214, 7–13. [Google Scholar] [CrossRef]
- Bai, X.; Yan, J.; Qin, J.C.; Yang, Z.Y. A multi-ion fluorescent probe for Mg2+/Zn2+ based on a novel chromone-dendron Schiff base. Inorg. Chim. Acta 2018, 474, 44–50. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mergu, N.; Kumawat, L.K.; Singh, A.K. Selective naked-eye detection of magnesium (II) ions using a coumarin-derived fluorescent probe. Sens. Actuators B 2015, 207, 216–223. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, T.; Sun, C.Y.; Li, W.J.; Chang, Z.D. A Schiff base based fluorescent probe for the quick detection of ClO− ions. Can. J. Chem. 2020, 98, 403–407. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 2014, 43, 1963–1981. [Google Scholar] [CrossRef]
- Yang, T.X.; Zuo, Y.J.; Zhang, Y.; Gou, Z.M.; Lin, W.Y. Novel polysiloxane-based rhodamine B fluorescent probe for selectively detection of Al3+ and its application in living-cell and zebrafish imaging. Spectrochim. Acta Part A 2019, 216, 207–213. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Niu, W.J.; Lin, Z.Q.; Cui, Y.H.X.; Li, Y.J. A novel “turn-off” fluorescent sensor for Al3+ detection based on quinolinecarboxamide-coumarin. Inorg. Chem. Commun. 2020, 121, 108168. [Google Scholar] [CrossRef]
- Kang, L.; Xing, Z.Y.; Ma, X.Y.; Liu, Y.T.; Zhang, Y. A highly selective colorimetric and fluorescent turn-on chemosensor for Al3+ based on naphthalimide derivative. Spectrochim. Acta Part A 2016, 167, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.P.; Liu, X.Y.; Wang, P.; Fu, J.X.; Yao, K.; Xu, K.X. A new fluorescent probe based on quinoline for detection of Al3+ and Fe3+ with “off–on–off” response in aqueous solution. RSC Adv. 2016, 6, 99933–99939. [Google Scholar] [CrossRef]
- Fu, J.X.; Li, B.; Mei, H.H.; Chang, Y.X.; Xu, K.X. Fluorescent schiff base probes for sequential detection of Al3+and F– and cell imaging applications. Spectrochim. Acta Part A 2020, 227, 117978. [Google Scholar] [CrossRef] [PubMed]
- Kolcu, F.; Kaya, İ. Carbazole-based Schiff base: A sensitive fluorescent ‘turn-on’ chemosensor for recognition of Al(III) ions in aqueous-alcohol media. Arabian J. Chem. 2022, 15, 103935. [Google Scholar] [CrossRef]
- Mohanty, P.; Behura, R.; Bhardwaj, V.; Dash, P.P.; Sahoo, S.K.; Jali, B.R. Recent advancement on chromo-fluorogenic sensing of aluminum(III) with Schiff bases. Trends Environ. Anal. Chem. 2022, 34, e00166. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Wang, J.F.; Niamnont, N.; Liu, B.; McDonald, L.; Pang, Y.; Sukwattanasinitt, M. Novel turn-on fluorescent sensors with mega stoke shifts for dual detection of Al3+ and Zn. ACS Sens. 2015, 1, 144–150. [Google Scholar] [CrossRef]
- Bharali, B.; Talukdar, H.; Phukan, P.; Das, D.K. A Nnew Schiff Base based fluorescent sensor for Al(III) based on 2-hydroxyacetophenone and o-phenylenediamine. J. Fluoresc. 2020, 30, 751–757. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.C.; Li, J.X.; Sun, C.Y.; Li, W.J.; Chang, Z.D.; Qi, D.D. Two Schiff-base fluorescent-colorimetric probes based on naphthaldehyde and aminobenzoic acid for selective detection of Al3+, Fe3+ and Cu2+ ions. J. Mol. Struct. 2022, 1255, 132431. [Google Scholar] [CrossRef]
- Shi, Z.C.; Zhao, Z.G. Microwave irradiation synthesis of novel indole triazole Schiff base fluorescent probe for Al3+ ion. Inorg. Chim. Acta. 2019, 498, 119135. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, L.X.; Bai, L.P.; Tian, S.H.; Zhang, L.T.; Huang, X.Y.; Zhu, Y.; Tao, F.R.; Wang, L.P.; Li, G. Water-soluble fluorescent chemosensor based on Schiff base derivative terminated PEG for highly efficient detection of Al3+ in pure aqueous media. Tetrahedron Lett. 2020, 61, 152335. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lim, C.; Suh, H.; Song, E.J.; Kim, C. A multifunctional sensor: Chromogenic sensing for Mn2+ and fluorescent sensing for Zn2+ and Al3+. Sens. Actuators B Chem. 2014, 201, 535–544. [Google Scholar] [CrossRef]
- Ding, W.H.; Cao, W.; Zheng, X.J.; Ding, W.J.; Qiao, J.P.; Jin, L.P. A tetrazole-based fluorescence "turn-on" sensor for Al(III) and Zn(II) ions and its application in bioimaging. Dalton Trans. 2014, 43, 6429–6435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, C.H.; Wu, A.T. A turn-on and reversible fluorescence sensor for Al3+ ion. Analyst 2012, 137, 5201–5203. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Gupta, N.; Singh, A.K. Fluorescent sensor for Al3+ ion in partially aqueous media using julolidine based probe. New J. Chem. 2016, 40, 7536–7541. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Xu, N.Z.; Zhang, M.Q.; Wu, G.Z.; Yao, C. A colorimetric and ratiometric fluorescent chemosensor based on furan-pyrene for selective and sensitive sensing Al3+. Chin. Chem. Lett. 2016, 27, 1673–1678. [Google Scholar] [CrossRef]
- Sen, S.; Mukherjee, T.; Chattopadhyay, B.; Moirangthem, A.; Basu, A.; Marek, J.; Chattopadhyay, P. A water soluble Al3+ selective colorimetric and fluorescent turn-on chemosensor and its application in living cell imaging. Analyst 2012, 137, 3975–3981. [Google Scholar] [CrossRef]
- Jeyanthi, D.; Iniya, M.; Krishnaveni, K.; Chellappa, D. A ratiometric fluorescent sensor for selective recognition of Al3+ ions based on a simple benzimidazole platform. RSC Adv. 2013, 3, 20984–20989. [Google Scholar] [CrossRef]
- Neupane, L.N.; Mehta, P.K.; Oh, S.; Park, S.H.; Lee, K.H. Ratiometric red-emission fluorescence detection of Al3+ in pure aqueous solution and live cells by a fluorescent peptidyl probe using aggregation-induced emission. Analyst 2018, 143, 5285–5294. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Zhang, C.J.; Zhang, X.; Xing, S.; Yao, J.S.; Qiao, C.D.; Liu, Q.A. A colorimetric, ultraviolet absorption and fluorescence three-signal probe based on biscarbazole for Al3+ detection and the application in cell imaging. J. Mol. Struct. 2019, 1188, 14–22. [Google Scholar] [CrossRef]
- Mu, Y.L.; Zhang, C.J.; Gao, Z.L.; Zhang, X.; Lu, Q.; Yao, J.S.; Xing, S. A highly selective colorimetric, absorption and fluorescence probe for Al3+ detection based on a new Schiff base compound. Synth. Met. 2020, 262, 116334. [Google Scholar] [CrossRef]
- Gamov, G.A.; Khodov, I.A.; Belov, K.V.; Zavalishin, M.N.; Kiselev, A.N.; Usacheva, T.R.; Sharnin, V.A. Spatial structure, thermodynamics and kinetics of formation in solution of hydrazones derived from pyridoxal 5′-phosphate and 2-furoic, thiophene-2-carboxylic hydrazides. J. Mol. Struct. 2019, 283, 825–833. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Pogonin, A.E.; Savenkova, M.A.; Gamov, G.A. Spatial structure and conformations of hydrazones derived from pyridoxal 50-phosphate and 2-, 3-pyridinecarbohydrazide in the light of NMR study and quantum chemical calculations. J. Mol. Struct. 2021, 342, 117372. [Google Scholar]
- Gamov, G.A.; Zavalishin, M.N.; Petrova, M.V.; Khodov, I.A.; Gashnikova, A.V.; Kiselev, A.N.; Sharnin, V.A. Interaction of pyridoxal-derived hydrazones with anions and Co2+, Co3+, Ni2+, Zn2+ cations. Phys. Chem. Liq. 2021, 59, 666–678. [Google Scholar] [CrossRef]



| Structure of Probes | Tested Media | LOD | Modes (Probe: Al) | Ref. |
|---|---|---|---|---|
 | DMF | 6.03 × 10−6 | 1:1 | [32] |
 | DMSO | 5.86 × 10−6 | 1:1 | [33] |
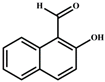 | EtOH/H2O | 3.28 × 10−6 | 1:1 | [34] |
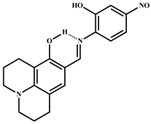 | MeOH/H2O | 1.5 × 10−6 | 1:1 | [35] |
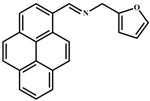 | EDTA | 1.04 × 10−6 | 2:1 | [36] |
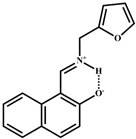 | DMSO/H2O | 6.03 × 10−7 | 1:1 | [37] |
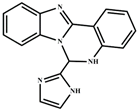 | DMSO | 5.3 × 10−7 | 1:1 | [38] |
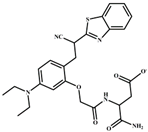 | CH3CN/H2O | 1.45 × 10−7 | 1:1 | [39] |
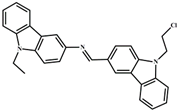 | EtOH | 7.4 × 10−8 | 1:1 | [40] |
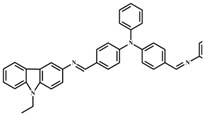 | EtOH | 6.3 × 10−8 | 2:1 | [41] |
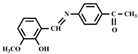 | DMSO/H2O | 1.98 × 10−8 | 2:1 | This work |
 | 4.79 × 10−8 | 2:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Meng, D.; Liu, J.; Duan, S.; Fan, J.; Gao, L.; Long, X. Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al3+ Ions. Molecules 2023, 28, 3090. https://doi.org/10.3390/molecules28073090
Pang Y, Meng D, Liu J, Duan S, Fan J, Gao L, Long X. Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al3+ Ions. Molecules. 2023; 28(7):3090. https://doi.org/10.3390/molecules28073090
Chicago/Turabian StylePang, Yanling, Desu Meng, Jian Liu, Shengxia Duan, Jingru Fan, Longyu Gao, and Xinshu Long. 2023. "Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al3+ Ions" Molecules 28, no. 7: 3090. https://doi.org/10.3390/molecules28073090
APA StylePang, Y., Meng, D., Liu, J., Duan, S., Fan, J., Gao, L., & Long, X. (2023). Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al3+ Ions. Molecules, 28(7), 3090. https://doi.org/10.3390/molecules28073090





